Abstract
Purpose
This study tested the hypothesis that γ- and δ-tocotrienols are more effective than α-tocotrienol and α-tocopherol in attenuating the signs of diet-induced metabolic syndrome in rats.
Methods
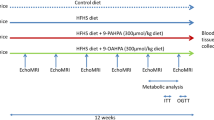
Five groups of rats were fed a corn starch-rich (C) diet containing 68 % carbohydrates as polysaccharides, while the other five groups were fed a diet (H) high in simple carbohydrates (fructose and sucrose in food, 25 % fructose in drinking water, total 68 %) and fats (beef tallow, total 24 %) for 16 weeks. Separate groups from each diet were supplemented with either α-, γ-, δ-tocotrienol or α-tocopherol (85 mg/kg/day) for the final 8 of the 16 weeks.
Results
H rats developed visceral obesity, hypertension, insulin resistance, cardiovascular remodelling and fatty liver. α-Tocopherol, α-, γ- and δ-tocotrienols reduced collagen deposition and inflammatory cell infiltration in the heart. Only γ- and δ-tocotrienols improved cardiovascular function and normalised systolic blood pressure compared to H rats. Further, δ-tocotrienol improved glucose tolerance, insulin sensitivity, lipid profile and abdominal adiposity. In the liver, these interventions reduced lipid accumulation, inflammatory infiltrates and plasma liver enzyme activities. Tocotrienols were measured in heart, liver and adipose tissue showing that chronic oral dosage delivered tocotrienols to these organs despite low or no detection of tocotrienols in plasma.
Conclusion
In rats, δ-tocotrienol improved inflammation, heart structure and function, and liver structure and function, while γ-tocotrienol produced more modest improvements, with minimal changes with α-tocotrienol and α-tocopherol. The most important mechanism of action is likely to be reduction in organ inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is excessive fat storage in the body and is associated with increased morbidity and mortality due to hypertension, diabetes, dyslipidaemia, and cardiovascular and liver diseases [1–4]. These metabolic risk factors interact with each other, resulting in chronic organ complications such as cardiovascular damage and increased fat deposition in hepatocytes [2, 5]. Cardiovascular damage due to remodelling of the heart and blood vessels causes cardiovascular complications including atherosclerosis and coronary artery disease [6, 7]. Obesity is characterised by chronic low-grade inflammation with permanently increased oxidative stress initiated and maintained by the release of adipokines from adipose tissue [8–10]. The World Health Organization estimates that at least 300 million adults worldwide are obese [11], including, in 2011–2012, approximately 42 % of Australian men and 28 % of women aged 18 and over [12]. In the UK, the prevalence of obesity increased to 26 % of men and 24 % of women in 2013 [13]. Similar changes are occurring in the developing world, with the prevalence of obese adults in Malaysia increasing from 5.5 % in 1996 to 14.0 % in 2006 [14].
Vitamin E is a group of closely related tocochromanol phytochemicals including the tocopherols and tocotrienols, for example, from edible plant products such as palm oil, rice bran and wheat germ, with potential cardiovascular and metabolic health-promoting properties [15]. They share a common chroman-6-ol ring with the tocopherols having a saturated phytyl side chain, differing from the geranylgeranyl side chain with three double bonds in the tocotrienols. Each group has α-, β-, γ- and δ-homologues [16]. More research has been performed on α-tocopherol in mammals than on the tocotrienols as α-tocopherol is more readily available [17].
Tocopherols and tocotrienols differ in their biological responses. Although they have similar antioxidant activities [18], tocotrienols have anti-inflammatory and anti-angiogenic activities, unlike the tocopherols [19]. These activities could play vital roles in attenuating metabolic syndrome. Our previous study on the therapeutic responses of tocotrienol-rich fraction (TRF), a commercial mixture of approximately equal proportions of α-tocopherol and α-, γ- and δ-tocotrienols, showed cardiovascular and liver protection with improved plasma glucose and lipid profiles in diet-induced obese rats [16]. Interpretation of these results was complicated by the possibility that different homologues produce different responses as well as possible interactions between tocopherols and tocotrienols. In the current study, we measured the changes following intervention with the same doses of the individual homologues present in TRF (α-tocopherol, α-, γ- and δ-tocotrienols) in a rat model of diet-induced obesity, as well as organ concentrations following chronic intake. Our hypothesis was that γ- and δ-tocotrienols are more effective than α-tocotrienol and α-tocopherol to reverse obesity-related metabolic changes to abdominal fat pads, systolic blood pressure, heart and liver structure and function, and inflammatory biomarkers in our rat model of metabolic syndrome.
Methods
Experimental rats
The previously described experimental protocol for diet-induced obesity in rats [16, 20] has been used with slight modifications. Male Wistar rats (aged 9–10 weeks; weighing 335 ± 3 g, n = 80) were obtained from The University of Queensland Biological Resources unit and individually housed at the University of Southern Queensland Animal House Facility. All experimental protocols were approved by the Animal Ethics Committees of the University of Southern Queensland and The University of Queensland, under the guidelines of the National Health and Medical Research Council of Australia. Rats were divided into 10 groups (n = 8/group). Separate groups of rats were treated with α-tocopherol (CAS Registry Number 59-02-9, 91.6 % purity), α-tocotrienol (CAS Registry Number 58864-81-6, 90.7 % purity), γ-tocotrienol (CAS Registry Number 14101-61-2, 95 % purity) or δ-tocotrienol (CAS Registry Number 25612-59-3, 90 % purity). The dietary interventions for these groups of rats were: (1) corn starch (C), (2) C + α-tocopherol (CαT), (3) C + α-tocotrienol (CαT3), (4) C + γ-tocotrienol (CγT3), (5) C + δ-tocotrienol (CδT3), (6) high carbohydrate, high fat (H), (7) H + α-tocopherol (HαT), (8) H + α-tocotrienol (HαT3), (9) H + γ-tocotrienol (HγT3) and (10) H + δ-tocotrienol (HδT3). The sample size was determined using Mead’s resource equation [21]. All diets were prepared in our laboratory with nutritional parameters meeting or exceeding the National Research Council, USA, nutrient requirements of laboratory animals [22]. Table 1 shows the composition of the diets. In addition, the drinking water for the H group was supplemented with 25 % fructose so that the carbohydrate intake in both C and H groups would be approximately equal at 68 %. All experimental groups were housed in a temperature-controlled, 12-h light/dark cycle environment with ad libitum access to water and food. Measurements of body weight and food and water intakes were taken daily to monitor the day-to-day health of the rats. Feed conversion efficiency (%) was calculated as:
Increase in body weight (%): body weight difference between day 56 (week 8) and day 112 (week 16).
