Abstract
Introduction
No evidence-based guidelines are available to determine the appropriate stem length, and whether or not to cement stems in revision total knee arthroplasty (TKA). Therefore, the objective of this study was to compare stresses and relative movement of cemented and uncemented stems of different lengths using a finite element analysis.
Materials and methods
A finite element model was created for a synthetic tibia. Two stem lengths (95 and 160 mm) and two types of fixation (cemented or press fit) of a hinged TKA were examined. The average compressive stress distribution in different regions of interest, as well as implant micromotions, was determined and compared during lunge and squat motor tasks.
Results
Both long and short stems in revision TKA lead to high stresses, primarily in the region around the stem tip. The presence of cement reduces the stresses in the bone in every region along the stem. Short stem configurations are less affected by the presence of cement than the long stem configuration. Press-fit stems showed higher micromotions compared to cemented stems.
Conclusions
Lowest stresses and micromotion were found for long cemented stems. Cementless stems showed more micromotion and increased stress levels especially at the level of the stem tip, which may explain the clinical phenomenon of stem-end pain following revision knee arthroplasty. These findings will help the surgeon with optimal individual implant choice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Revision TKA is necessary within 10–15 years in approximately 5–10 % of patients who underwent primary TKA, in increasing numbers due to an aging and active population [1, 2]. The demand for primary TKAs in the USA is projected to grow by 673 % between 2005 and 2030 to reach 3.48 million procedures in 2030 by some authors. TKA revisions in the USA are projected to grow from 38.300 in 2005 by 601 % to 268.000 in 2030, in a rather epidemic scenario [3]. The logical consequence predicts a comparable increase of re-revisions with continuously more challenging cases. Patients undergoing TKA today are quite demanding: having 20 % higher body weight, being physically more active and living longer than patients several decades ago [4]. This leads to a higher likelihood of later revision operations. However, the clinical outcome of revision TKA has not reached that of primary TKA. This is due to often poor bone stock, significant condylar bone defects and frequent ligament instability. In addition, surgical factors including fixation technique, level of prosthesis constraint, restoration of limb alignment [5] and choice of revision prosthesis must be considered [6]. In cases of several revisions, the bone loss is getting higher as well as the amount of ligamentous instability due to soft tissue degradation. This leads the surgeon to an implantation of a hinged revision arthroplasty.
Stemmed arthroplasty contributes to correct alignment and stability by better stress distribution [7–9]. Care should be taken to avoid excessive stress on a metaphysis with poor bone stock by distributing some of the load to the diaphysis of the tibia and femur [10]. To reach this goal, the surgeon can choose between different stem lengths and diameters. Stems can also be cemented (and augmented with antibiotics) or left uncemented. Relevant factors in these decisions include stress shielding, osteolysis, stem-end pain, implant wear, periprosthetic fracture and aseptic as well as septic loosening [10–13].
However, no evidence-based guidelines are available to standardize stem length and whether or not to use cement. With uncemented stems implant removal is supposed to be easier in revision scenarios and major cement reactions should be avoided [14]. However, an initial rigid bond between the prosthesis and bone has been considered a prerequisite for long-term success of a revision TKA [2, 12, 15]. Thus, the main advantage of cemented stems is a fast and good fixation of the meta- and diaphysis and good results in long-term studies [16]. Cementing is commonly recommended in patients with low bone quality or altered anatomy [17]. Due to several downsides of cementing including possible lethal allergic reactions, longer surgical time and demanding cement resection if revision is needed, the use of press-fit stems is increasing in recent years, despite several long-term studies showing reliable and durable results for cemented stems, even in huge bony defects [18].
To evaluate and compare the effect of the fixation technique (cemented or press-fit) and stem length (short or long) in a tibial bone with a hinged TKA, a three-dimensional numerical model was developed, combining rigid body kinematics simulation and finite element analysis (FEA). Finite element analysis enables detailed biomechanical investigations with the potential to detect effects of the different configurations on the bone that cannot be investigated in vivo, in cadaveric bones or by means of clinical studies [19–22]. The stress distribution in several regions of the tibial bone and the micromotions between the implant and bone during lunging and squatting were determined and compared among the different investigated configurations.
The intent of this study was to produce data that could assist the clinical decision on stem length and whether or not to cement a tibial stem in revision hinged TKA. It was hypothesized, that long cemented stems would deliver lowest micromotion and favorable stress distribution compared to shorter and uncemented stems.
