Abstract
The incorporation of monodisperse, polymer-modified silica into poly(methyl metharylate) to prepare polymer films containing particle array structure was investigated. The preparation was carried out by a two-step radical polymerization for gelation and solidification. The colloidal crystallization of poly(methyl metharylate)-modified silica, in 78 nm size, in acetonitrile and successive copolymerization of methyl methacrylate and 1,2-dimethacryloylethane by UV light irradiation gave the polymer gel containing the colloidal crystal structure. The exchange of acetonitrile in the gel with methyl methacrylate and further photo-radical polymerization gave the durable polymer film composed of silica particle array.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
For an aqueous or alcoholic colloidal suspension, it is well-known that monodisperse and spherical colloidal particles periodically array in a limited range of volume fractions [1–3]. The 3D particle array, the so called “colloidal crystals,” is based on an electrostatic repulsive interaction among the particles, arising from the spreading of the electric double layer [3–5]. In this respect, we have previously reported that monodisperse and polymer-modified silica forms the colloidal crystals in organic solvent, which is a polar and good solvent for grafted polymer [6, 7]. If it were possible to incorporate the colloidal crystal structure into polymer matrix, the polymer would lead to a noble functional material. For example, the periodic particles array, i.e. photonic crystal, has recently been receiving much attention with regard to application to optical devices, such as dielectric mirror or photonic filters [8–10]. Concerning the application of colloidal crystals to photonic crystals, the immobilization of the crystals into polymer film or sheet is one of the essential processes. Regarding the immobilization, some researchers have so far reported the immobilization of colloidal crystals into polymer hydrogels by a radical polymerization [11, 12]. However, it seems to be difficult to conduct further solidification of hydrogels by chemical reactions. In this sense, the colloidal crystals, formed in organic solvent, have advantages for the solidification, because it is possible to choose various organic reactions or polymerizations in organic media to solidify. As reported previously [13], in order to immobilize colloidal crystals of polymer-modified silica into polymer matrix, the incorporating should be carried out by means of a two-step polymerization, first gelation and successive solidification, because radical polymerization of a vinyl compound in the suspension containing colloidal crystals leads to phase separation between silica and polymer. We have reported that photo-irradiated radical copolymerization of methyl methacrylate (MMA) and 1,2-dimethacryloyloxyethane (EDM) in the poly(methyl methacrylate)-modified silica (PMMA/SiO2) successfully gave the polymer gels including the silica particles array, keeping the colloidal crystal structure intact [13]. In this case, a slow and lesser extent of cross-linking in the polymerization resulted in high maintenance of the crystal structure. In this paper, we describe the preparation of polymer films via the PMMA gels containing PMMA/SiO2 colloidal crystals by radical polymerization.
2 Experimental
2.1 Materials
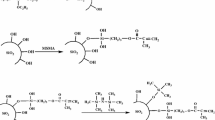
Monodisperse colloidal silica, containing 40% SiO2 of 78 nm diameter suspended in ethanol, was kindly offered by Catalysts & Chemicals Ind. Co, Japan. Trimethoxysilyl-terminated poly(methyl metharylate) (PMMA-Si(OMe)3) of number average molecular weight 8,100 was synthesized by the method reported previously [14]. The particles of PMMA/SiO2 were prepared by the reaction of the colloidal silica with PMMA-Si(OMe)3 in acetone [15].
2.2 Measurements
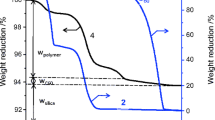
The amount of grafted polymer was estimated from the weight loss during temperature elevation from 100 to 800 °C, after maintaining at 100 °C for 1 h to remove moisture. The critical volume fraction (ϕ0) of PMMA/SiO2 in the colloidal crystallization was determined by the naked eye or a digital camera. Reflection spectra of colloidal crystals were recorded on a photonic multi-channel spectral analyzer, Hamamatsu Photonics PMA-111, employing a 150-W halogen lamp on Hayashi LA-150UX. Scanning electron micrographs were recorded on JEOL JSM-6320F.
2.3 Gelation by radical polymerization
Gelation of PMMA/SiO2 suspension of CH3CN was carried out by photo-radical polymerization. A typical run was as follows. The suspension of 0.25 cm3 acetonitrile solution composed of PMMA/SiO2(ϕ=0.0985), 22.6 wt% MMA, 3.7 wt% EDM, and 6 mmol/L 2,2′-azobis(isobutyronitrile) was put into a 2-mm thickness Pyrex cell and set up on a chamber, which was equipped with a cooling water jacket. The polymerization was carried out by photo-irradiation by a 500 W high pressure Hg lamp for 5~7 h. The resulting gel was taken out from the cell and supplied to the measurement of the reflection spectrum.
2.4 Solidification by radical polymerization
The above gel was put into the cell again. The cell was immersed into MMA at room temperature for 24 h to exchange CH3CN with MMA in the gel. The polymerization was carried out in the MMA vapor-saturated cell by UV light irradiation, as described above.
