Abstract
Cerebral amyloid angiopathy (CAA) is caused by the deposition of the amyloid β-protein (Aβ) in the wall of cerebral and leptomeningeal blood vessels and is related to Alzheimer’s disease (AD). Capillary Aβ deposition is observed in a subset of CAA cases and represents a distinct type of CAA named capillary CAA or CAA type 1. This type of CAA is strongly associated with the presence of the apolipoprotein E ε4 allele. CAA type 1-associated AD cases often exhibit a more severe Aβ plaque pathology but less widespread neurofibrillary tangle (NFT) pathology. The objective of this study was to analyze whether capillary CAA and its effects on cerebral blood flow have an impact on dementia. To address this objective, we performed neuropathological evaluation of 284 autopsy cases of demented and non-demented individuals. We assessed the presence of CAA and its subtypes as well as for that of hemorrhages and infarcts. Capillary CAA and CAA severity were associated with allocortical microinfarcts, comprising the CA1 region of the hippocampus. Allocortical microinfarcts, capillary CAA and CAA severity were, thereby, associated with cognitive decline. In conclusion, allocortical microinfarcts, CAA severity, and the capillary type of CAA were associated with one another and with the development of cognitive decline. Thus, AD cases with CAA type 1 (capillary CAA) appear to develop dementia symptoms not only due to AD-related Aβ plaque and NFT pathology but also due to hippocampal microinfarcts that are associated with CAA type 1 and CAA severity, and that damage a brain region important for memory function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral amyloid angiopathy (CAA) is characterized by the deposition of the amyloid β-protein (Aβ) in the wall of cerebral and leptomeningeal arteries, veins and cerebral capillaries [15, 31, 33, 53] and is related to Alzheimer’s disease (AD) [24]. Capillary Aβ deposition is observed in a subset of CAA cases, is strongly associated with the presence of the apolipoprotein E (APOE) ε4 allele [48], and has been shown to be associated with dementia [30]. Capillary CAA can be seen in CAA cases with beginning AD-related pathology, even in the absence of Aβ deposition in larger vessels. Therefore, capillary CAA is considered to be a distinct type of CAA, previously described as CAA type 1, which is different from those CAA cases that show Aβ deposits only in larger vessels (i.e., CAA type 2) [48]. In an animal model for CAA it was shown that capillary CAA can cause blood flow disturbances [46]. Moreover, increased levels of Aβ have been discussed to alter blood vessel function in APP-overexpressing mouse models even in the absence of CAA lesions [38, 39, 41].
CAA is associated with hemorrhages, microbleeds, and infarcts [17, 31]. However, it is not yet clear whether differences exist among the types of CAA in their potential to cause brain hemorrhages or infarcts and, if so, whether such differences influence cognition in cases with AD pathology.
To address these questions we performed a neuropathological study of 284 autopsy cases.
Materials and methods
Neuropathology
Human brains from 284 autopsy cases of both sexes (170 males and 114 females) with an average age of 68.8 years ± 13.5 years (average ± SD) were investigated (Table 1). The cases were consecutive samples from municipal and university hospitals in Germany (Bonn, Offenbach and Ulm). Autopsies were performed in accordance with German law and were used for this study after receiving a positive vote from the local ethical committees in Ulm/Germany (No. 86/13) and Leuven/Belgium (No. S-59295). Cases of familial AD, Down syndrome, Creutzfeldt–Jakob disease, familial CAA, inflammatory diseases of the brain or the vessels, large brain tumors, vascular malformations, and severe head traumata were excluded to ensure that these factors did not interfere with the assessment of vascular dementia or AD as the causes of cognitive and/or neurological symptoms.
The patients were examined at the time point of hospital admission (approximately 1–4 weeks prior to death) by clinicians with different specialties according to standardized protocols. The protocols included the assessment of cognitive function (orientation to place, time and person; specific cognitive or neuropsychiatric tests were not performed) and recorded the patients’ ability to care for themselves and to get dressed, eating habits, bladder and bowel continence, speech patterns, writing and reading ability, short-term and long-term memory, and orientation within the hospital setting. These data were used to retrospectively assess the clinical dementia rating (CDR) scores for each patient [21] without knowledge of the pathological diagnosis. For this purpose, the information from the clinical files was used to provide a CDR score according to the standard CDR protocol [21]. Sufficient data to estimate a CDR score were available for 234 patients. In the other cases the clinical information was not sufficient. Information about the presence or absence of hypertension was available in 277 cases, of myocardial infarction in 274 cases, of atrial fibrillation in 76 cases and of diabetes mellitus in 276 cases (Table 1).