Materials and methods
Geometry
A physiological three-dimensional tibial bone model was created from computer tomography images of a left, fourth generation composite tibia, size medium (# 3401, Sawbones, Pacific Research Laboratories Inc., Malmo, Sweden). Such bone models are widely used for numerical and experimental tests [19, 22–26]. The tibial bone model consists of three parts: cortical bone, cancellous bone and the intramedullary canal. An RT-PLUS Modular Rotating Hinge TKA (Smith & Nephew, Memphis, TN, USA) was considered for the numerical analysis. According to the dimensions of the tibial bone model, a size 6, left tibial tray with an 8 mm polyethylene insert was selected. Four different configurations were considered for analysis. Two stem lengths (95 and 160 mm) and two types of fixation (cemented or press-fit) were examined. The polyethylene insert size and the tibial tray size remained the same in all configurations. A review of the main geometric characteristics of the four configurations is shown in Table 1. The correct choice of the TKA components size was confirmed by an experiment in which an actual implant was placed in the real mechanical-equivalent synthetic tibia by an experienced orthopedic surgeon.
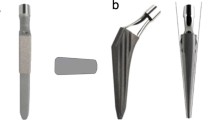
Each configuration was virtually inserted into the tibia following the manufacturer’s surgical technique in perfect positioning and rotation of the implant. To ream the intramedullary canal, numerical models of the surgical rasps were virtually created, to exactly reproduce all the steps of the actual procedure.
To define the stem position in the numerical models, cortex engagement of three stem teeth was accepted in the press-fit implants (Fig. 1a). In the cemented case, the stem was placed exactly in the center of the intramedullary canal surrounded by a homogeneous cement mantle (Fig. 1b). The cement mantle was defined by filling the previously reamed hole and subtracting the stem volume from that, simulating perfect cementing technique. The presence of cement was considered at the level of the bone cut with a penetration of 3 mm in agreement with literature [25].
Material properties
Implant materials (UHMWPE, Cement, CoCrMo and Titanium) and cancellous bone were assumed to be homogeneous, isotropic and linearly elastic according to prior studies [27–30], while the cortical bone was considered as transversely isotropic [22, 28], with properties varying along the mechanical axis of the tibia. The values of the material properties used for the model are shown in Table 2.
The coefficient of friction between the ultrahigh molecular weight polyethylene (UHMWPE) insert and the tibial tray was set at 0.05, between the titanium stem (press fit) and bone at 0.6, between the CoCrMo (tibial tray and cemented stem) and bone at 0.2 and between the CoCrMo and cement 0.25 [31].
Analysis of rigid body kinematics
Lunge and squat movements were reproduced numerically using a validated musculoskeletal modeling software (LifeMOD/KneeSIM 2008.1.0, LifeModeler Inc., San Clemente, CA, USA) [32–34]. A validated numerical model for contact forces [32, 35] as well as for reproduction of the kinematics was used [36, 10]. The development of the model followed strictly in all steps, from geometry definition, material, models and properties as well as load and boundary conditions with the mesh the previous model and the experimental validated model described earlier [37].
The program replicates an existing knee kinematics rig with regard to geometry, constraints, input and output (Fig. 2). Both movements were implemented with a range of flexion of 0° to 120°. A constant vertical hip load of 200 N was applied during the motor task. According to the Grood and Suntay convention [38], internal rotation and adduction at the maximum flexion angle (120.0°) were, respectively, 1.3° and 3.2°. All other settings and hardware parameters necessary to define rigid body kinematics were based upon prior studies [32, 36, 39, 40]. Medial and lateral tibiofemoral contact forces, patellar tendon forces and knee kinematics during both activities were extracted during the entire motion. Maximum force values obtained from the rigid body models are presented below (Table 3).
Finite element analysis
For ensuring that the numerical model of the bone has the same geometry of the bone that will be used in the experimental tests, a CT scan of the left fourth generation composite tibia, size medium (Pacific Research Laboratories) as template for the further studies was performed. For both the numerical rigid body kinematics and the FEA, the same TKA system, an RT-PLUS Modular Rotating Hinge TKA (Smith & Nephew, Memphis, TN, USA) [32], was used (Fig. 2). The full FEA model used for this study and a zoom of the proximal tibial part are presented in Fig. 3a, b.
Each model was meshed using tetragonal elements with an approximate element size of 4 mm. A refinement of the mesh, with an approximate element size of 0.5–1 mm, was performed in the contact area of the bone with the TKA stem. Abaqus/Standard version 6.10-1 (Dassault Systèmes, Vélizy-Villacoublay, France) was used to develop the models and perform all finite element simulations.
