Abstract
Objectives
Non-nutritive sweeteners (NNS) are widely used as replacements for table sugar in beverages and dessert. However, the metabolic effects of NNS remain controversial. This study aimed to investigate the effects of various sucralose loads on glucose metabolism and expression of sweet taste receptors (STR) and glucose transporters in a high-fat diet (HFD) rats.
Methods
Four-week-old male Sprague Dawley rats were fed a HFD for 8 weeks, then randomly divided into eight groups (6 in each group). All were gavaged with either saline, sucralose (0.54 mM or 0.78 mM), or sucrose (324 mM) with/without gurmarin, a sweet taste inhibitor, for 4 weeks, followed by an intragastric glucose tolerance test (IGGTT) with blood glucose, and plasma insulin, GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) measurements. In the following week, the rats were sacrificed and the small intestine was removed for measurement of sweet taste receptor and glucose transporter expression by quantitative Reverse Transcription-Polymerase Chain Reaction.
Results
In HFD rats, blood glucose levels were decreased at 30, 60, and 120 min during the IGGTT after 4 weeks supplementation with 0.78 mM sucralose. TIR3 expression was increased in the duodenum and TIR2 was increased in the ileum after 324 mM sucrose supplementation. T1R3 expression was increased after 0.54 mM and 0.78 mM sucralose in the ileum, but there was no change in the expression of TIRs in the duodenum after sucralose treatments. SGLT-1 expression was increased after both 0.78 mM sucralose and 324 mM sucrose in the ileum, and only increased in the duodenum after 324 mM sucrose supplementation.
Conclusions
The effects of sucralose on glucose metabolism in HFD rats are dose-dependent and related to enhanced expression of sweet taste receptors and glucose transporters. Further studies are needed to clarify the molecular mechanisms involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes, a growing worldwide challenge, is associated with increased consumption of sugars, particularly sucrose [1]. Non-nutritive sweeteners (NNS) mimic the taste of sucrose and were considered useful as substitutes to reduce energy intake. The U.S. Food and Drug Administration (FDA) has approved 6 artificial sweeteners including sucralose, aspartame, neotame, saccharin, acesulfame potassium and advantage for use in the food industry [2]. However, their metabolic effects remain controversial. Some studies indicated there were no significant effects of NNS on blood glucose levels [3, 4], but more recent evidence suggests that NNS may increase body mass index (BMI) and affect glucose metabolism in a variety of ways [5, 6]. Suez et al. even reported that NNS correlated with higher fasting blood glucose, haemoglobin (HbA1C%) and impaired glucose tolerance in non-diabetic individuals. Moreover, most healthy individuals demonstrated increased glycaemic responses 5–7 days after acceptable daily intake (ADI) NNS consumption compared to their glycaemic responses on days 1–4. In mice, an NNS dose equivalent to the ADI for humans also increased blood glucose levels compared to doses of glucose, sucrose or water [7]. Therefore, the effects of NNS on glucose homestasis should be re-evaluated.
Both sugars and NNS exert effects by binding to the sweet-taste receptor (STR)–a heterodimer, formed by the subunits T1R2 and T1R3. Sweeteners can selectively bind to one or both receptors in different proportions. Key elements of the taste transduction pathways include the G-protein, α-gustducin, and the transient receptor potential ion channel, TRPM5. The sweeteners effect can be blocked by STR inhibitors. According to the species, gurmarin is only effective in rodents but ineffective in humans, while lactisole is active on primates including human. The binding sites of gurmarin were identified in the open VFT (venus flytrap) of T1R3, the joint of T1R2 and T1R3 and the TM domain of T1R3, and that of lactisole were in the TM domain of T1R3 [8,9,10]. In addition to the tongue, STRs are expressed at numerous sites, including the small intestine, brain and bone. The activation of these extra-gustatory receptors has been proposed to regulate metabolic processes [11]. Sucralose is 450–600 times sweeter than sucrose, and its ADI in the US is 5 mg/kg. It has been shown that sucralose (from 0.04 mM, 0.2 mM, 1 mM to 5 mM) stimulated glucagon-like peptide-1 release (GLP-1) from human L cells in a concentration-dependent manner, and this effect could be blocked by sweet receptor antagonists [12]. However, acute exposure to sucralose by intragastric and intraduodenal infusion failed to increase plasma GLP-1 concentrations in healthy subjects [13, 14], although more prolonged exposure has been reported to do so [15].
