Abstract
The study sought to assess the impact of chronic kidney disease (CKD) on recurrences of ventricular tachyarrhythmias in implantable cardioverter defibrillator (ICD) recipients. Data regarding the outcome of patients with CKD in ICD recipients is limited. A large retrospective registry was used including consecutive ICD recipients surviving episodes of ventricular tachycardia (VT) or fibrillation (VF) from 2002 to 2016. CKD patients were compared to non-CKD patients. The primary endpoint was the first recurrence of ventricular tachyarrhythmias at 5 years. Secondary endpoints were ICD-related therapies, rehospitalization and all-cause mortality at 5 years. Kaplan–Meier, multivariable Cox regression and propensity score matching were applied. A total of 585 consecutive patients were included (non-CKD: 57%, CKD: 43%). CKD had higher rates of the primary endpoint of recurrent ventricular tachyarrhythmias compared to non-CKD patients (50% vs. 40%; log rank p = 0.008; HR = 1.398; 95% CI 1.087–1.770; p = 0.009), which was irrespective of a primary or secondary preventive ICD and mainly attributed to recurrent VF (11% vs. 5%; p = 0.007) and electrical storm (ES) (10% vs. 5%; p = 0.010). Accordingly, CKD patients had higher rates of the secondary endpoint of appropriate ICD therapies (41% vs. 30%; log rank p = 0.002; HR = 1.532; 95% CI 1.163–2.018; p = 0.002), mainly attributed to appropriate ICD shocks (19% vs. 11%; p = 0.005). After multivariable Cox regression CKD was associated with a 1.4-fold higher risk of appropriate device therapies (HR = 1.353; 95% CI 1.001–1.825; p = 0.049), but not with first recurrence of ventricular tachyarrhythmias (p = 0.177). Irrespective of propensity score matching, CKD was associated with increasing all-cause mortality at 5 years (p = 0.001). The presence of CKD is associated with increased rates of recurrent ventricular tachyarrhythmias, appropriate device therapies, mainly attributed to appropriate shock, and all-cause mortality in ICD recipients at 5 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The risk of ventricular tachyarrhythmias and sudden cardiac death (SCD) in patients with chronic kidney disease (CKD) is high and represents the leading cause of death in haemodialysis patients [1]. A reduced glomerular filtration rate (GFR) was shown to be associated with increased risk of death, cardiovascular events and rehospitalization [2]. Notably, CKD patients are usually excluded in about 80% from randomized controlled trials (RCT) [3,4,5]. The most common risk factor for the development of ventricular tachyarrhythmias is coronary artery disease (CAD) [6]. Several pathomechanisms for CAD in CKD patients are described. Progressive coronary calcification due to Monckeberg sclerosis or hyperparathyroidism might induce arterial wall thickening and stiffness up to ossification of vascular smooth muscle cells of coronary vessels and heart valves [7]. Increased cardiovascular risk factors such as diabetes and dyslipidemia are associated with CKD and in further consequence with CAD [8, 9]. Especially CKD patients suffer from ongoing oxidative stress and elevated homocysteine levels, which are also present in CAD and CKD patients [8, 9].
Implantable electronic cardiac devices are frequently used for the management of patients with cardiac arrhythmias [10]. Amongst these, implantable cardioverter defibrillators (ICD) have become therapeutic cornerstones for an effective primary and secondary prevention of ventricular tachyarrhythmias and SCD. They were shown to primarily decrease long-term mortality in patients with left ventricular ejection fraction (LVEF) < 35%, ischemic and dilated cardiomyopathy [11,12,13,14,15]. The prognostic benefit of an ICD was shown for patients suffering from channelopathies such as Brugada syndrome, long- and short-QT-syndrome, as well as cardiomyopathies, including hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy [16]. In secondary prevention, patients with prior haemodynamically relevant ventricular tachyarrhythmias were shown to benefit from ICD therapy, irrespective of the underlying disease [14, 17]. However, the potential survival benefit of an ICD is still unclear in CKD patients compared to the general population. Especially in primary prevention, ICD-related complications such as venous thromembolism and infections may further limit the assumed benefit [10, 18,19,20]. Furthermore, it is important to identify subgroups of patients at higher risk to develop recurrent ventricular tachyarrhythmias to ensure their optimal long-term survival.