Element size was chosen based on a convergence test to make sure that the selected mesh did not influence the result. No relative movements were considered between the cement mantle and the bone. The maximum forces determined by the rigid body kinematic analysis were applied on the FE models. Therefore, we defined a coordinate system using the mechanical axis of femur. The planes were defined as follows:
-
Frontal plane: plane containing the mechanical axis and parallel to the line joining the medial and lateral tibial condylar center.
-
Horizontal axis: the perpendicular line to the mechanical axis in the frontal plane, passing through the center of the tibial knee center.
-
Horizontal plane: perpendicular to the frontal plane and containing the tibial horizontal axis.
-
Sagittal axis: the line perpendicular to the mechanical axis and to the horizontal axis, passing through the tibial knee center.
For all four models we used the same references of the LifeMOD model. Load values and application points were defined by that coordinate system which is fixed to the polyethylene insert. For defining load values, kinematics of the knee during motions and TF contact area, we used the loading frame with strain gauge and the musculoskeletal modeling software (LifeMOD/KneeSIM 1.0, LifeModeler Inc. The values were extracted using previously validated models [32, 36].
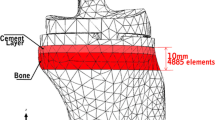
Patellar tendon force was applied on the tibial tubercle. The tibia was fully constrained at its distal part. Twenty regions of interest (ROI) were identified in the tibia. These ROIs were obtained by subdividing the cortical region of the tibia into regions of 10 mm thickness each. Each region is defined cutting the cortical bone with planes perpendicular to the mechanical axis of the tibia. The most interesting regions of the cortical bone were near the stem and under the tibial tray. For all models, each regions of interest had a width of 15 mm. Average compressive stresses were computed for each ROI and compared among the different configurations. The relative motion between the implant and bone was also computed and compared. The reliability of this method is proven in current literature [20, 22, 32, 37].
Results
For both the squatting and the lunge movement, the average compressive stress in each ROI was extracted and reported (Figs. 4, 5). In general, it increases along the stem with growing distance from the baseplate.
For each movement, the most stressed ROIs were situated around the stem tips, marked with two vertical black lines (Figs. 4, 5). The maximal average compressive stress was highest for the press fit long stem configuration (squat 18.2 MPa; lunge 17.7 MPa) and lowest for the cemented long stem configuration (squat 11.5 MPa; lunge 10.1 MPa). Also, for the short stems, the press-fit configuration showed a higher average compressive stress (squat 13.3 MPa; lunge 14.6 MPa) compared to the cemented configuration (squat 9.5 MPa; lunge 10.4 MPa) in the region situated around the stem tips. As visible on Figs. 4 and 5 for the short stems, the stress continues to rise underneath the tip and reaches a maximum value a little bit lower. Biomechanically and from the clinical perspective, the real maximal values of the stresses are relevant (short press fit: squat 15.4 MPa, lunge 16.4 MPa; short cemented: squat 15.6 MPa, lunge 16.6 MPa), because this is what the bone experiences. However, cemented and press-fit short stems showed similar maximal stresses below the stem tip.
For both movements, the presence of cement reduced the average compressive stresses along the bone–cement interface, compared to the press-fit configuration. Different activities induce the same stress distribution pattern with the load mainly situated at the stem tip. Both long and short stem configurations induce stress peaks in the region around the stem tip. The presence of cement reduces the stresses induced in every region along the stem, with a bigger difference in the region close to the stem tip. Short stem configurations are less affected by the presence of cement than the long stem configuration.
Von Mises stresses at the interface between the cortical bone and tibial tray are shown graphically in Figs. 6 and 7. The values of the stress are indicated by colors, with red for high and blue for low values, respectively. Numerical values are shown in Fig. 8. In cemented cases, different loads and stem lengths induce the same stress on the interface. Press-fit implants induce higher stresses than cemented implants; a higher difference is observed with the short stem, where the interaction area between stem and bone is smaller. There was no relevant difference found for the different movement activities.
The micromotions between the implant and the bone in the region of the tibial tray are shown in Fig. 8. Micromotions were considered as the total displacement of the tibial tray in the resection plane with respect to the tibial bone (Figs. 9 and 10). In the press-fit models, micromotions at the tray can be more than twice the value in the cemented models (long cemented: ~40 µm vs long press fit: ~100 µm).
Subsidence of the stem tip along the mechanical axis was also evaluated (Fig. 10). No notable differences were induced by different motions. The subsidence in the press-fit models is twice as large as in the cemented models [from ~30 to ~70 µm (long) and from ~40 to ~80 µm (short)].