Daily energy intake: average of daily energy intake from week 8 to week 16.
α-Tocopherol, α-tocotrienol, γ-tocotrienol or δ-tocotrienol dissolved in vitamin E-stripped palm olein was given for the final 8 weeks of the 16 weeks protocol by once-daily oral gavage. Palm olein (Malaysian Palm Oil Board) is the liquid fraction obtained from fractionation of palm oil. The fractionation process involves a physical process of cooling the oil under controlled conditions to low temperatures, followed by filtration of the crystals through membrane press. The liquid olein and solid stearin are products of fractionation. α-Tocopherol was donated by Golden Hope Bioganic (Sime Darby, Malaysia), α- and γ-tocotrienol (DavosLife Naturale3) were donated by Davos Life Science Pte Ltd, Singapore, while δ-tocotrienol (DeltaGold 70, containing ~90 % δ- and 10 % γ-tocotrienol) was donated by American River Nutrition, Inc., USA. In this study, 9.17 g of α-tocopherol (91.6 % purity), 9.26 g of α-tocotrienol (90.7 % purity, <1 % α-tocopherol), 8.84 g of γ-tocotrienol (95 % purity, <1 % α-tocopherol) and 13.33 g of δ-tocotrienol (90 % purity) were dissolved in 50 ml of vitamin E-stripped palm olein, respectively, to provide a dose of 85 mg/kg body weight/day. This dose was chosen as the reported oral no-observed-adverse-effects level in male rats given a similar tocotrienol–tocopherol mixture [23].
Echocardiography
Echocardiography was performed by trained cardiac sonographers at the Medical Engineering Research Facility, The Prince Charles Hospital, Brisbane, Australia. Rats were anaesthetised via intraperitoneal injection with Zoletil (tiletamine 15 mg/kg, zolazepam 15 mg/kg) and Ilium Xylazil (xylazine 10 mg/kg). Echocardiographic images were obtained using the Hewlett Packard Sonos 5500 (12 MHz frequency foetal transducer) at an image depth of 3 cm using two focal zones. Measurements of left ventricular posterior wall thickness and internal diameter were made using two-dimensional M-mode taken at mid-papillary level [24].
Body composition measurements
Dual-energy X-ray absorptiometric (DXA) measurements using a Norland XR36 DXA instrument (Norland Corp., Fort Atkinson, WI, USA) were performed on the rats after 16 weeks of feeding, 2 days before rats were killed for pathophysiological assessments. DXA scans were analysed using the manufacturer’s recommended software for use in laboratory animals (Small Subject Analysis Software, version 2.5.3/1.3.1; Norland Corp.) [25]. The precision error of lean mass for replicate measurements, with repositioning, was 3.2 %. Visceral adiposity index (%) was calculated from wet weights of fat pads at euthanasia as [26]:
Physiological parameters
Systolic blood pressure was measured after 0, 4, 8, 12 and 16 weeks under light sedation with intraperitoneal injection of Zoletil (tiletamine 15 mg/kg, zolazepam 15 mg/kg), using an MLT1010 Piezo-Electric Pulse Transducer (ADInstruments) and an inflatable tail cuff connected to a MLT844 Physiological Pressure Transducer (ADInstruments) and PowerLab data acquisition unit (ADInstruments, Sydney, Australia). Abdominal circumference was measured using a standard measuring tape under light sedation.
Oral glucose and insulin sensitivity tests
Oral glucose tolerance tests (OGTT) were performed after 0, 8 and 16 weeks of diet. After 12 h of food deprivation, including replacement of 25 % fructose in water with tap water, blood glucose concentrations were measured in blood samples taken from the tail vein. Subsequently, each rat was treated with glucose (2 g/kg) via oral gavage. Tail vein blood samples were taken every 30 min up to 120 min following glucose administration. The blood glucose concentrations were analysed with a Medisense Precision Q.I.D glucose meter (Abbott Laboratories, Bedford, MA, USA).
For insulin sensitivity testing (ITT), basal blood glucose concentrations were measured after 4–5 h of food deprivation as above. The rats were injected intraperitoneally with 0.33 IU/kg insulin-R (Eli Lilly Australia, West Ryde, NSW, Australia), and tail vein blood samples were taken at 0, 30, 60, 90 and 120 min. Rats were withdrawn from the test if the blood glucose concentrations dropped below 1.1 mmol/l, and 4 g/kg glucose was administered immediately by oral gavage to reverse hypoglycaemia.
Organ weights
Rats were killed with an intraperitoneal injection of pentobarbitone sodium (100 mg/kg). The heart, liver, kidneys, visceral fat pads and spleen were removed and blotted dry for weighing. All organ weights were normalised relative to tibial length at the time of removal with values presented in mg/mm. Tibial length is an independent variable in these age-matched rats, whereas body weight, the usual parameter to normalise organ weights, is not independent as the induction of obesity does not cause proportional changes to all organs in the body.
Histology of heart and liver
Immediately after removal, blotting dry and weighing, heart and liver tissues were fixed in 10 % buffered formalin with three changes of formalin every third day to remove traces of blood from the tissue. The samples were then dehydrated and embedded in paraffin wax. Thin sections (5 μm) of left ventricle and the liver were cut and stained with haematoxylin and eosin stain for determination of inflammatory cell infiltration. Collagen distribution was observed in the left ventricle following picrosirius red staining. Laser confocal microscopy (Zeiss LSM 510 upright Confocal Microscope) was used to determine the extent of collagen deposition in selected regions.
Organ bath studies
Changes in the responsiveness of thoracic aorta were defined using organ bath studies. Thoracic aortic rings (4 mm in length) were suspended in an organ bath chamber filled with Tyrode physiological salt solution bubbled with 95 % O2–5 % CO2 and maintained at 35 °C and allowed to stabilise at a resting tension of 10 mN. Cumulative concentration–response (contraction) curves were measured for noradrenaline (Sigma-Aldrich Australia); concentration–response (relaxation) curves were measured for acetylcholine (Sigma-Aldrich Australia) or sodium nitroprusside (Sigma-Aldrich Australia) after submaximal (70 %) contraction to noradrenaline [27].