Absorption of glucose in the gastrointestinal tract occurs via the Na+/glucose cotransporter (SGLT-1) and glucose transporter 2 (GLUT2) [16]. It has been proposed that in low concentrations, glucose is absorbed by SGLT-1, but at high concentrations GLUT2 at the apical location in the enterocyte becomes an important route of uptake [17, 18]. Long-term use of sucralose could increase the expression of glucose transporters and thereby enhance glucose absorption in the small intestine [19]. However, SGLT1 expression and glucose absorptive capacity are not enhanced by either dietary sugar or sucralose in T1R3 −/− or alpha-gustducin −/− mice, indicating the important role of STRs in this process [18].
From the above, it appears that the duration of exposure to sweeteners might determine their effects on glucose metabolism. Moreover, their concentration is likely to be important. The aim of the current study was to evaluate the effects of sucralose loads of various concentrations on glucose metabolism in vitro and in vivo, and on the expression of sweet taste receptors and glucose transporters in HFD rats.
Methods
Animals and experimental design
Four-week-old male Sprague–Dawley (SD) rats (Laboratory Animal Resources, Chinese Academy of Sciences) were housed in individual cages and kept in the controlled room temperature (20–23 °C) with a 12-h light–dark cycles and free access to water and food. After 1 week adaptation to the environment, rats were fed a high-fat diet (HFD; 45% of energy as fat) for 8 weeks. They were then randomized into eight groups and received intragastric infusion every morning for 4 weeks with either (1) 2 ml saline with or without gurmarin 20 μg/ml, (2) 2 ml 324 mM sucrose with or without gurmarin 20 μg/ml, or (3) 2 ml 0.54 mM sucralose with or without gurmarin 20 μg/ml, or (4) 2 ml 0.78 mM sucralose with or without gurmarin 20 μg/ml (n = 6 per group). Bodyweight was measured using an electronic balance every week and % body weight change was calculated. At the end of treatment, the rats were sacrificed after 12 h of fasting and the small intestine was rapidly removed and stored at − 80℃. The study (NSFC No. 81670728) got the ethic approval from the Institutional Animal Care and Use Committee of SLAC (IACUC) Guide for Care of Laboratory Animals.
Intragastric glucose tolerance test (IGGTT)
An IGGTT was performed after 4 weeks of treatment. After an overnight fast, all rats received intragastric infusion of glucose (2 g/kg body weight). Blood samples were taken from tails immediately before and 30 min, 60 min and 120 min after the glucose load. Blood glucose levels were measured with a glucometer (Roche). At each time point, 0.5 ml blood samples were also collected from the orbital venous plexus. These samples were centrifuged immediately (3000g, 4 °C, 10 min) and plasma was stored at − 80 °C for subsequent insulin, GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) assays.
ELISA
The plasma insulin, total GLP-1 and total GIP levels were measured by ELISAs according to the manufacturers’ procedures. All the ELISA kits were purchased from Crystal Chem. The optical density values were read at 450 nm. The sensitivities of the specific assays were 0.156 ng/ml for insulin, 1.24 pM for GLP-1 and 0.78 pM for GIP.
RNA extraction and quantitative RT-PCR
The intestinal tissues were placed in TRIzol reagent (Invitrogen) for total RNA extraction. 1 mg total RNA was converted into the first-strand cDNA with the first-strand cDNA synthesis kit (Takara) according to the manufacturer’s instructions. Quantitative RT-PCR was performed using SYBR Master Mix (Takara) and a LightCycler 480 System (Roche). A human GAPDH gene was used as an endogenous control for sample normalization. Results were presented as the fold expression relative to that of GAPDH.