The brains were fixed in a 4% aqueous solution of formaldehyde. After dissection of the brainstem and cerebellum from the forebrain, the forebrain was cut into 1-cm frontal slabs. Brain stem and cerebellum were cut perpendicular to the Meynert brain stem axis in 0.5-cm slabs. The gross-sections were inspected by two investigators (MH, DRT). The investigators were blinded with respect to the neuropathological diagnosis and assessed the brains independently. Due to the fact that macroscopic and microscopic samples permit visual perception of distinct pathologies (e.g., infarcts) while investigating others (e.g., vessel lesions such as CAA), a blinded pathological analysis only focusing on one single pathology was impossible. The clinical aspects of each case were known to the investigators at the time of assessment. Infarcts seen at the macroscopic level were only considered to be infarcts if they were confirmed histopathologically. In case of different opinions, the investigators discussed the findings with the help of microscopy and reached a consensus. The distribution of hemorrhages of all sizes, large infarcts (all infarcts lager than 10 mm in diameter), and lacunar infarcts (all infarcts measuring 5–10 mm in diameter) [18] as well as the severity of atherosclerosis (AS) in the circle of Willis were recorded. The severity of AS was assessed as previously published [26]: 0 = no macroscopically detectable AS lesions; severity grade 1 = no more than three AS plaques in an artery and no circular AS plaques; severity grade 2 = more than three plaques in at least one artery but no concentric plaques; severity grade 3 = more than three plaques in at least one vessel and concentric plaques. The expansion of cerebral small vessel disease (SVD)-affected brain vessels was staged histopathologically as previously published [47]: SVD stage 0 = no SVD; SVD stage 1 = SVD-affected vessels are limited to the basal ganglia region; SVD stage 2 = SVD-affected vessels are found in the basal ganglia, the white matter of cortical gyri, the thalamus and the cerebellum; SVD stage 3 = SVD disease-affected vessels expand into the brainstem and the midbrain.
Cases affected by large or lacunar infarcts were investigated using a standardized protocol: pictures of all gross-section slices were taken with a digital camera and saved as JPG files. The respective JPG files were assessed stereologically by measuring the infarcted area and the total area of each slice in a given brain with the ImageJ image analysis software (NIH, Bethesda, USA). To estimate the relative infarct volume we determined the percentage of the brain volume that was destroyed by the infarct(s):
For histological assessments tissue blocks from frontal, parietal, and occipital lobe (Area 17), cingulate gyrus, occipital lobe, hippocampus with temporal cortex, entorhinal cortex, hypothalamus, basal ganglia, amygdala, basal nucleus of Meynert, thalamus, midbrain, pons, medulla oblongata and cerebellum were dissected, embedded in paraffin and microtomed to 5–12 μm. For neuropathological diagnosis including the detection of large and lacunar infarcts, microinfarcts, hemorrhages, and microbleed paraffin sections were stained with hematoxylin and eosin (H&E) or aldehyde fuchsine–Darrow red for lipofuscin pigment and Nissl material. Neurofibrillary tangles (NFTs), neuropil threads and neuritic plaques were assessed using an antibody directed against abnormal phosphorylated τ-protein (AT-8, 1/1000, Thermo-Fisher, Waltham, MA, USA) [6, 12]. Aβ deposits were detected with an antibody directed against Aβ17–24 (4G8, 1/5000, formic acid pretreatment, Covance, Dedham, USA). Antibodies against the phosphorylated transactive response DNA-binding protein pTDP43 (polyclonal rabbit—pS409/410-2, 1/5000, microwave pretreatment, Cosmo Bio Co., Ltd, Tokyo, Japan) were used to detect TDP43 aggregates and to differentiate microinfarcts in the hippocampus from TDP43-related hippocampal sclerosis. These primary antibodies were marked with a biotinylated secondary antibody. The secondary antibodies were visualized with the ABC complex (Vectastain, Vector Laboratories, Burlingame, CA, USA) and 3,3-diaminobenzidine (DAB). Immunolabelled paraffin sections were counterstained with hematoxylin. Positive and negative controls were included. All tissue sections were viewed with an Olympus BX 51 or a Leica DMLB 2 light microscope. Digital photographs were taken with a Leica DC 500 or a Leica DFC 290 camera.
Phases of Aβ plaque deposition in the medial temporal lobe (Aβ-MTL phases) were assessed representing an appropriate estimate for Aβ-deposition in the entire brain as previously shown [51, 52]. Braak stages for NFT expansion throughout the brain were determined as previously described on the basis of anti-abnormal τ-protein-stained sections [6, 7]. The frequency of τ-positive neuritic plaques was assessed according to the recommendations of the consortium to establish a registry for AD (CERAD) [32]. The degree of AD pathology was determined according to Hyman et al. [22] on the basis of the Aβ-MTL phase, Braak NFT stage, and the CERAD score for neuritic plaque pathology.
CAA was diagnosed when Aβ deposits were found in the wall of cerebral and leptomeningeal blood vessels. The severity of the CAA-related vessel wall damage was rated according to Vonsattel et al. [55] as follows: 0 = no CAA; 1 = Aβ-positive material in vessel wall(s) without significant smooth muscle cell degeneration; 2 = Aβ-positive material in vessel wall(s) with significant destruction and fibrosis of media; and 3 = Aβ-positive material in vessel wall(s) with micro- or macrohemorrhages. The presence of capillary Aβ was employed to distinguish two types of CAA as previously reported [48]: CAA cases exhibiting capillary involvement were assessed as CAA type 1 and CAA cases lacking capillary CAA as CAA type 2.
