Abstract
Purpose
Most total knee arthroplasty tibial components are metal-backed, but an alternative tibial component made entirely of polyethylene (all-polyethylene design) exists. While several clinical studies have shown that all-poly design performs similarly to the metal-backed, the objective of this study is to perform a biomechanical comparison.
Methods
Loads, constraints and geometries during a squat activity at 120° of flexion were obtained from a validated musculoskeletal model and applied to a finite element model. Stresses in the tibia and micromotions at the bone–implant interface were evaluated for several implant configurations: (1) three different thicknesses of the cement penetration under the baseplate (2, 3 and 4 mm), (2) the presence or absence of a cement layer around the stem of the tibial tray and (3) three different bone conditions (physiological, osteopenic and osteoporotic bone).
Results
All-polyethylene tibial components resulted in significantly higher (p < 0.001) and more uneven stress distributions in the cancellous bone under the baseplate (peak difference: +128.4 %) and fivefold increased micromotions (p < 0.001). Performance of both implant designs worsened with poorer bone quality with peaks in stress and micromotion variations of +40.8 and +54.0 %, respectively (p < 0.001). Performance improvements when the stem was cemented were not statistically significant (n.s.).
Conclusion
The metal-backed design showed better biomechanical performance during a squat activity at 120° of flexion compared to the all-polyethylene design. These results should be considered when selecting the appropriate tibial component for a patient, especially in the presence of osteoporotic bone or if intense physical activity is foreseen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total knee arthroplasty (TKA) is a successful procedure with more than 600,000 surgeries performed in the USA each year [21]. Two types of tibial implants can be used today during TKA: a metal-backed modular device or an all-poly tibial implant. Both systems have their advantages and disadvantages. The all-poly solution presents three main benefits in comparison with the metal-backed. First, it eliminates the insert-metal interface and may therefore reduce backside wear and, consequently, risk of osteolysis [30, 32, 34]. Second, the use of an all-poly component reduces the cost of TKA surgeries by 20–50 % [5–8, 11, 25, 26]. Third, the implant can be used when patient is allergic to metal (in combination with ceramic femoral components [17]).
Metal-backed implants present different advantages: modularity, use of porous-coated implant, constrained or mobile liner stems or tibial augments, the possibility to replace only the insert during revision surgery, and, finally, easier removal of posteriorly extruded cement. Studies that have focused on biomechanical performance have stated that metal-backed components reduce the compressive stresses in cancellous bone underneath the tibial baseplate and distribute the load more uniformly over a larger area of the proximal tibia [2, 22, 36].
Metal-backed and all-poly components can be implanted with different thicknesses of cement penetration in tibial cancellous bone, which could alter the interactions at the implant–bone interface. Differences in stress and strain distributions in the tibial bone could lead to pain, subsidence or loosening [2]. Similarly, large micromotions at the bone–baseplate interface can increase the risk of loosening and failure of the implant [37]. While prior studies have assessed the clinical impact and patient outcomes comparing all-poly and metal-backed designs, they have not characterized differences in the stress distributions in the tibia and the interface micromotions.
For this reason, the aim of this study was to evaluate and compare the biomechanical performance of metal-backed and all-poly tibial components implanted in the same bone geometry during a high-flexion motor task using finite element analysis (FEA). Stresses and strains in the tibia and micromotion at the implant–bone interface were evaluated. A variety of cementing techniques and bone quality representing physiological, osteopenic and osteoporotic material properties were evaluated.
The objective of the current study was to address the following questions:
-
1.
What are the differences between metal-backed and all-poly tibial components in terms of mechanical effects (stress, strain and micromotion) on the bone during a high-demand motor task?
-
2.
How is the mechanical behaviour of the two implants influenced by different cementing techniques (thickness of the cement penetration and presence of cement around the stem)?
-
3.
How are mechanical effects of the two implants on the bone influenced by the condition of bone?
Materials and methods
Finite element analysis enables detailed biomechanical investigations with potential to detect effects of the two implants on the bone that cannot be investigated in vivo or by means of clinical studies [16, 35]. Therefore, finite element models were developed to investigate the stress and strain distributions in the tibial bone in which an all-poly or a metal-backed tibial component was implanted. These models were analysed at the maximum flexion angle of a deep knee squat and with different surgical and patient conditions in terms of cementing techniques and bone quality. Stress and micromotion were calculated and compared for a variety of conditions. The main features of the models are described in the following paragraphs.
