Abstract
Ischaemic preconditioning (IPC) provides myocardial resistance to ischaemia/reperfusion (I/R) injuries. The protection afforded by IPC is not limited to the target tissue but extends to remote tissues, suggesting a mechanism mediated by humoral factors. The aim of the present study was to identify the humoral factors that are responsible for the cardioprotection induced by the coronary effluent transferred from IPC to naïve hearts. Isolated rat hearts were submitted to IPC (three cycles of 5 min I/R) before 30-min global ischaemia and 60-min reperfusion. The coronary effluent (Efl_IPC) collected during IPC was fractionated by ultrafiltration in different molecular weight ranges (<3, 3–5, 5–10, 10–30, 30–50, and >50 kDa) and evaluated for cardioprotective effects by perfusion before I/R in naïve hearts. Only the <3, 5–10 and <10 kDa fractions of hydrophobic eluate reduced I/R injuries. The cardioprotective effect of the 5–10 fraction was blocked by KATP channel blockers and a PKC inhibitor. An Efl_IPC proteomic analysis revealed 14 cytoprotection-related proteins in 4–12 kDa peptides. HSP10 perfusion protected the heart against I/R injuries. These data provide insights into the mechanisms of cardioprotection in humoral factors released by IPC. Cardioprotection is afforded by hydrophobic peptides in the 4–12 kDa size range, which activate pathways that are dependent on PKC and KATP. Fourteen 4–12 kDa peptides were identified, suggesting a potential therapeutic role for these molecules in ischaemic diseases. One of these, HSP10, identified by mass spectrometry, reduced I/R injuries and may be a potential candidate as a therapeutic target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischaemic preconditioning (IPC) protects the myocardium against ischaemia/reperfusion (I/R) injuries [58]. The protection afforded by IPC is not limited to the target tissue but extends to neighbouring tissues and distant organs [65]. This “conditioning at a distance” is known as remote ischaemic preconditioning (rIPC) and provides a remote protective signal [62]. Beyond the heart, it also has been demonstrated in different organs and tissues with in vivo and ex vivo IPC [1, 3, 20, 22, 28, 38, 41, 62, 63]; however, it is an invasive procedure and requires direct, potentially harmful manipulation of organ vessels, which can cause coronary microembolisation and consecutive microvascular and myocardial injury [28, 30]. In addition, IPC represents an ischaemic non-lethal event, and this may present a problem in clinical settings [62]. Therefore, rIPC represents a good alternative to IPC against I/R injuries.
The rIPC by humoral signal transfer was first demonstrated in an ex vivo isolated heart bioassay, where IPC-induced cardioprotection was transferred between isolated rabbit hearts via coronary effluent from preconditioned hearts to virgin hearts [11]. Subsequent studies in vivo demonstrated the humoral transfer of cardioprotection from remotely conditioned donors to ex vivo acceptor hearts by the transfer of whole plasma or dialysate [84]. The protection can be transferred from the donor species after rIPC to virgin hearts, even among different species, and various studies have identified these humoral factors [19, 49, 70, 72]. Although many factors have been identified [28], surprisingly, the elimination of a single factor abrogates the whole protection. In this regard, not all important factors appear to be identified and the identity and characteristics of these unknown humoral factors remains largely enigmatic [35].
Initially, the first step to isolate and characterise functional molecular content is to identify the molecular weight range. The humoral factors in the rIPC effluent were first showed a molecular weight range smaller than 14 kDa since the protective effect of rIPC effluent remains even after dialysis with a 12–14 kDa cut-off membrane [19, 34,35,36, 54, 66, 73, 78]. Otherwise, Breivik et al. [2] found that the IPC coronary effluent had cardioprotective effects also in fractions above 30 kDa. However, the majority of studies have focused on molecular weights less than 15 kDa. A previous study from our lab showed two characteristics of these humoral factors: a molecular weight range greater than 3.5 kDa and hydrophobicity [71]. However, a low molecular weight range had been proposed and a narrow range of molecular weights for these humoral factors remains controversial.
The mass spectrometry approach has been used to identify the humoral factors released during rIPC. In fact, mass spectrometry is the most advanced approach for molecular identification [69]. The first study to try to identify humoral factors by mass spectrometry showed impaired identification. This limitation could be explained by contamination due to the use of serum [44]. In addition, some studies had already identified molecules released during rIPC [25, 26]. However, whether these molecules are involved in the cardioprotection by rIPC is unclear. Furthermore, the release of these molecules during rIPC was proposed to be randomised, creating difficulty in obtaining the same proteomic identification in different samples [25]. Therefore, reinforcing the hypothesis of the casual release and the activity of these humoral factors warrants investigation [28, 30].
Therefore, the aim of the present study were (1) to improve the characterisation of the cardioprotective factors released in the coronary IPC effluent (Efl_IPC) by fractionation into different molecular size ranges and to evaluate the cardioprotective effect of each fraction compared to the total IPC effluent and (2) to identify the alleged cardioprotective factors responsible for the humoral transfer of cardioprotection from rIPC to naïve hearts with a proteomic analysis.
Methods
Animals
Adult male Wistar rats (300–350 g) were used for the experiments. This study conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (8th edition, 2011) and the experimental protocols were approved by the local Institutional Animal Care and Use Committee (IBCCF194-07/16).
Ex vivo I/R experiments
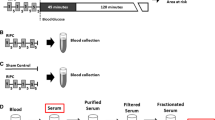
The I/R experiments were performed on isolated rat hearts as described previously [71]. The hearts were rapidly removed and cannulated through the aorta in a modified Langendorff apparatus and perfused at constant flow of 10 mL/min with Krebs–Henseleit buffer solution (in mmol/L: NaCl 118.0, NaHCO3 25.0, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, CaCl2 1.25, and glucose 11.0) at 37 °C and equilibrated with a gas mixture of 95% O2 and 5% CO2 (pH 7.4). A water-filled latex balloon was inserted into the left ventricle (LV) through the mitral valve and was connected to a pressure transducer and the PowerLab System (ADInstruments, Australia) for continuous LV pressure recording. The heart remained immersed in a buffer-filled, water-jacketed glass chamber and the end-diastolic pressure (LVEDP) was adjusted to 10 mmHg. The LV developed pressure (LVDP) was determined as the difference between the systolic peak and the LVEDP. After 20–30 min of stabilisation time, the hearts were subjected to the experimental protocols (Fig. 1) as described in a previous study [71]. The hearts were allocated to following groups: CTL (n = 5) control hearts were only subjected to the I/R protocol of normothermic no-flow global ischaemia for 30 min followed by reperfusion for 60 min. IPC (n = 10) hearts were subjected to the IPC protocol, which consisted of three consecutive cycles of 5 min of ischaemia and 5 min of reperfusion, immediately before the I/R protocol. Coronary effluent was collected from the IPC hearts during the three 5-min episodes of reperfusion in the IPC protocol and used fresh (used within 2 h) or stored between 0 and 2 °C for subsequent fractionation. Efl_IPC (n = 7) hearts were perfused with the fresh coronary effluent from the IPC hearts for 15 min before the I/R protocol.
Experimental protocols. All hearts were subjected to 30 min of global ischaemia followed by 60 min of reperfusion. IPC hearts were subjected to three cycles of 5-min global ischaemia/5-min reperfusion. IPC effluent (Efl_IPC) or its fractions were perfused in naíve hearts for 15 min prior to ischaemia/reperfusion. Chelerythrine (Chel), glibenclamide (Glib) or 5-hydroxydecanoate (5-HD) were administered for 20 min, starting 5 min prior to 5–10 kDa fraction perfusion
The preconditioned coronary effluent was filtered using ultrafiltration membranes (Amicon model 8200, Millipore) with different cut-offs to obtain fractions within the following molecular weight ranges: <3, 3–5, 5–10, <10, 10–30, 30–50, and >50 kDa. The preconditioned coronary effluent was maintained at 0–2 °C before ultrafiltration. The coronary effluent ultrafiltration and perfusion was performed on the same day of the collection. Each fraction was perfused within 2 h, including ultrafiltration and pH stabilisation. Hearts allocated to the groups <3, 3–5, 5–10, <10, 10–30, 30–50 and >50 (n = 5 per group) were perfused with the respective fractions for 15 min before the I/R protocol. The lyophilized coronary effluent was stable for use for up to 30 days as shown in our previous study [71]. The lyophilized coronary effluent was kept frozen at −80 °C. Furthermore, the hearts allocated to the groups Chel, Glib, and 5-HD (n = 5 per group) were perfused with the 5–10 kDa fraction in the presence of 10 μmol/L chelerythrine, 10 μmol/L glibenclamide, or 100 μmol/L 5-HD, respectively, before the I/R protocol.