The vascular brain tissue lesions were defined according to Grinberg and Thal [18]. The number and distribution of microinfarcts was assessed in H&E and/or aldehyde fuchsine–Darrow red-stained sections in all areas taken for histological analysis. A microinfarct was diagnosed when we observed well-delineated lesions with neuron loss and gliosis or fresh necrotic lesions with a diameter less than 0.5 cm in the gray or white matter, usually not seen macroscopically [4, 13, 14, 18, 29]. We subclassified microinfarcts as neocortical microinfarcts (seen in sections of the frontal, parietal, temporal, and occipital cortex); allocortical microinfarcts (in the hippocampus, entorhinal cortex, amygdala, and the cingulate gyrus); subcortical microinfarcts (in the basal ganglia, thalamus, hypothalamus, basal forebrain, midbrain, pons, medulla oblongata, and the cerebellum); and white matter microinfarcts (seen in any part of the white matter without affecting the cortical ribbon or subcortical gray matter). The category cortical microinfarcts included cases with both neo- and/or allocortical microinfarcts. Microbleeds were defined as hemorrhages limited to the perivascular space, whereas regardless of the size all blood extravasations into the brain parenchyma were defined as hemorrhages [18].
Statistical analysis
Statistical analysis was carried out with IBM-SPSS 24. To search for associations we performed logistic or linear regression analysis using simple model terms including only one independent variable. In the event that we found an association for a distinct independent variable we constructed model terms that were controlled for other variables that were considered to have an influence on a given pathology.
For the different types of infarction or hemorrhage the control variables were the cardiovascular risk factors assessed in this study, namely the severity of AS in the circle of Willis, the stage of SVD, and the presence/absence of hypertension, myocardial infarction, and diabetes mellitus. Since the presence or absence of atrial fibrillation was known in only 76 cases we decided not to include this parameter as additional covariate. The impact of atrial fibrillation on allocortical microinfarcts was only studied in a model with atrial fibrillation as the only independent variable.
In the event that there were multiple covariates used in a model, analysis of multicollinearity was performed for those variables, which showed an effect as covariate in a model term with only one covariate but not in a more complex model. This analysis of multicollinearity was carried out by testing the associations between the variables that had still an effect in the complex model and those that did no longer exhibit an association with the dependent variable by linear or binary logistic regression analysis. Multicollinearity was considered in the event that there was (a) a significant correlation between two or more independent variables used in a given model term that were (b) biologically linked with the same disease.
When analyzing the CDR score as the dependent variable, age, sex, and Braak NFT stage were used as control variables. For the analysis of the association of CAA type (including the group of cases without CAA) with CAA severity or the CDR score, we used the Mann–Whitney U test adjusted by the Bonferroni correction for multiple tests. Since three nominal groups (no CAA, CAA type 1, and CAA type 2) were compared with one another, linear or binary logistic regression could not be applied.
Results
The neuropathological analysis showed large infarcts in 12.7%, lacunar infarcts in 7.4%, neocortical microinfarcts in 4.6%, allocortical microinfarcts in 6.7%, white matter microinfarcts in 0.4% and subcortical microinfarcts in 3.5% of the cases. Hemorrhages were found in 15.5% of the cases. In our cohort, 58.8% of the cases exhibited amyloid plaque pathology. CAA was observed in 34.2% of the cases with a prevalence of CAA type 1 (capillary CAA) of 15.2% and CAA type 2 of 19.0%.
In 10 of the 19 cases that showed allocortical microinfarcts, the clinical files indicated signs of dementia. In six cases no signs of dementia were reported. No information about the cognitive status was available for the remaining three cases. 50% of the ten demented cases with allocortical microinfarcts showed capillary CAA (CAA type 1) whereas 40% had CAA type 2 and 10% had no CAA. When all 19 cases with allocortical microinfarcts were included, capillary CAA was seen in 42.1%, whereas CAA type 2 (26.3%) and cases without CAA (31.6%) were less frequently observed in this group of cases (Table 2).
Within the entire sample, 18.6% of the CAA type 1 cases, 9.3% of the CAA type 2 cases and 3.2% of the cases without CAA showed allocortical microinfarcts (Fig. 1).
CAA severity did not vary significantly between cases with CAA type 1 and cases with CAA type 2 assessed with the Mann–Whitney U test adjusted by the Bonferroni correction for multiple testing (p = 1). The distribution of CAA severity among the CAA types is shown in Table 2.
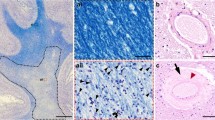
In all cases with allocortical microinfarcts the infarcts were located in the CA1 region of the hippocampus (Fig. 2). In one case there was an additional allocortical microinfarct in the entorhinal region, in another one in CA3, and in a third case there were additional neocortical and subcortical microinfarcts. The hippocampal lesions considered as microinfarcts exhibited no TDP43-related hippocampal sclerosis. A direct local co-occurrence of CAA and allocortical microinfarcts in the CA1 region was not observed. Parenchymal Aβ deposits were occasionally seen in the infarct region with some of these Aβ deposits showing the pattern of pericapillary amyloid (Fig. 2b, d). Capillary CAA was usually seen in the subiculum area but not in the infarct area in the CA1 sector (Fig. 2c, e).
