Abstract
Background
The study sought to assess the prognostic impact of chronic kidney disease (CKD) and renal replacement therapy (RRT) in patients with ventricular tachyarrhythmias and sudden cardiac arrest (SCA) on admission.
Methods
A large retrospective registry was used including all consecutive patients presenting with ventricular tachycardia (VT), fibrillation (VF) and SCA on admission from 2002 to 2016. Non-CKD vs. “CKD without RRT”, and “CKD without RRT” vs. “CKD with RRT” were compared applying multivariable Cox regression models and propensity-score matching for evaluation of the primary prognostic endpoint defined as long-term all-cause mortality at 2 years. Secondary prognostic endpoints were cardiac death at 24 h, in-hospital death at index and the composite endpoint of recurrent ventricular tachyarrhythmias, appropriate ICD therapies and cardiac death at 24 h.
Results
In 2686 unmatched high-risk patients with ventricular tachyarrhythmias and SCA, non-CKD was present in 46%, “CKD without RRT” in 46% and “CKD with RRT” in 8%. Each, VT and VF occurred in about one-third of CKD patients. Multivariable Cox regression models revealed that “CKD without RRT” (HR = 2.118; p = 0.001) and “CKD with RRT” (HR = 3.043; p = 0.001) patients were associated with the primary endpoint of long-term mortality at 2 years, which was also proven after propensity-score matching (non-CKD vs. “CKD without RRT”: 43% vs. 27%, log rank p = 0.001; HR = 1.847; “CKD without RRT” vs. “CKD with RRT”: 74% vs. 51%, log rank p = 0.001; HR = 2.129). The rates of secondary endpoints were higher for cardiac death at 24 h, in-hospital death at index and the composite of recurrent ventricular tachyarrhythmias, appropriate ICD therapies and cardiac death at 24 h, respectively, for “CKD without RRT” and “CKD with RRT” patients.
Conclusion
In patients presenting with ventricular tachyarrhythmias and aborted SCA on admission, the presence of CKD, especially combined with RRT, is independently associated with an increase of long-term all-cause mortality at 2 years, cardiac death at 24 h, in-hospital death and the composite of recurrent ventricular tachyarrhythmias, appropriate ICD therapies and cardiac death at 24 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compared to the general population, patients suffering from chronic kidney disease (CKD) are associated with a higher incidence of arterial wall thickening and stiffness leading to progressive coronary calcification [1,2,3]. This CKD-related coronary calcification may be caused by the development of patchy calcification of intimal atheromatous plaques and linear calcification of lipid depots in the media (i.e. Monckeberg’s sclerosis) [4]. Additionally, secondary hyperparathyroidism with consecutive hyperphosphatemia may induce ossification of vascular smooth muscle cells of coronary arteries and heart valves [5]. Furthermore, arterial hypertension, dyslipidemia, diabetes mellitus, oxidative stress and elevated homocysteine level may alleviate the risk for cardiac arrhythmias due to the induction of a coronary artery disease (CAD) in CKD patients [6, 7]. Most CKD patients are affected by malignant arterial hypertension due to poor volume control, which is the direct cause for left ventricular hypertrophy (LVH) [1, 8,9,10]. LVH itself is associated with an increased risk of sudden cardiac arrest (SCA) and more than 70% of hemodialysis patients are affected by this clinical condition [1, 8, 11,12,13,14,15]. A prior study reported about an association between heart rate variability and LVH, which may promote myocardial autonomic dysfunction and increase the risk of ventricular tachyarrhythmias and SCA [16].
SCA reflects an international health burden accounting for 15–20% of all deaths in the Western world [17]. SCA is commonly caused by CAD [1, 18,19,20,21,22]. Notably, the risk of ventricular tachyarrhythmias and SCA in CKD patients is high and represents a leading cause of death in hemodialysis patients [1, 20, 23]. However, patients suffering from chronic or terminal kidney disease were excluded from 80% of randomized controlled trials (RCT) [24, 25]. Additionally, it has never been investigated whether CKD with or without renal replacement therapy (RRT) may impact long-term prognosis of patients presenting with life-threatening ventricular tachyarrhythmias and SCA.
Therefore, this study evaluates the prognostic impact of CKD and RRT in patients presenting with ventricular tachyarrhythmias and aborted SCA on admission.
Methods
Study patients, design and data collection
The present study retrospectively included all consecutive patients presenting with ventricular tachyarrhythmias or SCA on hospital admission from 2002 until 2016 at the First Department of Medicine, University Medical Centre Mannheim, Germany. Using the hospital information system, all relevant clinical data related to the index event were documented.
Ventricular tachyarrhythmias comprised ventricular tachycardia (VT) and fibrillation (VF), as defined by current international guidelines [19]. Sustained VT was defined by duration of > 30 s or causing hemodynamic collapse within 30 s, non-sustained VT by duration of < 30 s both characterized by wide QRS complexes (≥ 120 ms) at a rate greater than 100 beats per minute [26]. Ventricular tachyarrhythmias were documented by 12-lead electrocardiogram (ECG), ECG tele-monitoring, implantable cardioverter defibrillator (ICD) or in case of unstable course or during cardiopulmonary resuscitation (CPR) by external defibrillator monitoring. Documented VF was treated by external defibrillation and in case of prolonged instability with additional intravenous anti-arrhythmic drugs during CPR.