Discussion
The hypothesis that long cemented stems would deliver lowest micromotion and favorable stress distribution compared to shorter and uncemented stems was proven correct. To date, there are hardly any evidence-based criteria to determine the appropriate stem length and whether or not to use cement in revision TKA. These data will support the surgeons’ decision making for the choice of stem length and fixation technique. The evaluation of micromotions and stresses in different regions of the tibia after revision TKA is difficult in in vivo as well as cadaveric models. These variables seem to be best determined by finite element analysis (FEA) with standardized sawbones having defined material properties for cortical and spongious bone. In summary, short cementless stems induce higher stresses in the tibia underneath the tibial tray and along the stem compared to long cemented stems. Cemented stems show less interface stress and micromotion compared to cementless stems.
Several clinical studies report on cementing of stems in TKA with excellent long-term results. Good clinical and biomechanical results have also been achieved when cementing a tibial stem during revision surgery, particularly where bone quality is poor [1, 13]. The advantages of cementing described are an increase of the contact area between the stem and the bone, better centralization of the stem in the tibial canal and the possibility of adding topical antibiotics in revision situations [41]. Other studies report that the cemented interface may break down gradually with time because of cyclic loading, producing component failure [42]. Other disadvantages of cemented stems are potentially lethal microembolism and allergic reactions, as well as difficulties encountered in cement removal and bone stock loss if subsequent surgery is required.
Most publications dealing with non-cemented stems present good mid-term but no long-term results [43]. Some authors report challenges in achieving a real press-fit fixation due to altered anatomy. This may lead to compromises in correct implant placement and may contribute to early loosening in former studies due to limited availability of modular implants [44, 45]. It is crucial to select the thickest possible stem in these modular systems, with the goal of achieving the best press-fit situations [46]. Several authors state that due to short- and middle-term studies in most modern press-fit tibial stems, cementing is not necessary [14, 41, 47, 48]. This may be also due to the prosthetic design as constrained implants have other biomechanical properties than hinged TKAs.
Micromotion and loosening of stems
The biomechanical effects of long intramedullary stems in revision TKA have been studied extensively [10, 11, 41, 49]. Rawlinson et al. carried out cadaveric tests and finite element (FE) analyses on nine paired tibiae to compare stemmed and unstemmed tibial trays. It was seen in the FE analysis that a stem reduced the stresses and strains in the bone beneath the tibial tray. However, due to the biological variability between specimens, results remained highly variable and the effect of the stem inconclusive [50]. Several studies indicate that stems significantly enhance fixation in the presence of large bone loss [51, 52] and that a long stem contributes to correct alignment and stability due to better stress distribution [7–9, 53]. Albrektsson et al. hypothesized that a long central stem would ‘guide’ migration predominantly along the vertical axis, thereby minimizing the risk of recurrent malalignment and loosening due to tilting [35].
Radiolucent lines correlated with higher micromotion between the prosthesis–bone interface and were found in 65 % of tibias in 123 cemented TKAs at 4.5 years [54]. There was no significant correlation between thin radiolucent lines and clinical outcome. Lines >2 mm were associated with poor results. Whiteside noted global sclerotic lines in 52 of 56 press-fit stemmed tibial components in revision TKA at 2 years [47]. While these lines were presumed to indicate micromotion, a significant loosening rate was not observed. In an RSA analysis, Albrektsson et al. showed an association of micromotion with time from surgery in press-fit stems. After 6 months, migration and micromotion were negligible for the stemmed press-fit implant. Continuous migration after 2 years seems to correlate with malalignment, which leads to bad clinical results [54, 55].
Completo et al. compared the load sharing of the cortical rim, the cancellous bone and the stem, as well as the stability of the cement–bone interface below the tibial components in cemented and press-fit stems in an FEA [18]. The press-fit stem reduced the relative motion by 19 % and the cemented stems by 23 % compared to a standard model without stem. In addition, Jazrawi et al. described a significant decrease in motion of the tibial tray with increasing press-fit stem length and increasing stem diameter in a cadaveric study [56]. Arbitrarily, Stern et al. showed in 30 cadaveric tibias that longer stems were associated with increased micromotion, especially under eccentric loading. Cemented implants seemed to have more stable fixation, compared with press-fit implants [52, 57]. Jazrawi et al. reported that cemented tibial stems showed significantly less tray motion than uncemented stems. The short cemented stems produced tray stability equivalent to long press-fit stems.