Geometry
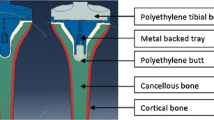
Computed tomography (CT) images of a left mechanical equivalent tibial Sawbone (fourth-generation left composite tibia, Pacific Research Laboratories, WA, USA), including cortical bone, cancellous bone and intramedullary canal, were used to obtain the bone geometry [1, 12, 16]. The implant design used in this study (for both metal-backed and all-poly) was the GENESIS II Total Knee System (Smith & Nephew, Memphis, TN, USA). Virtual cuts were performed on the model of the tibia, following the surgical procedures for this implant design. Size 4 was chosen for both the implant solutions according to the medial–lateral (M–L) and anterior–posterior (A–P) dimensions of the proximal tibial surface after the cut. This choice was confirmed by experimental implantation tests in a mechanical equivalent synthetic tibia.
The following implant conditions were modelled:
-
1.
Three different cement penetration thicknesses (2, 3 and 4 mm) in the tibial cancellous bone under the baseplate were selected, according to reported values of penetration [39]. Cement penetration of 2–3 mm is required to engage at least one level of trabeculae and sufficient bends in the vertical channels [43]. Thermal damage is likely to occur with a cement penetration of more than 5 mm [13]; therefore, a penetration depth between 2 and 5 mm seems to be ideal.
-
2.
The presence or absence of a 1-mm cement layer around the stem of the implant was also evaluated. Tibial trays with cement around the stem have undergone biomechanical analysis showing proximal stress shielding, distal cortical load transfer and improved fixation stability [18]. In order to model this condition, a larger 13-mm-diameter hole, instead of the traditional 11 mm, was created in the tibial cancellous bone and the space between the bone and stem was filled with cement.
-
3.
Three different bone conditions were selected in order to represent variability in bone quality: physiological, osteopenic and osteoporotic bone. TKA is often performed on older patients, whose bones may present a decrease in bone mass and density and a degradation of bony structure. This was represented in a FE model by weaker mechanical properties of the material. In particular, the Young’s moduli of the physiological cortical and cancellous bone (that were found in the literature [33]) were reduced by 32 and 66 %, respectively, to represent the osteoporotic bone, and by 16 and 33 % to represent the osteopenic bone [23].
A total of 36 configurations were obtained between all of the above conditions.
Material models and properties
Although cortical and cancellous bones show viscoelastic properties, the assumption of linear elasticity is adequate for most studies [12, 14, 16, 38]. Cortical bone was assumed to be linear elastic and orthotropic, while cancellous bone was assumed to be linear elastic and isotropic [14, 33, 38]. Isotropic linear elastic homogeneous models were used also for UHMWPE, titanium alloy (Ti6Al4V), cement (PMMA) and cobalt–chromium alloy (CoCrMo) [19, 29, 42]. Material properties are shown in Table 1.
Mechanical properties of the bone (physiological, osteopenic and osteoporotic conditions) were chosen according to the literature (Table 2) [23, 33]. Appropriate friction coefficients at the interfaces were also chosen according to the literature (Table 3) [4, 9, 18, 28, 44].
Simulated motor task
A numerically validated musculoskeletal model (LifeMOD/KneeSIM 2007.5.0, LifeModeler Inc., San Clemente, CA) [15, 31] was used to simulate a squat motor task up to 120° flexion of a knee with a GENESIS II TKA design. A constant vertical hip load of 200 N was applied during the motor task [15, 31, 40, 41]. According to the Grood and Suntay convention [10], internal rotation and adduction at the maximum flexion angle (120.0°) were, respectively, 1.3° and 3.2°. The contact forces between the femoral component and the polyethylene insert at this orientation were evaluated in two regions. One region consisted of the loads exchanged between the insert plateau and the lateral and medial condyles of the femoral component, and the other region consisted of the loads exchanged by the post of the insert and the cam of the femoral component. The quadriceps load, which was applied to the tibial bone through the patellar tendon at the tibial tuberosity, was also calculated (Table 4).
The femoral component was coupled with each of the tibial components in the relative position identified by the previously validated musculoskeletal model. The same relative position between the two components and the loads previously identified were applied to all 36 models.
In all simulations, the tibial bone model was cut and fixed at the distal end.
Finite element analysis
Each model was meshed with tetrahedral elements with an average edge length of approximately 5 mm. A refinement of the mesh was performed in the region of the bone in contact with the implant. Each model was composed of approximately 55,000 elements. The region of interest, where stresses and strains in the bone were analysed, was the 10-mm proximal region under the baseplate of the tibial component (Fig. 1).