Further data being documented contained baseline characteristics, prior medical history (PMH), prior medical treatment (PMT), length of index stay, detailed findings of laboratory values at baseline, data derived from all non-invasive or invasive cardiac diagnostics and device therapies, such as coronary angiography (CA), electrophysiological examination (EP), ICD, pacemaker (PM) or cardiac contractility modulation (CCM), as well as imaging modalities, such as echocardiography or cardiac magnetic resonance imaging (cMRI). The overall presence of an activated ICD comprises the total sum of all patients with either a prior implanted ICD before admission, those undergoing new ICD implantation at index stay, as well as those with ICD implantation at the complete follow-up period after index hospitalization, referring to sole transvenous ICD, subcutaneous-ICD (s-ICD) and cardiac resynchronization therapy with defibrillator function (CRT-D). Pharmacological treatment was documented according to the discharge medication of patients surviving index hospitalization. Rates of overall ICDs and of pharmacological therapies are referred to the number of surviving patients being discharged from index hospitalization.
Every re-visit at the outpatient clinic or rehospitalization was documented when related to recurrent ventricular tachyarrhythmias and adverse cardiac events. Adverse cardiac events comprised acute heart failure, CPR, cardiac surgery, recurrent percutaneous coronary intervention (re-PCI), new implants or upgrades of cardiac devices, worsening or improvement of left ventricular function.
Documentation period lasted from index event until 2016. Documentation of all medical data was performed by independent cardiologists at the time of the patients´ individual period of clinical presentation, being blinded to final data analyses.
The present study is derived from an analysis of the “Registry of Malignant Arrhythmias and Sudden Cardiac Death–Influence of Diagnostics and Interventions (RACE–IT)” and represents a single-center registry including consecutive patients presenting with ventricular tachyarrhythmias and aborted SCA being acutely admitted to the University Medical Center Mannheim (UMM), Germany (clinicaltrials.gov identifier: NCT02982473) from 2002 until 2016. The registry was carried out according to the principles of the declaration of Helsinki and was approved by the medical ethics committee II of the Medical Faculty Mannheim, University of Heidelberg, Germany.
The medical centre covers a general emergency department for emergency admission of traumatic, surgical, neurological and cardiovascular conditions. Interdisciplinary consultations is an in-built feature of this 24/7 service and connects to a stroke unit, 4 intensive care units with extracorporeal life support and a chest pain unit to alleviate rapid triage of patients. The cardiologic department itself include cardiac catheterization and electrophysiologic laboratories, a hybrid operating room and telemetry units.
Definition of study groups, inclusion and exclusion criteria
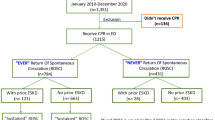
For the present analysis, risk stratification was performed according to the presence of non-CKD vs. “CKD without RRT”, and “CKD without RRT” vs. “CKD with RRT” according to the clinical practice guideline of the kidney disease improving global outcome (KDIGO) executive committee for the evaluation of chronic kidney disease [27]. Accordingly, CKD was defined as abnormalities of kidney function with implication for health accompanied with a GFR < 60 ml/min/1.73 m2 (GFR categories G3a-G5) and a duration > 3 months [28]. Patients reaching stage 5 of CKD (estimated GFR < 15 ml/min/1.73 m2) were defined as terminal kidney disease with the need for RRT [25]. RRT patients comprised those with a history of prior chronic hemodialysis before admission as well as those with continuous veno-venous hemodiafiltration (CVVHDF) during intensive care treatment.
Overall exclusion criteria comprised patients without complete follow-up data regarding mortality. Each patient was counted only once for inclusion when presenting with the first episode of ventricular tachyarrhythmias and aborted SCA.
Study endpoints
The primary prognostic outcome was all-cause mortality at long-term follow-up at 2 years. Secondary endpoints were cardiac death at 24 h, in-hospital death at index and the composite endpoint of recurrent VT/VF, appropriate ICD therapies and SCA at long-term follow-up. Early cardiac death was defined as occurring < 24 h after onset of ventricular tachyarrhythmias or an assumed unstable cardiac condition on index admission [26].
Overall follow-up period lasted until 2016. All-cause mortality was documented using our electronic hospital information system and by directly contacting state resident registration offices (“bureau of mortality statistics”) across Germany. Identification of patients was verified by place of name, surname, day of birth and registered living address. Lost to follow-up rate was 1.7% (n = 48) regarding survival until the end of the follow-up period.
