Abstract
Synthesis of fullerene (C60)-tethered polymer-grafted silica and colloidal crystallization of the particles was investigated. The particles were prepared by the reaction of C60 with 4-azidobenzoyl groups introduced in poly(methyl methacrylate-co-2-hydroxyethyl methacrylate), followed by esterification of 2-hydroxyethyl metharylate moieties with 4-azidobenzoyl chloride and grafting onto colloidal silica. The reaction afforded bindings of C60 in the range from 0.44 × 104 to 1.71 × 104 molecules/particle. The C60 amounts did not monotonously increase with 4-azidobenzoyl group on the particles, but decreased with mole fraction of methyl methacrylate in the copolymer. Colloidal crystallizations of the C60-tethered silica particles were observed in acetonitrile with critical volume fractions in the range from 0.018 to 0.024. Inter-sphere distances in the colloidal crystals were consistent with calculated values on assumption of face-centered cubic-closed packing, and then it was suggested that the crystallization took place due to electrostatic repulsion between the particles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Three-dimensional (3D) particle-arrayed structure, inter-sphere distance of which is comparable to visible light wave length, has been receiving much attention for the promising application to optical devices, such as wave guide, sensor, and so on [1–6]. So far, many approaches for fabrication of 3D particle arrays have been reported [1–22]. One of the attractive approaches is the fabrication employing colloidal crystals formed in solution due to easy tuning inter-sphere space by changing the volume fraction and exhibiting sharp and clear Bragg reflection. The fabrications have been mostly carried out by immobilization of colloidal crystals formed in aqueous solution in hydrogels [7, 10, 14–18]. However, in the practical application, it is quite difficult to utilize the hydrogels for optical devices because they contain much water. In this regard, we have successfully achieved colloidal crystallization of polymer-grafted silica in organic solvents [23–26] and then immobilization of the crystals in polymer matrix [19–22]. Colloidal crystallizations in organic solvents are favorable for fabrication of 3D particle-arrayed structure by immobilization in polymer matrixes because of being able to utilize various polymerization reactions.
Concerning colloidal crystallization in solution, in many cases, monodisperse colloidal silica, polystyrene, and poly(methyl methacrylate) are employed for colloidal particles because of giving stable crystallites. Among them, colloidal silica usually brings stable colloidal crystals in aqueous solution due to negatively high surface charge. However, fabrication of 3D particle-arrayed optical device from colloidal crystals of silica has major shortcomings stemming from original property of silica, comparatively low refractive index, and dielectric constant. In this respect, we have reported that introduction of ferrocenyl groups in polymer grafted onto silica particles effectively increases refractive index of colloidal crystal system [27].
Meanwhile, fullerenes have been attractive and highlighted materials due to spherical π-conjugated molecule exhibiting characteristic properties, i.e., electron accepting or releasing abilities, high dielectric constant, high heat conductivity, thermal stability, high refractive index, radical trapping, UV absorption, and so on. Thus, C60 and C60-based nanomaterials have been contributing to a variety of promising application to functional materials, such as high surface area particles and supports in catalysis [28], electron carriers in electronic devices [29], and semiconductors [30, 31]. Furthermore, Tu and coworkers have recently reported that grafting of C60 into polyesters elevates refractive index to give the maximum value of 1.79 [32]. Therefore, incorporation of C60 into colloidal crystals could lead not only to improvement of refractive index but also to challenging fabrication of new functional materials, exhibiting specific properties of C60. In this study, preparation of C60-tethered polymer-grafted silica (C60/polymer/SiO2) and colloidal crystallization of the composite particles in organic solvent was investigated.
Experimental
Materials
Colloidal silica aqueous sol, containing 20 wt% SiO2 of 134 nm in diameter with a polydispersity 0.030, was kindly gifted by Nikki Catalysts & Chemical Co. Ltd., Kanagawa, Japan. Fullerene (C60), Nanom purple ST, was purchased from Frontier Carbon Co. Ltd., Tokyo, Japan. Methyl methacrylate (MMA) and 2-hydroxyethyl methacrylate (HEMA), (3-mercaptopropyl)trimethoxysilane, 2,2′-azobis(isobutyronitrile) (AIBN), tetrahydrofuran (THF), N,N,N-triethylamine, diethyl ether, 1,2-dimethoxyethane (DME), acetonitrile, and toluene were obtained from Wako Chemicals Co. Ltd., Osaka, Japan. 4-Azidobenzoyl chloride was purchased from Kanto Chemicals Co. Ltd., Tokyo, Japan.