Hippocampus of an 89-year-old woman with Alzheimer’s disease stained with an antibody against Aβ17–24. a The overview magnification shows amyloid plaques in the subiculum, CA1 and CA2 sectors, and in the outer molecular layer of the dentate gyrus as well as in the presubiculum. The Ammon’s horn sector CA1 also shows an old gliotic infarct with neuron loss in this region (arrowheads). b At the higher magnification level the neuron loss is more evident (arrowheads). Parenchymal amyloid plaques were seen in the infarct area but no CAA-affected blood vessels. Only perivascular parenchymal Aβ was found focally (arrow). c CAA-affected capillaries were seen distant to the infarct region in the subiculum (small headed arrows). d High magnification of the perivascular Aβ, which is associated with the glia limitans next to small capillaries, which are free of amyloid deposits representing pericapillary Aβ [5]. e In capillary CAA in the subiculum, the vessel wall of the capillaries contains Aβ deposits. Calibration bar in e valid for a = 600 µm; b, c = 130 µm; d = 35 µm; e = 25 µm)
Capillary CAA and CAA severity are associated with allocortical microinfarcts
To study the relationships between CAA, its subtypes, the different types of infarcts and hemorrhages we performed binary logistic regression analysis with different model terms (Tables 3, 4). Only the presence of allocortical microinfarcts was associated with the presence of CAA type 1 (capillary CAA) and the severity grade of CAA (Tables 3, 4) even in a logistic regression model controlled for the severity of AS in the circle of Willis, the stage of SVD, and the presence/absence of hypertension, myocardial infarction, and diabetes mellitus. The presence of other types of infarcts (such as neocortical, subcortical or white matter microinfarcts, or large or lacunar infarcts) was not associated with CAA type 1, CAA type 2 or CAA severity (Table 3). The volume of large and lacunar infarcts was not related to CAA severity or type when assessed with linear regression analysis with one covariate (CAA severity, CAA type 1, or CAA type 2: p = 0.427 − 0.639).
To clarify whether CAA type 1 or the severity of CAA represents the leading factor in this scenario binary logistic regression models were analyzed that included CAA severity, presence of CAA type 1 and/or CAA type 2 as covariates and the presence/absence of allocortical microinfarcts as dependent variable. This analysis confirmed that CAA type 1 and not CAA type 2 cases were associated with allocortical microinfarcts although the severity of CAA dominated the association with allocortical microinfarcts in a model containing CAA severity and the presence CAA type 1 as covariates (Table 5).
Allocortical microinfarcts, CAA severity and the presence of capillary CAA correlate with the degree of dementia
To clarify whether CAA severity, the presence of CAA type 1, and/or allocortical microinfarcts had an impact on the development of dementia as measured by the CDR score we used linear regression models. First, the different types of infarcts or hemorrhage, respectively, were considered as independent variables and the CDR score was set as dependent variable. With this analysis we saw an association of allocortical and cortical (neocortical + allocortical) microinfarcts with CDR scores. Such an association was not observed for hemorrhage or for other types of infarcts, including those cases in which only neocortical microinfarcts were seen (Table 7, Fig. 3). To clarify whether the associations with allocortical and cortical microinfarcts were attributable to patients’ age and sex, we used linear regression models controlled for these variables. In these models, only the presence of allocortical microinfarcts showed an age- and sex-independent association with the CDR scores; this association remained stable when the Braak NFT stages were added in the model term (Tables 8, 9).
Relationship between the degree of dementia as represented by the CDR score and the different types of cerebral infarcts, CAA and the presence or absence of cerebral hemorrhages in the study sample. a The CDR score was significantly higher in individuals with allocortical microinfarcts (in our cohort, this group always included CA1 microinfarcts) than in those without. b Likewise, individuals with capillary CAA (CAA type 1) had higher CDR scores than those with CAA type 2 or without CAA. c–h No significant differences between cases with and without a given lesion were observed for large infarcts (c), lacunar infarcts (d), neocortical microinfarcts (e), with matter microinfarcts (f), subcortical microinfarcts (g) and hemorrhages (h). a *p < 0.05; ***p < 0.001 (linear regression analysis controlled for age and sex). b *p < 0.05; ***p < 0.001 (Mann–Whitney U test with Bonferroni correction for multiple testing)
The presence of CAA type 1 and CAA severity were also associated with increasing CDR scores in linear regression models (Tables 7, 8, 9): CAA type 1 cases had higher CDR scores than CAA type 2 and non-CAA cases (Fig. 3b). The presence of CAA type 2 was not associated with the CDR score (linear regression analysis: Table 7). The association between the CAA types (no CAA, CAA type 1, or CAA type 2) and the CDR score was analyzed with the Mann–Whitney U test with Bonferroni correction for multiple testing, which confirmed these findings (Fig. 3b).