Data is rare whether CKD may affect future recurrences of ventricular tachyarrhythmias in ICD recipients. Therefore, this study evaluates the impact of CKD on recurrences of ventricular tachyarrhythmias, device-related therapies, rehospitalization and all-cause mortality in ICD recipients surviving index episodes of ventricular tachyarrhythmias.
Methods
Data collection and documentation
The present study included all consecutive patients with an activated ICD presenting with ventricular tachyarrhythmias on admission from 2002 until 2016 at our institution. All relevant clinical data related to the index event, as well as to recurrences of ventricular tachyarrhythmias and rehospitalization—as being documented during routine clinical care by independent cardiologists and medical staff—was retrospectively derived from the electronic hospital information system, patient files, discharge letters, results from diagnostic testings and the laboratory system. Data was transferred into a standardized electronic database, where the quality and accuracy of documented data was re-assessed by two independent cardiologists (M.Be.) and (I.A.).
Ventricular tachyarrhythmias comprised ventricular tachycardia (VT) and fibrillation (VF), as defined by current international guidelines [21, 22]. Sustained VT was defined by the duration of more than 30 s or causing hemodynamic collapse within 30 s. Non-sustained VT was defined by a duration of less than 30 s. Both types of VT had wide QRS complexes ( ≥ 120 ms) at a rate greater than 100 beats per minute [23]. Ventricular tachyarrhythmias at index was documented by 12-lead electrocardiogram (ECG), ECG tele- monitoring, ICD or in case of unstable course or during cardiopulmonary resuscitation (CPR) by external defibrillator monitoring. Documented VF was treated by ICD-related shock or external defibrillation and in case of prolonged instability with additional intravenous anti-arrhythmic drugs during CPR. Electrical storm (ES) was defined as ≥ 3 episodes of ventricular tachyarrhythmias requiring appropriate device therapy and occurring during a period of 24 h [23, 24].
Clinical data comprised baseline characteristics, prior medical history, prior medical treatment, length of index stay, detailed findings of laboratory values at baseline, data derived from all non-invasive or invasive cardiac diagnostics and device therapies, such as coronary angiography, electrophysiological examination, 12-lead or holter ECG, echocardiography, cardiac magnetic resonance imaging (cMRI), coronary angiography, pharmacological therapy and ICD protocols.
The following device types were allowed: ICD, cardiac resynchronisation therapy with defibrillator (CRT-D) and subcutaneous ICD (s-ICD). ICD recipients routinely presented every 3–6 months for device check and unscheduled in case of noticed device interrogations at our clinic. Device settings and programming were performed according to current international guidelines by specialized cardiologists in electrophysiology during routine clinical care [21, 23, 25].
Every re-hospitalization of each patient—either ambulatory or in-hospital at our institution—was reviewed and documented for recurrent ventricular tachyarrhythmias, in-hospital death and upcoming relevant cardiac events.
The present study is derived from an analysis of the “Registry of Malignant Arrhythmias and Sudden Cardiac Death—Influence of Diagnostics and Interventions (RACE-IT)”, a single-center registry including consecutive patients presenting with ventricular tachyarrhythmias and sudden cardiac arrest being acutely admitted to the University Medical Center Mannheim (UMM), Germany (clinicaltrials.gov identifier: NCT02982473) from 2002 until 2016. The study was carried out according to the principles of the declaration of Helsinki and was approved by the medical ethics committee II of the Faculty of Medicine Mannheim, University of Heidelberg, Germany.
Inclusion and exclusion criteria
Only patients with an activated ICD were included (i.e. ICD recipients). All patients had a documented episode of ventricular tachyarrhythmias, which defines the index event. Each patient was counted only once for inclusion when presenting with the first episode of ventricular tachyarrhythmias. All analyzed patients had to survive index hospitalization.