This agrees with our results which show the lowest micromotions between implants and bone measured on the tibial base plate and at the stem tip for the long cemented configuration in comparison to the highest values in the short press-fit stems (up to 50 % more). Comparing our measured values of micromotions with literature, press-fit implants (long and short) show micromotions up to 120 µm due to softer cancellous bone in the metaphyseal area. This may result in considerable fibrous tissue formation in the contact area as mentioned above.
Stress shielding
Reilly et al. showed that a long cemented stem results in significant stress shielding of the proximal tibia with direct load transfer to the cortex at the tip of the stem [11]. They considered this stress shielding due to the ability of cemented tibial stems to carry up to 60 % of the axial load, which could be useful to unload a structurally compromised proximal tibia with significant bone defects. Furthermore, Brooks et al. pointed out the potential long-term risk of stress shielding such as cortical atrophy and tibial fracture at the tip of the stem and favored the use of tibial components that have either no stems or only short intramedullary stems [58]. In summary, cemented stems seem to reduce proximal stress, which is useful in considerable proximal bony defects but may result in proximal bone resorption in intact bones, contributing to tibial component loosening. However, a certain degree of proximal stress seems to be necessary to prevent bone loss, but should not be too high to result in loosening or fracture.
A better stress distribution in the tibial bone in cemented stems is also shown in our graphical evaluation of the stresses, computed directly below the tibial plateau. In both movements, the cemented stems showed higher stresses in that region than the press-fit implants, which seem to load more or less completely on the distal stem. These results suggest that the stress distribution in cemented stems is better divided over the longitudinal axis of the stem and the tibial bone.
Contact pressure and stem-end pain
Barrak et al. found that diaphyseal pain is present in 14 % of patients with press-fit tibial stems and in 19 % of patients with cemented tibial stems [12]. This coincides with the region of stem-end pain described in previous clinical and radiographical studies [12, 52, 59]. Several authors explain this pain as related to the load transfer or contact pressure between the implant and bone surface, [12, 52, 59]. Kim et al. found in an FEA after revision TKA that a longer stem length, larger stem diameter and stronger press fit increased peak contact pressures [60]. The contact pressure modeled in the present study was highest in the stem-end regions. There was an obvious difference in the peak pressure values at the stem-end in different fixation techniques. The cemented short and long stems had lower values suggesting a lower risk for stem-end pain. The higher peak in the long stemmed trial could be explained by the smaller intramedullary canal distally and the higher pressure acting on the smaller area. Kim et al. also suggested that the proximal cancellous bone surrounding short stems has a lower stiffness than the distal cortical bone when a longer stem was used [60].
The axial displacement of the stem tip was related to the fixation technique showing higher displacement for press-fit stems. A possible explanation is the improved stress distribution in the cemented tibiae, loading the cortical bone in a larger area than a focal stress of a press-fit stem, moving into the metaphyseal area and touching the cortical bone. No relevant differences were found in movements such as lunging or squatting. The present findings are contrary to those of Barrak [59]. Cemented stems show in the FEA lower stress values in the tip area, which would lead to less stem-end pain in these patients.
Several authors observed that the design of the tip of the stem is the most important factor in stem-end pain [12, 52, 59]. In a radiographic analysis, it was shown that a slot shape at the stem tip could reduce the velocity of loosening and stem-end pain due to much smaller peak contact pressures in designs with slots [59]. The implant investigated in this study is also designed with such a slot in the tip of the tibial stem. Comparative evaluations with other implants were not made.
Concerning the loads on the tibial tray, recent in vivo data of implants allowing measurements of contact forces by radio frequency showed higher forces on the medial side of the tibial tray [61]. This is opposite to our results, possibly due to a different implant design and a central pin used in our study of a rotating hinge arthroplasty.
Limitations
There were several limitations to this study. Heterogeneity of the cortical and cancellous bones, which could affect the stress results, were not considered in our model. Moreover, large bone defects or poor bone stock due to osteoporosis was not taken into consideration. In addition, only squatting and lunging were considered, despite the various loading situations (load pattern, load location and bone or implant condylar surface geometry) in the knee joint. FEA does not account for bony ingrowth and age of patients; it simulates the situation in the early phase following revision TKA. Recently discussed variations in the amount of anatomic tibial slope between racial groups may also affect results and is not taken into account in this study [62].
In actual surgery, it may be difficult to place a fully press-fit stem into the tibia due to an eccentric canal with respect to the tibial plateau. Some surgeons may compromise the position of the tibial tray to obtain a truly press-fit stem if modularity of the system is not forgiving enough. Furthermore, optimal cementing of the stem is not always achieved in real surgery. However, analysis of the data in this study presumed optimal press-fit placement and optimal (4th generation) cementing technique.
