Abstract
Objective
This study aimed to assess the relative efficacy and safety of olokizumab at different dosages in patients with active rheumatoid arthritis (RA).
Methods
We performed a Bayesian network meta-analysis to combine direct and indirect evidence from randomized controlled trials (RCTs) to examine the efficacy and safety of olokizumab administered intravenously to RA patients at 64 mg/kg every 2 or 4 weeks (Q2 or Q4W).
Results
Five RCTs comprising 2609 patients met the inclusion criteria. Both olokizumab Q2 and Q4W treatments achieved a significant American College of Rheumatology 20% response (ACR20) compared with the placebo (odds ratio [OR] 3.21, 95% credible interval [CrI] 2.53–4.09; OR 3.05, 95% CrI 2.43–3.86). However, olokizumab Q2W was associated with the most favorable surface using the cumulative ranking curve (SUCRA) for the ACR20 response rate. The ranking probability based on the SUCRA indicated that olokizumab Q2W had the highest probability of being considered the best treatment option for achieving the ACR20 response rate, followed by olokizumab Q4W, adalimumab, and placebo. The ACR50 and 70 response rates showed a similar distribution pattern to the ACR20 response rate, except that olokizumab Q4W had a higher-ranking probability than olokizumab Q2W for ACR50. The SUCRA rating likelihood of adverse events (AEs) and withdrawal due to AEs showed that a placebo was likely to be the best intervention.
Conclusion
Both olokizumab Q2 and Q4W were efficacious and well-tolerated treatments for active RA.
Zusammenfassung
Ziel
Ziel der vorliegenden Studie war es, die relative Wirksamkeit und Sicherheit von Olokizumab in verschiedenen Dosierungen bei Patienten mit aktiver rheumatoider Arthritis (RA) zu untersuchen.
Methoden
Es wurde eine Bayes-Netzwerk-Metaanalyse zur Kombination direkter und indirekter Evidenz aus randomisierten kontrollierten Studien (RCT) durchgeführt, um die Wirksamkeit und Sicherheit von Olokizumab in der Dosierung von 64 mg/kg alle 2 Wochen (Q2W) oder alle 4 Wochen (Q4W) als i.v.-Gabe bei Patienten mit aktiver rheumatoider Arthritis (RA) zu untersuchen.
Ergebnisse
Die Einschlusskriterien wurden von 5 RCT mit 2609 Patienten erfüllt. Sowohl die Therapie mit Olokizumab Q2W als auch Q4W erzielte eine signifikante Therapieantwort von 20% gemäß American College of Rheumatology (ACR20) im Vergleich zu Placebo (Odds Ratio, OR: 3,21; 95%-Glaubwürdigkeitsintervall, „95% credible interval“, 95%-CrI: 2,53–4,09; OR 3,05; 95%-CrI: 2,43–3,86). Jedoch war Olokizumab Q2W mit der günstigsten Oberfläche bei Einsatz der kumulativen Rangfolgekurve (SUCRA, „surface using the cumulative ranking curve“) für die ACR20-Ansprechrate vergesellschaftet. Die Ranking-Wahrscheinlichkeit auf der Grundlage der SUCRA zeigte, dass Olokizumab Q2W die höchste Wahrscheinlichkeit aufwies, als beste Therapieoption zur Erzielung der ACR20-Ansprechrate zu gelten, danach folgten Olokizumab Q4W, Adalimumab und Placebo. Die ACR50- und ACR70-Ansprechraten wiesen ein ähnliches Verteilungsmuster wie die ACR20-Ansprechrate auf, außer, dass Olokizumab Q4W eine höhere Ranking-Wahrscheinlichkeit für ACR50 besaß als Olokizumab Q2W. Die SUCRA-Bewertungs-Wahrscheinlichkeit für unerwünschte Ereignisse (AE) und Therapieabbruch aufgrund von AE zeigte, dass ein Placebo am ehesten die beste Intervention darstellte.