Measurements
Number average of molecular weight (M n) of the synthesized polymers were determined by a gel permeation chromatography (GPC) on the columns, TSK gel G4000H6, and G5000H6, Tosoh Co. Ltd., Yamaguchi, Japan, at 35 °C using THF as an eluent at the flow rate of 0.8 mL/min calibrated with a polystyrene standard. 1H NMR spectra for solution samples and 13C CP/MAS NMR spectra for solid state samples were recorded on a Bruker AVANCE 400 (400 MHz) and 300 (300 MHz) using a 7-mm rotor prove, Karisruhe, Germany respectively. Amounts of grafted polymer and C60 were determined by a thermal-gravimetric analysis on TG-50, Shimadzu Co. Ltd., Kyoto, Japan, during elevating temperature up to 800 °C at heating rate 10 °C/min. Particle size and its distribution were determined by a dynamic light scattering (DLS) on an Otsuka Electronics DLS-7000 spectrophotometer, equipped with a He–Ne laser (10 mW, 633 nm), Osaka, Japan. Reflection spectra of colloidal crystals were recorded on a multichannel spectrometer, Hamamatsu Photonics PMA-11, Shizuoka, Japan.
Synthesis of trimethoxysilyl-capped poly(MMA-co-HEMA)(1)
A typical run was as follows. A mixture of 12.0 mL (112 mmol) MMA, 0.68 mL (5.6 mmol) HEMA, 10 mg (0.07 mmol) (3-mercaptopropyl)trimethoxysilane, 16 mg (0.17 mmol) AIBN, and 20 mL dry THF was put into a 50-mL flask and stirred at 70 °C for 10 h in N2 atmosphere. After evaporation of THF from the mixture and precipitation with diethyl ether, drying under reduced pressure gave 7.2 g copolymer 1, of Mn 24,000 and MMA/HEMA mole ratio 14.7:1.0. The mole ratio was determined by the area ratio of resonance peak at 3.63 ppm assigned to methyl protons of MMA moiety to peaks at 3.87 and 4.15 ppm assigned to methylene protons of HEMA moiety on 1H NMR spectrum (Fig. 2a). 1H NMR (CDCl3): 0.87, 1.05, 1.23 (m, CH3), 1.76–2.15 (broad, CH 2 , CH), 3.63 (s, OCH 3 ), 3.87 (broad, COOCH 2 ), and 4.15 ppm (broad, CH 2 OH).
Synthesis of trimethoxysilyl-capped poly(methyl methacrylate-co-2-(4-azidobenzoyloxy)ethyl metharylate) (2)
Into a 50-mL flask, 2.0 mL N,N,N-triethylamine, 3.0 g 1, and 30 mL dry chloroform were put, and the mixture was cooled on an ice bath. Chloroform solution 2.0 mL containing 3 g (17 mml) 4-azidobenzoyl chloride was added dropwise to the solution, followed by stirring for 6 h at room temperature. Filtration, evaporation of solvent, and precipitation with diethyl ether gave 2.45 g 2. 1H NMR (CDCl3): 0.87, 1.05, 1.23 (m, CH 3 ), 1.76–2.15 (broad, CH 2 , CH), 3.63 (s, OCH 3 ), 3.87 (broad, COOCH 2 ), 4.15 (broad, CH 2 OH), 4.32 (broad, COOCH 2 CH2OC=OC6H4N3), 4.55 (broad, CH 2 OC=OC6H4N3), 7.18 (broad, o−CH 2 (C=OC6H4N3)), and 8.09 ppm (board, m–CH 2 (C=OC6H4N3)).