Conclusion
In case of cemented stems, both long and short stems offer good stability with little micromotion and acceptable stress levels in the bone. Press-fit stems show more micromotion, higher stress levels and more stress peaks in the bone. This should be considered in the clinical decision process when performing revision TKA.
References
Murray PB, Rand JA, Hanssen AD (1994) Cemented long-stem revision total knee arthroplasty. Clin Orthop Relat Res 309:116–123
Rand JA, Trousdale RT, Ilstrup DM, Harmsen WS (2003) Factors affecting the durability of primary total knee prostheses. J Bone Joint Surg Am 85-A(2):259–265
Kurtz S, Ong K, Lau E, Mowat F, Halpern M (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89(4):780–785
Crowninshield RD, Rosenberg AG, Sporer SM (2006) Changing demographics of patients with total joint replacement. Clin Orthop Relat Res 443:266–272
Bieger R, Huch K, Kocak S, Jung S, Reichel H, Kappe T (2014) The influence of joint line restoration on the results of revision total knee arthroplasty: comparison between distance and ratio-methods. Arch Orthop Trauma Surg 134(4):537–541
Insall JN, Dethmers DA (1982) Revision of total knee arthroplasty. Clin Orthop Relat Res 170:123–130
Conditt MA, Parsley BS, Alexander JW, Doherty SD, Noble PC (2004) The optimal strategy for stable tibial fixation in revision total knee arthroplasty. J Arthroplasty 19(7 Suppl 2):113–118
Fehring TK, Odum S, Olekson C, Griffin WL, Mason JB, McCoy TH (2003) Stem fixation in revision total knee arthroplasty: a comparative analysis. Clin Orthop Relat Res 416:217–224
Gofton WT, Tsigaras H, Butler RA, Patterson JJ, Barrack RL, Rorabeck CH (2002) Revision total knee arthroplasty: fixation with modular stems. Clin Orthop Relat Res 404:158–168
Bourne RB, Finlay JB (1986) The influence of tibial component intramedullary stems and implant-cortex contact on the strain distribution of the proximal tibia following total knee arthroplasty. An in vitro study. Clin Orthop Relat Res 208:95–99
Reilly D, Walker PS, Ben-Dov M, Ewald FC (1982) Effects of tibial components on load transfer in the upper tibia. Clin Orthop Relat Res 165:273–282
Barrack RL, Rorabeck C, Burt M, Sawhney J (1999) Pain at the end of the stem after revision total knee arthroplasty. Clin Orthop Relat Res 367:216–225
Lee RW, Volz RG, Sheridan DC (1991) The role of fixation and bone quality on the mechanical stability of tibial knee components. Clin Orthop Relat Res 273:177–183
Haas SB, Insall JN, Montgomery W 3rd, Windsor RE (1995) Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am 77(11):1700–1707
Ducheyne P, Kagan A 2nd, Lacey JA (1978) Failure of total knee arthroplasty due to loosening and deformation of the tibial component. J Bone Joint Surg Am 60(3):384–391
Completo A, Simoes JA, Fonseca F (2009) Revision total knee arthroplasty: the influence of femoral stems in load sharing and stability. Knee 16(4):275–279
Schlegel UJ, Bruckner T, Schneider M, Parsch D, Geiger F, Breusch SJ (2015) Surface or full cementation of the tibial component in total knee arthroplasty: a matched-pair analysis of mid- to long-term results. Arch Orthop Trauma Surg 135(5):703–708
Completo A, Simoes JA, Fonseca F, Oliveira M (2008) The influence of different tibial stem designs in load sharing and stability at the cement-bone interface in revision TKA. Knee 15(3):227–232
Brihault J, Navacchia A, Pianigiani S, Labey L, De Corte R, Pascale V, Innocenti B (2016) All-polyethylene tibial components generate higher stress and micromotions than metal-backed tibial components in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 24:2550–2559
Innocenti B, Truyens E, Labey L, Wong P, Victor J, Bellemans J (2009) Can medio-lateral baseplate position and load sharing induce asymptomatic local bone resorption of the proximal tibia? A finite element study. J Orthop Surg Res 4:26
Soenen M, Baracchi M, De Corte R, Labey L, Innocenti B (2013) Stemmed TKA in a femur with a total hip arthroplasty: is there a safe distance between the stem tips? J Arthroplasty 28(8):1437–1445