Statistical methods
Quantitative data are presented as mean ± standard error of mean (SEM), median and interquartile range (IQR), and ranges depending on the distribution of the data and were compared using the Student’s t test for normally distributed data or the Mann–Whitney U test for nonparametric data. Deviations from a Gaussian distribution were tested by the Kolmogorov–Smirnov test. Spearman’s rank correlation for nonparametric data was used to test univariate correlations. Qualitative data are presented as absolute and relative frequencies and compared using the χ2 test or the Fisher’s exact test, as appropriate.
First, overall data of consecutive patients on admission are given for the entire unmatched cohort to present the real-life character of health-care supply at our institution in between 2002 and 2016. Here, multivariable Cox regression models were applied for the evaluation of the primary prognostic endpoint within the total study cohort for “CKD without RRT” and “CKD with RRT”. Then multivariable Cox regression models were applied for the primary prognostic endpoint in the sub-groups of non-CKD, “CKD without RRT” and “CKD with RRT” patients. Multivariable Cox regression models were adjusted for the following covariables: age, sex, diabetes, CAD, CPR, acute myocardial infarction, (non-) ST segment myocardial infarction ((N)STEMI), left ventricular ejection fraction (LVEF) < 35%, cardiogenic shock, index ventricular tachyarrhythmia (VT/VF) and overall ICD.
Second, propensity score matching was applied. There is a relevant and increasing demand from patients, clinicians and within the health care system in general for growing evidence from non-randomized studies. There are simply too many medically relevant questions/hypotheses, which will never be investigated within randomized controlled trials (RCT) due to several reasons (i.e. funding, recruitment, difficult study settings, high-risk patients, etc.). Therefore, we felt that the method of propensity matching would be a reasonable additional statistical method beside multivariable Cox regression models for the purpose of the present study evaluating the prognostic impact of non-CKD, “CKD with and without RRT” in high-risk patients presenting with ventricular tachyarrhythmias and SCA on admission. These high-risk patients are usually excluded from RCT. In RCT, patients with or without a specific treatment would have a 50% chance to be treated and balanced measured and unmeasured baseline characteristics would be expected. However, patients with different disease entities may not be randomized in real-life (such as non-CKD versus “CKD without RRT”, or “CKD without RRT” versus “CKD with RRT”) due to different pathophysiologies and treatment recommendations. An observational study usually recruits consecutive real-life patients without randomization resulting in varying chances between 0 and 100% to receive imbalances in baseline characteristics and treatments. Therefore, differences of outcomes in specific disease groups might be explained by heterogeneous distribution of baseline characteristics and applied therapies. To further reduce this selection bias, we used 1:1 propensity-scores for “CKD without RRT” versus non-CKD, respectively, “CKD with RRT” versus “CKD without RRT” patients, to assemble matched cohorts, in which patients would be well-balanced regarding all measured baseline characteristics. 1:1 propensity score matching was performed including the entire study cohort and in CKD patients only, applying a non-parsimonious multivariable logistic regression model using “CKD without RRT” and “CKD with RRT” patients as the dependent variables [29, 30].
Propensity scores were created according to the presence of the following independent variables: age, sex, diabetes, CAD, acute myocardial infarction, (non-) ST segment myocardial infarction ((N)STEMI), left ventricular ejection fraction (LVEF) < 35%, cardiogenic shock, CPR, index ventricular tachyarrhythmia (i.e., VT/VF) and overall ICD. Based on the propensity score values counted by logistic regression, for each patient in the “CKD without RRT” group (“CKD with RRT” group, respectively) one patient in the control group with a similar propensity score value was found (accepted difference of propensity score value < 5%). Propensity scores were calculated for the following comparative analyses: (1) non-CKD versus “CKD without RRT” (2) “CKD without RRT” versus “CKD with RRT”. Uni-variable stratification was performed using the Kaplan–Meier method with comparisons between groups using uni-variable hazard ratios (HR) given together with 95% confidence intervals, according to the presence of non-CKD, “CKD without RRT” and “CKD with RRT” within the propensity-matched cohorts.
Follow-up periods for the evaluation of long-term all-cause mortality were set at 2 years according to the median survival time of CKD patients to guarantee complete follow-up of at least 50% of patients. Patients not meeting long-term follow-up were censored.
Finally, a study-specific risk score (RACE-IT-CKD-score) was developed within the unmatched cohort to illustrate the risk of the primary prognostic endpoint of all-cause mortality at 2 years and the secondary endpoint of cardiac death at 24 h. The risk score includes the following variables derived from significant adverse predictors evaluated in the multivariable Cox regression model from the total unmatched cohort: age > 66 years, male sex, LVEF < 35%, cardiogenic shock, CPR, “CKD without RRT” and “CKD with RRT”. Each predictive variable was given 1 point revealing a total minimum of 0 to a maximum of 7 points. Risk categories were set at 0–1 points for lowest risk, 2–4 points for intermediate risk and 5–7 points for high-risk for both endpoints. Uni-variable stratification was performed using the Kaplan–Meier method. The maximum risk for each endpoint corresponded to the number of deaths related to the total number of patients in each risk-group.