Preparation of poly(methyl methacrylate-co-2-(4-azidobenzoyloxy)ethyl metharylate)-grafted silica (3)
Colloidal silica suspended in ethanol was prepared by solvent exchanging with azeotropic evaporation of water after addition of ethanol to the original aqueous sol. A mixture of 0.5 g 2, 50 mL colloidal silica ethanol suspension, containing 1.0 g SiO2, and 50 mL DME was put into a 100-mL flask. After sonication for 30 m, the suspension was stirred at 90 °C for 5 h along with azeotropical removal of ethanol. Centrifugal washing with THF eight times and drying under reduced pressure gave 1.0 g 3, with 47.8 mg/g grafted polymer. 13C CP/MAS NMR: 10.3–25.2 (broad, −CH2–C(CH3)(C=O)−), –CH2–C(CH3)(C=O)–), 44.5 (–CH2–C(CH3)(C=O)−), 40.8–62.0 (broad, O–CH3, O–CH2 CH2–O), and 177.8 ppm (C=O).
Reaction of C60 with 3
Into 20 mL toluene, 50 mg C60 and 1.0 g 3 were put, and the mixture was stirred at 110 °C under a nitrogen atmosphere for 24 h. Centrifugal separation of resulting particles with toluene and drying under reduced pressure gave 0.88 g 4. 13C CP/MAS NMR: 9.9–26.8 (broad, –CH2–C(CH3)(C=O)–), –CH2–C(CH3)(C=O)–), 44.6 (–CH2–C(CH3)(C=O)–), 39.1–70.3 (broad, O–CH3, O–CH2 CH2–O), 107.7–153.0 (broad, –C 60), and 177.3 ppm (C=O).
Determination of grafted polymer and C60 on silica
Amounts of grafted polymer on 3 and 4 were determined by weight decrease (Wpolymer) during elevation from 170 to 420 °C on a thermogravimetric analysis. The amount of C60 tethered on 4 was also determined by weight loss (Wc60), corresponding to C60 ignition, during elevation from 550 to 800 °C. Typical thermograms of 4, along with those of poly(MMA-co-HEMA) 2 with mole ratio of MMA/HEMA = 1.9/1.0 and C60, are shown in Fig. 1.
Observation of colloidal crystallization and determination of inter-sphere distance
Colloidal crystallization of silica composite particles in organic solvent was observed by naked eyes and a digital camera. Inter-sphere distance (d cal) in colloidal crystal was calculated from the volume fraction on assumption of face centered cubic (fcc) closed packing by Eq. (1) [33]:
where ϕ is volume fraction of polymer-grafted silica, d cal is neighboring inter-sphere distance, and r is diameter of the particle. The inter-sphere distance (d obs) in the crystals was also determined according to Bragg formula by following the equation [34]:
where λ p is the peak top wavelength on a reflection spectrum and n is average refractive index of the suspension system calculated by Eq. (3)
where n silica and n sol are refractive index of silica and solvent, respectively, and ϕ is volume fraction of silica. Equations (1), (2), and (3) are given in detail in Online resource.
Results and discussion
Synthesis of C60/polymer/SiO2 (4)
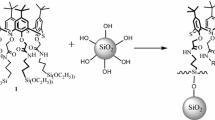
In Scheme 1, the synthetic route of 4 was shown. Trimethoxysilyl-terminated poly(MMA-co-HEMA), 1, was synthesized by a radical copolymerization of MMA and HEMA in the presence of (3-mercaptopropyl)trimethoxysilane of a chain transfer reagent using AIBN as an radical initiator. The polymers of Mn = 11,000–24,000 with mole ratios of MMA/HEMA in the range from 1.9:1.0 to 14.7:1.0 were obtained by changing feed ratio of the monomers (Table 1). The reaction of 4-azidobenzoyl chloride with 1 was carried out in the presence of N,N,N-triethylamine at 4 °C. In Fig. 2, 1H NMR spectra of respective 1 and 2 were shown. The introduction of 4-azidobenzoyl group into HEMA moieties in 2 was confirmed by the appearance of resonance peaks at 4.32 and 4.55 ppm on 1H NMR spectrum, assignable to protons in ethylene group of HEMA moiety, and at 7.18 and 8.09 ppm, assignable to protons in 4-azidobenzoyl group (Fig. 2). Amounts of 4-azidobenzoyl group were determined by area ratio of peaks at 7.18 and 9.08 ppm to peaks at 3.87 and 4.15 ppm, assignable to unreacted methylene groups of HEMA moiety. The amounts of 4-azidobenzoyl group unexpectedly increased with decreasing HEMA fraction in 1 (Table 1). Polymer chains of 1 with high MMA