Development of the models and all the finite element simulations were performed using Abaqus/Standard 6.11-1 (Dassault Systèmes, Vélizy-Villacoublay, France). The extracted outputs were (1) von Mises stress distribution at the tibial proximal cut, (2) mean compressive stress in the bone and (3) micromotion at the bone–baseplate interface, which was calculated as M–L and A–P relative displacements between the prosthesis and bone.
Statistical analysis
Statistical difference between sets of results was checked by performing independent two-sample Student’s t tests. Significance was set at p < 0.001.
Results
Qualitative comparison of the von Mises stress at the bone–implant interface
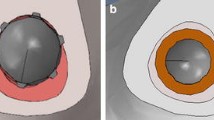
Comparing the von Mises stress distribution at the implant–bone interface, the results show that the metal-backed design distributes the stress more uniformly throughout the bone compared to the all-poly solution (Fig. 2). The all-poly solution resulted in stress concentrated in smaller regions beneath the two loads applied by the femoral condyles. Conversely, the metal-backed solution presented a more uniform distribution beneath the baseplate. This finding was expected due to the higher stiffness of the metal-backed component compared to the stiffness of the all-poly component.
Stress analysis in the bone: effect of cement penetration thickness under the baseplate
When the thickness of the cement penetration under the baseplate changed from 2 to 4 mm in the different configurations, an average compressive stress variation of +8 % was calculated in the region of interest of the bone (Fig. 3). The maximum stress variation observed was +15.9 %. Stress variation obtained by changing the cement thickness was not consistent and not statistically significant (n.s.). For this reason, the differences in the stress values due to the change of the cement thickness were ignored in the following comparisons.
Stress analysis in the bone: effect of implant design
Comparing the mechanical effects in the bone between metal-backed and all-poly implants, the following results were obtained: in cortical bone, no significant compressive stress variations were measured (1.6 % mean variation, n.s.); in cancellous bone, the compressive stress variation ranged from a minimum of +74.7 % to a maximum of +128.4 % and statistical difference was found (p < 0.001). The mean variation in stress obtained for the all-poly compared to metal-backed design was +75.5 % without cement around the stem, and +114.4 % with cement around the stem (Fig. 4).
Stress analysis in the bone: effect of cement layer around the stem
When the mechanical effects obtained with and without the cement layer around the stem were compared, the following results were obtained: for the metal-backed analysis, the presence of the cement layer reduced average stresses both in the cancellous bone (−4.6 % of average) and in the cortical bone (−2.0 % of average), but these changes were not statistically significant (n.s.). For the all-poly analysis, the cement increased significantly stresses in the cancellous bone (+16.5 % on average, p < 0.001), but decreased stresses in cortical bone (−2.5 % on average) but without statistical difference (n.s.). Figure 5 shows the variations, in terms of average von Mises stress at the tibial proximal cut, while Fig. 6 shows the compressive stress results in the region of interest of the bone.
Stress analysis in the bone: effect of varying bone quality
Evaluating the differences in bone quality, a statistical difference (p < 0.001) in the stress variation in the cortical bone was found: in particular, the mean stress variation in the cortical bone between physiological and osteoporotic bone was +38.1 %, while the maximum variation observed was 40.8 % (Fig. 5). In cancellous bone, the variation was less significant (n.s.) with an average of +10.2 %. The von Mises stress variations at the tibial proximal cut can be seen in Fig. 7.
Micromotion analysis: effect of cement penetration thickness under the baseplate
The micromotion results followed similar trends: when the cement penetration thickness under the baseplate is changed from 2 to 4 mm, micromotion variation is slightly higher than stress variation. In fact, the micromotion variation in the A–P direction is the most relevant considering the boundary conditions applied to the models and increases from 1.3 to 24.7 %. The mean A–P micromotion variation obtained by changing the cement penetration thickness was +11.0 %, but again it was not consistent and there was no statistical difference (n.s.). An example of the effects on micromotion obtained when the cement penetration was changed is shown in Fig. 8.
In the M–L direction, the percentage variation analysis could be misleading because, especially for metal-backed, the values are so small that a small variation causes a large percentage difference. In fact, as most of the load was applied to the model along the A–P direction, the most meaningful results from these analyses are those that refer to the A–P direction. However, since differences in the A–P micromotion variation were not consistent and thereby not meaningful, the differences obtained by changing the thickness of the cement penetration were ignored in the following comparisons, as well as for the stress analysis.