3 Results and discussion
As reported previously, the addition of low polar compounds, such as MMA or EDM, made the colloidal crystals unstable to increase ϕ0[9]. In Fig. 1, dependences of MMA and EDM addition on ϕ0 for the colloidal crystallization of PMMA/SiO2 in CH3CN are shown. Addition of EDM to the suspension system remarkably increased ϕ0 and then the crystallization did not take place above 11 wt% EDM concentration. Addition of MMA also resulted in increasing ϕ0, but the crystallization was observed at less than 50 wt% MMA concentrations. These results showed that the gelation should be carried out under the critical concentration of MMA and EDM in order to keep crystals stable. On the basis of these results, we optimized polymerization conditions for the formation of gel, and then successfully made the gel incorporated the colloidal crystals by the polymerization in PMMA/SiO2 suspension of the volume fraction (ϕ)=0.098–0.157, 19.5–22.2 wt% MMA and 3.3–3.9 wt% EDM in acetonitrile.
In Figs. 2 and 3, typical reflection spectra of the gel formed using a 2-mm and a 1-mm cell are shown, respectively. In these cases, we could observe the reflection peaks based on Bragg reflection. When the gelation was carried out in a 2-mm cell, the resulting gel showed reflection peaks separated and shifted from the one before gelation. The reflection peaks indicate that the space between neighboring particles becomes long in the front side of the cell and short in the rear side. A difference in the particle space between the front and rear sides of the cell might arise from the heterogeneous cross-linking during the polymerization. On the other hand, the polymerization in a 1-mm cell gave the gel, which showed less shifted reflection peaks of the front and rear side, as compared with the one before polymerization. From these reflection peaks, it was estimated that average space distances between neighboring particles were 230 and 236 nm at the front and rear side on an assumption of face-centered cubic array, respectively, while the average distance before the gelation was 235 nm. Therefore, it is suggested that polymerization employing a 1-mm cell gave the polymer gel containing less distorted particle array structure due to relatively homogeneous polymerization. However, the intensity of the reflection peak of the gel was lower than that of the peak before the gelation. The lowering of the intensity suggested that the polymerization decreased the number of crystallites.
The exchange of acetonitrile with MMA in the gel and successive UV light irradiation in each cell gave a durable film showing slight iridescent color due to Bragg light reflection. A typical photograph of a PMMA film prepared using a 1-mm cell is shown in Fig. 4. In this case, the refractive index of PMMA is 1.490 [16], being very close to that of silica, and therefore the resulting PMMA film was translucent. In Fig. 5, a SEM photograph of the cross-section of the PMMA film containing PMMA/SiO2 is shown. The micrograph showed the silica particles periodically arrayed in the cross section, while some damages due to the electron irradiation during SEM measurement were observed. The average distance between neighboring particles was 163 nm, which was 73 nm shorter than that in the gel. These results showed that the shrinking took place during the second polymerization, but relatively homogeneous polymerization occurred in the gel. In this sense, the procedure of the particles array structure immobilization in polymer gel and solidification would be applicable to the preparation of a new material.
In conclusion, the gelation by radical copolymerization of MMA and EDM in PMMA/SiO2 acetonitrile suspension, which caused colloidal crystallization, successfully afforded PMMA gels containing particle array structure. Further photo-irradiation to the gel, which was thoroughly swelled with MMA, gave a solid film having the array structure. Investigation on the preparation of a polymer matrix that includes much large sized colloidal crystals for the practical application is now in progress.
References
Aastuen DJW, Clark NA, Cotter LK, Ackerson BJ (1986) Phys Rev Lett 57:1733
Pulsey PN, van Mengen W (1986) Nature 320:340
Okubo T (1988) Acc Chem Rev 21:281
Stevens MJ, Falk ML, Robins MO (1990) J Chem Phys 104:5209
Okubo T (1993) Prog Polym Sci 28:481
Yoshinaga K, Chiyoda M, Yoneda A, Nishida H, Komatsu M (1999) Colloid Polym Sci 28:481
Yoshinaga K, Chiyoda M, Ishiki H, Okubo T (2002) Colloid Surfaces A 204:285
Zubrzycki WH, Hou H, Alleman A (2000) Nature 407:983
Wong S, Kitaev V, Ozin GA (2003) J Am Chem Soc 125:15589
Lu Y, Mclellan J, Xia YN (2004) Langmuir 20:3464
Iwayama Y, Yamanaka J, Taniguchi Y, Takasaka M, Ito K, Shinohara T, Sawada T, Yonese M (2003) Langmuir 19:977
Lellig C, Hartl W, Wagner J, Hempelmann R(2002) Angew Chem Int Ed Engl 41:102
Yoshinaga K, Fujiwara K, Tanaka Y, Nakanishi M, Takesue M (2003) Chem Lett 32:1082
Yoshinaga K, Horie R, Saigoh F, Kito T, Enomoto N, Nishida H, Komatsu M (1992) Polym Adv Technol 3:91
Yoshinaga K, Tani Y, Tanaka Y (2002) Colloid Polym Sci 280:85
Altmann RB, Renge I, Kador L, Haarer D (1992) J Chem Phys 97(8):5316
Acknowledgements
This work was supported by Grant-in-Aid for Science Research (No. 14350499) from Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshinaga, K., Mouri, E., Ogawa, J. et al. Preparation of poly(methyl methacrylate) films containing silica particle array structure from colloidal crystals. Colloid Polym Sci 283, 340–343 (2004). https://doi.org/10.1007/s00396-004-1202-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1202-4