fraction were probably favorable to react with 4-azidobenzoyl chloride due to highly affinity with chloroform. The reaction of 2 with colloidal silica particles was conducted in DME at 90 °C for 5 h along with azeotropical removal of ethanol. Average particle sizes of 3 were in the range from 145 to 150 nm, summarized in Table 1, being 11–16 nm larger than that of the original silica. Particle sizes of 3 slightly became larger by polymer grafting, but distributions of particle size were still narrow, as shown in Fig. 3. Therefore, we confirmed that aggregation between the particles scarcely occurred during the reaction. A 13C CP/MAS NMR spectrum of 3 distinctly indicated grafting of 2 on silica particles by the appearance of resonance peaks at 10.3–25.2, 44.5, 40.8–62.0, and 177.8 ppm assigned to CH2 on polymer backbone and branched CH3, quaternary carbon, OCH3 of MMA moieties, and OCH2 CH2O– of HEMA in HEMA moieties and carbonyl respectively, as shown in Fig. 4. The grafting reactions resulted in bindings of 4-azidobenzoyloxy groups in the range from 10.5 to 51.5 mmol/g-SiO2 on 3 (Table 1). Amounts of 4-azobenzoyl group on 3, calculated from grafted polymer on 2, decreased with increasing of MMA fraction in 1. The reaction of C60 with 3 was carried out in toluene at 110 °C under a nitrogen atmosphere for 30 h to give bindings of C60 from 1.90 to 7.37 mg/g-SiO2, that is, from 2.63 to 10.2 μmol/g-SiO2. The bindings of C60 on 4 were confirmed by the appearance of resonance peeks at 107.7–153.0 ppm, assignable to carbon atoms in C60, on a 13C CP/MAS NMR spectrum in Fig. 4. Particle sizes of 4 were 157–167 nm, being ca. 15 nm larger than those of 3, but those distributions were still narrow (Fig. 3c). Thus, it was observed that aggregation between particles of 3 scarcely took place during the reaction of 3 with C60. Interestingly, amounts of tethered C60 decreased with mole ratio of MMA/HEMA in 1, not simply with amounts of 4-azidobenzoyl group on 3. Probably, polymer chains with 4-azidobenzoyl groups on 3, prepared from high mole fraction of HEMA in 1, might have high flexibility during the reaction of C60 with 3 in toluene. In other words, 4-azidobenzoyl group in grafted polymer composed of high mole fraction of MMA moiety on 3 could be less active for C60 bindings due to shrinking of polymer chains in toluene.
Colloidal crystallization
The authors have reported that poly(methyl methacrylate)-grafted silica particles formed colloidal crystals in polar solvents, such as CH3CN, acetone, and N,N-dimethylformamide [23–25]. When spheres of 4 were dispersed in CH3CN, formation of colloidal crystals was observed. Typical photographs and reflection spectra of the crystals were shown in Fig. 5. Color of the crystals of 4 with much amount of tethered C60 was dark green, which gradually became pale green with decrease of the C60 amount, probably due to absorption of C60 at near ultraviolet light region. In Table 2, critical volume fractions of 4, ϕo, being minimal volume fraction in the crystallization in CH3CN were listed. Values of ϕo for colloidal crystallization in CH3CN were in the range from 0.018 to 0.022, being mostly comparable in the crystallization of poly(MMA)-grafted silica [23, 24]. Values of ϕo in CH3CN were independent on C60 amounts on silica. The reasons for the phenomenon were still unclear.
In Table 3, inter-sphere distances, d obs and d cal, in colloidal crystals of 4 in CH3CN were summarized. The observed values of d obs estimated by Eq. (2) were well coincident with d cal, which were evaluated on postulation of fcc-closed packing from volume fraction of the particles by Eq. (1). Therefore, these results indicated that the colloidal crystallization took place based on electrostatic repulsion between the particles to form fcc-closed packing, as well as ones of colloidal silica in aqueous solution [34]. Colloidal crystallization of spherical particles in solution predominantly holds stable fcc structure rather than bcc packing [34].