Micromotion analysis: effect of implant design
The comparison between all-poly and metal-backed implant designs showed a marked difference: micromotion with all-poly was far larger than with metal-backed. In particular, the average A–P micromotion variation obtained by changing the implant solution from metal-backed to all-poly was 2.5 times higher (+258.7 %, p < 0.001), reaching a peak of 5 times higher (+502 %) in one of the analysed configurations (Fig. 9).
Micromotion analysis: effect of cement layer around the stem
When the cement layer around the stem was added, the micromotion in the A–P direction decreased for all of the configurations. The mean micromotion decreased by 5.5 % in the A–P direction when the cement layer around the stem was added and a maximum variation of −13.8 % was measured (Fig. 10), but these variations were not statistically significant (n.s.).
Micromotion analysis: effect of varying bone quality
When the bone properties were changed from physiological to osteoporotic, the mean A–P micromotion variation was +34.2 %, with a peak variation of +54.0 % in one analysis (Figs. 9, 10). This variation was statistically significant (p < 0.001) for both all-poly and metal-backed designs.
Discussion
The most important finding of the present study was that all-poly components resulted in higher and less uniform stress distributions, especially in the cancellous bone, and increased micromotion up to 5 times at the interface compared to the metal-backed component. Moreover, the performance of both implant designs worsened with poorer bone quality, and performance improvements are shown to be not statistically significant when the stem was cemented. More in details, the all-poly design resulted in concentrated stresses in regions near the applied condylar loads. In addition, compressive stress values in the cancellous bone were clearly higher with the all-poly solution. This confirms the results from previous studies that compared all-poly and metal-backed performances [2, 36]. These findings were predictable, since the stiffness of the metal component is much higher than the stiffness of polyethylene. With the metal-backed design, the loads were transferred from the polyethylene insert to the metal, which carried the bulk of the load and generated lower peak stress in the cement layer beneath the tibial component. Although exact correlations with clinical outcomes are unknown, these effects on tibial bone could lead to an increase of pain and risk of implant loosening [2, 3]. What was observed in the current study was also observed by Taylor et al. [36] on cementless TKA tibial component: higher cancellous bone stresses are correlated with migration and survivorship data, with the implant generating the highest cancellous bone stresses, migrating the most and having the poorest survival rates at 5 years. The progressive failure of cancellous bone seems to be a shared mechanism of implant migration, regardless of the method of fixation and the implantation site.
Micromotions with all-poly were higher than those with metal-backed. Although the friction coefficient between polyethylene and cement is higher than the friction coefficient between titanium and cement, the deformability of polyethylene caused far greater micromotion with all-poly because of the higher strains in the polyethylene component. High micromotions increase risk of loosening and consequent failure [37]. This result contradicts a previous study performed by means of a radiostereophotometric analysis (RSA), which showed that all-poly components’ migration is equal or lower than that in the case of metal-backed components [27]. This difference could be motivated by the fact that the current study analyses the behaviour of the two implants during a high-flexion motor task, while the cited work performed a comparison with older patients with a mean age of 69 years, who would unlikely perform activities such as a full squat. Moreover, the current study focused on the determination of micromotion due to the application of a single loading condition, while RSA measures migration that is a long-term effect influenced by many loading cycles at the joint.
Changing the thickness of the cement penetration under the baseplate did not result in significant variations in terms of stress and micromotion. This could be explained considering that a minimum level of penetration (2–3 mm) is required to engage at least one level of transverse trabeculae [43] and a maximum value (5 mm) is necessary in order to avoid thermal injury [13]. Therefore, a penetration depth between 2 and 5 mm is ideal, and within this range, there is little performance variation.
The use of cement around the stem in metal-backed tibial components in primary TKA has been shown in previous studies to increase fixation stability [18]. The results of this study confirmed better fixation, showing a reduction of micromotion at the bone–baseplate interface. However, they confirmed a stress decrease in cancellous bone only with metal-backed. In the case of the all-poly design, the presence of the additional cement layer caused increased compressive stress values in cancellous bone. In particular, the cement reduced stresses in the anterior part of the bone, where most of the cement is situated, but increased stresses at the wedge–bone interface, where there is no cement. This difference could be caused only by the greater cement Young’s modulus compared to the cancellous bone that may increase stress values but not strain values.
When the bone properties are changed from physiological to osteoporotic, most of the stress increase was observed in the cortical bone with both all-poly and metal-backed. The same increasing trends were identified when micromotion variation was evaluated. These results show that the performances of both implant designs worsen when bone quality is reduced.