Statistical analyses
SPSS version 19.0 and Graph Pad Prism 7.0 were applied in the statistical calculations. Student’s t test or the Mann–Whitney U test was performed to compare the two groups. Differences between multiple groups were tested by one-way ANOVA and post hoc comparisons were performed with Tukey’s test. The area under the curve (AUC) of the OGTT and plasma insulin levels over time were calculated. Data are presented as mean ± SD. p < 0.05 was considered significant.
Results
Sucralose improved glucose tolerance in HFD rats (Fig. 1)
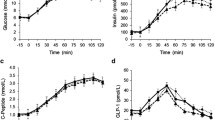
Blood glucose levels in rats at different time points during IGGTT. a Glucose response and area under the curve (AUC) in HFD rats after 4 weeks sweetener exposure; (b–e) comparison of glucose response and AUC between sweetener groups and the groups with the sweet taste inhibitor, gurmarin. Data are mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
During the 4 weeks, there were no differences in food intake or bodyweight between the groups. Neither sucrose nor sucralose treatments altered fasting glucose significantly. Compared to the control group, blood glucose levels were significantly lower at t = 30, 60 and 120 min after the glucose load in the 0.78 mM sucralose group, with an associated reduction in AUC, and lower at t = 120 min in the 0.54 mM sucralose group. There was no difference in the blood glucose levels between sucrose and control groups at any time point after the glucose load (Fig. 1a).
The murine sweet taste receptor antagonist, gurmarin, was used to confirm whether sweeteners function via STRs (Fig. 1b–e). It was shown that the improvement of glucose tolerance in the 0.78 mM sucralose exposure group could be reversed when gurmarin was added.
Sucralose increased insulin secretion
Both sucrose and sucralose treatments tended to increase fasting insulin levels, but this was significant only for the 0.54 mM sucralose group, in whom the increase was blocked by gurmarin (Fig. 2a). Both homeostasis assessment of insulin resistance (HOMA-IR) and β-cell function (HOMA-β) after exposure to sucralose were higher in 0.54 mM group than the control group, and these were reversed with gurmarin (Fig. 2b, c). No differences were found at any time point in the insulin response after the glucose load, nor did the insulin AUC differ between any groups (Supplementary Fig. 1).
Fasting insulin levels, HOMA-IR and HOMA-β in different groups. a Fasting plasma insulin levels, (b and c) homeostasis assessment of insulin resistance (HOMA-IR) and β-cell function (HOMA-β) in HFD rats after 4 weeks sweeteners exposure with or without the sweet taste inhibitor, gurmarin. Data are mean ± SD. *p < 0.05, **p < 0.01 control compared with sucralose group; ##p < 0.001 sucralose group compared with corresponding inhibitor group
Effect of sweeteners on GLP-1 secretion in HFD rats (Fig. 3)
The sucrose treatment was associated with decreased GLP-1 levels 30 min after the glucose load in the HFD rats, and this change was blocked by gurmarin. There were no significant changes in GLP-1 in any other group, not were there any changes in GIP levels (Supplementary Fig. 2).
Effect of sucralose on sweet taste receptors and glucose transporters in the small intestine in HFD rats
Sucrose treatment was associated with increased TIR3 and TRPM5 levels in the duodenum and increased TIR2, α-gustducin and TRPM5 expression in the ileum. After treatment with 0.54 mM sucralose and 0.78 mM sucralose, T1R3 and α-gustducin expression were higher in the ileum and TRPM5 expression was also increased in the 0.78 mM group. However, sucralose had no effect on the expression of TIRs in the duodenum despite 0.54 mM group had higher α-gustducin and TRPM5 level. All these effects could be reversed by gurmarin (Figs. 4a–d and 5a–d).