Isolated heart preparation
The left ventricular function of the rats in all treatment groups was assessed using the Langendorff heart preparation [28]. Terminal anaesthesia was induced via intraperitoneal injection of pentobarbitone sodium (100 mg/kg); heparin (1000 IU) was then injected into the right femoral vein. The heart was removed and perfused with modified Krebs–Henseleit bicarbonate buffer, containing (in millimolar): NaCl, 119.1; KCl, 4.75; MgSO4, 1.19; KH2PO4, 1.19; NaHCO3, 25.0; glucose, 11.0; and CaCl2, 2.16. Buffer was bubbled with 95 % O2–5 % CO2 and maintained at 35 oC. Isovolumetric ventricular function was measured by inserting a latex balloon into the left ventricle connected to a Capto SP844 MLT844 physiological pressure transducer with Chart software on a MacLab system. All left ventricular end-diastolic pressure values were measured by pacing the heart at 250 beats per minute using an electrical stimulator. End-diastolic pressure was obtained starting from 0 mmHg up to 30 mmHg. The right and left ventricles were separated and weighed. Diastolic stiffness constant (κ, dimensionless) was calculated [29].
Lipid profile and liver enzyme analyses
Blood was collected from the abdominal aorta following euthanasia and centrifuged at 5000g for 15 min within 30 min of collection into heparinised tubes. Plasma was separated and transferred to Eppendorf tubes for storage at −20 °C before analysis. Plasma concentrations of total cholesterol, triglycerides (TG) and non-esterified fatty acids (NEFA), and activities of plasma alanine transaminase (ALT) and aspartate transaminase (AST) were determined according to manufacturer’s protocols using an Olympus AU400 analyser with kits and controls supplied by Olympus Corporation, Tokyo, Japan: ALT, Olympus OSR6107 kinetic UV test; AST, Olympus OSR6109 kinetic UV test; total plasma cholesterol, Olympus OSR6516 enzymatic colour test; plasma triglycerides, Olympus OSR6133 enzymatic colour test. NEFA were determined using a commercial kit (Wako, Osaka, Japan).
Extraction of vitamin E from diet, tissue and plasma
3 g of diets was used for extraction according to Sundram and Nor [30]. Organ samples were collected, cut into pieces, weighed (0.5–0.8 g) and kept at −80 °C prior to extraction. During extraction, samples were thawed and 1 ml of water was added into each sample. Samples were homogenised. For adipose tissues, samples were homogenised in tissue lysis buffer. Diets, plasma, heart and liver samples were spiked with 10 μl of 0.5 mg/ml 2,2,5,7,8-pentamethyl-6-chromanol (PMC, dissolved in hexane, Aldrich, USA) [31], while retroperitoneal and epididymal adipose tissues were spiked with 10 μl 0.5 mg/ml δ-tocopherol dissolved in hexane as internal standard. We could not detect PMC in adipose tissue and hence δ-tocopherol was selected as an alternative internal standard because of its high purity (97 %) that eliminates the overestimation of other vitamin E homologues. In addition, it has a different retention time to other homologues which rules out overlapping peaks. Mixture was then vortexed for 10 sec. Next, 1 ml t-butyl-hydroxytoluene in ethanol (0.1 g/l; 0.01 % BHT) to minimise the oxidation of target analytes and 2 ml hexane were added to samples. Samples were vortexed for 5 min. Samples were then centrifuged at 15,000 rpm for 10 min. Extraction steps were repeated twice for the remaining samples with 2 ml of hexane. At least 1.5 ml of supernatant was transferred, if not all, in each extraction to achieve highest recovery of vitamin E in solvent liquid extraction. The organic solution in the pooled supernatant was evaporated using Buchi rotavapor R-205 (Flawil, Switzerland). Dried samples were reconstituted with 300 μl hexane (adipose tissue samples with 600 μl hexane). For determination in plasma, 0.1 ml plasma was aliquoted into the 5-ml test tube; 100 μl 0.01 % BHT in ethanol and 1.5 ml hexane were added into plasma and extraction was performed as mentioned above. Dried samples were reconstituted with 100 μl hexane.
Tocotrienol and tocopherol analysis with high-performance liquid chromatography
The α-tocopherol and tocotrienol homologues were analysed with normal phase high-performance liquid chromatography (HPLC). 10 μl of sample was injected into an Agilent 1100 Series HPLC System (Agilent, Santa Clara, Calif., USA). The chromatographic separation was carried out using a Zorbax Silica 60 (5 μm; 250 × 4 mm internal diameter) analytical column. The mobile phase consisting of 97 % hexane: 2.5 % dioxane: 0.5 % isopropanol (v/v) was delivered at 1 ml/min flow rate. The absorbances of α-tocopherol and tocotrienol homologues were detected at an excitation wavelength of 290 nm and an emission wavelength of 330 nm. PMC and δ-tocopherol were used as internal standards. Both methods for internal standards were validated for specificity, linearity, precision, accuracy, limit of detection (LOD) and limit of quantitation (LOQ). For specificity validation, mobile phase solutions showed no interference. Spiked PMC or δ-tocopherol showed that all known substance was eluted at different retention times from other studied vitamin E homologues. For linearity validation, PMC and delta δ-tocopherol were tested at least at 5 concentrations (0, 1, 10, 100 and 1000 ppm). Within this range, values of concentration vs peak area were linear with R 2 > 0.9. For accuracy validation, biological samples were spiked with PMC or δ-tocopherol, across the range of 80–120 % concentration, in triplicate. Report % recovery was 100 ± 3 %. This accuracy was only performed when both specificity and linearity were established. For precision validation, both system and method precision tests were conducted. System precision was determined by 5 repeated PMC or δ-tocopherol (100 ppm) injections to the system with repeatability (relative standard deviation) <5 %. Method precision was determined with three concentrations of PMC or δ-tocopherol with three repetitions each, showing relative standard deviation of <5 %. The LOD and LOQ for both PMC and δ-tocopherol were 1 and 10 ppm, respectively.
Statistical analyses
All data sets are represented as mean ± standard error of mean (SEM) to allow comparison with our previous papers and most of the literature. Comparisons of findings between groups were made via statistical analysis of data sets using two-way ANOVA. When interaction and/or the main effects were significant, means were compared using Newman–Keuls multiple-comparison post hoc test. A p value of <0.05 was considered as statistically significant. All statistical analyses were performed using Graph Pad Prism version 5.00 for Windows.
Results
Dietary intake and adiposity indices
Vitamin E homologues were given by oral gavage at 85 mg/kg/day. These homologues were also ingested from the food at approximately 1000-fold lower doses ranging from 35 to 130 μg/day, which is approximately 0.1–0.3 mg/kg/day (Table 2). Food and water intake were decreased in H rats compared to C rats. Treatment with α-tocopherol or individual tocotrienols did not change food or water intake compared with their respective controls except CγT3 which had lower food intake compared with C rats. H rats had higher body weight with higher fat mass and total visceral adipose tissue (retroperitoneal, epididymal and omental fat pads) than C rats (Table 2). The visceral adiposity index and abdominal circumference of H rats were higher than in C rats. Total fat mass, abdominal circumference, adiposity index, and retroperitoneal and epididymal fat pads of HδT3 were lower than H rats (Table 2).