Conclusions
Fullerene(C60)-tethered polymer-grafted silica spheres were successfully synthesized via reaction of C60 with 4-azidobenzoyl group in poly(MMA-co-HEMA) grafted on silica. Bindings of C60 on poly(MMA-co-HEMA)-grafted silica were confirmed by appearance of characteristic resonance peaks at 107–153 ppm, assignable to carbon atoms of C60, on a 13C CP/MAS NMR spectrum. The reaction afforded bindings of C60 in the range from 2.63 to 10.2 μmol/g-SiO2, corresponding to 0.44 × 104 to 1.71 × 104 molecules/particle, on the polymer-grafted silica. Colloidal crystallization of C60/polymer/SiO2 particles was observed in CH3CN, and critical volume fraction in the crystallization was in the range from 0.018 to 0.024. Inter-sphere distances in the colloidal crystals mostly agreed with calculated values on assumption of fcc-closed packing. Therefore, it was suggested that the crystallization occurred due to electrostatic repulsion between the particles as well as those of colloidal silica particles in aqueous solution.
References
Holtz JH, Asher SA (1997) Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 389:829–832
Reese CE, Mikhonin AV, Kamenjicki M, Tikhonov A, Asher SA (2004) Nanogel nanosecond photonic crystal optical switching. J Am Chem Soc 126:1493–1496
Muscatello MMW, Stunja LE, Thareja P, Wang L, Bohn JJ, Velankar SS, Asher SA (2009) Dependence of photonic crystal nanocomposite elasticity on crystalline colloidal array particle size. Macromolecules 21:4403–4406
Xia Y, Gates B, Yin Y, Lu Y (2000) Monodispersed colloidal spheres: old materials with new applications. Adv Mater 12:693–713
Lawrence JR, Ying Y, Yiang P, Foulger SH (2006) Dynamic tuning of organic lasers with colloidal crystals. Adv Mater 18:300–303
Park JH, Ohoi WS, Koo HY, Kim DY (2005) Colloidal photonic crystal with graded refractive-index distribution. Adv Mater 17:879–885
Zhou J, Cai T, Tang S, Marquez M, Hu Z (2006) Growth of columnar hydrogel colloidal crystals in water−organic solvent mixture. Langmuir 22:863–866
Li Y, Kunitake T, Fujikawa S, Ozasa K (2007) Photoluminescence modification in 3D-ordered films of fluorescent microspheres. Langmuir 23:9109–9113
Zhou Z, Yan Q, Li Q, Zhao XS (2007) Fabrication of binary colloidal crystals and non-close-packed structures by a sequential self-assembly method. Langmuir 23:1473–1477
Hosein ID, Lindell CM (2007) Homogeneous, core−shell, and hollow-shell ZnS colloid-based photonic crystals. Langmuir 23:2892–2897
Weekes SM, Ogrin FY, Murray WA, Keatley PS (2007) Macroscopic arrays of magnetic nanostructures from self-assembled nanosphere templates. Langmuir 23:1057–1060
Camargo PH, Lee YH, Jeong U, Zou Z, Xia Y (2007) Cation exchange: a simple and versatile route to inorganic colloidal spheres with the same size but different compositions and properties. Langmuir 23:2985–2992
Nakamura H, Ishii M (2005) Effects of compression and shearing on the microstructure of polymer-immobilized non-close-packed colloidal crystalline arrays. Langmuir 21:11578–11581
Nakamura H, Mitsuoka T, Ishii M (2006) Microstructures and optical features of polymer-immobilized non close-packed colloidal crystalline array. J Appl Polym Sci 102:2308–2314
Kumada M, Watanabe M, Takeoka Y (2006) Preparations and optical properties of ordered arrays of submicron gel particles: interconnected state and trapped state. Langmuir 22:4403–4407
Sakai T, Takeoka Y, Seki T, Yoshida R (2007) Organized monolayer of thermosensitive microgel beads prepared by double-template polymerization. Langmuir 23:8651–8654
Toyotama A, Yamanaka J, Shinohara M, Onda S, Sawada T, Yonese M, Uchida F (2009) Gel immobilization of centimeter-sized and uniform colloidal crystals formed under temperature gradient. Langmuir 25:589–593