Risk stratification was performed according to the presence of CKD and non-CKD according to the clinical practice guideline of the “kidney disease improving global outcome” (KDIGO) executive committee for the evaluation of chronic kidney disease [26]. According to the KDIGO guideline a CKD was defined as abnormalities of kidney function with implication for health. Patients with GFR < 60 ml/min/1.73 m2 (GFR categories G3a-G5) and a duration > 3 months were included [27].
Patients without a prior history of CKD or no evidence of renal function at index presentation were excluded. Furthermore, patients on hemodialysis were excluded.
Primary and secondary outcomes
Follow-up period was set at 5 years for all outcomes. The primary endpoint was the first recurrence of ventricular tachyarrhythmias (VT or VF) as documented within ICD protocols. Secondary endpoints were overall recurrences at follow-up, recurrences per patient, associated appropriate or inappropriate device therapies (first, overall, per patient), first re-hospitalization and all-cause mortality at follow-up. Further stratification was performed into subgroups of primary or secondary prevention and appropriate or inappropriate device therapies.
Appropriate device therapy was defined as device interrogation in the presence of VT or VF including antitachycardia pacing (ATP), ICD-related shock or both ATP and shock. Inappropriate device therapy was defined as ATP or ICD shock in the absence of VT or VF. First re-hospitalization comprised rehospitalizations due to VT, VF, acute myocardial infarction (AMI), acute heart failure and inappropriate device therapy.
All-cause mortality was documented using our electronic hospital information system and by directly contacting state resident registration offices (“bureau of mortality statistics”) all across Germany. Identification of patients was verified by place of name, surname, day of birth and registered living addresses.
Statistical methods
CKD patients were compared to non-CKD patients. Quantitative data are presented as mean ± standard error of mean (SEM), median and interquartile range (IQR), and ranges depending on the distribution of the data. Data were compared using the Student’s t test for normally distributed data or the Mann–Whitney U test for nonparametric data. Deviations from a Gaussian distribution were tested by the Kolmogorov–Smirnov test. Spearman’s rank correlation for nonparametric data was used to test univariate correlations. Qualitative data are presented as absolute and relative frequencies and compared using the Chi2 test or the Fisher’s exact test, as appropriate.
Firstly, univariable Kaplan–Meier method was applied to evaluate differences in primary and secondary endpoints within the entire unmatched cohort between CKD and non-CKD patients. Furthermore, differences were tested in subgroups of primary versus secondary prevention. Hazard ratios (HR) are given together with 95% confidence intervals (CI). Secondly, multivariable Cox regression models were developed using the “forward selection” option in the unmatched cohort, where only statistically significant variables (p < 0.05) or clinically relevant variables were included and analyzed simultaneously. Predefined variables being used for multivariable Cox regressions included: age, diabetes, CAD, CPR, beta-blocker, CKD. Thirdly, Kaplan–Meier analyses were repeated in propensity matched cohorts for primary and secondary endpoints. Details on propensity-score matching are outlined below. Patients without complete follow-up were censored (accepted lost-to follow-up rate < 10%).
The result of a statistical test was considered significant for p < 0.05, a statistical trend was defined as p < 0.10. SAS, release 9.4 (SAS Institute Inc., Cary, NC, USA) and SPSS (Version 25, IBM, Armonk, New York) were used for statistics.