Cardiovascular structure and function
After 16 weeks, H rats showed cardiac remodelling with marked cardiac hypertrophy, as shown by higher left ventricular wet weight relative to body weight and left ventricular mass derived from echocardiography (Table 3). H rats developed eccentric hypertrophy, characterised by higher left ventricular weight and internal diameter in diastole (LVIDd), while relative wall thickness remained unchanged, with higher stroke volume and cardiac output (Table 3) than C rats. H rats showed lower systolic function seen as lower fractional shortening with higher systolic wall stress. This is supported by lower contractility, measured as maximal rate of positive rise of pressure (+dP/dt) and negative rise of pressure (−dP/dt), and left ventricular developed pressure in the isolated heart of H rats compared with C rats. Diastolic function, estimated from mitral flow rates calculated as the ratio of the maximal E- (early filling velocity) and A-wave (atrial filling velocity), was lower with lower E/A ratio [ratio of the early (E) to late (A) ventricular filling velocities] in H compared with C rats. δ-Tocotrienol normalised eccentric hypertrophy shown by lower LVIDd (left ventricular internal diameter during diastole), stroke volume and cardiac output in HδT3 compared with H rats. In vivo, systolic function of hearts from γ- and δ-tocotrienol-treated rats (HγT3 and HδT3) was higher with higher fractional shortening and lower systolic wall stress. Ex vivo, the hearts of HαToc, HαT3, HγT3 and HδT3 rats showed higher contractility with higher +dP/dt and −dP/dt and LV-developed pressures, and lower diastolic stiffness compared with H rats. No changes were seen in hearts from CαT, CαT3, CγT3 and CδT3 rats compared with C rats.
In the left ventricle, the number of inflammatory cells in H rats was higher than in C rats (Fig. 1). These cells were usually found in clusters of cells located at scar sites and throughout the interstitium and the areas of fibrosis. Collagen content in the heart was higher in H rats (Fig. 2). Treatment with α-tocopherol, α-, γ- and δ-tocotrienols reduced inflammatory cell infiltration in HαT, HαT3, HγT3 and HδT3 predominantly due to the lower area of scar tissue as shown by lower collagen within the heart compared with H rats (Fig. 2).
Haematoxylin and eosin staining of left ventricle (×20) showing inflammatory cells (labelled as ‘i’) as dark spots near the myocytes in C, CαT, CαT3, CγT3, CδT3, HαT, HαT3, HγT3 and HδT3 rats. C, corn starch; CαT, corn starch + α-tocopherol; CαT3, corn starch + α-tocotrienol; CγT3, corn starch + γ-tocotrienol; CδT3, corn starch + δ-tocotrienol; H, high carbohydrate, high fat; HαT, high carbohydrate, high fat + α-tocopherol; HαT3, high carbohydrate, high fat + α-tocotrienol; HγT3, high carbohydrate, high fat + γ-tocotrienol; HδT3, high carbohydrate, high fat + δ-tocotrienol
Picrosirius red staining of left ventricular interstitial collagen deposition (×40) in C, CαT, CαT3, CγT3, CδT3, HαT, HαT3, HγT3 and HδT3 rats; collagen deposition is labelled as ‘col’. C, corn starch; CαT, corn starch + α-tocopherol; CαT3, corn starch + α-tocotrienol; CγT3, corn starch + γ-tocotrienol; CδT3, corn starch + δ-tocotrienol; H, high carbohydrate, high fat; HαT, high carbohydrate, high fat + α-tocopherol; HαT3, high carbohydrate, high fat + α-tocotrienol; HγT3, high carbohydrate, high fat + γ-tocotrienol; HδT3, high carbohydrate, high fat + δ-tocotrienol
H rats exhibited higher systolic blood pressure compared to C rats. Supplementation with γ- or δ-tocotrienol normalised blood pressures in HγT3 and HδT3 rats (Table 3). Vascular smooth muscle dysfunction was shown as lower contractile responses to noradrenaline and lower relaxant responses to sodium nitroprusside together with endothelial dysfunction, defined as lower relaxation responses to acetylcholine in isolated thoracic aortic rings of H rats compared to C rats (Fig. 3). Following γ- or δ-tocotrienol supplementation, thoracic aortic contraction responses to noradrenaline and relaxation responses to sodium nitroprusside and acetylcholine were higher in HγT3 and HδT3 compared with H rats. Blood pressures and vascular responses in CαT, CαT3, CγT3 and CδT3 rats compared with C rats, and HαT and HαT3, compared to H rats, were unchanged.
Cumulative concentration–response curves for noradrenaline, sodium nitroprusside and acetylcholine in thoracic aortic rings from α-tocopherol-treated group (A, B, C), α-tocotrienol-treated group (D, E, F), γ-tocotrienol-treated group (G, H, I) and δ-tocotrienol-treated group (J, K, L). Data shown as means ± SEM. Labelled means in a row with superscripts without a common letter differ, n = 8/group. C, corn starch; CαT, corn starch + α-tocopherol; CαT3, corn starch + α-tocotrienol; CγT3, corn starch + γ-tocotrienol; CδT3, corn starch + δ-tocotrienol; H, high carbohydrate, high fat; HαT, high carbohydrate, high fat + α-tocopherol; HαT3, high carbohydrate, high fat + α-tocotrienol; HγT3, high carbohydrate, high fat + γ-tocotrienol; HδT3, high carbohydrate, high fat + δ-tocotrienol
Lipid profile, liver function and structure
H rats had higher total cholesterol, triglyceride and NEFA plasma concentrations than C rats (Table 4). δ-Tocotrienol reduced total cholesterol, NEFA and triglyceride concentrations in HδT3, while γ-tocotrienol reduced plasma NEFA in HγT3 rats compared with H rats. H rats had higher liver weights, approximately twofold higher plasma ALT and 1.4-fold higher plasma AST activities than C rats (Table 4). Liver histology showed presence of lipid droplets with portal inflammatory cell infiltration in livers of H rats compared with C rats (Fig. 4). Treatment with vitamin E homologues for 8 weeks attenuated the degree of liver injury in HαT, HαT3, HγT3 and HδT3 rats, as demonstrated by lower plasma ALT and AST activities, less infiltration of inflammatory cells and decreased lipid droplets (Fig. 4). Lipid profile, liver structures and function of CαT, CαT3, CγT3 and CδT3 rats remained unchanged from C rats.