Effect of sucrose (324 mM) and sucralose (0.54 mM and 0.78 mM) on taste transduction pathways and glucose transporters in the ileum of rats. a TIR2, b TIR3, c TRPM5, d α-gustducin, e SGLT1 and f GLUT2 mRNA levels in the ileum of HFD rats fed with different sweeteners with or without the sweet taste inhibitor, gurmarin. Data are mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 control compared with sweetener group; #p < 0.05, ##p < 0.01, sweetener group compared with corresponding inhibitor group
Effect of sucrose (324 mM) and sucralose (0.54 mM and 0.78 mM) on taste transduction pathways and glucose transporters in the duodenum of rats. a TIR2, b TIR3, c TRPM5, d α-gustducin, e SGLT1 and f GLUT2 mRNA levels in the duodenum of HFD rats fed with different sweeteners with or without the sweet taste inhibitor, gurmarin. Bars show the mean ± SD. *p < 0.05, ***p < 0.001 control compared with sweetener group; #p < 0.05, ##p < 0.01, ###p < 0.001 sweetener group compared with corresponding inhibitor group
Both sucrose and 0.78 mM sucralose treatments were associated with upregulated SGLT-1 expression, but there were no effects on GLUT2 expression in the ileum (Fig. 4e, f). In the duodenum, only sucrose group had higher SGLT-1 expression (Fig. 5e, f).
Discussion
Our data first indicate that continuous dietary supplementation with sucralose can affect glucose metabolism in a load-dependent manner in HFD rats. Four-weeks dosing with 0.78 mM sucralose improved glucose tolerance at all the time points compared to control, while 0.54 mM sucralose decreased glucose levels only at 120 min, and 324 mM sucrose had no effect. The effects of sucralose on glucose tolerance were blocked by the rodent STR inhibitor, gurmarin, consistent with the involvement of the sweet taste signaling pathway in these effects.
We delivered sucralose by intragastric infusion to HFD obese rats over 4 weeks and found that there were no significant changes in GLP-1 in either the 0.78 mM (0.624 mg daily) or 0.54 mM (0.432 mg daily) sucralose groups after the IGGTT. This is consistent with the report that daily gavage with 0.52 mg sucralose for 8 weeks did not affect GLP-1 levels in HFD C57BL/6 wild type mice [20]. A higher oral sucralose load (about 0.9 g daily) and longer feeding time (4 months) did increase GLP-1 levels, but this was accompanied by elevated serum glucose [21]. Hence, both duration and concentration/load of sucralose exposure may be key factors affecting GLP-1 secretion. Although sucralose did not increase GLP-1 concentrations significantly in our study, it increased fasting insulin levels, especially in the 0.54 mM group. Further analysis found that HOMA-IR was increased in the 0.54 mM group, and the insulin secretion index was also increased. Similar results were obtained in a randomized controlled study of human [15]. Steensels et al. also reported a similar phenomenon in C57BL/6 mice, whereby sucralose increased serum insulin profiles independently of GLP-1 levels, in an α-gustducin-dependent manner [20]. This phenomenon could be attributable to stimulation of STRs on pancreatic beta cells, leading via intracellular signal transduction to insulin release [22, 23], although the mechanism still needs further investigation.
Both human and animal studies have demonstrated that STRs and gustducin appear to co-localize in GLP-1 secreting L cells [24]. In obese rats, the STR inhibitor, gurmarin, prevented the increase in fasting insulin levels induced by 4 weeks exposure to 0.54 mM sucralose. Our former study reported that TIR2/T1R3, α-gustducin and TRPM5 were expressed in rat small intestine, particularly the ileum [25]. These may explain the different expression of taste molecules between the ileum and duodenum after sweeteners treatments. Interestingly, the current study showed that sucralose only increased TIR3 levels, but not TIR2 expression. It is apparent that the subunits of the STR bind to different kinds of sweeteners, with T1R2 being required to recognize aspartame and neotame, and T1R3 to recognize cyclamate [10]. All these data demonstrate that sucralose affects glucose metabolism, at least in part, through the STR and its downstream pathways.