Propensity score matching
In RCT patients with or without a specific disease (such as CKD and non-CKD) would have a 50% chance to be treated. Also balanced measured and unmeasured baseline characteristics would be expected. In an observational study, recruiting real-life patients, no randomization results in varying chances between 0 and 100% resulting in imbalances of baseline characteristics. Consecutively, differences of outcomes in specific disease groups might, therefore, also be explained by heterogenous distribution of baseline characteristics. To reduce this selection bias, we used 1:1 propensity score for the presence of CKD to assemble a matched cohort in which CKD and non-CKD patients would be well balanced on all measured baseline characteristics. 1:1 propensity score matching was performed including the entire study cohort performing a non-parsimonious multivariable logistic regression model using patients with CKD as the dependent variable [28, 29]. Propensity scores were created according to the presence of the following independent variables: age, gender, diabetes, left ventricular dysfunction and underlying ventricular tachyarrhythmias (i.e. VT/VF) on admission. Based on the propensity score values counted by logistic regression, for each patient in the CKD group one patient in the non-CKD group (control group) with a similar propensity score value was found (accepted difference of propensity score value < 5%).
Results
Study population
A total of 585 consecutive ICD recipients (CKD: 57%; non-CKD: 43%) surviving an episode of ventricular tachyarrhythmias were included (Table 1). Most patients were males. VT was more common than VF (68–70% vs. 30–33%) at index in both groups. CKD patients were older and had higher rates of diabetes, CAD, CPR, LVEF < 35%, atrial fibrillation (statistical trend) and beta-blockers. No further differences were seen in both groups. Table 2 outlines ICD-related data of the study population. Most patients had an activated transvenous ICD (89–93%), whereas CRT-D or subcutaneous ICD were present in minor part (3–8%). Indication for ICD implantation was equally distributed (about 42% primary and 58% secondary prevention). The median detection thresholds for VT (171 bpm) and for VF (214 bpm) were similar in both groups, as well as the median cycle length of VT 280 ms (Table 2).
Follow-up data, primary and secondary endpoints
At least 90% of patients were followed-up regularly within the follow-up period of 5 years (1825 days) with at least one ICD check-up every 6–12 months.
The primary endpoint of first recurrence of ventricular tachyarrhythmias was increased in CKD patients (50% vs. 40%, log-rank p = 0.008; HR = 1.398; 95% CI 1.087–1.770; p = 0.009) (Table 2 and Fig. 1, left panel), irrespective of the presence of primary and secondary preventive ICD indication (primary: 45% vs. 36%; log rank p = 0.057; HR = 1.468; 95% CI 0.986–2.186; p = 0.059; secondary: 53% vs. 44%; log rank statistical trend p = 0.089; HR = 1.306; 95% CI 0.959–1.778; p = 0.090) (Fig. 1, middle and right panel). Differences of recurrences of ventricular tachyarrhythmias were attributed to higher rates of VF (11% vs. 5%) and ES (10% vs. 5%).
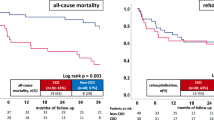
Regarding secondary endpoints, freedom from first appropriate device therapy was decreased in CKD patients (41% vs. 30%, log rank p = 0.002; HR = 1.532; 95% CI 1.163–2.018; p = 0.002) (Fig. 2, left panel), whereas no difference was found for inappropriate device therapies (Fig. 2, right panel). The difference of first appropriate device therapies was driven by increasing rates of appropriate ICD shocks (19% vs. 11%). No differences were seen for overall rehospitalization at 5 years in both groups, whereas CKD patients had higher rates of all-cause mortality compared to non-CKD patients (30% vs. 14%, p = 0.001; HR = 2.451;95% CI 1.707–3.519; p = 0.001) (Table 2).
Multivariable cox regression models
After multivariable adjustment, CKD patients were not associated with first recurrences of ventricular tachyarrhythmias (HR = 1.201; 95% CI 0.921–1.568; p = 0.177) (Table 3). However, there was a 1.4-fold higher risk of appropriate ICD therapy (HR = 1.353; 95% CI 1.001–1.825; p = 0.049) (Table 3) in CKD patients. Patients ≥ 74 years were associated with a 1.5-fold higher risk and patients with an LVEF < 35% were associated with a 1.4-fold higher risk of appropriate ICD therapy.