Furthermore, we evaluated the cardioprotective activity of the mitochondrial 10 kDa heat shock protein (HSP10), one of the proteins previously identified in our proteomic analysis. The hearts were perfused with 0.1, 0.3, 0.5, and 1 µM of HSP10 (Sigma Aldrich) for 15 min before the I/R protocol in the absence or presence of 0.2 µM PUH71 (6-amino-8-[(6-iodo-1,3-benzodioxol-5-yl)thio]-N-(1-methylethyl)-9H-purine-9-propanamine, TOCRIS), a non-selective heat shock protein inhibitor. In another set of experiments, we perfused the 5–10 kDa fraction in the presence of PUH71 for 15 min before the I/R protocol.
Infarct size measurement
At the end of the 60-min reperfusion period, the hearts were removed and sliced into 1.5 mm cross-sections from the apex to the base and were incubated in 1% triphenyl-tetrazolium chloride (TTC) for 4 min at 37 °C, followed by incubation in a 10% (v/v) formaldehyde solution for 24 h to improve the contrast between the stained (viable) and the unstained (necrotic) tissues. The slices were placed between two glass slides and scanned (imaged). The infarct size was determined by planimetry using ImageJ 1.22 software (NIH, USA). The infarct size was expressed as a percentage of the area at risk (total). Although some concern exists in terms of the reperfusion duration necessary to determine the infarct size by TTC staining [67], other studies have shown that the 60-min reperfusion duration in ex vivo perfused hearts is sufficient to assess valid measures of the infarct size [17].
The effect of the dilution or the concentration of Efl_IPC on the cardioprotective response
To assess the effect of the concentration or the dilution of the humoral transfer factors on the cardioprotection induced by the Efl_IPC, the fluid was concentrated (twofold) or diluted (75, 50, 25%) as described below. To obtain the twofold concentrated sample, the Efl_IPC collected from two hearts (300 mL) was dialysed (3 kDa cut-off) to wash out salts and the retained sample was frozen and lyophilised (used within 30 days), followed by resuspension in 150 mL of filtered Krebs solution (within 2 h). To obtain the diluted samples, fresh Efl_IPC was diluted to 25, 50, and 75% with Krebs solution. After pH adjustment, the samples were perfused for 15 min before the ischaemia period. The diluted samples were perfused in other hearts within 2 h after collection, dilution, filtration and pH stabilisation.
The cardioprotective effect of hydrophobic and hydrophilic fractions of the Efl_ 5–10 kDa
The Efl_ 5–10 kDa samples were applied in an adsorbent cartridge (Sep-Pak C-18, 20 mg, Water Corp., Milford, MA) at a constant rate of 3 mL/min using a peristaltic pump (Minipuls3, Gilson, Middleton, WI). The cartridge was previously conditioned with acetonitrile (100%), followed by washing with water (MilliQ). After sample application, the cartridge was washed with water to remove the unbound fraction (hydrophilic compounds). The hydrophobic compounds bound in the C-18 silica were eluted using 35% acetonitrile. The organics phases were removed by lyophilisation. This protocol was performed within 2 h, including coronary effluent collection and ultrafiltration, to preserve the cardioprotective effects. Both fractions were lyophilised and stored frozen at −80 °C to be used within 30 days for the later analysis.
Electrophoresis analysis
Lyophilised samples of Efl_IPC, Efl_ >10 kDa and Efl 5–10 kDa were solubilised (60 µg/mL) with buffer (0.5 mM Tris, pH 6.8, 1% SDS, 20% glycerol, 0.5% mercaptoethanol) and boiled at 100 °C for 5 min. After centrifugation at 14,000×g for 4 min at 4 °C, the supernatant was subjected to electrophoresis in discontinuous vertical polyacrylamide gel containing SDS. The proteins in the stacking gel (4% polyacrylamide) were subjected to a current of 10 mA and the proteins in the separating gel (15% polyacrylamide) were subjected to a current of 20 mA. Bands were visualised by staining with a silver nitrate solution. The gels were scanned using the Labscan image scanning programme.
Efl_IPC preparation under reducing and alkylating conditions
Two independent Efl_IPC samples were obtained at different times. Each sample was constituted by a pool of coronary effluent collected from five IPC hearts. The Efl_IPC sample was filtered (Amicon, 3 kDa cut-off), lyophilised, and resuspended in 50 µL of water. The amounts of protein in the sample were quantified using the Pierce BCA protein assay kit (Thermo Scientific). Then, 10 μg of Efl_IPC proteins were precipitated by the TCA protocol and resuspended in an ammonium bicarbonate solution (50 mM) buffer. The reduction of the proteins in the sample was performed with the addition of 2.5 µL of dithiothreitol (DTT) 100 mM followed by incubation for 30 min at 60 °C. The alkylation was carried out with the addition of 2.5 μL of iodoacetamide 300 mM followed by incubation for 30 min at room temperature in darkness. The digestion of the proteins was performed with the addition of trypsin solution (Promega) to establish a ratio of 1:50 (trypsin:protein), followed by incubation for 14 h at 37 °C. The tryptic peptides were dried in a speed vac and cleaned/concentrated with the use of OASYS (Waters Corporation, UK), with elution in 100% methanol. After drying the material a second time in the speed vac, the peptides were resuspended in a solution of 0.1% formic acid and 3% acetonitrile for a mass spectrometry analysis. Three replicates of each Efl_IPC sample were analysed by LC–MS/MS.
LC–MS/MS analysis and data analysis
Two microliters of peptides solution (0.8 μg of proteins) were used for the nanoLC-based separation, combined with mass spectrometry analysis on a UPLC–ESI-Q-TOF Micro MS/MS instrument (Waters Co., Williford, USA) by the data-dependent acquisition (DDA) mode. Peptide separation was performed in a NanoAcquity system equipped with a Symmetry C18 5 μm, 5 mm × 300 precolumn and an Atlantis 100 × 100, 1.7 μm analytical reverse phase C18 column, with a solution gradient of 5–50% mobile phase acetonitrile over 50 min at a flow rate of 350 nL/min. Column temperature was maintained at 35 °C. The lock mass used was phosphoric acid, delivered by the auxiliary pump at a flow rate of 600 nL/min. The conditions for peptide ionization included a source temperature of 80 °C, capillary voltage at 3500 V, positive polarity and a sample cone at 35 V. Mass spectra were acquired with the TOF mass analyser operating in V-mode and the spectra were integrated over 1 s of scan and 0.1 s interscan intervals. The MS/MS mass spectra were acquired using 50–1700 m/z with the reference mass acquired and continuous fragmentation mode in 10 eV collision energy.
The DDA raw data were processed and searched by the Peaks 7 software server search engine (Bioinformatics solutions, Inc., Waterloo Canada), Mascot Distiller (http://www.matrixscience.com/) and ProteinLinx 2.5.1 (Waters, Inc. Willilford, USA) using a tolerance up to ±0.1 Da for precursor and fragment ions. A maximum of one missed cleavage site and carbamidomethyl (C) and oxidation (M) to fixed and variable modifications were, respectively, selected. Protein identification was performed by searching for the Rattus norvegicus species in the reverse databases of UNIPROT with FDR <1%. Only proteins that were identified by three proteomic software programmes were considered as valid hits.
All results were manually checked considering only those valid IDs whose protein hits presented three or more different peptides or, if less than three peptides were identified, the presence of a peptide with at least seven amino acid residues sequenced consecutively in the series y or b. Valid identification was also accepted for a protein with less than three peptides if at least five residues were indicated in bold red by Mascot or for those that had at least one peptide only if 100% of the protein’s identification was performed on the UNIPROT database. The cluster analysis was performed using the STRING (Search Tool for the Retrieval of Interacting Genes) tool to evaluate possible interactions between the related proteins in mass spectrometry.