The current analysis presents some limitations. First, the results pertain to a high-flexion squat and may not extend to other activities. This activity was chosen because of the necessity to evaluate implant performance during high-demand motor tasks. This necessity was driven by the anticipated demand for primary TKA since current trend in patients’ lifestyle shows increased activity in medium- and high-impact sports [20, 24]. Second, direct validation of the results obtained in this study was not performed. If the absolute values of stress and micromotion had been the main results of this study, a more rigorous validation would have been necessary; however, this was a comparative analysis of the stress and micromotion ratios between all-poly and metal-backed implants. Moreover, the boundary conditions used in this study were previously validated [15, 31].
Clinically, the results of this study should be considered in addition to the already known advantages and disadvantages of the two implant designs when selecting the appropriate tibial baseplate option for a patient, especially in the presence of osteoporotic bone or if intense physical activity is anticipated.
Conclusion
In conclusion, even if metal-back and all-poly should be viewed as complementary approach, the all-poly components resulted in higher and less uniform stress distributions in the bone and increased micromotion up to 5 times at the interface compared to the metal-backed component. The all-poly design shows larger mechanical effects on the bone that could potentially lead to an increase of pain and risk of failure of the implant. Moreover, the performances of both the implant designs worsened when the bone quality was reduced; however, they both improved with a cemented stem.
References
Au AG, James Raso V, Liggins AB et al (2007) Contribution of loading conditions and material properties to stress shielding near the tibial component of total knee replacements. J Biomech 40(6):1410–1416
Bartel DL, Burstein AH, Santavicca EA et al (1982) Performance of the tibial component in total knee replacement. J Bone Joint Surg Am 64(7):1026–1033
Bourne RB, Finlay JB (1986) The influence of tibial component intramedullary stems and implant-cortex contact on the strain distribution of the proximal tibia following total knee arthroplasty. An in vitro study. Clin Orthop Relat Res 208:95–99
Completo A, Simoes JA, Fonseca F et al (2008) The influence of different tibial stem designs in load sharing and stability at the cement–bone interface in revision TKA. Knee 15(3):227–232
Gioe TJ, Bowman KR (2000) A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res 380:108–115
Gioe TJ, Killeen KK, Mehle S et al (2006) Implementation and application of a community total joint registry: a twelve-year history. J Bone Joint Surg Am 88(6):1399–1404
Gioe TJ, Maheshwari AV (2010) The all-polyethylene tibial component in primary total knee arthroplasty. J Bone Joint Surg Am 92(2):478–487
Gioe TJ, Sinner P, Mehle S et al (2007) Excellent survival of all-polyethylene tibial components in a community joint registry. Clin Orthop Relat Res 464:88–92
Godest AC, Beaugonin M, Haug E et al (2002) Simulation of a knee joint replacement during a gait cycle using explicit finite element analysis. J Biomech 35(2):267–275
Grood ES, Suntay WJ (1983) A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng 105(2):136–144
Healy WL, Iorio R, Ko J et al (2002) Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am 84(3):348–353
Heiner AD, Brown TD (2001) Structural properties of a new design of composite replicate femurs and tibias. J Biomech 34(6):773–781
Huiskes R, Slooff TJ (1981) Thermal injury of cancellous bone, following pressurized penetration of acrylic cement. Trans Orthop Res Soc 6:134
Innocenti B, Bilgen OF, Labey L et al (2014) Load sharing and ligament strains in balanced, overstuffed and understuffed UKA. A validated finite element analysis. J Arthroplasty 29(7):1491–1498
Innocenti B, Pianigiani S, Labey L et al (2011) Contact forces in several TKA designs during squatting: a numerical sensitivity analysis. J Biomech 44(8):1573–1581
Innocenti B, Truyens E, Labey L et al (2009) Can medio-lateral baseplate position and load sharing induce asymptomatic local bone resorption of the proximal tibia? A finite element study. J Orthop Surg Res 4:26
Innocenti M, Carulli C, Matassi F et al (2014) Total knee arthroplasty in patients with hypersensitivity to metals. Int Orthop 38(2):329–333
Janssen D, Mann KA, Verdonschot N (2008) Micro-mechanical modeling of the cement–bone interface: the effect of friction, morphology and material properties on the micromechanical response. J Biomech 41(15):3158–3163