Propensity score matching
After propensity score matching similar baseline characteristics were achieved in both subgroups (Table 1; right panel). CKD was not associated with the the primary endpoint of recurrences of ventricular tachyarrhythmias (41% vs. 48%; log rank p = 0.111) (Fig. 3, left panel), but with the secondary endpoint of appropriate device therapies (39% vs. 33%, log rank statistical trend p = 0.076; HR = 1.329; 95% CI 0.965–1.823; p = 0.077) (Fig. 3, right panel) and appropriate ICD shocks (26% vs. 14%, log rank p = 0.001, HR = 2.249; 95% CI 1.444–3.502; p = 0.001).
Discussion
The present study evaluates the prognostic impact of CKD on recurrences of ventricular tachyarrhythmias, device-related therapies, rehospitalization and all-cause mortality at five years of follow-up in consecutive ICD recipients surviving episodes of ventricular tachyarrhythmias. This study suggests, that CKD may decrease freedom from first recurrent ventricular tachyarrhythmias (mainly attributed to VF and ES), as well as from first appropriate device-related therapies (predominantly ICD related shock). The prognostic impact of CKD on first appropriate device therapy was seen also after multivariable adjustment and propensity score matching. Furthermore, CKD patients revealed higher all-cause mortality at 5 years. Both CKD and LVEF < 35% were associated with a higher risk of appropriate ICD therapy.
CKD is a well-known cardiovascular risk factor for heart failure and CAD, which themselves are common risk factors for the development of ventricular tachyarrhythmias [6, 30]. In particular, LVEF < 35% increases the risk of ventricular tachyarrhythmias [31]. In the present study both, the presence of CKD and LVEF < 35%, were associated with a 1.4-fold higher risk of first appropriate ICD therapy. In turn, LVEF < 35% is associated with CKD and in this context reflects the cardio-renal (CRS) or reno-cardiac syndrome (RCS), where heart and kidney dysfunctions overlap [32]. The cardio-renal syndrome can be subdivided in five types. Type I and II are caused by acute or chronic heart failure with limitation of kidney function by decreased renal blood flow due to cardiac low output [32]. Type III and IV are caused by an acute or chronic kidney failure with vascular and myocardial damages by oxidative stress, inflammation and increased volume-dependant pre- and afterload [32,33,34]. Type V CRS describes the simultaneous occurrence of cardiac and renal injury [32,33,34]. The different types of CRS cannot be identified exactly in this retrospective cohort. However both LVEF < 35% and CKD appear to have relevant and significant impact in patients presenting with ventricular tachyarrhythmias.
Treatment with ICD has become a therapeutic cornerstone for an effective primary and secondary prevention of ventricular tachyarrhythmias and SCD. It was shown to effectively decrease long-term mortality in patients with LVEF < 35%, irrespective of the underlying disease [11,12,13,14,15]. ICD therapy in the chronic post-infarct period (≥ 30 days) was shown to be associated with decreased long-term mortality in patients with ischemic cardiomyopathy and LVEF < 30% [15, 35], whereas the prognostic benefit of an ICD in the acute postinfarct period ( < 30 days) is limited [15, 35]. Whether CKD patients may be associated with a prognostic benefit related to ICD therapy has not yet been completely understood. In contrast, CKD patients with ICD were shown to be associated with higher rates of device-related complications including central venous thrombosis and bloodstream infections [10, 18,19,20, 36].
The Cleveland clinic CKD registry included 631 pairs of CKD patients with and without an implanted ICD. At a median follow-up of 2.9 years, the presence of an ICD was associated with lower risk of death among patients with an estimated GFR 45–49 ml/min, which was not observed in patients with a GFR < 30 ml/min [37]. Beyond, the potential benefit of ICD in non-dialysis CKD patients is still unclear and concise studies evaluating the risk of recurrent ventricular tachyarrhythmias in CKD patients are rare at all.