Statistical analysis
The data are presented as the mean ± SEM. Statistical differences were determined by a one-way ANOVA followed by a Bonferroni post hoc test. The differences were considered statistically significant at P < 0.05.
Results
Cardioprotective effects of the coronary effluent collected during IPC
After stabilisation, no significant differences in the baseline LVDP among the groups were observed (Supplementary material online, Table S1). All LVDP results were expressed as a percentage of the baseline values. Under the ischaemic condition, all hearts exhibited a fast reduction of LVDP to zero (Fig. 2a) and an increased LVEDP (Fig. 2b) after a few minutes of ischaemia. During reperfusion, an additional increase in LVEDP was observed in CTL hearts but not in Efl_IPC hearts. The IPC hearts exhibited a reduction in LVEDP (Fig. 2b). The post-ischaemic recovery of LVDP was poor in CTL hearts but improved in the hearts subjected to IPC or in those preconditioned with pre-ischaemia perfusion of coronary effluent (Efl_IPC) collected from IPC hearts (Fig. 2a).
Effect of preconditioned coronary effluent (Efl_IPC) on post-ischaemic cardiac performance. a Time course of changes in left ventricular developed pressure (LVDP) during 30 min of global ischaemia and 60 min of reperfusion. b Time course of changes in left ventricular end-diastolic pressure (LVEDP) during ischaemia/reperfusion protocol. c Dilution or concentration effects of Efl_IPC on post-ischaemic recovery of LVDP. Efl_IPC was collected from IPC hearts during ischaemic preconditioning. Efl_IPC hearts were perfused with preconditioned coronary effluent before ischaemia. Data are mean ± SEM. Number in each colunm is n of hearts. ***P < 0.001 vs. CTL; ### P < 0.001 and ## P < 0.01 vs. IPC
The dilution and concentration effect of the Efl_IPC on the post-ischaemic LVDP recovery (Fig. 2c) was evaluated by dilution (25, 50, and 75%) or concentration (twofold) of the coronary effluent collected during IPC. The improvement on the LVDP recovery was abolished by Efl_IPC dilution to 25 or 50%. Additionally, the twofold increase in the Efl_IPC concentration was not able to increase the cardioprotective effect.
Cardioprotective factors are present in the <3 and 5–10 kDa fractions of coronary effluent collected during IPC
The coronary effluent collected during IPC was fractionated by molecular size (<3, 3–5, 5–10, <10, 10–30, 30–50 and >50 kDa), and each fraction was perfused for 15 min before ischaemia in the hearts subjected to I/R. The fractions <3, 5–10, and <10 kDa improved the post-ischaemic recovery of LVDP (Fig. 3a). Conversely, the 3–5, 10–30, 30–50, and >50 kDa fractions had no significant effect on the post-ischaemic recovery of LVDP (Fig. 3b) compared to CTL hearts. The LVDP recovery at 60 min of reperfusion (Fig. 3c) was approximately 4.5-fold higher in IPC hearts compared to CTL hearts. The fractions 5–10 and <10 kDa induced post-ischaemic LVDP recovery similar to IPC hearts. The hearts preconditioned with Efl_IPC and the <3 kDa fraction showed greater LVDP recovery than CTL, but less than IPC hearts. The fractions 3–5, 10–30, 30–50 and >50 kDa had no effect on the LVDP recovery compared to CTL hearts.
Effect of different molecular weight fractions of preconditioned coronary effluent on post-ischaemic cardiac performance. a, b Time course of changes in left ventricular developed pressure (LVDP) during ischaemia/reperfusion protocol in hearts submitted to ischaemic preconditioning (IPC) or perfused with total (Efl_IPC) or fractioned coronary effluent from IPC hearts. Post-ischaemic LVDP recovery (c) and end-diastolic pressure (d) at 60 min of reperfusion. LVDP was expressed as percentage of pre-ischaemic basal values. Data are mean ± SEM. Number in each colunm is n of hearts. ***P < 0.001 and **P < 0.01 vs. CTL; ### P < 0.001 and ## P < 0.01 vs. IPC
The mean value of LVEDP measured at 60 min of reperfusion is shown in Fig. 3d. The <3, 5–10, and <10 kDa fractions decreased the LVEDP during reperfusion. However, the hearts perfused with the 5–10 kDa fraction before I/R presented LVEDPs similar to the IPC hearts, and the hearts pre-treated with the <3 or <10 kDa fractions exhibited LVEDPs similar to the Efl_IPC hearts. All other groups (3–5, 10–30, 30–50 and >50) had a mean value of LVEDP at 60 min of reperfusion similar to the CTL group.
The myocardial infarct size measured at 60 min of reperfusion decreased in the hearts pre-treated with the Efl_IPC one or twofold concentrated, or diluted to 75% (Fig. 4a). The cardioprotection was abolished by Efl_IPC dilution to 25 or 50%. Additionally, the twofold increase in the Efl_IPC concentration was not able to increase the cardioprotective effects. The myocardial infarct size decreased in the hearts pre-treated with the <3, 5–10 and <10 kDa fractions to a level similar to that observed in IPC and Efl_IPC hearts (Fig. 4b). However, the other Efl_IPC fractions (3–5, 10–30, 30–50 and >50 kDa) had no significant effect on infarct size.
Infarct size in isolated rat hearts perfused with preconditioned coronary effluent (Efl_IPC) before 30 min of global ischaemia and 60 min of reperfusion. a effect of dilution or concentration of Efl_IPC on myocardial infarct size. b Effects of different molecular weight of Efl_IPC on myocardial infarct size. Representative images of TTC-stained heart sections from each of the groups are shown beneath each bar in the graphs. Data are mean ± SEM. Number in each column is n of hearts. ***P < 0.001 vs. CTL
Cardioprotective effects of the 5–10 kDa fraction are dependent on PKC and KATP channels
As previously demonstrated in the Efl_IPC [71], we found that the cardioprotective effect of the 5–10 kDa fraction was abrogated by treatment with chelerythrine, a PKC inhibitor, and by the KATP channel blockers, glibenclamide and 5-HD. Figure 5a, b show that the post-ischaemic recovery of LVDP was enhanced by perfusion of the 5–10 kDa fraction prior to ischaemia. However, when the hearts were perfused with the 5–10 kDa fraction in the presence of chelerythrine, glibenclamide or 5-HD, the post-ischaemic recovery of LVDP was diminished to levels similar to the CTL group. Treatment with chelerythrine, glibenclamide, or 5-HD also abrogated the effects of the 5–10 kDa fraction on the post-ischaemic LVEDP (Fig. 5c) and myocardial infarct size (Fig. 5d).
Inhibition of PKC and blocking of KATP channels abolishes the cardioprotection induced by the 5–10 kDa fraction of IPC coronary effluent. (a) Time course of changes in left ventricular developed pressure (LVDP) during ischaemia/reperfusion protocol in hearts preconditioned with 5–10 kDa fraction, in absence or presence of chelerythrine (Chel), glibenclamide (Glib), or 5-HD. LVDP recovery (b), end-diastolic pressure (c), and infarct size (d), after 60 min of reperfusion. LVDP was expressed as percentage of pre-ischaemic basal values. Representative images of TTC-stained heart sections are shown beneath each bar. Data are mean ± SEM. Number in each column is n of hearts. ***P < 0.001 vs. CTL; ### P < 0.001 and # P < 0.05 vs. 5–10 kDa fraction
Characteristics of the humoral factors present in the 5–10 kDa fraction
We also evaluated the cardioprotective effects of hydrophobic and hydrophilic fractions obtained by passing the lyophilised extract of the 5–10 kDa fraction through a Sep-Pak C18 column. The hydrophobic analyte was effective in improving the post-ischaemic recovery of LVDP (Fig. 6a) and reducing the infarct area (Fig. 6b) of the hearts subjected to I/R. The hydrophilic eluate fraction exhibited a shorter but significant improvement in LVDP recovery but had no significant effect on the infarct size. Figure 7 shows a representative gel of a tenfold concentrated sample of the 5–10 kDa fraction, suggesting the presence of peptides with molecular sizes in the range of 4–11 kDa.