Schlussfolgerung
Sowohl Olokizumab in der Dosierung Q2W als auch in der Dosierung Q4W war eine wirksame und gut verträgliche Behandlung der aktiven RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease that causes persistent synovial joint inflammation, resulting in disability and loss of quality of life [1, 2]. Interleukin 6 (IL-6) is a multifunctional cytokine involved in inflammatory reactions and immune response regulation, including B and T cell development [3]. IL‑6 is overexpressed in RA-afflicted tissues [4]. Higher IL‑6 levels in blood and synovial fluid are associated with synovitis, systemic inflammation, bone metabolism, and joint damage [5]. Tocilizumab and sarilumab, humanized anti-human IL‑6 receptor (IL-6R) monoclonal antibodies, have been developed to inhibit IL‑6 signaling [6]. Unlike IL-6R inhibitors, sirukumab is a human monoclonal antibody that binds to IL‑6 with high affinity and specificity, inhibiting IL‑6 from interacting with IL-6Rs [7]. IL‑6 and IL-6R inhibitors have been used effectively to treat RA, since IL‑6 overexpression is not per say a cause of RA.
Olokizumab, a new humanized monoclonal antibody specific for IL‑6, has been investigated for the treatment of RA [8]. It prevents the interaction of IL‑6 and the IL‑6 receptor dimer with the receptor complex’s signal-transducing receptor subunit glycoprotein 130 [8]. In RA clinical trials, olokizumab was significantly more efficacious than placebo [9, 10]. Olokizumab has been studied in phase II and III investigations of active RA patients who did not respond to methotrexate (MTX) and/or biologics [9,10,11,12,13]. However, the comparative effectiveness and safety of olokizumab at various doses remain unknown, owing to the lack of adequate multiple comparisons.
Unlike typical meta-analyses, network meta-analyses integrate direct and indirect evidence of relative treatment effects [14, 15]. Thus, even without head-to-head comparisons, the network meta-analysis enhances statistical power and accuracy by analyzing the comparative efficacy of various therapies and pooling data across a network of randomized controlled trials (RCTs) [16]. Using a network meta-analysis, the current study examined the effectiveness and safety of olokizumab administered every 2 or 4 weeks (Q2 or Q4W) to patients with active RA.
Methods
Identification of eligible studies and data extraction
We searched exhaustively for studies examining the efficacy and safety of olokizumab in patients with active RA. We used MEDLINE, EMBASE, the Cochrane Controlled Trials Register, the American College of Rheumatology (ACR), and the European League Against Rheumatism (EULAR) conference proceedings to identify available articles (up to September 2022), employing the keywords “olokizumab” and “rheumatoid arthritis.” All references cited in the studies were reviewed to identify additional reports that were excluded from electronic databases. The present study included RCTs meeting the following criteria: the study 1) compared olokizumab or tocilizumab with a placebo for the treatment of active RA; 2) provided endpoints for the clinical efficacy and safety of olokizumab at 24 weeks; and 3) included patients diagnosed with RA based on the ACR criteria for RA [17] or the 2010 ACR/EULAR classification criteria [18]. The studies that 1) included duplicate data and 2) did not contain adequate data for inclusion were excluded. The primary endpoint for efficacy was the number of patients who achieved an ACR 20% (ACR20) response rate as a preferred outcome measure for testing efficacy. The primary safety outcome was the number of patients with adverse events (AEs), which is crucial for assessing risks. The secondary endpoint for efficacy was the number of patients who achieved an ACR 50% (ACR50) or 70% (ACR70) response rate. Data were extracted from the original studies by two independent reviewers. The secondary endpoint for efficacy was the number of patients who withdrew owing to AEs. Any discrepancies between reviewers were resolved by consensus. The following information was extracted from each study: first author; year of publication; country of the study; doses of JAK inhibitor, IL‑6 inhibitor, and adalimumab; time of outcome evaluation; and efficacy and safety outcomes at 24 weeks. We quantified the methodological qualities of the three included studies using Jadad scores, with the quality classified as high (score of 3–5) or low (score of 0–2), and conducted a network meta-analysis following the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [19].