Evanoff DD, Hayes SE, Ying Y, Shim GH, Lawrence JR, Carroll JB, Roeder RD, Houchins JM, Huebner CF, Foulger SH (2007) Functionalization of crystalline colloidal arrays through click chemistry. Adv Mater 19:3507–3512
Yoshinaga K, Mouri E, Ogawa J, Nakai A, Ishii M, Nakamura H (2004) Preparation of poly(methyl methacrylate) films containing silica particle array structure from colloidal crystals. Colloid Polym Sci 283:340–343
Yoshinaga K, Fujiwara K, Mouri E, Ishii M, Nakamura H (2005) Stepwise controlled immobilization of colloidal crystals formed by polymer-grafted silica particles. Langmuir 21:4471–4477
Yoshinaga K, Satoh S, Mouri E, Nakai A (2006) Immobilization of colloidal crystals, formed by polymer-grafted silica in organic solvent, in physical gels. Colloid Polym Sci 285:694–698
Ma Z, Watanabe M, Mouri E, Nakai A, Yoshinaga K (2011) Effects of particle volume fraction on distortion of particle-arrayed structure during immobilization of colloidal crystals formed by poly(methyl methacrylate)-grafted silica in acetonitrile. Colloid Polym Sci 289:85–91
Yoshinaga K, Chiyoda M, Yoneda A, Nishida H, Komatsu M (1999) Formation of colloid crystals from polymer-modified monodisperse colloidal silica in organic solvents. Colloid Polym Sci 277:479–482
Yoshinaga K, Chiyoda M, Ishiki H, Okubo T (2002) Colloidal crystallization of monodisperse and polymer-modified colloidal silica in organic solvents. Colloids Surf A 204:285–293
Yoshinaga K, Fujiwara K, Tanaka Y, Nakanishi M, Takesue M (2003) Immobilization of colloidal crystals, formed from polymer-modified silica in organic solvent, in polymer gel with radical polymerization. Chem Lett 32:1082–1083
Yoshinaga K, Shigeta M, Komune S, Mouri E, Nakai A (2007) Colloidal crystallization of colloidal silica modified with ferrocenyl group-containing polymers in organic solvents. Colloids Surf B 54:108–113
Ma Z, Watanabe M, Mori E, Yoshinaga K (2010) Effects of ferrocenyl group on refractive index of colloidal crystal system formed by polymer-grafted silica in organic solvent. Colloid Polym Sci 288:519–525
Dresselhaus MS, Dresehaus G, Eklund PC (1996) Science of fullerenes and carbon nanotubes. Academic, San Diego
Bonifazi D, Enger D, Diedrich F (2007) Supramolecular [60] fullerene chemistry on surfaces. Chem Soc Rev 36:390–444
Lee JK, Ma WL, Brabec CJ, Yuen J, Moon JS, Kim JY, Lee K, Bazan GC, Heeger AJ (2008) Processing additives for improved efficiency from bulk heterojunction solar cells. J Am Chem Soc 130:3619–3623
Fernandez G, Sanchez L, Veldman D, Wienk MM, Atienza C, Guldi DM, Janssen RAJ, Martin N (2008) Tetrafullerene conjugates for all-organic photovoltaics. J Org Chem 73:9–3196
Yan H, Chen S, Lu M, Zhu X, Li X, Wu D, Tu Y, Zhu X (2014) Side-chain fullerene polyesters: a new class of high refractive index polyesters. Mater Horiz 1:247–250
Okubo T, Okada S (1998) Kinetic analyses of colloidal crystallization in alcoholic organic solvents and their aqueous mixtures as studied by reflection spectroscopy. J Colloid Interface Sci 204:198–204
Okubo T (1996) Importance of electrical double layers in structural and diffusional properties of deionized colloidal suspensions. Colloid Surf A 109:77–88
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 132 kb)
Rights and permissions
About this article
Cite this article
Yang, Y., Ohno, T. & Yoshinaga, K. Colloidal crystallization of C60/polymer-grafted silica particles in organic solvent. Colloid Polym Sci 293, 2075–2081 (2015). https://doi.org/10.1007/s00396-015-3601-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3601-0