The result of a statistical test was considered significant for p < 0.05, p values ≤ 0.1 were defined as a statistical trend. SAS, release 9.4 (SAS Institute Inc., Cary, NC, USA) and SPSS (Version 25, IBM Armonk, New York, USA) were used for statistics.
Results
Entire unmatched real-life cohort
In the entire unmatched real-life cohort including 2684 high-risk patients, the prevalence of “CKD without RRT” was 46%, “CKD with RRT” 8%, and non-CKD 46%. As shown in Table 1, the rate of VT was significantly higher in non-CKD patients (57% vs. 35% vs. 30%) attributed to both sustained and non-sustained VT, whereas the rate of VF was equally distributed (29% vs. 28% vs. 25%). The rates of early cardiac death were significantly highest in “CKD with RRT” patients (45% vs. 37% vs. 14%) (Table 1).
Most patients were of male sex. Compared to non-CKD patients, “CKD without RRT” patients were older and had higher rates of cardiovascular risk factors, prior heart failure, CAD, obstructive pulmonary diseases, acute myocardial infarction, respectively, (N)STEMI, cardiogenic shock, in- and out-of hospital CPR and LVEF < 35% (Table 1). Compared to “CKD without RRT”, “CKD with RRT” patients were older and had higher rates of diabetes, prior heart failure, acute myocardial infarction, respectively, (N)STEMI, cardiogenic shock and in- and out-of hospital CPR, with an equally distributed LVEF (Table 1). 49% of RRT patients were on chronic haemodialysis before admission with a mean RRT duration of 4.6 years (range 1–35 years). 51% of RRT patients were treated with CVVHDF during intensive care treatment. Non-CKD patients showed the highest rate of EP and ablation therapy followed by “CKD without RRT” and “CKD with RRT” patients (33% vs. 13% vs. 7%; 7% vs. 3% vs. 0,5%) (Table 1). No difference regarding medication at discharge was shown, especially for anti-arrhythmic drugs.
Median follow-up time was 18 months (IQR 2 days to 6 years). Multivariable Cox regression analyses within the entire unmatched real-life cohort revealed “CKD without RRT” (HR = 2.118, 95% CI 1.743–2.573, p = 0.001) and “CKD with RRT” patients (HR = 3.043, 95% CI 2.797–4.031, p = 0.001) to be significantly associated with the primary endpoint of long-term all-cause mortality at 2 years (Fig. 1a).
a Forrest blots of a multivariable Cox regression model showing significant predictors of the primary prognostic endpoint of long-term all-cause mortality at 2 years within the entire unmatched real-life cohort. b Forrest blots of a multivariable Cox regression models showing significant predictors of the primary prognostic endpoint of long-term all-cause mortality at 2 years in the subgroups of non-CKD (left panel), “CKD without RRT” (middle panel) and “CKD with RRT” (right panel). Bold type indicates statistical significance (p < 0.05)
Focusing on the sub-groups of non-CKD, “CKD without RRT” and “CKD with RRT” patients (Fig. 1b, left, middle, and right panels), multivariable Cox regressions revealed age, LVEF < 35%, cardiogenic shock and CPR as the strongest predictors of long-term all-cause mortality at 2 years across all sub-groups, whereas the presence of an overall ICD was consistently beneficial. Particularly in non-CKD patients, age and male sex were significantly associated with the primary endpoint. Contrastively, presence of STEMI was a protective treatable risk factor in non-CKD and “CKD without RRT” patients (Fig. 1B, left, middle, right panels).
Propensity matched cohorts
After applying propensity-score matching for the comparison of non-CKD versus “CKD without RRT” patients (589 matched pairs) and CKD without versus “CKD with RRT” patients (136 matched pairs) comparable sub-groups with similar rates were achieved for age, sex, diabetes, prior heart failure, prior CAD, prior AMI, LVEF, cardiogenic shock, CPR and overall ICDs. A higher rate of induced VT was shown for “CKD without RRT” compared to non-CKD patients. Early cardiac death was significantly higher in “CKD with RRT” compared to “CKD without RRT” patients (26% vs. 20%, p = 0.046) and higher in “CKD without RRT” compared to non-CKD patients (18% vs. 11%, p = 0.001) (Table 2).
Non-CKD patients revealed a higher rate of EP, but not of ablation therapy compared to “CKD without RRT” patients (29% vs. 20%, p = 0.001) (Table 2). “CKD without RRT” patients showed higher rates of EP and ablation therapy compared to “CKD with RRT” patients (21% vs. 9%, p = 0.006; 6% vs. 0.7%, p = 0.036) (Table 2). No difference regarding the medication at discharge was shown. Only slight differences were still seen after matching for smoking, cardiac family history and atrial fibrillation (Table 2).