Innocenti B, Bellemans J, Catani F (2015) Deviations from optimal alignment in TKA: is there a biomechanical difference between femoral or tibial component alignment? J Arthroplasty 31(1):295–301
Au AG, Raso VJ, Liggins AB, Otto DD, Amirfazli A (2005) A three-dimensional finite element stress analysis for tunnel placement and buttons in anterior cruciate ligament reconstructions. J Biomech 38(4):827–832
Bougherara H, Zdero R, Mahboob Z, Dubov A, Shah S, Schemitsch EH (2010) The biomechanics of a validated finite element model of stress shielding in a novel hybrid total knee replacement. Proc Inst Mech Eng H 224(10):1209–1219
Vanlommel J, Luyckx JP, Labey L, Innocenti B, De Corte R, Bellemans J (2011) Cementing the tibial component in total knee arthroplasty: which technique is the best? J Arthroplasty 26(3):492–496
van de Groes S, de Waal-Malefijt M, Verdonschot N (2013) Probability of mechanical loosening of the femoral component in high flexion total knee arthroplasty can be reduced by rather simple surgical techniques. Knee 21(1):209–215
Godest AC, Beaugonin M, Haug E, Taylor M, Gregson PJ (2002) Simulation of a knee joint replacement during a gait cycle using explicit finite element analysis. J Biomech 35(2):267–275
Heiner AD (2008) Structural properties of fourth-generation composite femurs and tibias. J Biomech 41(15):3282–3284
Oguz Kayabasi FE (2006) Finite element modelling and analysis of a new cemented hip prosthesis. Adv Eng Softw 37:477–483
Wannasri SVP, Ivanova LR, Kornienko LA, Piriyayon S (2009) Increasing wear resistance of UHMWPE by mechanical activation and chemical modification combined with addition of nanofibers. Procedia Eng 1:67–70
Simpson DJ, Little JP, Gray H, Murray DW, Gill HS (2009) Effect of modular neck variation on bone and cement mantle mechanics around a total hip arthroplasty stem. Clin Biomech (Bristol, Avon) 24(3):274–285
Innocenti B, Pianigiani S, Labey L, Victor J, Bellemans J (2011) Contact forces in several TKA designs during squatting: a numerical sensitivity analysis. J Biomech 44(8):1573–1581
Al Nazer R, Rantalainen T, Heinonen A, Sievanen H, Mikkola A (2008) Flexible multibody simulation approach in the analysis of tibial strain during walking. J Biomech 41(5):1036–1043
Innocenti B, Follador M, Salerno M, Bignardi C, Wong P, Labey L (2009) Experimental and numerical analysis of patello-femoral contact mechanics in TKA, vol 22. In: IFMBE proceedings—4th European Conference of the International Federation for Medical and Biological Engineering ECIFMBE 2008, 12 edn, 23–27 November 2008, Antwerp, Springer, Berlin
Albrektsson BE, Ryd L, Carlsson LV, Freeman MA, Herberts P, Regner L, Selvik G (1990) The effect of a stem on the tibial component of knee arthroplasty. A roentgen stereophotogrammetric study of uncemented tibial components in the Freeman-Samuelson knee arthroplasty. J Bone Joint Surg Br 72(2):252–258
Pianigiani S, Chevalier Y, Labey L, Pascale V, Innocenti B (2012) Tibio-femoral kinematics in different total knee arthroplasty designs during a loaded squat: a numerical sensitivity study. J Biomech 45(13):2315–2323
Innocenti B, Bilgen OF, Labey L, van Lenthe GH, Sloten JV, Catani F (2014) Load sharing and ligament strains in balanced, overstuffed and understuffed UKA. A validated finite element analysis. J Arthroplasty 29(7):1491–1498
Grood ES, Suntay WJ (1983) A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng 105(2):136–144
Victor J, Van Doninck D, Labey L, Van Glabbeek F, Parizel P, Bellemans J (2009) A common reference frame for describing rotation of the distal femur: a ct-based kinematic study using cadavers. J Bone Joint Surg Br 91(5):683–690
Victor J, Labey L, Wong P, Innocenti B, Bellemans J (2010) The influence of muscle load on tibiofemoral knee kinematics. J Orthop Res 28(4):419–428
Gustke KA (2004) Cemented tibial stems are not requisite in revision. Orthopedics 27(9):991–992
Santare MH, Keer LM, Lewis JL (1987) Cracks emanating from a fluid filled void loaded in compression: application to the bone-implant interface. J Biomech Eng 109(1):55–59
Shannon BD, Klassen JF, Rand JA, Berry DJ, Trousdale RT (2003) Revision total knee arthroplasty with cemented components and uncemented intramedullary stems. J Arthroplasty 18(7 Suppl 1):27–32