A prospective study from Brazil focused on ventricular tachyarrhythmias in 76 ICD recipients with CKD and non-ischemic cardiomyopathy (LVEF < 35%) at 12 months of follow-up [38]. Patients with LVEF > 35%, ischemic heart disease and valvular heart disease were excluded [38]. The study suggested that the risk of SCD or recurrent ventricular tachyarrhythmias increased with advanced stages of CKD [38]. In contrast to previous studies, the strength of the present study is the longer follow-up period of 5 years, the larger sample size and the comparison to non-CKD patients.
Besides the CRS, different approaches, explaining the association between CKD and ventricular tachyarrhythmias do exist. Firstly, CKD patients are at risk for QTc-prolongation of more than 500 ms due to an impairment of cardiac repolarization [39]. QTc-prolongation is a risk factor of ventricular tachyarrhythmias, affecting two-thirds of all CKD patients [39]. Secondly, CKD and hemodialysis patients are affected by electrolyte shifts, such as sudden potassium and calcium shifts and rapid changes of volume and blood pressure. This might also sustain a milieu of mechanical and electrical imbalance of myocytes, which might alleviate the onset of ventricular tachyarrhythmias [40]. Thirdly, the electrical imbalance might be influenced by oxidative stress, elevated homocysteine levels, hyper-phosphatemia, and the accumulation of several cardiotoxic substances such as b2-microglobulin, nucleosides, parathyroid hormone and many more [41, 42].
Whether CKD patients may significantly benefit from ICD implantation is still unclear, even when focussing on primary or secondary preventive indication. It may be speculated whether the risk of device-related complications may justify the potential, not yet proven, benefits for the prevention of ventricular tachyarrhythmias in CKD patients compared to the general population. Accordingly, the present study contributes to a better understanding of this important high-risk sub-group. The use of epicardial or subcutaneous leads may further prevent central venous thrombosis and device-related infections in future [10].
Conclusion
The present study demonstrates that ICD recipients with CKD are associated with an increased risk for recurrent ventricular tachyarrhythmias, appropriate ICD therapies and higher all-cause mortality at five years.
Study limitations
This observational and retrospective registry-based analysis reflects a realistic picture of consecutive health-care supply of high-risk patients presenting with ventricular tachyarrhythmias. Lost to follow-up rates regarding the evaluated endpoint of all-cause mortality was minimal. To minimize lost to follow-up rates, all patients not meeting ICD follow-up for at least once after discharge were excluded from the present analysis. All clinical data was documented reliably by individual cardiologists during routine clinical care being blinded to final analyses, alleviating the use of an independent clinical event committee. The effect of CKD is only based on the assessment of CKD at index presentation. CKD development at follow-up was not documented. Therefore, the present results need to be re-evaluated within even larger and more representative multi-centre registries, especially focusing on the impact of CKD in selected subgroups with ICD therapies.
References
Zannad F, Rossignol P (2017) cardiovascular outcome trials in patients with advanced kidney disease: time for action. Circulation 135(19):1769–1771
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351(13):1296–1305
Zannad F, Alonso Garcia MLA, Borer JS, Stough WG, Clutton-Brock T, Rosenberg Y, Packer M (2017) Role of payers in the development of cardiovascular therapeutics: misalignment between approval and reimbursement. J Am Coll Cardiol 70(22):2822–2830
Charytan D, Kuntz RE (2006) The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int 70(11):2021–2030
Coca SG, Krumholz HM, Garg AX, Parikh CR (2006) Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 296(11):1377–1384
McElwee SK, Velasco A (2016) Doppalapudi, mechanisms of sudden cardiac death. J Nucl Cardiol 23(6):1368–1379
National Kidney F. KDOQI (2015) Clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 66(5):884–930