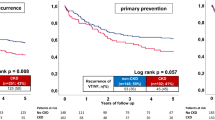
Figure 2a (left panel) and Table 3 illustrate the significantly adverse prognosis for the primary endpoint of long-term all-cause mortality at 2 years in non-CKD compared to “CKD without RRT” patients (primary endpoint, all-cause mortality at 2 years: 43% vs. 27%; log rank p = 0.001; HR = 1.848; 95% CI 1.521–2.24; p = 0.001). Moreover, Fig. 2a (right panel) and Table 3 illustrate adverse prognosis for the primary study endpoint in “CKD without RRT” compared to “CKD with RRT” patients (primary endpoint, all-cause mortality at 2 years: 74% vs. 51%; log rank p = 0.001; HR = 1.944; 95% CI 1.428–2.646; p = 0.001).
a After propensity score matching, Kaplan Meier survival curves still demonstrate increasing rates of the primary prognostic endpoint of long-term all-cause mortality at 2 years between non-CKD versus “CKD without RRT” (left panel) and “CKD without RRT” versus “CKD with RRT” patients (right panel). b After propensity score matching, Kaplan Meier survival curves still demonstrate increasing rates of the secondary 2-years composite endpoint of recurrent ventricular tachyarrhythmias, appropriate ICD therapies and cardiac death at 24 h between non-CKD versus “CKD without RRT” (left panel) and “CKD without RRT” versus “CKD with RRT” patients (right panel)
Both “CKD without RRT” and “CKD with RRT” patients revealed significantly higher rates of the secondary prognostic endpoints of cardiac death at 24 h (18% and 36%) and in-hospital death at index (31% and 57%) (Table 3).
Figure 2b shows significantly adverse prognosis for the secondary composite endpoint of recurrent ventricular tachyarrhythmias, appropriate ICD therapies at 2 years and cardiac death at 24 h, which was already observed in non-CKD versus “CKD without RRT” patients (secondary composite endpoint: 32% vs. 27%; log rank p = 0.026; HR = 1.255; 95% CI 1.018–1.548; p = 0.033) (left panel), as well as in “CKD without RRT” versus “CKD with RRT” patients (secondary composite endpoint: 68% vs. 47%; log rank p = 0.009; HR = 1.577; 95% CI 1.088–1.291; p = 0.016) (right panel).
RACE-IT-CKD-score
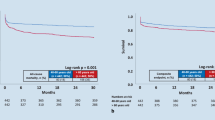
Patients with a high score were associated with highest all-cause mortality at 2 years compared to patients with an intermediate and low risk score (Fig. 3, Kaplan–Meier curves) (corresponding to maximum risk at 2 years of 84% in the high-risk group, 39% in the intermediate and 5% in the low-risk group) (Fig. 3). Similar risk scores were obtained for cardiac death at 24 h (high risk 47% vs. intermediate risk 16% vs. low risk 1%) (p < 0.05 for each group comparison) (Fig. 3).
The RACE-IT-CKD-Score describes the maximum associated risk of all-cause mortality at 2 years and the risk of death at 24 h when presenting with ventricular tachyarrhythmias and aborted cardiac arrest. The high risk group was associated with the highest risk of mortality at 2 years and with death at 24 h, followed by the intermediate and low risk group (p < 0.05 for each group comparison)
Discussion
The present study evaluates the prognostic impact of the presence of CKD either without or with RRT in consecutive high-risk patients presenting with ventricular tachyarrhythmias and aborted SCA on admission.
This real-world data suggests that these high-risk patients presenting reveal highest long-term all-cause mortality in the presence of “CKD with RRT”, followed by “CKD without RRT” and non-CKD patients. Respectively, increasing rates of secondary endpoints, including cardiac death at 24 h, in-hospital death at index as well as the composite endpoint of recurrent ventricular tachyarrhythmias, appropriate ICD therapies and cardiac death at 24 h, were seen in CKD with and without RRT compared to non-CKD patients. Prognostic differences were demonstrated even within multivariable Cox regression models and after propensity-score matching. The strongest predictors of long-term mortality across all analyzed subgroups consisted in age, LVEF < 35%, cardiogenic shock and CPR, whereas the presence of an ICD was consistently beneficial. This study identifies the presence of CKD both with and without RRT as robust predictors of adverse secondary outcomes in patients presenting with ventricular tachyarrhythmias and aborted SCA straight from the admission scenario, which is also reflected within the novel description of the so-called “RACE-IT-CKD-score”.
It is currently under debate whether CKD patients may reveal a higher risk for ventricular tachyarrhythmias favoring SCA, which has been shown to be a leading cause of death in hemodialysis patients [1, 23]. Data about the prevalence of ventricular tachyarrhythmias and CKD and their influence on mortality are rare [23] and usually derived from sub-analyses of randomized controlled trials (RCT) evaluating patients in need for hemodialysis only with regard to different types of applied hemodialysis, whereas patients with compensated CKD and patients without CKD were not included.