To clarify whether Braak NFT stage, AβMTL phase, allocortical microinfarcts, the presence of CAA type 1, and/or the CAA severity had independent effects on the dementia we analyzed model terms that contained all of these variables and reduced then the number of variables after analyzing multicollinearity effects by testing the association between distinct variables used as covariates in the first model containing all variables. In the model that includes all variables only the Braak NFT stage was associated with the CDR score (Table 10, model nos. 1 and 2). The Braak NFT stage was, thereby, strongly associated with the AβMTL phase, the CAA severity and the presence of CAA type 1 indicative of multicollinearity of these AD-related variables. Moreover, as shown before the presence of allocortical microinfarcts was strongly associated with the CAA type 1 and CAA severity pointing to multicollinearity among these vessel disease-related variables as well.
Since it is well known that multicollinearity can hide specific effects of distinct variables [1] we decided in a first step to exclude one of the two parameters describing CAA, resulting in a second parameter that was associated with the CDR score (either CAA severity or the presence of CAA type 1) in addition to the Braak NFT stage (Table 10, model nos. 3–6). Allocortical microinfarcts became evident as a parameter that influences the CDR score after reducing the model term to allocortical microinfarcts and Braak NFT stages (with or without controlling for age and sex) without other variables that showed associations with these two parameters (Table 10, model nos. 7 and 8). This analysis indicated that only model terms containing one AD-related (Braak NFT stage) and one CAA/allocortical microinfarct-related parameter allowed an interpretation of the results without multicollinearity-induced bias.
Relationship of allocortical microinfarcts with cardiovascular risk factors
To exclude cardiovascular causes as explanation for the occurrence of allocortical microinfarcts we performed binary logistic regression analysis. There was no significant association between allocortical microinfarcts and the severity of AS in the circle of Willis, the stage of cerebral SVD, hypertension, myocardial infarction, atrial fibrillation, and diabetes mellitus (Table 11). However, the p values for arterial hypertension (p = 0.068) and atrial fibrillation (p = 0.076) show a statistically borderline relationship of allocortical microinfarcts with these two parameters.
Discussion
The main finding of this study was an association of allocortical microinfarcts located in the CA1 region of the hippocampus with cognition as measured by the CDR score. CA1 microinfarcts were, thereby, seen most frequently in cases with severe and capillary CAA. An association of dementia with neocortical microinfarcts alone was not seen in our sample.
In contrast to our study, most studies reporting an association of cortical microinfarcts with dementia actually classified hippocampal microinfarcts as cortical microinfarcts [3, 4, 16, 23]. If we classify hippocampal microinfarcts as cortical microinfarcts in our dataset we also see a very mild association of cortical (neocortical + allocortical) microinfarcts with dementia (Table 7). In comparison with studies focusing on microinfarcts in general [28, 43] or on neocortical microinfarcts alone [43, 57], we distinguished neocortical from allocortical microinfarcts, including those in the hippocampal CA1 region. In our dataset, neocortical microinfarcts (if allocortical microinfarcts were excluded) did not exhibit a significant association with cognition in contrast to the results of Skrobot et al. [43]. However, Skrobot et al. [43] included only 113 cases and the regression models that were used differentiated only between patients with or without dementia. Other potential sources of differences in our results compared with those of other investigators include: 1) we studied a hospital-based sample whereas most of the other studies investigated population-based cohorts [3, 23, 28] and 2) we determined CDR scores retrospectively, whereas other authors either classified patients simply as having or not having dementia [23, 43] or carried out neuropsychological testing prior to death, which likely provides a more accurate assessment of cognitive status than a retrospective analysis [3, 8, 28]. In addition, we used immunohistochemical labeling of phosphorylated TDP43 to differentiate infarct lesions from TDP43-related hippocampal sclerosis [2]. Epilepsy-related hippocampal sclerosis was excluded by the morphology of the lesion because epilepsy-related Ammon’s horn sclerosis usually covers the entire CA1 sector and often extends into the CA3 and CA4 sector [58] whereas microinfarcts only partially destroy the hippocampal architecture of a given hippocampal subfield (Table 12).
Taken together, the varying approaches different investigators use to analyze the impact of microinfarcts on dementia are not fully comparable, which could explain the different, presumably more precise results obtained by our approach. Here, we focused on the role of specific allocortical regions, especially on the hippocampus, which is well known to be critically involved in memory function and orientation [19, 37, 44]. We found that microinfarcts in this specific memory-related brain region are associated with dementia. Thus, it is tempting to speculate that the association of microinfarcts with dementia is critically linked to the functional relevance of the brain region affected in accordance with the concept of strategic infarct dementia [54]. This hypothesis is also supported by the finding of Ince et al. [23] that subcortical microinfarcts in anatomical correlatives relevant for movement function were associated with impaired mobility.