Evaluation of statistical associations for network meta-analysis
Results from the different arms of RCTs that compared multiple doses of olokizumab were analyzed simultaneously. The efficacy and tolerability of olokizumab and placebo in the different arms were arranged based on the probability that the treatment would be the best-performing regimen. We adopted a Bayesian fixed-effects model for network meta-analysis using NetMetaXL [20] and the WinBUGS statistical analysis program, version 1.4.3 (MRC Biostatistics Unit, Institute of Public Health, Cambridge, UK). We used the Markov chain Monte Carlo method to obtain the pooled effect sizes [16]. All chains were run with 10,000 burn-in iterations followed by 10,000 monitoring iterations. Data on the relative effects were converted into a probability that a particular treatment was best, second-best, and so on, or into a ranking for each treatment based on the “surface under the cumulative ranking curve” (SUCRA) [21]. SUCRA is expressed as a percentage (e.g., a value of 100% for SUCRA would be obtained when a particular treatment is guaranteed to be the best, and a value of 0% would guarantee that it is the worst treatment). League tables were used to organize summary estimates by ranking treatments according to the strength of their impact on the outcome based on their respective SUCRA values [21]. We reported the pairwise odds ratio (OR) and 95% credible interval (CrI or Bayesian confidence interval) and adjusted them for multiple-arm trials. Pooled results were considered statistically significant when the span of the 95% CrI did not include 1.
Test for inconsistency
Inconsistency is the disagreement between direct and indirect evidence [22]. Therefore, an inconsistency assessment is crucial when conducting a network meta-analysis [23]. To assess the network inconsistency between the direct and indirect estimates in each loop, we plotted the posterior mean deviance of individual datapoints in the inconsistency model against their posterior mean deviance in the consistency model [24].
Results
Studies included in the meta-analysis
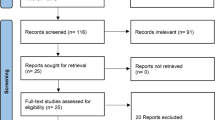
One hundred and eighty-three studies were identified through an electronic or manual search and 12 were selected for full-text review based on the title and abstract details. However, seven studies were excluded because they were duplicates or irrelevant. Thus, 5 RCTs, which included 2609 patients, met the inclusion criteria. The search results contained 6 pairwise comparisons, including 6 direct comparisons and 4 interventions. Various dosages of the biologics were reported: olokizumab, at 64 mg/kg, was administered intravenously every (q) 2 or 4 weeks (Q2 or Q4W); tocilizumab 8 mg, administered subcutaneously every (q) 2 weeks; and adalimumab 40 mg, administered subcutaneously every 2 weeks. All patients received conventional synthetic disease-modifying antirheumatic drug (csDMARD) therapy. The Jadad scores of the studies were between 3 and 5, indicating high-quality studies (Table 1). The relevant features of the studies in the meta-analysis are listed in Table 1 and 2.
Network meta-analysis of olokizumab efficacy in RCTs
Olokizumab Q2W is listed at the top left of the diagonal of the league table because it was associated with the most favorable SUCRA for the ACR20 response rate (Fig. 1). All of the olokizumab Q2W, olokizumab Q4W, and adalimumab treatments achieved a significant ACR20 response compared to that of the placebo (OR 3.21, 95% CrI 2.53–4.09; OR 3.05, 95% CrI 2.43–3.86; OR 2.60, 95% CrI 1.97–3.47; Figs. 1, 2, 3). SUCRA simplifies information on the effect of each treatment into a single number to guide the decision-making process. The ranking probability based on the SUCRA indicated that olokizumab Q2W had the highest probability of being considered the best treatment option for achieving the ACR20 response rate, followed by olokizumab Q4W, adalimumab, and placebo (Table 3). The ACR50 and 70 response rates showed a distribution pattern similar to that of the ACR20 response rate, except that olokizumab Q4W had a higher-ranking probability than olokizumab Q2W for the ACR50 (Table 3).
Bayesian network meta-analysis of randomized controlled trials examining the relative effectiveness of olokizumab at different dosages according to the number of patients achieving the American College of Rheumatology 20% response rate ACR20 (a), ACR50 (b), and ACR70 (c). O.R. odds ratio, Cr.I. credible interval
Network meta-analysis of olokizumab safety in RCTs
The SUCRA rating likelihood showed that the placebo was likely to be the best intervention in terms of AEs and withdrawal due to AEs (Fig. 2 and Table 4). However, the number of patient withdrawals owing to AEs did not differ significantly between the treatments, except for placebo vs. olokizumab Q4W for withdrawals owing to AEs (Table 4, Fig. 4). Withdrawals due to AEs were significantly lower in the placebo group than in the olokizumab Q4W group (OR 0.51, 95% CrI 0.26–0.93) (Table 4, Fig. 4).