As mentioned above, “CKD with RRT” patients were associated with the secondary composite endpoint of recurrent ventricular tachyarrhythmias, appropriate ICD therapies and cardiac death at 24 h. “CKD with RRT” patients also showed the lowest rate of EP and ablation therapy, and the highest rates of cardiac death at 24 h due to VT associated with increasing CPR rates. The lower rates of EP and ablation therapy might be explained by a higher co-morbidity of “CKD with RRT” and frailty compared to the other groups. This theory is supported by the fact, that non-CKD patients showed the lowest rate of cardiac death at 24 h as well as the highest rate of EP. Whether invasive EP and ablation therapy may affect long-term all-cause mortality in CKD patients with and without RRT, may not be explained by the present study. However, CKD patients are at risk for recurrent ventricular tachyarrhythmias and might, therefore, benefit from EP and VT ablation in apparently daily active patients. Therefore, it would be desirable if further studies would investigate the effect on long-term all-cause mortality of VT ablation therapy in “CKD with RRT” patients.
Data from the HEMO study including 1,846 patients with different types of hemodialysis found an overall SCA rate of 22% according to a total of 808 patients with SCA during follow-up period of 2.5 years [31]. Rates of SCA, non-SCA, and non-cardiac death were 22%, 17%, and 61%, respectively [31]. Neither non-CKD nor “CKD without RRT” patients were included, as well as no rates of ventricular tachyarrhythmias were reported in this study. In line, the prospective and placebo-controlled study of heart and renal protection (SHARP) assigned 4,650 patients to receive simvastatin plus ezetimibe and 4,620 to placebo all suffering from CKD including 33% patients being on hemodialysis, whereas patients with a history of AMI or coronary revascularization were excluded. Beside a significant reduction of adverse clinical events (i.e. non-fatal myocardial infarction or coronary death, non-hemorrhagic stroke, or any arterial revascularization procedure) due to simvastatin plus ezetimibe, an overall mortality was reported at 24.3% during 4.9 years of follow-up. Cardiovascular diseases including SCD accounted for 27.9% of total mortality [32]. Patients without CKD as well as patients with ventricular tachyarrhythmias from the outset were not included and data on rates of ventricular tachyarrhythmias is not given. The US renal data system (USRDS) collects mortality data based on end-stage kidney disease. According to the 2012 USRDS annual report ventricular tachyarrhythmias and cardiac arrest accounted for 63% of cardiovascular and 26.5% of all-cause mortality in prevalent dialysis patients [14]. However, the individual outcome of patients specifically presenting with ventricular tachyarrhythmias was also not documented.
According to Sherif et al. the progression of CKD is associated with an impairment of cardiac repolarization as being expressed by two-thirds of CKD patients with QTc-prolongation, of which 20% revealed QTc intervals of more than 500 ms. The prolongation was independent of electrolyte disturbance, drug intake or underlying structural heart disease [33]. Moreover, sudden potassium and calcium shifts during hemodialysis are associated with pro-arrhythmogenic side-effects [1, 2]. These shifts combined with rapid changes of blood pressure and volume load may sustain a milieu of mechanical and electrical imbalance of myocytes, potentially increasing the risk of ventricular tachyarrhythmias [16, 34, 35]. Additionally, dyslipidemia, oxidative stress, elevated homocysteine level, hyper-parathyreoidism, hyper-phosphataemia and electrolyte imbalance may also alleviate the development of ventricular tachyarrhythmias [1]. Of note, accumulation of numerous toxins, such as b2-microglobulin, guanidines, phenols, indoles, aliphaticamines, furans, polyols, nucleosides, leptin, parathyroid hormone and erythropoiesis inhibitors may aggravate cardio-toxicity, which is nowadays not clarified in detail [36, 37]. Whether genomic alterations may influence the occurrence of ventricular tachyarrhythmias has not yet been completely understood and is a matter for further studies in future [38, 39].
Supply with activated ICDs for primary and secondary prevention in patients with severe systolic heart failure is based on a class I, level of evidence A recommendation [40]. An alternative to the transvenous ICD is the subcutaneous implantable defibrillator (s-ICD) in patients with systolic heart failure when antitachycardia or antitbradycardia pacing is not additionally needed (class of recommendation II, level of evidence A) [41,42,43,44,45,46]. Nevertheless, the implantation of an ICD or s-ICD is an invasive procedure. Therefore, it is important to identify high risk subgroups of patients that may benefit from an ICD-therapy [47,48,49,50].
However, whether especially CKD patients may benefit from an activated ICD is still under ongoing debate [51]. This present study demonstrated that CKD patients presenting with ventricular tachyarrhythmias and aborted SCA are still associated with increasing mortality despite the presence of an activated ICD compared to patients without CKD. The presence of an ICD increased long-term survival, especially in ’CKD with RRT” patients, however, these patients were still associated with highest mortality compared to “CKD without RRT” and non-CKD patients. Multivariable Cox regression models demonstrated a prognostic benefit of the presence of an activated ICD both in VT and VF patients, as well as in CKD with and without RRT and non-CKD patients. It might be speculated, whether an activated ICD may be of prognostic benefit even for primary prevention especially in “CKD with RRT” patients. Results of the prospective ICD-2-study from Leiden University Medical Center are awaited and will address this important topic [51, 52]. In summary, the present study demonstrates highest secondary all-cause mortality at 2 years in “CKD with RRT” followed by “CKD without RRT” patients compared to non-CKD patients, when presenting with ventricular tachyarrhythmias and aborted SCA on admission. In line, secondary prognostic endpoints of cardiac death at 24 h, in-hospital death and the composite of recurrent ventricular tachyarrhythmias, appropriate ICD therapies and early cardiac death at 24 h were increased in “CKD with RRT”, followed by “CKD without RRT” and non-CKD patients. Future RCT should address, whether implantation of an ICD may be indicated in high-risk CKD patients presenting with ventricular tachyarrhythmias.