Although it is well known that CAA is associated with microinfarcts [11, 27] we could extend this knowledge insofar as we show here for the first time that the presence of allocortical microinfarcts in the hippocampus is associated with the presence of capillary CAA (CAA type 1) and with the severity of CAA in the brain. Based on our findings we think that CAA type 1 and CAA severity represent the crucial CAA-related factors for the association with allocortical/hippocampal microinfarcts whereas CAA type 2 showed no significant impact on the development of allocortical microinfarcts. The arguments supporting this conclusion are: 1) only CAA type 1 cases but not CAA type 2 cases were associated with the presence of allocortical microinfarcts in several logistic regression models; 2) both the presence of CAA type 1 and the CAA severity were associated with allocortical microinfarcts and logistic regression models whereby CAA severity showed the leading effect in a model term that includes both parameters; 3) CAA type 1 affects capillaries in the subiculum [49] whereas CAA type 2 is by far less prominent in the medial temporal lobe regardless of the severity grade. Since the severity of CAA represents the degree of CAA-induced damage of the walls of affected blood vessels it is tempting to speculate that moderately or severely affected blood vessels could more easily give rise to blood flow disturbances than less strongly affected ones because autoregulation of the blood flow will be altered in relation to vessel wall damage [42]. For the development of hippocampal microinfarcts such moderately to severely CAA-affected vessels are expected to occur in the medial temporal lobe, which is the case in CAA type 1 cases exhibiting prominent capillary CAA in the subiculum region [49].
It is known from animal experiments that capillary CAA can cause blood flow disturbances [46]. However, severe capillary CAA was found distant from the allocortical microinfarcts located in the CA1 sector of the hippocampus. This finding is in accordance with observations of other authors that hippocampal and frontal cortex microinfarcts were mainly associated with morphologically intact vessels [25]. However, we did find perivascular/pericapillary Aβ [5] at the site of such microinfarcts. Although a direct, local effect by capillary Aβ deposits on the development of microinfarcts may not be very likely, the presence of capillary CAA may indicate a generally impaired clearance of Aβ in the perivascular drainage channels of the brain [9, 10, 34, 56]. Accordingly, Aβ and/or its diffusible aggregates may exist in a certain, increased concentration capable of inducing alterations of the vasomotoric function of blood vessels as shown in transgenic mouse brain [38, 39, 41]. These observations could explain Aβ-related blood flow disturbances even in the absence of local CAA-related Aβ deposits [38, 39, 41]. Thus, in the light of these results from transgenic mouse studies it is tempting to speculate that in patients with capillary CAA the perivascular clearance of Aβ is impaired leading to increased concentrations of diffusible Aβ even in brain areas distant from the sites of capillary CAA, finally resulting in an Aβ-related impairment of vasomotor function. Another possible explanation for CAA-related blood flow disturbances as a cause for distant hippocampal microinfarcts is that capillary CAA may lead to a general impairment of cerebral blood flow even in brain areas distant from affected vessels. Therefore, vascular adaptation during hypoperfusion or mild hypoxia may be altered in patients with capillary CAA, possibly resulting in microinfarcts in the most vulnerable brain regions (such as the CA1 sector of the hippocampus) as soon as hypoxia lasts 2 min or more [45]. An alternative explanation is that hypoxia itself causes alterations of the basement membrane as described experimentally [20], thereby stimulating vascular Aβ deposition. In a mouse model of Aβ deposition such an effect of hypoperfusion on the development of microinfarcts and CAA has already been reported [40] supporting the concept that the presence of Aβ in the perivascular space increases not only the likelihood of capillary CAA development but also that of hypoxia/hypoperfusion-related microinfarcts in vulnerable brain regions. Moreover, the fact that hypoxia can stimulate vascular Aβ deposition may indicate a vicious cycle ultimately resulting in the development of microinfarcts.
The association between cognitive status and allocortical/hippocampal microinfarcts as well as between allocortical microinfarcts and the presence of CAA type 1 indicates that such microinfarcts appear to be related to AD and Aβ-toxicity and may argue for the existence of subgroups of AD cases based on the CAA type, as previously suggested [50]. Accordingly, CAA type 1-related AD would be characterized by capillary CAA, a strong association with the APOE ε4 allele, predominant plaque pathology [50] and a higher likelihood for development of microinfarcts in allocortical brain regions (namely the CA1 region of the hippocampus). As for infarcts/microinfarcts in other locations (neocortex, subcortical nuclei of the brain, white matter) we did not find such an association with CAA type 1 or CAA severity according to Vonsattel et al. [55] in our sample. The distinction of different subgroups of AD based upon their vascular contribution to the disease by CAA may also have implications for future treatment strategies and for the planning of clinical trials for testing novel treatments.
The limitations of this study are the retrospective evaluation of the CDR scores, the hospital-based sample, and the fact that multiple statistical tests were performed. The retrospective evaluation of the CDR score was necessary because of the autopsy-based method of case recruitment in which cases were recruited only in the event that a permission for autopsy of the brain or the entire body was granted. This strategy also explains differences in the composition of this case cohort in comparison to that of population-based studies. The problem of multiple testing cannot be neglected. Despite these limitations, we provide a cohort of 284 cases with robust neuropathological and retrospectively collected clinical data that suggest that allocortical/hippocampal microinfarcts associated with CAA type 1 and the severity of CAA play a role in a specific subtype of AD cases by the destruction of a brain area that is a key player in memory function.