Kayabasi O, Erzincanli F (2006) Finite element modelling and analysis of a new cemented hip prosthesis. Adv Eng Softw 37(7):477–483
Kurtz SM, Lau E, Ong K et al (2009) Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res 467(10):2606–2612
Kurtz SM, Ong KL, Lau E et al (2011) International survey of primary and revision total knee replacement. Int Orthop 35(12):1783–1789
Lewis JL, Askew MJ, Jaycox DP (1982) A comparative evaluation of tibial component designs of total knee prostheses. J Bone Joint Surg Am 64(1):129–135
Little JP, Taddei F, Viceconti M et al (2007) Changes in femur stress after hip resurfacing arthroplasty: response to physiological loads. Clin Biomech 22(4):440–448
Mayr HO, Reinhold M, Bernstein A et al (2015) Sports activity following total knee arthroplasty in patients older than 60 years. J Arthroplasty 30(1):46–49
Muller SD, Deehan DJ, Holland JP et al (2006) Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br 88(12):1596–1602
Najibi S, Iorio R, Surdam JW et al (2003) All-polyethylene and metal-backed tibial components in total knee arthroplasty: a matched pair analysis of functional outcome. J Arthroplasty 18:9–15
Nouta KA, Verra WC, Pijls BG et al (2012) All-polyethylene tibial components are equal to metal-backed components. Clin Orthop Relat Res 470(12):3549–3559
Oosterom R, van Ostayen RAJ, Antonelli V et al (2005) Effect of interface conditions between ultrahigh molecular weight polyethylene and polymethyl methacrylate bone cement on the mechanical behaviour of total shoulder arthroplasty. Proc Inst Mech Eng H 219:425–435
Panin SV, Wannasri S, Piriyayon S et al (2010) Increasing wear resistance of UHMW-PE based composite materials by adding micro- and nanofillers, mechanical activation, chemical modification and ion implantation. J Iron Steel Res Int 17:62–70
Parks NL, Engh GA, Topoleski LD et al (1998) The Coventry Award. Modular tibial insert micromotion. A concern with contemporary knee implants. Clin Orthop Relat Res 356:10–15
Pianigiani S, Chevalier Y, Labey L et al (2012) Tibio-femoral kinematics in different total knee arthroplasty designs during a loaded squat: a numerical sensitivity study. J Biomech 45(13):2315–2323
Rao AR, Engh GA, Collier MB et al (2002) Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg Am 84(10):1849–1855
Rho JY (1996) An ultrasonic method for measuring the elastic properties of human tibial cortical and cancellous bone. Ultrasonics 34(8):777–783
Rodriguez JA, Baez N, Rasquinha V et al (2001) Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res 392:174–183
Soenen M, Baracchi M, De Corte R et al (2013) Stemmed TKA in a femur with a total hip arthroplasty: is there a safe distance between the stem tips? J Arthroplasty 28(8):1437–1445
Taylor M, Tanner KE, Freeman MA (1998) Finite element analysis of the implanted proximal tibia: a relationship between the initial cancellous bone stresses and implant migration. J Biomech 31(4):303–310
Tissakht M, Eskandari H, Ahmed AM (1995) Micromotion analysis of the fixation of total knee tibial component. Comput Struct 56:365–375
van Jonbergen HP, Innocenti B, Gervasi GL et al (2012) Differences in the stress distribution in the distal femur between patellofemoral joint replacement and total knee replacement: a finite element study. J Orthop Surg Res 7:28
Vanlommel J, Luyckx JP, Labey L et al (2011) Cementing the tibial component in total knee arthroplasty: which technique is the best? J Arthroplasty 26(3):492–496
Victor J, Labey L, Wong P et al (2010) The influence of muscle load on tibiofemoral knee kinematics. J Orthop Res 28(4):419–428
Victor J, Van Doninck D, Labey L et al (2009) A common reference frame for describing rotation of the distal femur: a ct-based kinematic study using cadavers. J Bone Joint Surg Br 91(5):683–690
Waanders D, Janssen D, Mann KA et al (2010) The mechanical effects of different levels of cement penetration at the cement–bone interface. J Biomech 43(6):1167–1175
Walker PS, Soudry M, Ewald FC et al (1984) Control of cement penetration in total knee arthroplasty. Clin Orthop Relat Res 185:155–164
Xiong DS, Gao Z, Jin ZM (2007) Friction and wear properties of UHMWPE against ion implanted titanium alloy. Surf Coat Technol 201(15):6847–6850
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brihault, J., Navacchia, A., Pianigiani, S. et al. All-polyethylene tibial components generate higher stress and micromotions than metal-backed tibial components in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 24, 2550–2559 (2016). https://doi.org/10.1007/s00167-015-3630-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3630-8