Effects of hydrophobic (Hphob) and hydrophilic (Hphil) compounds from the Efl_ 5–10 kDa fraction on developed pressure recovery (a) and infarct size (b), after 60 min of reperfusion. Representative images of TTC-stained heart sections are shown beneath each bar of the graph b. Data are mean ± SEM. Number in each column is n of hearts. ***P < 0.001 vs. CTL; ## P < 0.01 and # P < 0.05 vs. 5–10 kDa
Representative results from gel electrophoresis analysis (SDS–PAGE) of proteins present in >10 and 5–10 kDa fractions of the IPC coronary effluent. Samples were lyophilized and desalted before protein extraction. M1 and M2: protein marker. Lane 1 fivefold concentrated protein extract from the >10 kDa fraction. Lane 2 tenfold concentrated protein extract of the 5–10 kDa fraction. Silver nitrate staining
Mass spectrometry and cluster analysis
The mass spectrometry analysis of the Efl_IPC revealed the presence of 60 cytoprotection-related proteins, 14 of which had molecular weights below 12 kDa (Table 1), 12 proteins ranging from 12 to 20 kDa (Supplementary material online, Table S2) and 34 proteins had molecular weights greater than 20 kDa (Supplementary material online, Table S3). Additionally, 38 putative pro-apoptotic proteins were identified (Supplementary material online, Table S4), but all of them were larger than 14 kDa.
The 14 identified proteins with molecular weights below 12 kDa were analysed by STRING from UNIPROT in a cluster analysis (Figure S1) to identify relevant interactions to understand the role of these molecules in the cardioprotection mechanism. The cluster analysis showed a direct interaction of HSP10 with heat shock protein 60 (HSP60, score 0.993), heat shock protein beta-7 (HSPB7, score 0.179), and Bcl-2 (score 0.172). HSP60 (score 0.240), HSPB7 (score 0.254) and Bcl-2 (score 0.998) interact with the cellular tumour antigen (p53). In fact, p53 appears to be a common target for interaction with several proteins identified in our analysis. p53 interacts with insulin-like factor 1 (score 0.454), protein S100a4 (score 0.973), protein S100a10 (score 0.168), pneumo-secretory protein 2 (score 0.151), and the protein Ash2l (score 0.330) to assemble with DPY30 (score 0.989) to form the regulatory module of H3K4 methyltransferase complexes. Spermatid nuclear transition protein 1 (Tnp1) showed an interaction (score 0.316) with protamine-3 (Prm3). The guanine nucleotide-binding protein subunit gamma (Gng4) interacted (score 0.966) with guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit γ-12 (Gnb3). Our analysis not showed interactions among apolipoprotein CII (Apoc2), DCM5 protein (Dcm5), CAMPATH antigen (Cd52), or the retinal cone rhodopsin-sensitive cGMP 3′,5′-cyclic phosphodiesterase, gamma subunit (Pde6h).
Cardioprotective effects of the HSP10
The perfusion of 0.1 µM, 0.3 µM or 0.5 µM HSP10 prior to I/R not revealed cardioprotective effects against I/R injuries (Supplementary material online, Fig. S2). However, at 1 µM, HSP10 elicited cardioprotective effects, as evidenced by increased post-ischaemic LVDP recovery (Fig. 8a, b) and reduced LEVDP (Fig. 8c) after 60 min of reperfusion. In addition, HSP10 perfusion reduced the infarct size from these hearts (Fig. 8d). The non-selective heat shock protein antagonist PUH71 (0.2 µM) abolished the cardioprotective effects of HSP10. However, this antagonist attenuated, but did not abolish, the cardioprotective effect of the 5–10 kDa fraction.
Cardioprotection conferred by exogenous HSP10 perfusion. a Time course of changes in left ventricular developed pressure (LVDP) during ischaemia/reperfusion protocol in hearts preconditioned with 1 µM HSP10 or 5–10 kDa fraction, in absence or presence of 0.2 µM PU-H71. PU-H71 prevented HSP10 or 5–10 kDa fraction-mediated improvement in post-ischaemic functional recovery and infarct-limiting effect. LVDP recovery (b), end-diastolic pressure (c), and infarct size (d), after 60 min of reperfusion. Representative images of TTC-stained heart sections are shown beneath each bar in the graph d. LVDP was expressed as percentage of the pre-ischaemic basal value. Data are mean ± SEM. Number in each colunm is n of hearts. *P < 0.05 and ***P < 0.001 vs. CTL; ## P < 0.01 and ### P < 0.001 vs. 5–10 kDa fraction; ††† P < 0.001 vs. HSP-10
Discussion
The novel findings of the present study are (1) that the cardioprotection induced by the transfer of coronary effluent from IPC to virgin rat hearts is afforded by humoral factors present in two fractions of the Efl_IPC: one sized below 3 kDa and the other sized between 5 and 10 kDa, as defined by the cut-off values of the ultrafiltration membranes. Both fractions allowed for greater recovery of the developed pressure and decreased diastolic pressure and infarct size after I/R; (2) that the proteomic analysis of the Efl_IPC identified 14 peptides within a molecular weight range of the 5–10 kDa fraction, which are potential candidates as cardioprotective factors present in the 5–10 kDa fraction; (3) that the humoral factors of the 5–10 kDa fraction induced cardioprotection by mechanisms that involve the activation of PKC and KATP channels; and (4) that the HSP10 protein, identified by mass spectrometry, improved the developed pressure, decreased the diastolic pressure, and reduced the infarct size after I/R when perfused in isolated hearts.
In the present study, we demonstrated in buffer-perfused ex vivo rat hearts that the cardioprotection afforded by local IPC can be transferred to other hearts by transferring coronary effluent or fractions <3 and 5–10 kDa from the IPC donor to virgin receptor hearts prior to I/R. In contrast to our previous study [71], which did not evaluate the dialysate of the coronary IPC effluent (fraction below 3.5 kDa), our present data demonstrate that the <3 kDa fraction also induced cardioprotection against I/R injury. The cardioprotective effects induced by this fraction were probably afforded by autacoids such as adenosine (267 Da), opioids (500–800 Da), bradykinin (1060 Da), and others signalling molecules with sizes below 3 kDa that are released during IPC [28]. Cytoprotection by these autacoids had been previously demonstrated [28, 48, 70, 83], but the focus of the present study was only on the characterisation and identification of the cardioprotective humoral factors present in the 5–10 kDa fraction. Our results are consistent with data from our previous study [71], which showed cardioprotective effects provided by retained content (fractions above 3.5 kDa) in the pre-dialysed IPC coronary effluent. Our findings also agree with results from rIPC studies that showed cardioprotective effects of dialysate from plasma dialysed through 12–14 kDa cut-off membranes [19, 34,35,36, 54, 66, 73, 78]. However, our results partially disagree with those of Breivik et al. [2] who found protective effects not only from the fractions below 10 kDa but also from the fractions above 10 kDa and below 30 kDa. Our data showed that the 10–30 kDa fraction not elicited cardioprotection, although the proteomic analysis of the IPC coronary effluent identified some cytoprotection-related proteins within this range of molecular weight, such as the heat shock protein beta-2 [21], interleukin-3 [10], Bcl-2 [51], and apolipoprotein A-1 [39]. Additionally, other proteins greater than 10 kDa in size, not identified in our proteomic analysis, have been described as cardioprotective, such as IL-10 [5], urocortin [69], and leptin [77]. However, some of these exerted cardioprotective effects only with exogenous administration (for review, Heusch [28]).
Our previous study [71] showed that the cardioprotective effect of the IPC effluent was due to hydrophobic molecules. This result was confirmed by Breivik et al. [2] and by our present data, which demonstrate that hydrophobic molecules in the 5–10 kDa fraction elicit cardioprotection similar to the total 5–10 kDa fraction. Furthermore, to determine whether the cardioprotection elicited by the 5–10 kDa fraction is dependent on the activation of PKC, as previously demonstrated for the IPC effluent [71], we perfused naïve hearts with this fraction in the presence of an inhibitor prior to I/R. The PKC inhibition abrogated the cytoprotective effect of the 5–10 kDa fraction. The involvement of PKC in the mechanism of IPC has been widely demonstrated and is considered a target for different triggers of cytoprotection [13, 47, 64, 68, 80, 85]. Activation of subtype PKCε is known to block the mitochondrial permeability transition pore structuring, activate mitochondrial KATP channels and increase the expression of functional Kir6.2-containing KATP channels in the mitochondrial inner membrane [18]. Indeed, the blocking of KATP channels by glibenclamide or 5-HD abrogated the cytoprotective effect of the 5–10 kDa fraction. Therefore, we can suggest that the activation of PKC and mitochondrial KATP channels has an important role in the cardioprotection induced by the factors present in the 5–10 kDa fraction. Furthermore, is related an interaction between PKC and HSP during IPC. However, there is no evidence whether PKC could interact with mitochondrial HSP10 [52].