It has been reported that persistent consumption of sweeteners can increase the abundance of STRs which can, in turn, regulate SGLT1 expression [18, 21]. Consistent with this, sucralose and sucrose exposure promoted the expression of SGLT1 in the ileum in the current study. We did not, however, find significant changes in GLUT2 expression, despite previous evidence of an increased abundance of GLUT2 in rat enterocytes after sucrose or sucralose treatment. Possibly this difference is accounted for by differing concentrations of sweeteners. The relevance of SGLT-1 expression to glycemic control is demonstrated by the observation that metformin, a hypoglycemic drug, can restore the down-regulation of SGLT1 expression that is observed after HFD feeding in rats, mediated by alterations of the intestinal microbiota, and associated with increased GLP-1 release [26]. Although there were no significant changes in GLP-1 during the IGGTT in our study, we found that 0.78 mM sucralose tended to increase the GLP-1 levels at 30 min after glucose load (p = 0.06), suggesting that sucralose at a suitable concentration may have a similar hypoglycemic effect to metformin.
There are some limitations to our study. First, the limited number of groups makes it difficult to determine precisely the optimum concentration of sucralose for improvement of blood glucose. Second, we only measured GLP-1 levels at 30 min after glucose load, so may have missed any early increases in GLP-1 levels. Third, we did not assess GLP-1 levels in the portal vein which might be more reliable than that of peripheral blood. Finally, although we measured the expression of glucose transporters, we did not directly evaluate glucose absorption.
Conclusions
Our study demonstrated that persistent supplementation with sucralose decreased blood glucose in obese rats. In addition, these sweeteners activated STRs and the downstream signaling pathway in the small intestine. Furthermore, we found that sucralose and sucrose exposure increased SGLT-1 expression. Our findings revealed that different sucralose concentrations can have different effects on glucose metabolism in a HFD rat model, suggesting that perhaps an optimal ADI of sucralose could be selected that was favorable for glucose metabolism in obese patients. However, further studies need to be conducted to clarify the mechanisms of the effects we have observed.
References
Pereira MA (2014) Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk. Adv Nutr 5:797–808. https://doi.org/10.3945/an.114.007062
Chattopadhyay S, Raychaudhuri U, Chakraborty R (2014) Artificial sweeteners: a review. J Food Sci Technol 51:611–621. https://doi.org/10.1007/s13197-011-0571-1
Renwick AG, Molinary SV (2010) Sweet-taste receptors, low-energy sweeteners, glucose absorption and insulin release. Br J Nutr 104:1415–1420. https://doi.org/10.1017/s0007114510002540
Fernstrom JD (2015) Non-nutritive sweeteners and obesity. Annu Rev Food Sci Technol 6:119–136. https://doi.org/10.1146/annurev-food-022814-015635
Swithers SE (2013) Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab 24:431–441. https://doi.org/10.1016/j.tem.2013.05.005
Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, Mann A, Jeyaraman MM, Reid AE, Fiander M, MacKay DS, McGavock J, Wicklow B, Zarychanski R (2017) Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 189:E929–e939. https://doi.org/10.1503/cmaj.161390
Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, Kuperman Y, Harmelin A, Kolodkin-Gal I, Shapiro H, Halpern Z, Segal E, Elinav E (2014) Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514:181–186. https://doi.org/10.1038/nature13793
Sigoillot M, Brockhoff A, Meyerhof W, Briand L (2012) Sweet-taste-suppressing compounds: current knowledge and perspectives of application. Appl Microbiol Biotechnol 96:619–630. https://doi.org/10.1007/s00253-012-4387-3
Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LM, Osman R, Margolskee RF, Max M (2005) Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J Biol Chem 280:15238–15246. https://doi.org/10.1074/jbc.M414287200
Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X (2004) Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci USA 101:14258–14263. https://doi.org/10.1073/pnas.0404384101