References
Agresti A (2002) Categorical data analysis. Wiley, Hoboken
Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445
Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA (2017) The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol 27:77–85
Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA (2011) Microinfarct pathology, dementia, and cognitive systems. Stroke 42:722–727
Attems J, Yamaguchi H, Saido TC, Thal DR (2010) Capillary CAA and perivascular Aβ-deposition: two distinct features of Alzheimer’s disease pathology. J Neurol Sci 299:155–162
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Brenowitz WD, Hubbard RA, Keene CD, Hawes SE, Longstreth WT Jr, Woltjer RL, Kukull WA (2017) Mixed neuropathologies and associations with domain-specific cognitive decline. Neurology 89:1773–1781
Calhoun ME, Burgermeister P, Phinney AL, Stalder M, Tolnay M, Wiederhold KH, Abramowski D, Sturchler-Pierrat C, Sommer B, Staufenbiel M, Jucker M (1999) Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc Natl Acad Sci USA 96:14088–14093
Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO (2013) Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol Appl Neurobiol 39:593–611
Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP, Viswanathan A, Greenberg SM (2017) Emerging concepts in sporadic cerebral amyloid angiopathy. Brain 140:1829–1850
Dickson DW (1997) The pathogenesis of senile plaques. J Neuropathol Exp Neurol 56:321–339
Erkinjuntti T, Haltia M, Palo J, Sulkava R, Paetau A (1988) Accuracy of the clinical diagnosis of vascular dementia: a prospective clinical and post-mortem neuropathological study. J Neurol Neurosurg Psychiatry 51:1037–1044
Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD (1999) Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet 354:919–920
Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890
Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E (2007) Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain 130:2830–2836
Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM (2009) Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 8:165–174
Grinberg LT, Thal DR (2010) Vascular pathology in the aged human brain. Acta Neuropathol 119:277–290
Hartley T, Lever C, Burgess N, O’Keefe J (2014) Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc Lond B 369:20120510
Hawkes CA, Michalski D, Anders R, Nissel S, Grosche J, Bechmann I, Carare RO, Hartig W (2013) Stroke-induced opposite and age-dependent changes of vessel-associated markers in co-morbid transgenic mice with Alzheimer-like alterations. Exp Neurol 250:270–281
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140:566–572
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13
Ince PG, Minett T, Forster G, Brayne C, Wharton SB, Medical Research Council Cognitive F, Ageing Neuropathology S (2017) Microinfarcts in an older population-representative brain donor cohort (MRC CFAS): Prevalence, relation to dementia and mobility, and implications for the evaluation of cerebral Small Vessel Disease. Neuropathol Appl Neurobiol 43:409–418
Joachim CL, Morris JH, Selkoe DJ (1988) Clinically diagnosed Alzheimer’s disease: autopsy results in 150 cases. Ann Neurol 24:50–56
Kovari E, Herrmann FR, Gold G, Hof PR, Charidimou A (2017) Association of cortical microinfarcts and cerebral small vessel pathology in the ageing brain. Neuropathol Appl Neurobiol 43:505–513
Larionov S, Dedeck O, Birkenmeier G, Orantes M, Ghebremedhin E, Thal DR (2006) The intronic deletion polymorphism of the a2-macroglobulin gene modulates the severity and extent of atherosclerosis in the circle of Willis. Neuropathol Appl Neurobiol 32:451–454
Lauer A, van Veluw SJ, William CM, Charidimou A, Roongpiboonsopit D, Vashkevich A, Ayres A, Martinez-Ramirez S, Gurol EM, Biessels GJ, Frosch M, Greenberg SM, Viswanathan A (2016) Microbleeds on MRI are associated with microinfarcts on autopsy in cerebral amyloid angiopathy. Neurology 87:1488–1492
Launer LJ, Hughes TM, White LR (2011) Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol 70:774–780
Lee JH, Olichney JM, Hansen LA, Hofstetter CR, Thal LJ (2000) Small concomitant vascular lesions do not influence rates of cognitive decline in patients with Alzheimer disease. Arch Neurol 57:1474–1479
Makela M, Paetau A, Polvikoski T, Myllykangas L, Tanskanen M (2016) Capillary amyloid-beta protein deposition in a population-based study (Vantaa 85 +). J Alzheimers Dis 49:149–157
Mandybur TI (1986) Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol 45:79–90
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Morel F (1950) Petite contribution a l’etude d’une angiopathie apparemment dyshorique et topistique. Monatsschr Psychiatr Neurol 120:352–357
Morris AW, Sharp MM, Albargothy NJ, Fernandes R, Hawkes CA, Verma A, Weller RO, Carare RO (2016) Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol 131:725–736
Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43:2412–2414
Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C (1989) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39:1159–1165