Westrich GH, Haas SB, Insall JN, Frachie A (1995) Resection specimen analysis of proximal tibial anatomy based on 100 total knee arthroplasty specimens. J Arthroplasty 10(1):47–51
Lotke PA, Ecker ML (1977) Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am 59(1):77–79
Vince KG, Long W (1995) Revision knee arthroplasty. The limits of press fit medullary fixation. Clin Orthop Relat Res 317:172–177
Whiteside LA (1993) Cementless revision total knee arthroplasty. Clin Orthop Relat Res 286:160–167
Mow CS, Wiedel JD (1994) Noncemented revision total knee arthroplasty. Clin Orthop Relat Res 309:110–115
Sanguineti F, Mangano T, Formica M, Franchin F (2014) Total knee arthroplasty with rotating-hinge Endo-Model prosthesis: clinical results in complex primary and revision surgery. Arch Orthop Trauma Surg 134(11):1601–1607
Rawlinson JJ, Peters LE, Campbell DA, Windsor R, Wright TM, Bartel DL (2005) Cancellous bone strains indicate efficacy of stem augmentation in constrained condylar knees. Clin Orthop Relat Res 440:107–116
Nazarian DG, Mehta S, Booth RE Jr (2002) A comparison of stemmed and unstemmed components in revision knee arthroplasty. Clin Orthop Relat Res 404:256–262
Stern SH, Wills RD, Gilbert JL (1997) The effect of tibial stem design on component micromotion in knee arthroplasty. Clin Orthop Relat Res 345:44–52
Taylor M, Tanner KE, Freeman MA (1998) Finite element analysis of the implanted proximal tibia: a relationship between the initial cancellous bone stresses and implant migration. J Biomech 31(4):303–310
Ecker ML, Lotke PA, Windsor RE, Cella JP (1987) Long-term results after total condylar knee arthroplasty. Significance of radiolucent lines. Clin Orthop Relat Res 216:151–158
Ryd L, Albrektsson BE, Herberts P, Lindstrand A, Selvik G (1988) Micromotion of noncemented Freeman-Samuelson knee prostheses in gonarthrosis. A roentgen-stereophotogrammetric analysis of eight successful cases. Clin Orthop Relat Res 229:205–212
Jazrawi LM, Bai B, Kummer FJ, Hiebert R, Stuchin SA (2001) The effect of stem modularity and mode of fixation on tibial component stability in revision total knee arthroplasty. J Arthroplasty 16(6):759–767
Luring C, Perlick L, Trepte C, Linhardt O, Perlick C, Plitz W, Grifka J (2006) Micromotion in cemented rotating platform total knee arthroplasty: cemented tibial stem versus hybrid fixation. Arch Orthop Trauma Surg 126(1):45–48
Brooks PJ, Walker PS, Scott RD (1984) Tibial component fixation in deficient tibial bone stock. Clin Orthop Relat Res 184:302–308
Barrack RL, Stanley T, Burt M, Hopkins S (2004) The effect of stem design on end-of-stem pain in revision total knee arthroplasty. J Arthroplasty 19(7 Suppl 2):119–124
Kim YH, Kwon OS, Kim K (2008) Analysis of biomechanical effect of stem-end design in revision TKA using Digital Korean model. Clin Biomech (Bristol, Avon) 23(7):853–858
Bergmann G, Bender A, Dymke J, Duda G, Damm P (2014) Standardized loads acting in hip implants. PLoS One 11(5):e0155612
Shen Y, Li X, Fu X, Wang W (2015) A 3D finite element model to investigate prosthetic interface stresses of different posterior tibial slope. Knee Surg Sports Traumatol Arthrosc 23(11):3330–3336
Acknowledgments
The study was performed in the European Centre for Knee Research, Leuven, Belgium, and funded by Smith and Nephew.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no competing interests in relation to this study from: Nelson Fanciullacci. Bilal Farouk El-Zayat, Thomas J. Heyse and Susanne Fuchs-Winkelmann are paid consultants, speakers and instructors to Smith and Nephew and received research support. Luc Labey and Bernardo Innocenti are former paid employees to Smith and Nephew.
Rights and permissions
About this article
Cite this article
El-Zayat, B.F., Heyse, T.J., Fanciullacci, N. et al. Fixation techniques and stem dimensions in hinged total knee arthroplasty: a finite element study. Arch Orthop Trauma Surg 136, 1741–1752 (2016). https://doi.org/10.1007/s00402-016-2571-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-016-2571-0