Laffitte A, Neiers F, Briand L (2014) Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr Opin Clin Nutr Metab Care 17:379–385. https://doi.org/10.1097/mco.0000000000000058
Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104:15069–15074. https://doi.org/10.1073/pnas.0706890104
Ma J, Bellon M, Wishart JM, Young R, Blackshaw LA, Jones KL, Horowitz M, Rayner CK (2009) Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 296:G735–G739. https://doi.org/10.1152/ajpgi.90708.2008
Ma J, Chang J, Checklin HL, Young RL, Jones KL, Horowitz M, Rayner CK (2010) Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr 104:803–806. https://doi.org/10.1017/s0007114510001327
Lertrit A, Srimachai S, Saetung S, Chanprasertyothin S, Chailurkit LO, Areevut C, Katekao P, Ongphiphadhanakul B, Sriphrapradang C (2018) Effects of sucralose on insulin and glucagon-like peptide-1 secretion in healthy subjects: a randomized, double-blind, placebo-controlled trial. Nutrition 55–56:125–130. https://doi.org/10.1016/j.nut.2018.04.001
Kellett GL, Brot-Laroche E, Mace OJ, Leturque A (2008) Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr 28:35–54. https://doi.org/10.1146/annurev.nutr.28.061807.155518
Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP (2005) Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33:302–305. https://doi.org/10.1042/bst0330302
Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP (2007) T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104:15075–15080. https://doi.org/10.1073/pnas.0706678104
Shi Q, Zhu X, Deng S (2019) Sweet taste receptor expression and its activation by sucralose to regulate glucose absorption in mouse duodenum. J Food Sci. https://doi.org/10.1111/1750-3841.14586
Steensels S, Cools L, Avau B, Vancleef L, Farre R, Verbeke K, Depoortere I (2017) Supplementation of oligofructose, but not sucralose, decreases high-fat diet induced body weight gain in mice independent of gustducin-mediated gut hormone release. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201600716
Sanchez-Tapia M, Martinez-Medina J, Tovar AR, Torres N (2019) Natural and artificial sweeteners and high fat diet modify differential taste receptors, insulin, and TLR4-mediated inflammatory pathways in adipose tissues of rats. Nutrients. https://doi.org/10.3390/nu11040880
Nakagawa Y, Nagasawa M, Mogami H, Lohse M, Ninomiya Y, Kojima I (2013) Multimodal function of the sweet taste receptor expressed in pancreatic beta-cells: generation of diverse patterns of intracellular signals by sweet agonists. Endocr J 60:1191–1206. https://doi.org/10.1507/endocrj.ej13-0282
Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I (2009) Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE 4:e5106. https://doi.org/10.1371/journal.pone.0005106
Young RL, Chia B, Isaacs NJ, Ma J, Khoo J, Wu T, Horowitz M, Rayner CK (2013) Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes 62:3532–3541. https://doi.org/10.2337/db13-0581
Feng R, Qian C, Liu Q, Jin Y, Liu L, Li S, Liao Y, Zhou H, Liu W, Rayner CK, Ma J (2017) Expression of sweet taste receptor and gut hormone secretion in modelled type 2 diabetes. Gen Comp Endocrinol 252:142–149. https://doi.org/10.1016/j.ygcen.2017.08.008
Bauer PV, Duca FA, Waise TMZ, Rasmussen BA, Abraham MA, Dranse HJ, Puri A, O'Brien CA, Lam TKT (2018) Metformin alters upper small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. Cell Metab 27:101–117.e5. https://doi.org/10.1016/j.cmet.2017.09.019
Acknowledgements
This study was supported by the grants from the National Natural Science Foundation of China (No.81670728 and No. 81800693) and sponsored by the Shanghai Pujiang Program (2019PJD027), Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20181807) and 2019 management and construction project of hospital (CHDI-2019-A-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no duality of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qian, C., Qi, Y., Feng, R. et al. Sucralose can improve glucose tolerance and upregulate expression of sweet taste receptors and glucose transporters in an obese rat model. Eur J Nutr 60, 1809–1817 (2021). https://doi.org/10.1007/s00394-020-02375-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02375-1