Moser EI, Moser MB, McNaughton BL (2017) Spatial representation in the hippocampal formation: a history. Nat Neurosci 20:1448–1464
Niwa K, Carlson GA, Iadecola C (2000) Exogenous A beta1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab 20:1659–1668
Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C (2000) Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci USA 97:9735–9740
Okamoto Y, Yamamoto T, Kalaria RN, Senzaki H, Maki T, Hase Y, Kitamura A, Washida K, Yamada M, Ito H, Tomimoto H, Takahashi R, Ihara M (2012) Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol 123:381–394
Park L, Koizumi K, El Jamal S, Zhou P, Previti ML, Van Nostrand WE, Carlson G, Iadecola C (2014) Age-dependent neurovascular dysfunction and damage in a mouse model of cerebral amyloid angiopathy. Stroke 45:1815–1821
Shin HK, Jones PB, Garcia-Alloza M, Borrelli L, Greenberg SM, Bacskai BJ, Frosch MP, Hyman BT, Moskowitz MA, Ayata C (2007) Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain 130:2310–2319
Skrobot OA, Attems J, Esiri M, Hortobagyi T, Ironside JW, Kalaria RN, King A, Lammie GA, Mann D, Neal J, Ben-Shlomo Y, Kehoe PG, Love S (2016) Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain 139:2957–2969
Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601
Smith ML, Auer RN, Siesjo BK (1984) The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol 64:319–332
Thal DR, Capetillo-Zarate E, Larionov S, Staufenbiel M, Zurbruegg S, Beckmann N (2009) Capillary cerebral amyloid angiopathy is associated with vessel occlusion and cerebral blood flow disturbances. Neurobiol Aging 30:1936–1948
Thal DR, Ghebremedhin E, Orantes M, Wiestler OD (2003) Vascular pathology in Alzheimer’s disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol 62:1287–1301
Thal DR, Ghebremedhin E, Rüb U, Yamaguchi H, Del Tredici K, Braak H (2002) Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol 61:282–293
Thal DR, Griffin WST, De Vos RAI, Ghebremedhin E (2008) Cerebral amyloid angiopathy and its relationship to Alzheimer’s disease. Acta Neuropathol 115:599–609
Thal DR, Papassotiropoulos A, Saido TC, Griffin WS, Mrak RE, Kölsch H, Del Tredici K, Attems J, Ghebremedhin E (2010) Capillary cerebral amyloid angiopathy identifies a distinct APOE epsilon4-associated subtype of sporadic Alzheimer’s disease. Acta Neuropathol 120:169–183
Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of Abeta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Thal DR, Rüb U, Schultz C, Sassin I, Ghebremedhin E, Del Tredici K, Braak E, Braak H (2000) Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol 59:733–748
Vinters HV (1992) Cerebral amyloid angiopathy. In: Barnett HJM, Mohr JP, Stein BM, Yatsu FM (eds) Stroke Pathophysiology, diagnosis and management. Churchill Livingstone, New York, pp 821–858
Vinters HV, Ellis WG, Zarow C, Zaias BW, Jagust WJ, Mack WJ, Chui HC (2000) Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol 59:931–945
Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP Jr (1991) Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol 30:637–649
Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE (1998) Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol 153:725–733
White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR (2002) Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann NY Acad Sci 977:9–23
Wyler AR, Dohan FC, Schweitzer JB, Berry AD III (1992) A grading system for mesial temporal pathology (hippocampal sclerosis) from anterior temporal lobectomy. J Epilepsy 5:220–225
Acknowledgements
This study was funded by grants from Alzheimer Forschung Initiative (AFI) Grant no.: #13803 (DRT); Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO- G0F8516 N Odysseus) (DRT), Vlaamse Impulsfinanciering voor Netwerken voor Dementie-onderzoek (IWT 135043) (DRT), the German Federal Ministry of Education and Research (FTLDc O1GI1007A) (MO), the EU (FAIRPARKII 633190) (MO), the foundation of the state Baden-Württemberg (D.3830) (MO), Thierry Latran Foundation (MO), ALS Association (MO), and BIU(MO). The authors gratefully thank Ms. Alicja Ronisz, Marta Koper, Irina Kosterin, Christine Schneider, Kathrin Pruy, Daniela Demharter, and Alice Yeates for technical help. We acknowledge the help of Ms. Jill Holbrook and Sandra Tomé for reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DRT received consultant honorary from GE-Healthcare and Covance Laboratories and collaborated with Novartis Pharma AG, and Janssen Pharmaceutical Companies. CAFVA received research support from Roche Diagnostics, Biologische Heilmittel Heel, and ViaMed, honoraria from serving on the scientific advisory board of Nutricia, and received speaker honoraria from Nutricia, Lilly Germany, Desitin Arzneimittel, Biogen and Dr. Willmar Schwabe GmbH&Co.KG.
Rights and permissions
About this article
Cite this article
Hecht, M., Krämer, L.M., von Arnim, C.A.F. et al. Capillary cerebral amyloid angiopathy in Alzheimer’s disease: association with allocortical/hippocampal microinfarcts and cognitive decline. Acta Neuropathol 135, 681–694 (2018). https://doi.org/10.1007/s00401-018-1834-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-018-1834-y