Haematoxylin and eosin staining of hepatocytes (×20) showing inflammatory cells around the portal region (labelled as ‘i’) and lipid droplet (labelled as ‘f’) in C, CαT, CαT3, CγT3, CδT3, HαT, HαT3, HγT3 and HδT3 rats. C, corn starch; CαT, corn starch + α-tocopherol; CαT3, corn starch + α-tocotrienol; CγT3, corn starch + γ-tocotrienol; CδT3, corn starch + δ-tocotrienol; H, high carbohydrate, high fat; HαT, high carbohydrate, high fat + α-tocopherol; HαT3, high carbohydrate, high fat + α-tocotrienol; HγT3, high carbohydrate, high fat + γ-tocotrienol; HδT3, high carbohydrate, high fat + δ-tocotrienol
Plasma glucose concentrations, glucose tolerance and insulin sensitivity
H rats showed lower glucose utilisation by week 16 with higher fasting plasma glucose concentrations at 16 weeks compared with C rats (Table 4). The area under the curve was calculated to reflect the total rise in blood glucose concentration following an oral glucose tolerance or insulin sensitivity test (glucoseAUC). The plasma glucose response to oral glucose loading was greater in H rats than C rats with glucoseAUC of H rats approximately 35 % higher than C rats at 16 weeks (Table 4), indicating impaired glucose and insulin tolerance in H rats. δ-Tocotrienol normalised the fasting plasma glucose concentrations in HDT rats. In addition, HDT rats cleared postprandial glucose from the blood with greater efficiency than H rats during OGTT shown as lower glucoseAUC at week 16 (Table 4). In insulin sensitivity testing, HδT3 rats had lower glucoseAUC than H rats (Table 4).
Plasma and tissue concentrations of α-tocopherol, α-, γ- and δ-tocotrienols
Supplementation of either α-, γ-, δ-tocotrienol or α-tocopherol for 8 weeks increased the concentration of the individual compound in liver, heart and adipose tissues (Table 5). However, only α-tocopherol and α-tocotrienol were detected in plasma. In this study, α-tocopherol and α-tocotrienol detected in tissues of γ- and δ-tocotrienols-treated rats may have been derived from the basal diet. α-Tocopherol and the tocotrienols were found in all organs; α-tocopherol and α-tocotrienol concentrations were highest in the liver, while γ- and δ-tocotrienol concentrations were highest in adipose tissue. Most of the body’s stores of tocotrienols and tocopherols were in the visceral fat pads. The concentrations of α- and δ-tocotrienols were higher than γ-tocotrienol in adipose tissue.
Discussion
α-Tocopherol and tocotrienols have been extensively studied in cells, animal models and humans as a potential treatment for chronic human diseases including metabolic syndrome and cancer [19, 32–34]. Earlier studies suggested that tocotrienols were better antioxidants in membrane systems [35]. However, α-tocopherol and α-tocotrienol are co-localised in the same place in membranes and they exert substantially the same mobility in liposomal membranes [18]. Tocopherols and tocotrienols have the same reactivities towards radicals and exert the same antioxidant activities against lipid peroxidation in solution. In addition, the physical effect of tocopherols on the fluidity of the membrane interior is greater than tocotrienols, but less than cholesterol, but these effects at the membrane surface are similar. Hence, in terms of antioxidant activity, tocopherol and tocotrienols are similar [18]. However, this study has tested whether vitamin E homologues differentially affect the signs of metabolic syndrome as studies in cancer have shown that the biological relevance of tocotrienols goes beyond antioxidant activity [19]. Using our rat model of diet-induced metabolic syndrome, we previously demonstrated that palm TRF, a mixture of α-tocopherol and α-, γ- and δ-tocotrienols, protected the heart and liver and improved plasma glucose and lipid profiles with minimal changes in abdominal obesity [16]. We have now extended this study to investigate the responses to the individual compounds present in TRF on cardiovascular and liver structure and function, and metabolic changes in the same rat model of diet-induced metabolic syndrome, and further we have shown that these compounds are present in the heart, liver and adipose tissue.
α-Tocopherol, α-, γ- and δ-tocotrienols reduced inflammatory cell infiltration and improved contractility ex vivo in the hearts of rats fed a high-carbohydrate, high-fat diet. However, only γ- and δ-tocotrienols improved heart structure, improved cardiac function in vivo and normalised blood pressure. These cardiovascular responses could be mediated through normalisation of sympathovagal balance. Dysregulation of sympathetic nervous system signalling is associated with diabetes mellitus, obesity and cardiovascular disease [36, 37] possibly by the increased activation of the sympathetic nervous system by angiotensin II [38, 39]. Further, glucose, insulin and NEFA potently stimulate sympathetic activity and noradrenaline release [40]. α-Tocopheryl acetate altered cardiac sympathovagal balance in patients with type 2 diabetes by increasing the high-frequency component, an index of vagal efferent activity, and decreasing the low-frequency component of heart rate variability, an index of vasomotor sympathetic activity [41]. TRF from palm oil, but not α-tocopherol, inhibited the increased plasma noradrenaline concentrations in rats exposed to restraint stress [42]. Tocotrienol mixtures from rice bran oil increased IkB kinase complex-associated protein and monoamine oxidase A transcripts, key enzymes responsible for degrading biogenic and dietary monoamines, hence reduced autonomic crises and exaggerated hypertension in familial dysautonomia [43]. While we did not measure the noradrenaline concentrations in plasma, the noradrenaline reuptake transporter expression in the heart or the low-frequency to high-frequency ratio of sympathovagal balance, the data from echocardiography (in vivo) and isolated Langendorff hearts (ex vivo) suggested a plausible association between tocotrienols and α-tocopherol, and the balance between sympathetic and parasympathetic activity, since in vivo echocardiography measures the innervated heart while the ex vivo Langendorff heart measures intrinsic heart function. Whether α-tocopherol and tocotrienols directly affect the sympathovagal balance warrants further investigation.
In the present study, δ-tocotrienol markedly reduced total body and abdominal fat, while γ-tocotrienol produced modest reductions, in agreement with previous studies [44–46]. We previously reported that TRF did not reduce obesity in rats [16]. This could be due to the possible physiological antagonism between α-tocopherol and tocotrienols in TRF. α-Tocopherol may decrease the responses to tocotrienols as the body prefers to absorb α-tocopherol rather than tocotrienols [47]. Further, preferential absorption has been reported for α-tocotrienol over γ-tocotrienol, δ-tocotrienol and α-tocopherol in thoracic duct-cannulated rats [48]. The very low amount of α-tocopherol acetate intake from the C and H diets (0.1–0.3 mg/kg compared with 85 mg/kg of α-tocopherol or tocotrienols as interventions) is unlikely to affect the delivery or responses of tocotrienol homologues to the vital organs, and hence the effects of dietary α-tocopherol acetate are considered negligible in this study. The use of high-purity tocotrienol homologues as interventions in this study allows their individual responses to be measured with minimal interference from α-tocopherol or α-tocotrienol from the diet or as impurities in the preparations.