Study limitations
This observational and retrospective registry-based analysis reflects a realistic picture of consecutive health-care supply of high-risk patients presenting with ventricular tachyarrhythmias and SCA. Lost to follow-up rate regarding the evaluated endpoint of all-cause mortality was minimal. Additionally, heterogeneity within the study population was controlled by a stepwise statistical approach including multivariable adjustment for several important comorbidities and risk factors, both within the entire and propensity matched cohorts. Patients not surviving out of hospital CPR and not being transferred to the heart centre were, therefore, not included in this study. All clinical data was documented reliably by individual cardiologists during routine clinical care being blinded to final analyses, alleviating the use of an independent clinical event committee.
Conclusions
The presence of CKD with and without RRT is an independent predictor of long-term all-cause mortality at 2 years, cardiac death at 24 h, in-hospital death at index and the 2-year composite endpoint of recurrent ventricular tachyarrhythmias, appropriate ICD therapies and cardiac death at 24 h in patients presenting with ventricular tachyarrhythmias and aborted SCA on admission.
References
Green D, New RP, Kalra D (2011) Sudden cardiac death in hamodialysis patients—an in depth review Am J Kidney Dis 57:921–929 P.
Singh TK, Arya V, Navaratnarajah N (2014) Chronic kidney disease and cardiovascular disease: a focus on primary care. Cardiovasc Hematol Disord Drug Targets 14(3):212–218
Emrich IE et al (2018) Symmetric dimethylarginine (SDMA) outperforms asymmetric dimethylarginine (ADMA) and other methylarginines as predictor of renal and cardiovascular outcome in non-dialysis chronic kidney disease. Clin Res Cardiol 107(3):201–213
Amann K (2008) Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol 3(6):1599–1605
Olgaard K, Lewin E, Silver J (2011) Calcimimetics, vitamin D and ADVANCE in the management of CKD-MBD. Nephrol Dial Transpl 26(4):1117–1119
Shivendra S (2014) Cardiovascular disease in chronic kidney disease. Nephrology (3):20–29
Ronco C (2011) The Cardiorenal syndrome: basis and common ground for a multidisciplinary patient-oriented therapy. Cardiorenal Med 1(1):3–4
Haider AW et al (1998) Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 32(5):1454–1459
Ukena C et al (2016) Renal denervation for treatment of ventricular arrhythmias: data from an International Multicenter Registry. Clin Res Cardiol 105(10):873–879
Rigopoulos AG et al (2016) Low occurrence of ventricular arrhythmias after alcohol septal ablation in high-risk patients with hypertrophic obstructive cardiomyopathy. Clin Res Cardiol 105(11):953–961
Franczyk-Skora B et al (2015) Sudden cardiac death in CKD patients. Int Urol Nephrol 47(6):971–982
Bleyer AJ et al (2006) Characteristics of sudden death in hemodialysis patients. Kidney Int 69(12):2268–2273
Burghardt A et al (2018) Risk marker profiles in patients treated with percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy. Clin Res Cardiol 107(6):479–486
Sedaghat-Hamedani F et al (2018) Clinical outcomes associated with sarcomere mutations in hypertrophic cardiomyopathy: a meta-analysis on 7675 individuals. Clin Res Cardiol 107(1):30–41
Frommeyer G et al (2016) Long-term follow-up of subcutaneous ICD systems in patients with hypertrophic cardiomyopathy: a single-center experience. Clin Res Cardiol 105(1):89–93
Chan CT et al (2010) Determinants of cardiac autonomic dysfunction in ESRD. Clin J Am Soc Nephrol 5(10):1821–1827
Hayashi M, Shimizu W, Albert CM (2015) The spectrum of epidemiology underlying sudden cardiac death. Circ Res 116(12):1887–1906
Yousuf O et al (2015) Clinical management and prevention of sudden cardiac death. Circ Res 116(12):2020–2040
Priori SG et al (2016) 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology. G Ital Cardiol (Rome) 17(2):108–170
Marx N et al (2018) Mechanisms of cardiovascular complications in chronic kidney disease: research focus of the Transregional Research Consortium SFB TRR219 of the University Hospital Aachen (RWTH) and the Saarland University. Clin Res Cardiol 107(Suppl 2):120–126
Stahli BE et al (2017) Outcomes of patients with periprocedural atrial fibrillation undergoing percutaneous coronary intervention for chronic total occlusion. Clin Res Cardiol 106(12):986–994
Coiro S et al (2017) Association of digitalis treatment with outcomes following myocardial infarction in patients with heart failure or evidence of left ventricular dysfunction: an analysis from the high-risk myocardial infarction database initiative. Clin Res Cardiol 106(9):722–733