To identify the putative cardioprotective humoral factors present in the 5–10 kDa fraction, we performed a proteomic analysis of the preconditioned coronary effluent. The key result from this study was the identification of 14 proteins with molecular weights in the range of 4–12 kDa, which may be the humoral factors responsible for the cardioprotective effects of the 5–10 kDa fraction. This result was verified by the presence of a 4–12 kDa band in the electrophoresis gel of a sample of the 5–10 kDa fraction. The mitochondrial 10 kDa heat shock protein (HSP10) was one of the proteins identified in our proteomic analysis that is less than 12 kDa in size. The cluster analysis showed a direct interaction between HSP10 and HSP60, HSPB7, and Bcl-2. HSP10 is known to interact with HSP60 to form a chaperonin complex that is important for mitochondrial protein folding and function [44]. The overexpression of both HSP10 and HSP60 was found to protect cardiomyocytes against apoptosis induced by ischaemia [44], I/R [46], or doxorubicin [72]. Indeed, the overexpression of HSP10 and HSP60 in doxorubicin-treated cardiomyocytes increased the expression of the anti-apoptotic Bcl-2 protein family and reduces the expression of the pro-apoptotic Bax [72]. In the present study, to evaluate the functional relevance of HSP-10 in the cardioprotective effect of the 5–10 kDa fraction, we evaluated this fraction in the presence of an HSP inhibitor. Our results showed that the HSP inhibitor PU-H71 attenuated the cardioprotective effect of the 5–10 kDa fraction. Additionally, we demonstrated that the exogenous administration of HSP-10 prior to I/R also reduced the I/R injury and this effect was abrogated by the inhibitor PU-H71. The minor effect of HSP10 compared to the 5–10 kDa fraction may suggest that other factors, in addition to HSP10, contribute to the cardioprotective effect of the 5–10 kDa fraction or perhaps a higher concentration of HSP10 is necessary.
Another identified protein below 12 kDa in size with the potential for cardioprotection transfer is HSPB7, a member of the α-crystallin-related small heat shock proteins [40]. In addition to interacting with HSP10, it also interacts directly with p53. The translocation of cytoplasmic p53 to the mitochondria in response to stress signalling has been shown to activate apoptosis and chromatin degradation [81]. However, no study has demonstrated the involvement of HSPB7 in local or remote IPC, and the increased expression of the homologue HSP20 has been related to cardioprotection against I/R injury [16].
Hearts subjected to I/R showed p53 upregulation and Bcl-2 downregulation [51]. Moreover, IPC induces p53 downregulation, Bcl-2 upregulation, and a reduction in apoptotic cells [51, 55]. In fact, the cluster analysis showed that protein p53 is a common target for interaction with several proteins identified in our analysis. The pre-pro insulin-like growth factor is a precursor of insulin-like factors (IGF). IGF-1 was demonstrated to reduce the infarction area when administered exogenously and IGF1 interacts with p53 by upregulating the Mdm2 protein, which induces p53 degradation [27] and elicits cardioprotection [4, 8, 45]. However, no evidence is available to show that endogenous IGF-1 is involved in local or remote IPC. Our cluster analysis also showed interactions of S100a4 and S100a10 proteins with p53. Both proteins are members of the S100 protein family [12]. S100-A4 was reported to interact with p53 in the nucleus and promote p53 degradation [60]. In addition, S100A4 overexpression protects myocytes against apoptosis [14]. The other identified S100 protein, S100a10, was reported to bind to annexin 2 to form the annexin A2-S100A10 complex [57], which is translocated to the plasma membrane by hypoxia-induced intracellular acidification [56]. However, to our knowledge, no evidence is available to show whether the annexin A2-S100A10 complex participates in the IPC or the rIPC mechanism. The protein DPY30 was shown to assemble with Ash2L and two other subunits to form the regulatory module of H3K4 methyltransferase complexes, which regulate histone 3-lysine 4 methylation and activate target gene transcription [7, 15, 37]. However, whether DPY30 or Ash2l has any important role in the IPC or the rIPC mechanism remains unknown. Furthermore, the cluster analysis not identified any relevant interactions among the proteins: pneumo-secretory protein 2 (Scgb3a1), apolipoprotein CII (Apoc2), guanine nucleotide-binding protein subunit gamma (Gng4), spermatid nuclear transition protein 1 (Tnp1), protamine-3 (Prm3), DCM5 protein (DCM5), CAMPATH antigen (Cd52), and retinal cone rhodopsin-sensitive cGMP 3′,5′-cyclic phosphodiesterase, gamma subunit (Pde6h). However, we cannot exclude the alleged involvement of one or more of these proteins in the IPC or the rIPC mechanism.
The proteomic analysis of the IPC effluent also identified another 84 proteins with sizes greater than 12 kDa, with 46 of these reported as cytoprotective proteins and the other 38 identified as pro-apoptotic proteins. Among the 46 cytoprotective proteins, 12 had molecular weights between 12 and 20 kDa (Table S2) and the other 34 had molecular weights greater than 20 kDa (Table S3). Among these proteins, apolipoprotein A-1 [33], pro-opiomelanocortin [61], heat shock protein beta-2 [16], and insulin-like growth factor II [82] were demonstrated to elicit cardioprotection when administered exogenously. However, we not observed any cardioprotective effect of fractions above 10 kDa. Since all pro-apoptotic proteins identified were larger than 14 kDa, we can suggest that the presence of pro-apoptotic proteins in the fractions larger than 12 kDa antagonised the effects of the cytoprotective proteins. On the other hand, the proteomic analysis of the IPC effluent not identified some proteins, such as stromal cell-derived factor-1α (SDF-1α) and interleukin 10 (IL-10), that were previously reported as cardioprotective cytokines that are upregulated by rIPC [6, 9]. However, these proteins were found in the plasma analysis and could not have been secreted by cardiac tissue, which is different from our results that analysed humoral factors secreted from isolated hearts. SDF-1α (10,103 Da) levels increased in the plasma of rats undergoing rIPC and blocking the SDF-1α receptor with AMD3100 abrogated the cardioprotective effect of rIPC [9]. The rIPC-induced upregulation of IL-10 (18,700 Da) expression promoted late protection against myocardial I/R in mice [6]. The cardioprotective effect of rIPC was lost in IL-10 knockout mice but was recovered by the exogenous administration of IL-10 [6]. The absence of IL-10 in the IPC effluent can be explained by a delay in the upregulation of IL-10 in response to rIPC.