Glucose utilisation and insulin sensitivity in obese rats were improved by δ-tocotrienol treatment. These changes most likely follow adipose tissue reduction and hence reduced proinflammatory microenvironment. Several factors commonly present with excess adiposity are involved in diabetes mellitus and cardiovascular dysfunction. These include increased adipokines, proinflammatory cytokines and inflammatory lipid mediators secreted from adipose tissue that initiate vascular dysfunction, cardiac fibrosis, impaired glucose metabolism [49–51] and insulin signalling [52, 53], hyperinsulinaemia [54] and activation of the renin-angiotensin system [55]. Inflammatory cells infiltrate into adipose tissue to activate inflammatory pathways [56]. This action precedes or is associated with the development of insulin resistance and ectopic lipid accumulation in obese animals and humans [56], suggesting the role of infiltrated macrophages in the pathophysiology of obesity. Assuming that macrophages in adipose tissue are the source of the mediators of chronic low-grade inflammation [57], reduction in adipose tissue would decrease the proinflammatory microenvironment in this tissue. As δ-tocotrienol reduced the infiltration of inflammatory cells in the heart and liver, this action in combination with reduction in fat depots may improve the metabolic disorders in treated obese rats [58]. Further, liver function was not compromised in δ-tocotrienol-treated rats despite continuation of the high intake of saturated fats and simple sugars.
The dose of tocopherol or tocotrienols given to the rats in this study (85 mg/kg/day) corresponds to a human dose of around 800 mg/day based on body surface area comparisons between rats weighing 500 g and humans weighing 60 kg [59]. This dose could be provided through a daily oral supplement as it is not reasonably achievable through dietary consumption of tocotrienol sources [19]. Tocotrienols are generally recognised as safe (GRAS) with no indication of significant adverse effects related to tocotrienols consumption at the dose used in this study. The European Food Safety Authority in 2008 published a no-observed-adverse-effect level (NOAEL) of tocotrienol (120 mg/kg/day for males and 130 mg/kg/day for females) in a subchronic study in rats using purified vitamin E mixtures from palm oil [60]. In a chronic study in rats using purified vitamin E mixtures from palm oil, the NOAEL values were 303 mg/kg/day for males and 473 mg/kg/day for females [61]. The effects observed at these doses were not considered to be adverse [60]. In an on-going phase I dose-escalation clinical trial in pancreatic cancer patients using δ-tocotrienol (ClinicalTrials.gov Identifier: NCT00985777), preliminary findings showed that a dosage of up to 800 mg/day was well tolerated with further escalation to 3200 mg/day being planned [62]. It is important to point out that the no-adverse-effect dose for tocotrienols in humans has not been defined but it is assumed to be the same as the tolerable upper intake level for tocopherol of 1000 mg/day for adults [60]. Reports on metabolism of tocotrienols are scarce, with only few reporting the postprandial distribution and pharmacokinetics of tocotrienols, using single-dose studies with the mixture of TRF derived from palm oil [63–66]. Half-lives of tocotrienols were approximately fourfold to fivefold lower than that of tocopherols (4 vs 20 h) in humans [67]. However, caution is needed in extrapolating the pharmacokinetics of individual tocopherol and tocotrienols dosed as a mixture in the TRF formulation. In addition, confusion arose when the tocopherol–tocotrienol ratios were unspecified, varied or altered in studies [63–66]. This study provides new results supporting the oral absorption and distribution of α-tocopherol, α-, γ- and δ-tocotrienols to plasma, heart, liver and adipose tissues using the individual homologues, unlike previous studies that used tocotrienol mixtures [66–70]. The animal studies on bioavailability of tocotrienols have used differing modes of delivery, sources and types of α-tocopherol, α-tocotrienol, γ-tocotrienol or even mixtures, durations of supplementation and diet conditions [71], making any comparison inherently difficult. This study showed that orally supplemented tocotrienols reached all vital organs even if they were not detected in plasma, as with γ- and δ-tocotrienols. Delivery of oral tocotrienols to vital organs is the key determinant of the overall efficacy of oral tocotrienols in these tissues, rather than the concentrations in plasma. Significant amounts of tocotrienols delivered to the vital organs indicated effective tocotrienol transport systems in vivo, independent of α-tocopherol transfer protein [15, 47]. The evidence that tocotrienols accumulated in vital organs supports future studies to identify specific mechanisms of tissue delivery and metabolism of tocotrienols.
In summary, the biological responses to γ- and δ-tocotrienols were more pronounced than with α-tocopherol and α-tocotrienol in this rat model of metabolic syndrome. All homologues improved liver structure and function. Only δ-tocotrienol enhanced glucose metabolism associated with obesity, although both γ- and δ-tocotrienols improved cardiovascular structure and function, and reduced adiposity. These effects may be associated with the sympathovagal balance and reduction in proinflammatory microenvironment, which may differentiate the biological functions of tocopherol and tocotrienol homologues. Their distribution to vital organs is an important prerequisite to biological activity of the tocotrienols. Hence, increasing intake of δ-tocotrienol and, to a lesser extent, γ-tocotrienol may serve as a complementary dietary strategy in managing metabolic syndrome.