Zannad F, Rossignol P, Cardiovascular outcome trials in patients with advanced kidney disease time for action. Circulation 2017. 135:1769–1771
Zannad F, Rossignol P (2017) Cardiovascular outcome trials in patients with advanced kidney disease: time for action. Circulation 135(19):1769–1771
National Kidney F (2015) KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 66(5):884–930
Priori SG et al (2015) ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015. 36(41):2793–2867
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 76(113):S1–S130
Andrassy KM (2013) Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int 84(3):622–623
Ferdinand D, Otto M, Weiss C (2016) Get the most from your data: a propensity score model comparison on real-life data. Int J Gen Med 9:123–131
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46(3):399–424
Shastri S et al (2012) Predictors of sudden cardiac death: a competing risk approach in the hemodialysis study. Clin J Am Soc Nephrol 7(1):123–130
Baigent C et al (2011) The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377(9784):2181–2192
Sherif KA et al (2014) Cardiac repolarization abnormalities among patients with various stages of chronic kidney disease. Clin Cardiol 37(7):417–421
Dinshaw L et al (2018) The T-peak-to-T-end interval: a novel ECG marker for ventricular arrhythmia and appropriate ICD therapy in patients with hypertrophic cardiomyopathy. Clin Res Cardiol 107(2):130–137
Pereira R et al (2017) Short QT syndrome in pediatrics. Clin Res Cardiol 106(6):393–400
Fort J, Chronic renal failure: a cardiovascular risk factor. Kidney Int Suppl 2005(99):S25–s29
Schiffrin EL, Lipman ML, Mann JF (2007) Chronic kidney disease: effects on the cardiovascular system. Circulation 116(1):85–97
Kayvanpour E et al (2017) Genotype-phenotype associations in dilated cardiomyopathy: meta-analysis on more than 8000 individuals. Clin Res Cardiol 106(2):127–139
Chua HC et al (2018) Unexplained cardiac arrest: a tale of conflicting interpretations of KCNQ1 genetic test results. Clin Res Cardiol 107(8):670–678
Writing Committee M et al (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128(16):e240–e327
Priori SG, Blomstrom-Lundqvist C (2015) European Society of Cardiology Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death summarized by co-chairs. Eur Heart J 36(41):2757–2759
Bettin M et al (2018) Right versus left parasternal electrode position in the entirely subcutaneous ICD. Clin Res Cardiol 107(5):389–394
Bettin M et al (2018) Follow-up of the first patients with a totally subcutaneous ICD in Germany from implantation till battery depletion. Clin Res Cardiol. https://doi.org/10.1007/s00392-018-1296-1
Kobe J et al (2017) Posttraumatic stress and quality of life with the totally subcutaneous compared to conventional cardioverter-defibrillator systems. Clin Res Cardiol 106(5):317–321
Erath JW et al (2017) The wearable cardioverter-defibrillator in a real-world clinical setting: experience in 102 consecutive patients. Clin Res Cardiol 106(4):300–306
Wolff G et al (2017) Implantable cardioverter/defibrillators for primary prevention in dilated cardiomyopathy post-DANISH: an updated meta-analysis and systematic review of randomized controlled trials. Clin Res Cardiol 106(7):501–513
Omran H et al (2018) Characteristics and circadian distribution of cardiac arrhythmias in patients with heart failure and sleep-disordered breathing. Clin Res Cardiol. https://doi.org/10.1007/s00392-018-1269-4
Linz D et al (2016) Impact of obstructive and central apneas on ventricular repolarisation: lessons learned from studies in man and pigs. Clin Res Cardiol 105(8):639–647
Duncker D et al (2014) Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function-value of the wearable cardioverter/defibrillator. Eur J Heart Fail 16(12):1331–1336
Lachmann V et al (2017) Aborted sudden cardiac death: ICD or no ICD. Clin Res Cardiol 106(9):760–763
Shen L et al (2017) Declining risk of sudden death in heart failure. N Engl J Med 377(1):41–51
De Bie MK et al (2008) Prevention of sudden cardiac death: rationale and design of the implantable cardioverter defibrillators in Dialysis patients (ICD2) TRIAL–a prospective pilot study. Curr Med Res Opin 24(8):2151–2157
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Rights and permissions
About this article
Cite this article
Weidner, K., Behnes, M., Schupp, T. et al. Prognostic impact of chronic kidney disease and renal replacement therapy in ventricular tachyarrhythmias and aborted cardiac arrest. Clin Res Cardiol 108, 669–682 (2019). https://doi.org/10.1007/s00392-018-1396-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1396-y