Shastri S, Tangri N, Tighiouart H, Beck GJ, Vlagopoulos P, Ornt D, Eknoyan G, Kusek JW, Herzog C, Cheung AK, Sarnak MJ (2012) Predictors of sudden cardiac death: a competing risk approach in the hemodialysis study. Clin J Am Soc Nephrol 7(1):123–130
Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Gronhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R, Investigators S (2011) The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet 377(9784):2181–2192
Saad TF, Hentschel DM, Koplan B, Wasse H, Asif A, Patel DV, Salman L, Carrillo R, Hoggard J, Workgroup ACPC (2013) Cardiovascular implantable electronic device leads in CKD and ESRD patients: review and recommendations for practice. Semin Dial 26(1):114–123
Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M (1996) Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia, multicenter automatic defibrillator implantation trial investigators. N Engl J Med 335(26):1933–1940
Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators (1997) A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med 337(22):1576–1583
John RM, Tedrow UB, Koplan BA, Albert CM, Epstein LM, Sweeney MO, Miller AL, Michaud GF, Stevenson WG (2012) Ventricular arrhythmias and sudden cardiac death. Lancet 380(9852):1520–1529
Jung W, Andresen D, Block M, Bocker D, Hohnloser SH, Kuck KH, Sperzel J, V. Deutschen Gesellschaft fur Kardiologie–Herz- und Kreislaufforschung e, (2006) Guidelines for the implantation of defibrillators. Clin Res Cardiol 95(12):696–708
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML (2002) Multicenter automatic defibrillator implantation trial, prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346(12):877–883
Galie N, Torbicki A, Barst R, Dartevelle P, Haworth S, Higenbottam T, Olschewski H, Peacock A, Pietra G, Rubin LJ, Simonneau G, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie M, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, McGregor K, Morais J, Oto A, Smiseth OA, Barbera JA, Gibbs S, Hoeper M, Humbert M, Naeije R, Pepke-Zaba J, Task F (2004) Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The task force on diagnosis and treatment of pulmonary arterial hypertension of the European Society of Cardiology. Eur Heart J 25(24):2243–2278
Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C (2006) Guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death, executive summary. Rev Esp Cardiol 59(12):1328
Charytan DM, Patrick AR, Liu J, Setoguchi S, Herzog CA, Brookhart MA, Winkelmayer WC (2011) Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis 58(3):409–417
Cuculich PS, Sanchez JM, Kerzner R, Greenberg SL, Sengupta J, Chen J, Faddis MN, Gleva MJ, Smith TW, Lindsay BD (2007) Poor prognosis for patients with chronic kidney disease despite ICD therapy for the primary prevention of sudden death. Pacing Clin Electrophysiol 30(2):207–213
Robin J, Weinberg K, Tiongson J, Carnethon M, Reddy M, Ciaccio C, Quadrini M, Hsu J, Fan J, Choi P, Kadish A, Goldberger J, Passman R (2006) Renal dialysis as a risk factor for appropriate therapies and mortality in implantable cardioverter-defibrillator recipients. Heart Rhythm 3(10):1196–1201
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Hlatky MA, Granger CB, Hammill SB, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL (2017) AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 138:e272–e391
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ (2015) ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Kardiol Pol 73(10):795–900
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ, E.S.C.S.D. Group, (2015) 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 36(41):2793–2867
Deneke T, Israel CW, Krug J, Nentwich K, Müller P, Mügge A, Schade A (2013) Indikationen zur Katheterablation bei ventrikulärer Tachykardie. Dtsch med Wochenschr 138(39):1952–1956
Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J, Ponikowski P, Priori SG, Sutton R, van Veldhuisen DJ, ESC Committee for Practice Guidelines (2010) Focused update of ESC guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Europace 12(11):1526–1536
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group (2009) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9(Suppl 3):S1–S155
Andrassy KM (2013) Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int 84(3):622–623
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46(3):399–424