References
Eckel RH, Grundy SM, Zimmet PZ (2005) The metabolic syndrome. Lancet 365:1415–1428
Fabbrini E, Sullivan S, Klein S (2010) Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51:679–689
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH (2006) Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Circulation 113:898–918
Tesauro M, Cardillo C (2011) Obesity, blood vessels and metabolic syndrome. Acta Physiol (Oxf) 203:279–286
Nikolopoulou A, Kadoglou NP (2012) Obesity and metabolic syndrome as related to cardiovascular disease. Expert Rev Cardiovasc Ther 10:933–939
Guerra F, Mancinelli L, Angelini L, Fortunati M, Rappelli A, Dessì-Fulgheri P, Sarzani R (2011) The association of left ventricular hypertrophy with metabolic syndrome is dependent on body mass index in hypertensive overweight or obese patients. PLoS One 6:e16630
Nestel PJ, O’Brien R, Nelson M (2008) Management of dyslipidaemia—evidence and practical recommendations. Aust Fam Physician 37:521–527
Scarpellini E, Tack J (2012) Obesity and metabolic syndrome: an inflammatory condition. Dig Dis 30:148–153
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G, Gitto E, Arrigo T (2015) Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci 16:378–400
Ahima RS, Flier JS (2000) Leptin. Annu Rev Physiol 62:413–437
National Heart Foundation of Australia. Factsheet: overweight and obesity statistics 2012
Health Survey of England. Adult anthropometric measures, overweight and obesity 2013
Khambalia AZ, Seen LS (2010) Trends in overweight and obese adults in Malaysia (1996–2009): a systematic review. Obes Rev 11:403–412
Weng-Yew W, Brown L (2011) Nutrapharmacology of tocotrienols for metabolic syndrome. Curr Pharm Des 17:2206–2214
Wong WY, Poudyal H, Ward LC, Brown L (2012) Tocotrienols reverse cardiovascular, metabolic and liver changes in high carbohydrate, high fat diet-fed rats. Nutrients 4:1527–1541
Traber MG, Atkinson J (2007) Vitamin E, antioxidant and nothing more. Free Radic Biol Med 43:4–15
Yoshida Y, Niki E, Noguchi N (2003) Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects. Chem Phys Lipids 123:63–75
Aggarwal BB, Sundaram C, Prasad S, Kannappan R (2010) Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol 80:1613–1631
Panchal SK, Poudyal H, Arumugam TV, Brown L (2011) Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J Nutr 141:1062–1069
Festing MFW, Altman DG (2002) Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J 43:244–258
National Research Council (1995) Nutrient requirements of laboratory animals, fourth revised edition, 1995. The National Academies Press, Washington
Nakamura H, Furukawa F, Nishikawa A, Miyauchi M, Son HY, Imazawa T, Hirose M (2001) Oral toxicity of a tocotrienol preparation in rats. Food Chem Toxicol 39:799–805
Brown L, Fenning A, Chan V, Loch D, Wilson K, Anderson B, Burstow D (2002) Echocardiographic assessment of cardiac structure and function in rats. Heart Lung Circ 11:167–173
Ward LC, Battersby KJ (2009) Assessment of body composition of rats by bioimpedance spectroscopy validation against dual-energy X-ray absorptiometry. Scand J Lab Anim Sci 36:253–261
Jeyakumar SM, Vajreswari A, Giridharan NV (2006) Chronic dietary vitamin A supplementation regulates obesity in an obese mutant WNIN/Ob rat model. Obesity 14:52–59
Chan V, Hoey A, Brown L (2006) Improved cardiovascular function with aminoguanidine in DOCA-salt hypertensive rats. Br J Pharmacol 148:902–908
Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R (2007) Isolated heart perfusion according to Langendorff—still viable in the new millennium. J Pharmacol Toxicol Methods 55:113–126
Brown L, Duce B, Miric G, Sernia C (1999) Reversal of cardiac fibrosis in deoxycorticosterone acetate-salt hypertensive rats by inhibition of the renin-angiotensin system. J Am Soc Nephrol 10(Suppl 11):S143–S148
Sundram K, Nor R (2002) Analysis of tocotrienols in different sample matrixes by HPLC. In: Amstrong D (ed) Methods in molecular biology: oxidative stress biomarkers and antioxidant protocols. Humana Press Inc, Totowa, pp 221–232
Ueda T, Igarashi O (1987) New solvent system for extraction of tocopherols from biological specimens for HPLC determination and evaluation of 2,2,5,7,8-pentamethyl-6-chromanol as an internal standard. J Micronutr Anal 3:185–198
Traber MG, Frei B, Beckman JS (2008) Vitamin E revisited: do new data validate benefits for chronic disease prevention? Curr Opin Lipidol 19:30–38
Yang C, Suh N (2013) Cancer prevention by different forms of tocopherols. In: Pezzuto JM, Suh N (eds) Natural products in cancer prevention and therapy. Springer, Berlin, pp 21–33
Ahsan H, Ahad A, Iqbal J, Siddiqui WA (2014) Pharmacological potential of tocotrienols: a review. Nutr Metab (Lond) 11:52
Kamal-Eldin A, Appelqvist L-Å (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31:671–701
Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G (2010) Mechanisms of obesity-induced hypertension. Hypertens Res 33:386–393
Lambert E, Sari CI, Dawood T, Nguyen J, McGrane M, Eikelis N, Chopra R, Wong C, Chatzivlastou K, Head G, Straznicky N, Esler M, Schlaich M, Lambert G (2010) Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension 56:351–358
Dendorfer A, Raasch W, Tempel K, Dominiak P (1998) Interactions between the renin-angiotensin system (RAS) and the sympathetic system. Basic Res Cardiol 93:24–29
Ishii H, Niioka T, Izumi H (2009) Circulating adrenaline released by sympathoadrenal activation elicits acute vasodilatation in the rat masseter muscle. Arch Oral Biol 54:486–494
Thackeray JT, Radziuk J, Harper ME, Suuronen EJ, Ascah KJ, Beanlands RS, Dasilva JN (2011) Sympathetic nervous dysregulation in the absence of systolic left ventricular dysfunction in a rat model of insulin resistance with hyperglycemia. Cardiovasc Diabetol 10:75
Manzella D, Barbieri M, Ragno E, Paolisso G (2001) Chronic administration of pharmacologic doses of vitamin E improves the cardiac autonomic nervous system in patients with type 2 diabetes. Am J Clin Nutr 73:1052–1057
Nur Azlina MF, Nafeeza MI (2008) Tocotrienol and a-tocopherol reduce corticosterone and noradrenalin levels in rats exposed to restraint stress. Pharmazie 63:890–892
Anderson SL, Rubin BY (2005) Tocotrienols reverse IKAP and monoamine oxidase deficiencies in familial dysautonomia. Biochem Biophys Res Commun 336:150–156
Ima-Nirwana S, Suhaniza S (2004) Effects of tocopherols and tocotrienols on body composition and bone calcium content in adrenalectomized rats replaced with dexamethasone. J Med Food 7:45–51