Study limitations
The bioassay approach chosen in the present study differs from functional rIPC in clinical settings. Remote ischaemic conditioning by transient I/R of the limb has emerged as a therapeutic non-invasive method for cardioprotection in patients undergoing cardiac surgery or acute infarct [34, 43]. However, the translation from experimental studies to the clinical settings has failed to produce the expected results, as shown in the recent multi-centre phase III trials ERICCA and RIPHeart [23, 24, 30, 31, 53]. The failure of these trials to improve clinical outcome in patients undergoing rIPC before cardiac surgery has been attributed to confounding variables such as co-morbidities, anaesthesia or comedications, which may have prevented the cardioprotective effects [5, 23, 24, 29, 32, 42, 76]. Therefore, more investigations on the mechanisms of cardioprotection by ischaemic conditioning are needed, with more insight into the identification of circulating cardioprotective factors and the pathways activated by the conditioning stimulus. Signal transduction in rIPC represents a complex interaction of neuronal and humoral signalling cascades [28, 30] and rIPC signal generation participates in different organs and their innervation [35, 50, 59]. Therefore, we chose a bioassay approach that was not influenced by other organs, blood-borne factors, cofounders, or signals from either the central or the autonomic nervous system. We utilised ex vivo rat hearts in a constant flow Langendorff perfusion setup to investigate the humoral factors responsible for the cardioprotection afforded by the transfer of coronary effluent from donor hearts undergoing IPC to receptor hearts prior to ischaemia. The main limitation of the constant flow mode is that under regional ischaemia, the same volume of perfusate may be forced through a smaller vascular bed [79]. However, we avoid this limitation using no-flow global ischaemia in all I/R protocols. Moreover, the Langendorff perfusion under constant flow enabled controlled conditions, in which the amount of perfusate delivered to the whole heart was not altered by changes in heart rate or force contraction, nor vascular auto regulatory mechanisms or sheer-mediated endothelium-derived relaxing factors [74, 79]. Although we have identified some proteins candidates in the IPC effluent as cardioprotective humoral transfer factors with potential therapeutic applications, their translation into the clinical setting has limitations due to species differences in the transduction of cardioprotective signals [75]. Moreover, patients differ from health laboratory animals used in the study since the cardioprotective responses of patients to rIPC is confounded by risk factors, comorbidity, and comedications [36, 42].
In conclusion, our findings demonstrate that cardioprotection induced by the transfer of preconditioned coronary effluent is afforded by humoral factors with molecular weights below 3 kDa or in the range of 4–12 kDa. The 4–12 kDa factors are hydrophobic molecules that activate cardioprotective pathways that are dependent on PKC and ATP-sensitive K channels, similar to those previously demonstrated for the Efl_IPC [71]. Our proteomic analysis assessed the protein content of the preconditioned coronary effluent and identified at least 14 peptides in the range of 4–12 kDa, which may be potential candidates as cardioprotective transfer factors, suggesting a potential therapeutic role for these molecules in the prevention of cardiac ischaemic injury. The HSP10, identified by mass spectrometry analysis, reduces the cardiac injuries by I/R, might be a new therapeutic target against I/R injuries.
References
Birnbaum Y, Hale SL, Kloner RA (1997) Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation 96:1641–1646. doi:10.1161/01.CIR.96.5.1641
Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK (2011) Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol 106:135–145. doi:10.1007/s00395-010-0133-0
Brzozowskia T, Konturekb PC, Kontureka SJ, Pajdoa R, Kwieciena S, Pawlika M, Drozdowicza D, Sliwowskia Z, Pawlik WW (2004) Ischemic preconditioning of remote organs attenuates gastric ischemia–reperfusion injury through involvement of prostaglandins and sensory nerves. Eur J Pharmacol 499:201–213. doi:10.1016/j.ejphar.2004.07.072
Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, Lefer AM (1995) Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci USA 92:8031–8035
Cabrera-Fuentes HA, Aragones J, Bernhagen J, Boening A, Boisvert WA, Bøtker HE, Bulluck H, Cook S, Di Lisa F, Engel FB, Engelmann B, Ferrazzi F, Ferdinandy P, Fong A, Fleming I, Gnaiger E, Hernández-Reséndiz S, Kalkhoran SB, Kim MH, Lecour S, Liehn EA, Marber MS, Mayr M, Miura T, Ong SB, Peter K, Sedding D, Singh MK, Suleiman MS, Schnittler HJ, Schulz R, Shim W, Tello D, Vogel CW, Walker M, Li QO, Yellon DM, Hausenloy DJ, Preissner KT (2016) From basic mechanisms to clinical applications in heart protection, new players in cardiovascular diseases and cardiac theranostics: meeting report from the third international symposium on “New frontiers in cardiovascular research”. Basic Res Cardiol 111:69. doi:10.1007/s00395-016-0586-x
Cai ZP, Parajuli N, Zheng X, Becker L (2012) Remote ischemic preconditioning confers late protection against myocardial ischemia–reperfusion injury in mice by upregulating interleukin-10. Basic Res Cardiol 107:277–288. doi:10.1007/s00395-012-0277-1
Chen Y, Cao F, Wan B, Dou Y, Lei M (2012) Structure of the SPRY domain of human Ash2L and its interactions with RbBP5 and DPY30. Cell Res 22:598–602. doi:10.1038/cr.2012.9
Davani EY, Brumme Z, Singhera GK, Côté HC, Harrigan PR, Dorscheid D (2003) Insulin-like growth factor-1 protects ischemic murine myocardium from ischemia/reperfusion associated injury. Crit Care 7:176–183. doi:10.1186/cc2375
Davidson SM, Selvaraj P, He D, Boi-Doku C, Yellon RL, Vicencio JM, Yellon DM (2013) Remote ischaemic preconditioning involves signalling through the SDF-1α/CXCR4 signalling axis. Basic Res Cardiol 108:377–386. doi:10.1007/s00395-013-0377-6
del Peso L, González-Garcia M, Page C, Herrera R, Nuñez G (1997) Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687–689
Dickson EW, Lorbar M, Porcaro WA, Fenton RA, Heard SO, Reinhardt CP, Gysembergh A, Przyklenk K (1999) Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol 277:2451–2457
Donato R (1999) Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta 1450:191–231
Dorn GW, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, Csukai M, Wu G, Lorenz JN, Mochly-Rosen D (1999) Sustained in vivo cardiac protection by a rationally designed peptide that causes ε protein kinase C translocation. Proc Natl Acad Sci USA 96:12798–12803. doi:10.1073/pnas.96.22.12798
Doroudgar S, Quijada P, Konstandin M, Ilves K, Broughton K, Khalafalla FG, Casillas A, Nguyen K, Gude N, Toko H, Ornelas L, Thuerauf DJ, Glembotski CC, Sussman MA, Völkers M (2016) S100A4 protects the myocardium against ischemic stress. J Mol Cell Cardiol 100:54–63. doi:10.1016/j.yjmcc.2016.10.001
Ernst P, Vakoc CR (2012) WRAD: enabler of the SET1-family of H3K4 methyltransferases. Brief Funct Genomics 11:217–226. doi:10.1093/bfgp/els017
Fan G-C, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones K, Chu G, Kranias EG (2005) Novel cardioprotective role of a small heat-shock protein, HSP20, against ischemia/reperfusion injury. Circulation 111:1792–1799. doi:10.1161/01.CIR.0000160851.41872.C6
Ferrera R, Benhabbouche S, Bopassa JC, Li B, Ovize M (2009) One hour reperfusion is enough to assess function and infarct size with TTC staining in Langendorff rat model. Cardiovasc Drugs Ther 23:327–331. doi:10.1007/s10557-009-6176-5
Garg V, Hu K (2007) Protein kinase C isoform-dependent modulation of ATP-sensitive K+ channels in mitochondrial inner membrane. Am J Physiol Heart Circ Physiol 293:322–332. doi:10.1152/ajpheart.01035.2006
Gedik N, Maciel L, Schulte C, Skyschally A, Heusch G, Kleinbongard P (2017) Cardiomyocyte mitochondria as targets of humoral factors released by remote ischemic preconditioning. Arch Med Sci 13:448–458. doi:10.5114/aoms.2016.61789
Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD (1996) Myocardial protection by brief ischemia in noncardiac tissue. Circulation 94:2193–2200. doi:10.1161/01.CIR.94.9.2193
Grose JH, Langston K, Wang X, Squires S, Mustafi SB, Hayes W, Neubert J, Fischer SK, Fasano M, Saunders GM, Dai Q, Christians E, Lewandowski ED, Ping P, Benjamin IJ (2015) Characterization of the cardiac overexpression of HSPB2 reveals mitochondrial and myogenic roles supported by a cardiac HspB2 interactome. PLoS One. doi:10.1371/journal.pone.0133994
Harkin DW, Barros D’Sa AAB, McCallion K, Hoper M, Campbell FC (2002) Ischemic preconditioning before lower limb ischemia–reperfusion protects against acute lung injury. J Vasc Surg 35:1264–1273. doi:10.1067/mva.2002.121981
Hausenloy DJ, Barrabes JA, Bøtker HE, Davidson SM, Di Lisa F, Downey J, Engstrom T, Ferdinandy P, Carbrera-Fuentes HA, Heusch G, Ibanez B, Iliodromitis EK, Inserte J, Jennings R, Kalia N, Kharbanda R, Lecour S, Marber M, Miura T, Ovize M, Perez-Pinzon MA, Piper HM, Przyklenk K, Schmidt MR, Redington A, Ruiz-Meana M, Vilahur G, Vinten-Johansen J, Yellon DM, Garcia-Dorado D (2016) Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol 111:70. doi:10.1007/s00395-016-0588-8
Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM, Trial Investigators ERICCA (2015) Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 373:1408–1417. doi:10.1056/NEJMoa1413534
Helgeland E, Breivik LE, Vaudel M, Svendsen ØS, Garberg H, Nordrehaug JE, Berven FS (2014) Exploring the human plasma proteome for humoral mediators of remote ischemic preconditioning—a word of caution. PLoS One 9:e109279. doi:10.1371/journal.pone.0109279
Hepponstall M, Ignjatovic V, Binos S, Monagle P, Jones B, Cheung MH, d´Udekem Y, Konstantinov IE (2012) Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. PLoS One 7:e48284. doi:10.1371/journal.pone.0048284
Héron-Milhavet L, LeRoith D (2002) Insulin-like growth factor I induces MDM2-dependent degradation of p53 via the p38 MAPK pathway in response to DNA damage. J Biol Chem 277:15600–15606. doi:10.1074/jbc.M111142200
Heusch G (2015) Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 116:674–699. doi:10.1161/CIRCRESAHA.116.305348
Heusch G (2017) Critical issues for the translation of cardioprotection. Circ Res 120:1477–1486. doi:10.1161/CIRCRESAHA.117.310820
Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D (2015) Remote ischemic conditioning. J Am Coll Cardiol 65:177–195. doi:10.1016/j.jacc.2014.10.031
Heusch G, Gersh BJ (2016) ERICCA and RIPHeart: two nails in the coffin for cardioprotection by remote ischemic conditioning? Probably not! Eur Heart J 37:200–202. doi:10.1093/eurheartj/ehv606
Heusch G, Rassaf T (2016) Time to give up on cardioprotection? A critical appraisal of clinical studies on ischemic pre-, post-, and remote conditioning. Circ Res 119:676–695. doi:10.1161/CIRCRESAHA.116.308736
Hibert P, Prunier-Mirebeau D, Beseme O, Chwastyniak M, Tamareille S, Lamon D, Furber A, Pinet F, Prunier F (2013) Apolipoprotein A-I is a potential mediator of remote ischemic preconditioning. PLoS One 8:e77211. doi:10.1371/journal.pone.0077211
Hildebrandt HA, Kreienkamp V, Gent S, Kahlert P, Heusch G, Kleinbongard P (2016) Kinetics and signal activation properties of circulating factor(s) from healthy volunteers undergoing remote ischemic pre-conditioning. JACC Basic Transl Sci 1:3–13. doi:10.1016/j.jacbts.2016.01.007
Jensen RV, Støttrup NB, Kristiansen SB, Bøtker HE (2012) Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol 107:285–293. doi:10.1007/s00395-012-0285-1
Jensen RV, Zachara NE, Nielsen PH, Kimose HH, Kristiansen SB, Bøtker HE (2013) Impact of O-GlcNAc on cardioprotection by remote ischaemic preconditioning in non-diabetic and diabetic patients. Cardiovasc Res 97:369–378. doi:10.1093/cvr/cvs337jiang2011
Jiang H, Shukla A, Wang X, Chen W, Bernstein BE, Roeder RG (2011) Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains. Cell 144:513–525. doi:10.1016/j.cell.2011.01.020
Johnsen J, Pryds K, Salman R, Løfgren B, Kristiansen SB, Bøtker HE (2016) The remote ischemic preconditioning algorithm: effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res Cardiol 111:10. doi:10.1007/s00395-016-0529-6
Kalakech H, Hibert P, Prunier-Mirebeau D, Tamareille S, Letournel F, Macchi L, Pinet F, Furber A, Prunier F (2014) RISK and SAFE signaling pathway involvement in apolipoprotein A-I-induced cardioprotection. PLoS One 9:e107950. doi:10.1371/journal.pone.0107950
Kappé G, Franck E, Verschuure P, Boelens WC, Leunissen JA, De Jong WW (2003) The human genome encodes 10 alpha-crystallin-related small heat shock proteins: hspB1-10. Cell Stress Chaperones 8:53–61. doi:10.1379/1466-1268(2003)8<53:THGECS>2.0.CO;2
Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R (2002) Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106:2881–2883. doi:10.1161/01.CIR.0000043806.51912.9B
Kleinbongard P, Neuhauser M, Thielmann M, Kottenberg E, Peters J, Jakob H, Heusch G (2016) Confounders of cardioprotection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. Cardiology 133:128–133. doi:10.1159/000441216
Kleinbongard P, Skyschally A, Heusch G (2017) Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch 469:159–181. doi:10.1007/s00424-016-1922-6
Lang SC, Elsässer A, Scheler C, Vetter S, Tiefenbacher CP, Kübler W, Katus HA, Vogt AM (2006) Myocardial preconditioning and remote renal preconditioning—identifying a protective factor using proteomic methods? Basic Res Cardiol 101:149–158. doi:10.1007/s00395-005-0565-0
Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, Kajstura J, Baserga R, Anversa P (1997) Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest 100:1991–1999. doi:10.1172/JCI119730
Lin KM, Lin B, Lian IY, Mestril R, Scheffler IE, Dillmann WH (2001) Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia–reoxygenation. Circulation 103:1787–1792. doi:10.1161/01.CIR.103.13.1787
Liu GS, Cohen MV, Mochly-Rosen D, Downey JM (1999) Protein kinase C-ε is responsible for the protection of preconditioning in rabbit cardiomyocytes. J Mol Cell Cardiol 31:1937–1948. doi:10.1006/jmcc.1999.1026
Liu GS, Thornton J, Van Winkle DM, Stanley AWH, Olsson RA, Downey JM (1991) Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation 84:350–356. doi:10.1161/01.CIR.84.1.350
Mastitskaya S, Basalay M, Hosford PS, Ramage AG, Gourine A, Gourine AV (2016) Identifying the source of a humoral factor of remote (pre)conditioning cardioprotection. PLoS One 11:e0150108. doi:10.1371/journal.pone.0150108
Mastitskaya S, Marina N, Gourine A, Gilbey MP, Spyer KM, Teschemacher AG, Kasparov S, Trapp S, Ackland GL, Gourine AV (2012) Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res 95:487–494. doi:10.1093/cvr/cvs212
Maulik N, Engelman RM, Rousou JA, Flack JE III, Deaton D, Das DK (1999) Ischemic preconditioning reduces apoptosis by upregulating anti-death gene Bcl-2. Circulation 100:II-369–II-375. doi:10.1161/01.CIR.100.suppl_2.II-369
Meldrum KK, Meldrum DR, Sezen SF, Crone JK, Burnett AL (2001) Heat shock prevents simulated ischemia-induced apoptosis in renal tubular cells via a PKC-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 281:R359–R364
Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Böning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schön J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K (2015) A multicenter trial of remote ischemic preconditioning for heart surgery. New Engl J Med 373:1397–1407. doi:10.1056/NEJMoa1413579
Michelsen MM, Støttrup NB, Schmidt MR, Løfgren B, Jensen RV, Tropak M, St-Michel EJ, Redington AN, Bøtker HE (2012) Exercise-induced cardioprotection is mediated by a bloodborne, transferable factor. Basic Res Cardiol 107:260–268. doi:10.1007/s00395-012-0260-x
Mocanu MM, Yellon DM (2003) p53 down-regulation: a new molecular mechanism involved in ischaemic preconditioning. FEBS Lett 555:302–306. doi:10.1016/S0014-5793(03)01260-2
Monastyrskaya K, Tschumi F, Babiychuk EB, Stroka D, Draeger A (2008) Annexins sense changes in intracellular pH during hypoxia. Biochem J 409:65–75. doi:10.1042/BJ20071116