Inconsistency and sensitivity analysis
Inconsistency plots were used to assess network inconsistencies between direct and indirect estimates, revealing a low possibility of inconsistencies that might significantly affect the network meta-analysis results. This finding was confirmed using random- and fixed-effects models, indicating that the results of this network meta-analysis were robust (Figs. 1 and 2).
Discussion
Therapeutic targeting of IL-6R is a significant step forward in treating RA because IL‑6 is involved in the development and clinical symptoms of the disease. Owing to the efficacy of tocilizumab in treating RA, novel biologics targeting IL‑6 or IL-6R have been developed. Olokizumab is a novel direct inhibitor of interleukin‑6 ligand, which differs from previously approved IL‑6 receptor inhibitors [8]. Although the existing data are not optimal, they are currently the best available for this specific study topic, awaiting additional conclusive RCTs.
We performed a network meta-analysis of patients with active RA to examine the efficacy and safety of olokizumab Q2 and Q4W. Olokizumab Q2W was more likely to be the optimal therapy for achieving an ACR20 and ACR70 response than olokizumab Q4W, even though no statistically significant difference in the ACR response rates was detected between these dosages. No significant differences in the number of AEs and withdrawals due to AEs were observed between groups, except that withdrawals due to AEs were significantly lower in the placebo group than in the olokizumab Q4W group; safety between the different olokizumab dosages was comparable.
However, our findings should be regarded with caution because of the limitations of the present investigation. First, a 6-month follow-up of the safety profile of IL-6-blocking biologics is deemed insufficient for evaluating all significant safety issues associated with biologicals, especially for examining unusual occurrences or events requiring longer exposure durations. Second, the included studies differed in their designs and clinical features. Consequently, these inter-study discrepancies may have influenced our findings. Third, the efficacy and safety outcomes of the biologicals were not adequately examined in this investigation. We only looked at treatment effectiveness (the number of patients who obtained ACR responses) and safety/tolerability (the number of AEs and withdrawals due to AEs) without looking at other outcomes. Because of their low frequency, the number of withdrawals due to AEs may not be adequate for safety outcome measures.
In contrast, this meta-analysis has several benefits. First, the RCTs included in this network meta-analysis were of high quality and yielded reliable results. Second, the number of patients in each sample varied from 125 to 1648, totaling 2609 patients in this study. Third, a network meta-analysis combines all relevant data to allow straightforward head-to-head comparisons of the different treatment modalities. In contrast to individual testing, statistical analysis and high resolution were used to obtain more reliable conclusions by merging independent study data [25,26,27,28]. To the best of our knowledge, this is the first Bayesian network meta-analysis to examine olokizumab Q2 and Q4W in patients with active RA.
In conclusion, using a Bayesian network meta-analysis encompassing five RCTs, we documented that olokizumab Q2 and Q4W were effective therapies for active RA and had similar effectiveness and safety in patients. Long-term trials are required to evaluate the effectiveness and safety of olokizumab in a larger number of individuals with active RA.