Burdeos G, Nakagawa K, Kimura F, Miyazawa T (2012) Tocotrienol attenuates triglyceride accumulation in HepG2 Cells and F344 rats. Lipids 47:471–481
Uto-Kondo H, Ohmori R, Kiyose C, Kishimoto Y, Saito H, Igarashi O, Kondo K (2009) Tocotrienol suppresses adipocyte differentiation and Akt phosphorylation in 3T3-L1 preadipocytes. J Nutr 139:51–57
Gee P (2010) Unleashing the untold and misunderstood observations on vitamin E. Genes Nutr 6:5–16
Ikeda I, Imasato Y, Sasaki E, Sugano M (1996) Lymphatic transport of alpha-, gamma- and delta-tocotrienols and alpha-tocopherol in rats. Int J Vitam Nutr Res 66:217–221
Haffner SM (2006) The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol 97:3–11
Shimizu T (2009) Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol 49:123–150
Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L (2010) Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol 6:71–82
Shulman GI (2000) Cellular mechanisms of insulin resistance. J Clin Invest 106:171–176
Virkamaki A, Korsheninnikova E, Seppala-Lindroos A, Vehkavaara S, Goto T, Halavaara J, Hakkinen AM, Yki-Jarvinen H (2001) Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes 50:2337–2343
Steinberg HO, Baron AD (2002) Vascular function, insulin resistance and fatty acids. Diabetologia 45:623–634
Hayden MR, Sowers KM, Pulakat L, Joginpally T, Krueger B, Whaley-Connell A, Sowers JR (2011) Possible mechanisms of local tissue renin-angiotensin system activation in the cardiorenal metabolic syndrome and type 2 diabetes mellitus. Cardiorenal Med 1:193–210
Suganami T, Tanaka M, Ogawa Y (2012) Adipose tissue inflammation and ectopic lipid accumulation. Endocr J 59:849–857
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444:860–867
Zhao L, Kang I, Fang X, Wang W, Lee MA, Hollins RR, Marshall MR, Chung S (2015) Gamma-tocotrienol attenuates high-fat diet-induced obesity and insulin resistance by inhibiting adipose inflammation and M1 macrophage recruitment. Int J Obes (Lond) 39:438–446
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661
EFSA (2008) Opinion on mixed tocopherols, tocotrienol tocopherol and tocotrienols as sources for vitamin E added as a nutritional substance in food supplements. Scientific Opinion of the Panel on Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (Question No EFSA Q-2005-146, Q-2005-172, Q-2006-265) EFSA J 604:1–34
Tasaki M, Umemura T, Inoue T, Okamura T, Kuroiwa Y, Ishii Y, Maeda M, Hirose M, Nishikawa A (2008) Induction of characteristic hepatocyte proliferative lesion with dietary exposure of Wistar Hannover rats to tocotrienol for 1 year. Toxicology 250:143–150
Springett GM, Neuger AM, Centeno BA, Hutchinson T, Jump H, Lush R, Sebti S, Malafa MP (2011) Abstract 1299: a phase I dose-escalation study of the safety, PK, and PD of vitamin E delta-tocotrienol administered to subjects with resectable pancreatic exocrine neoplasia. Cancer Res 71:1299
Khosla P, Patel V, Whinter JM, Khanna S, Rakhkovskaya M, Roy S, Sen CK (2006) Postprandial levels of the natural vitamin E tocotrienol in human circulation. Antioxid Redox Signal 8:1059–1068
Fairus S, Nor RM, Cheng HM, Sundram K (2006) Postprandial metabolic fate of tocotrienol-rich vitamin E differs significantly from that of alpha-tocopherol. Am J Clin Nutr 84:835–842
Fairus S, Nor RM, Cheng HM, Sundram K (2012) Alpha-tocotrienol is the most abundant tocotrienol isomer circulated in plasma and lipoproteins after postprandial tocotrienol-rich vitamin E supplementation. Nutr J 11:5
Yap SP, Yuen KH, Lim AB (2003) Influence of route of administration on the absorption and disposition of alpha-, gamma- and delta-tocotrienols in rats. J Pharm Pharmacol 55:53–58
Yap SP, Yuen KH, Wong JW (2001) Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J Pharm Pharmacol 53:67–71
Shibata A, Nakagawa K, Shirakawa H, Kobayashi T, Kawakami Y, Takashima R, Ohashi A, Sato S, Ohsaki Y, Kimura F, Kimura T, Tsuduki T, Komai M, Miyazawa T (2012) Physiological effects and tissue distribution from large doses of tocotrienol in rats. Biosci Biotechnol Biochem 76:1805–1808
Uchida T, Abe C, Nomura S, Ichikawa T, Ikeda S (2012) Tissue distribution of alpha- and gamma-tocotrienol and gamma-tocopherol in rats and interference with their accumulation by alpha-tocopherol. Lipids 47:129–139
Yamashita K, Ikeda S, Iizuka Y, Ikeda I (2002) Effect of sesaminol on plasma and tissue alpha-tocopherol and alpha-tocotrienol concentrations in rats fed a vitamin E concentrate rich in tocotrienols. Lipids 37:351–358
Frank J, Chin XW, Schrader C, Eckert GP, Rimbach G (2012) Do tocotrienols have potential as neuroprotective dietary factors? Ageing Res Rev 11:163–180
Hubbell RB, Mendel LB, Wakeman AJ (1937) A new salt mixture for use in experimental diets. J Nutr 14:273–285
Acknowledgments
We thank Sime Darby Foods & Beverages Marketing Sdn. Bhd., Malaysia, for the supply of α-tocopherol, Davos Life Science Pte Ltd, Singapore, for the supply of α- and γ-tocotrienol, American River Nutrition, Inc., USA, for the supply of δ-tocotrienol, and The Malaysian Palm Oil Board for the supply of vitamin E-stripped palm olein. We thank Jason Brightwell, The Prince Charles Hospital, Brisbane, Australia, for the acquisition of echocardiographic images. We thank Suet Hoay Lee from Davos Life Science Pte Ltd, Singapore, and Ghazali Abdul Razak from The Malaysian Palm Oil Board for the HPLC analyses. We thank our colleagues R. Senthil Arun Kumar, Nikolas Jin Wang, Shazini Ramli, Maharshi Bhaswant, Hemant Poudyal and Sunil Panchal for technical assistance. Weng-Yew Wong and Lindsay Brown developed the original study aims; Weng-Yew Wong conducted the experiments and carried out the data analyses; data were interpreted by all authors. Leigh C. Ward assisted with dual-energy X-ray absorptiometry and provided nutritional advice. Chee Wai Fong and Wei Ney Yap assisted with detection and evaluation of vitamin E concentrations. Weng-Yew Wong and Lindsay Brown prepared manuscript drafts, with all authors contributing to the final version. Lindsay Brown has been the corresponding author throughout the writing process. This study was supported by the Strategic Research Fund of the University of Southern Queensland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wong, WY., Ward, L.C., Fong, C.W. et al. Anti-inflammatory γ- and δ-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur J Nutr 56, 133–150 (2017). https://doi.org/10.1007/s00394-015-1064-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1064-1