Muimo R (2009) Regulation of CFTR function by annexin A2-S100A10 complex in health and disease. Gen Physiol Biophys 28:F14–F19
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136. doi:10.1161/01.CIR.74.5.1124
Olenchock BA, Moslehi J, Baik AH, Davidson SM, Williams J, Gibson WJ, Chakraborty AA, Pierce KA, Miller CM, Hanse EA, Kelekar A, Sullivan LB, Wagers AJ, Clish CB, Heiden MGV, Kaelin WG Jr (2016) EGLN1 inhibition and rerouting of α-ketoglutarate suffice for remote ischemic protection. Cell 164:884–895. doi:10.1016/j.cell.2016.02.006
Orre LM, Panizza E, Kaminskyy VO, Vernet E, Gräslund T, Zhivotovsky B, Lehtiö J (2013) S100A4 interacts with p53 in the nucleus and promotes p53 degradation. Oncogene 32:5531–5540. doi:10.1038/onc.2013.213
Ottani A, Galantucci M, Ardimento E, Neria L, Canalini F, Calevro A, Zaffe D, Novellino E, Grieco P, Giuliani D, Guarini S (2013) Modulation of the JAK/ERK/STAT signaling in melanocortin-induced inhibition of local and systemic responses to myocardial ischemia/reperfusion. Pharmacol Res 72:1–8. doi:10.1016/j.phrs.2013.03.005
Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B (1997) Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol Heart Circ Physiol 273:H1707–H1712
Pell TJ, Baxter GF, Yellon DM, Drew GM (1998) Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol 275:H1542–H1547
Povlsen JA, Løfgren B, Dalgas C, Jespersen NR, Johnsen J, Bøtker HE (2014) Frequent biomarker analysis in the isolated perfused heart reveals two distinct phases of reperfusion injury. Int J Cardiol 171:9–14. doi:10.1016/j.ijcard.2013.11.035
Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P (1993) Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87:893–899. doi:10.1161/01.CIR.87.3.893
Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, Dai X, Manlhiot C, Li J, Redington AN (2012) Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol 107:241–250. doi:10.1007/s00395-011-0241-5
Rossello X, Hall AR, Bell RM, Yellon DM (2016) Characterization of the Langendorff perfused isolated mouse heart model of global ischemia–reperfusion injury: impact of ischemia and reperfusion length on infarct size and LDH release. J Cardiovasc Pharmacol Ther 21:286–295. doi:10.1177/1074248415604462
Saurin AT, Pennington DJ, Raat NJ, Latchman DS, Owen MJ, Marber MS (2002) Targeted disruption of the protein kinase C epsilon gene abolishes the infarct size reduction that follows ischaemic preconditioning of isolated buffer-perfused mouse hearts. Cardiovasc Res 55:672–680. doi:10.1016/S0008-6363(02)00325-5
Schulman D, Latchman DS, Yellon DM (2002) Urocortin protects the heart from reperfusion injury via upregulation of p42/p44 MAPK signaling pathway. Am J Physiol Heart Circ Physiol 283:H1481–H1488. doi:10.1152/ajpheart.01089.2001
Schultz JE, Rose E, Yao Z, Gross GJ (1995) Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am J Physiol Heart Circ Physiol 268:H2157–H2161
Serejo FC, Rodrigues-Junior LF, Tavares KCS, Campos de Carvalho AC, Nascimento JHM (2007) Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol 49:214–220. doi:10.1097/FJC.0b013e3180325ad9
Shan YX, Liu TJ, Su HF, Samsamshariat A, Mestril R, Wang PH (2003) Hsp10 and Hsp60 modulate bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells. J Mol Cell Cardiol 35:1135–1143. doi:10.1016/S0022-2828(03)00229-3
Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN (2009) Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci 117:191–200. doi:10.1042/CS20080523
Skrzypiec-Spring M, Grotthus B, Szeląg A, Schulz R (2007) Isolated heart perfusion according to Langendorff—still viable in the new millennium. J Pharmacol Toxicol Methods 55:113–126. doi:10.1016/j.vascn.2006.05.006
Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G (2015) Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res 117:279–288. doi:10.1161/CIRCRESAHA.117.306878
Sloth AD, Schmidt MR, Munk K, Schmidt M, Pedersen L, Sørensen HT, Bøtker HE (2015) Impact of cardiovascular risk factors and medication use on the efficacy of remote ischaemic conditioning: post hoc subgroup analysis of a randomised controlled trial. BMJ Open 5:e006923. doi:10.1136/bmjopen-2014-006923
Smith CCT, Dixon RA, Wynne AM, Theodorou L, Ong S-G, Subrayan S, Davidson SM, Hausenloy DJ, Yellon DM (2010) Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol 299:H1265–H1270. doi:10.1152/ajpheart.00092.2010
Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, Redington A (2010) Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol 299:1598–1603. doi:10.1152/ajpheart.00396.2010
Sutherland FJ, Hearse DJ (2000) The isolated blood and perfusion fluid perfused heart. Pharmacol Res 41:613–627. doi:10.1006/phrs.1999.0653
Tong H, Chen W, Steenbergen C, Murphy E (2000) Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res 87:309–315. doi:10.1161/01.RES.87.4.309
Vaseva AV, Moll UM (2009) The mitochondrial p53 pathway. Biochim Biophys Acta 1787:414–420. doi:10.1016/j.bbabio.2008.10.005
Vogt AM, Htun P, Kluge A, Zimmermann R, Schaper W (1997) Insulin-like growth factor-II delays myocardial infarction in experimental coronary artery occlusion. Cardiovasc Res 33:469–477
Wall TM, Sheehy R, Hartman JC (1994) Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther 270:681–689
Wang L, Oka N, Tropak M, Callahan J, Lee J, Wilson G, Redington A, Caldarone CA (2008) Remote ischemic preconditioning elaborates a transferable blood-borne effector that protects mitochondrial structure and function and preserves myocardial performance after neonatal cardioplegic arrest. J Thorac Cardiovasc Surg 136:335–432. doi:10.1016/j.jtcvs.2007.12.055
Ytrehus K, Liu Y, Downey JM (1994) Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol 266:1145–1152
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Sources of funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [Grant 483639/2013-3] and the Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ) [Grant E-26/112.085/2012]. JHMN is a research fellow from CNPq. LM received fellowships from CNPq and FAPERJ. DFO received a fellowship from CNPq.
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
395_2017_641_MOESM1_ESM.tif
Supplementary material 1 (TIFF 11799 kb) Figure S1 Protein–protein interactions from the STRING interaction database. Proteins smaller than 12 kDa identified by mass-spectrometry analysis of the preconditioned coronary effluent. Rat pre-pro-insulin-like growth factor (Igf1), Heat shock protein beta-7 (Hspb7), Spermatid nuclear transition protein 1 (Tnp1), Protein dpy-30 homolog (Dpy30), DCM5 protein (Dcm5), Pneumo secretory protein 2 (Scgb3a1), CAMPATH-1 antigen (Cd52), Guanine nucleotide-binding protein, gamma subunit (Gnb3), 10 kDa heat shock protein, mitochondrial (Hspe1), Protein S100-A10, Protein S100-A4, Retinal cone rhodopsin-sensitive cGMP 3’,5’-cyclic phosphodiesterase, gamma subunit (Pde6 h), Protamine-3 (Prm3), and Apolipoprotein C-II (Apoc2). proteins are represented as nodes
395_2017_641_MOESM2_ESM.tif
Supplementary material 2 (TIFF 47941 kb) Figure S2 Effect of the perfusion of different concentrations of exogenous HSP10 on post-ischaemic cardiac performance and infarct size. a Left ventricular developed pressure after 60-min reperfusion in hearts preconditioned with 0.1, 0.3, 0.5, and 1 µM HSP10. Effects of HSP10 concentrations on the end-diastolic pressure (b) and infarct size (c), after 60-min reperfusion. Representative images of TTC-stained heart sections are shown beneath each bar in the graph c. Developed pressure was expressed as percentual of the basal value. Data are mean ± SEM. Number in each colunm is n of hearts. *P < 0.05 vs. CTL
Rights and permissions
About this article
Cite this article
Maciel, L., de Oliveira, D.F., Verissimo da Costa, G.C. et al. Cardioprotection by the transfer of coronary effluent from ischaemic preconditioned rat hearts: identification of cardioprotective humoral factors. Basic Res Cardiol 112, 52 (2017). https://doi.org/10.1007/s00395-017-0641-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-017-0641-2