References
Kim H, Sung Y‑K (2021) Epidemiology of rheumatoid arthritis in Korea. J Rheum Dis 28(2):60–67
Lee Y‑K, Bae S‑C (2021) Mortality in Korean patients with rheumatoid arthritis. J Rheum Dis 28(3):113–118
Fonseca JE, Santos MJ, Canhao H, Choy E (2009) Interleukin‑6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev 8(7):538–542
Nurnberg W, Haas N, Schadendorf D, Czarnetzki BM (1995) Interleukin‑6 expression in the skin of patients with lupus erythematosus. Exp Dermatol 4(1):52–57
Srirangan S, Choy EH (2010) The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv Musculoskelet Dis 2(5):247–256
Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A et al (2008) IL‑6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 67(11):1516–1523
Xu Z, Bouman-Thio E, Comisar C, Frederick B, Van Hartingsveldt B, Marini JC et al (2011) Pharmacokinetics, pharmacodynamics and safety of a human anti-IL‑6 monoclonal antibody (sirukumab) in healthy subjects in a first-in-human study. Br J Clin Pharmacol 72(2):270–281
Shaw S, Bourne T, Meier C, Carrington B, Gelinas R, Henry A et al (2014) Discovery and characterization of olokizumab: a humanized antibody targeting interleukin‑6 and neutralizing gp130-signaling. Mabs: Taylor Francis p:773–781
Nasonov E, Fatenejad S, Feist E, Ivanova M, Korneva E, Krechikova DG et al (2022) Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis 81(4):469–479
Smolen JS, Feist E, Fatenejad S, Grishin SA, Korneva EV, Nasonov EL et al (2022) Olokizumab versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 387(8):715–726
Genovese MC, Fleischmann R, Furst D, Janssen N, Carter J, Dasgupta B et al (2014) Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised phase IIb study. Ann Rheum Dis 73(9):1607–1615
Takeuchi T, Tanaka Y, Yamanaka H, Amano K, Nagamine R, Park W et al (2016) Efficacy and safety of olokizumab in asian patients with moderate-to-severe rheumatoid arthritis, previously exposed to anti-TNF therapy: results from a randomized phase II trial. Mod Rheumatol 26(1):15–23
Feist E, Fatenejad S, Grishin S, Korneva E, Luggen ME, Nasonov E et al (2022) Olokizumab, a monoclonal antibody against interleukin‑6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by tumour necrosis factor inhibitor therapy: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis. https://doi.org/10.1136/ard-2022-222630
Catalá-López F, Tobías A, Cameron C, Moher D, Hutton B (2014) Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int 34(11):1489–1496
Song GG, Lee YH (2016) Comparison of disease activity score 28 using C‑reactive protein and disease activity score 28 using erythrocyte sedimentation rate in assessing activity and treatment response in rheumatoid arthritis: a meta-analysis. J Rheum Dis 23(4):241–249
Caldwell DM, Ades A, Higgins J (2005) Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331(7521):897
Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F (1992) The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 35(5):498–502
Aletaha D, Landewe R, Karonitsch T, Bathon J, Boers M, Bombardier C et al (2008) Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Arthritis Care Res 59(10):1371–1377
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269
Brown S, Hutton B, Clifford T, Coyle D, Grima D, Wells G et al (2014) A Microsoft-Excel-based tool for running and critically appraising network meta-analyses—an overview and application of NetMetaXL. Syst Rev 3(1):110
Salanti G, Ades A, Ioannidis JP (2011) Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 64(2):163–171
Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades A (2013) Evidence synthesis for decision making 4 inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 33(5):641–656
Higgins J, Jackson D, Barrett J, Lu G, Ades A, White I (2012) Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 3(2):98–110
Valkenhoef G, Lu G, Brock B, Hillege H, Ades A, Welton NJ (2012) Automating network meta-analysis. Res Syn Meth 3(4):285–299
Lee YH, Bae SC, Song GG (2011) The efficacy and safety of rituximab for the treatment of active rheumatoid arthritis: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int 31(11):1493–1499
Lee YH, Bae SC, Choi SJ, Ji JD, Song GG (2012) Associations between interleukin-10 polymorphisms and susceptibility to rheumatoid arthritis: a meta-analysis. Mol Biol Rep 39(1):81–87
Song GG, Bae SC, Lee YH (2014) Association of the MTHFR C677T and A1298C polymorphisms with methotrexate toxicity in rheumatoid arthritis: a meta-analysis. Clin Rheumatol 33(12):1715–1724
Lee YH (2018) An overview of meta-analysis for clinicians. Korean J Intern Med 33(2):277–283. https://doi.org/10.3904/kjim.2016.195
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y.H. Lee and G.G. Song declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information
Redaktion
Ulf Müller-Ladner, Bad Nauheim
Uwe Lange, Bad Nauheim

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Lee, Y.H., Song, G.G. Comparison of the efficacy and safety of olokizumab at different dosages in patients with active rheumatoid arthritis: a network meta-analysis of randomized controlled trials. Z Rheumatol 83 (Suppl 1), 107–114 (2024). https://doi.org/10.1007/s00393-023-01367-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-023-01367-w