Ferdinand D, Otto M, Weiss C (2016) Get the most from your data: a propensity score model comparison on real-life data. Int J Gen Med 9:123–131
Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, Herrmann-Lingen C, Hoes A, Humphries S, Knapton M, Perk J, Priori SG, Pyorala K, Reiner Z, Ruilope L, Sans-Menendez S, Op Reimer WS, Weissberg P, Wood D, Yarnell J, Zamorano JL, Walma E, Fitzgerald T, Cooney MT, Dudina A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Funck-Brentano C, Filippatos G, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Altiner A, Bonora E, Durrington PN, Fagard R, Giampaoli S, Hemingway H, Hakansson J, Kjeldsen SE, Larsen L, Mancia G, Manolis AJ, Orth-Gomer K, Pedersen T, Rayner M, Ryden L, Sammut M, Schneiderman N, Stalenhoef AF, Tokgozoglu L, Wiklund O, Zampelas A, C. European Society of, P. European Association for Cardiovascular, Rehabilitation, N. Council on Cardiovascular, D. European Association for Study of, E. International Diabetes Federation, I. European Stroke, M. Society of Behavioural, H. European Society of, W. Europe, N. European Heart, S. European Atherosclerosis (2007) European guidelines on cardiovascular disease prevention in clinical practice: full text Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil 14(Suppl 2):S1–S113
Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JR, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology/American Heart Association Task, G. European Society of Cardiology Committee for Practice, A. European Heart Rhythm, S. Heart Rhythm, ACC/AHA/ESC (2006) Guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114(10):e385–e484
Di Lullo L, Bellasi A, Barbera V, Russo D, Russo L, Di Iorio B, Cozzolino M, Ronco C (2017) Pathophysiology of the cardio-renal syndromes types 1–5: an uptodate. Indian Heart J 69(2):255–265
Schwenger V, Remppis BA, Westenfeld R, Weinreich T, Brunkhorst R, Schieren G, Krumme B, Haller H, Schmieder R, Schlieper G, Frye B, Hoppe UC, Hoyer J, Keller T, Blumenstein M, Schunkert H, Mahfoud F, Rump LC (2014) Dialysis and ultrafiltration therapy in patients with cardio-renal syndrome: recommendations of the working group “heart-kidney” of the German Cardiac Society and the German Society of Nephrology. Dtsch Med Wochenschr 139(7):e1–e8
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM (2016) European guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts. Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation. G Ital Cardiol (Rome) 18(7):547–612
Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ (2004) Investigators, Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med 351(24):2481–2488
Buiten MS, BieMK De, Van der Heijden AC, Rotmans JI, Bootsma M, Groeneveld JHM, Wolterbeek R, Rabelink TJ, Jukema JW, Schalij MJ, Van Erven L (2014) Chronic kidney disease and implantable cardioverter defibrillator related complications: 16 years of experience. J Cardiovasc Electrophysiol 25(9):998–1004
Nakhoul GN, Schold JD, Arrigain S, Harb SC, Jolly S, Wilkoff BL, Nally JV, Navaneethan SD (2015) Implantable cardioverter-defibrillators in patients with CKD: a propensity-matched mortality analysis. Clin J Am Soc Nephrol 10(7):1119–1127
Kiuchi MG, Chen S, Purerfellner H (2017) Incidence of ventricular arrhythmic events in CKD patients with ICD. Int J Cardiol 227:312–317
Sherif KA, Abo-Salem E, Panikkath R, Nusrat M, Tuncel M (2014) Cardiac repolarization abnormalities among patients with various stages of chronic kidney disease. Clin Cardiol 37(7):417–421
Chan CT, Levin NW, Chertow GM, Larive B, Schulman G, Kotanko P (2010) Frequent hemodialysis network daily Trial, determinants of cardiac autonomic dysfunction in ESRD. Clin J Am Soc Nephrol 5(10):1821–1827
Fort J (2005) Chronic renal failure: a cardiovascular risk factor. Kidney Int Suppl 99:S25–S29
Schiffrin EL, Lipman ML, Mann JF (2007) Chronic kidney disease: effects on the cardiovascular system. Circulation 116(1):85–97
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kathrin Weidner and Michael Behnes contributed equally to this work.
Rights and permissions
About this article
Cite this article
Weidner, K., Behnes, M., Weiß, C. et al. Impact of chronic kidney disease on recurrent ventricular tachyarrhythmias in ICD recipients. Heart Vessels 34, 1811–1822 (2019). https://doi.org/10.1007/s00380-019-01415-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-019-01415-z