Abstract
Aims
Sleep-disordered breathing (SDB), in particular obstructive sleep apnoea, is associated with an increased risk of onset or recurrence of atrial fibrillation (AF) and atrial flutter. This prospective study investigated the relationship between restoration of sinus rhythm and SDB in patients with AF or atrial flutter.
Methods and results
138 consecutive patients (age 67.8 ± 10.3 years, 67.4 % male) with AF (86.2 %) or atrial flutter (13.8 %) were enrolled and underwent multichannel cardiorespiratory polygraphy the night before and immediately after electrical cardioversion (CV). None of the patients was treated with ventilation therapy before or during the study. Overall prevalence of SDB [apnoea–hypopnoea index (AHI) ≥5/h] was 92 % and prevalence of moderate-to-severe SDB (AHI) ≥15/h was 64 %. Within the first night after CV, AHI decreased from 23.4 ± 16.3 to 16.3 ± 11.5/h, p < 0.001. This was due to a significant decrease in central respiratory events, with a total reduction of patients showing central sleep apnoea (n = 53 at baseline vs n = 23 immediately after CV; p < 0.001).
Conclusions
In conclusion, SDB represents a highly prevalent comorbidity in patients with atrial arrhythmias. Through cardioversion, an immediate reduction of SDB can be detected due to a significant reduction in central respiratory events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is growing interest in an association between sleep-disordered breathing (SDB) and atrial fibrillation (AF)/atrial flutter. Both SDB and atrial arrhythmias have important effects on health status, quality of life and healthcare resource use [1–3], and the two conditions have been strongly linked in epidemiological studies [4, 5]. One form of SDB, obstructive sleep apnoea (OSA), has been shown to be an independent risk factor for AF [5–14]. Literature on central sleep apnoea (CSA) and AF or even atrial flutter is scarce. An association of AF and CSA has been shown in a cardiac healthy population, but not in an all comer collective [15].
To date most data are available for OSA only [16]. Early publications estimated that 3–7 % of adults in the US were affected by OSA. While these numbers are probably an underestimate, OSA still is a prevalent and underdiagnosed condition [17–19]. More recent studies report an estimated SDB prevalence (AHI ≥5/h) of 9 % in women and 24 % in men [20]. Data from the Wisconsin Sleep Cohort show prevalence estimates for moderate-to-severe SDB (AHI ≥15/h) of 10 % for 30- to 49-year-old men, 17 % for 50- to 70-year-old men, 3 % for 30- to 49-year-old women and 9 % for 50- to 70-year-old women [21]. Furthermore, interest in SDB among cardiologists is increasing [22], with implantable cardiac devices able to detect and treat the condition [23–26]. Correlations between AF and OSA have been reported in several studies, and OSA has been shown to be an independent risk factor for cardiac arrhythmias, including AF [6–11, 27]. There is overlap in the risk factors for AF, SDB and OSA. Risk factors for OSA include obesity, large neck circumference, male gender, increasing age, alcohol use, smoking, menopausal status, and Black race [28, 29]. The Sleep Heart Health Study reported that the risk of AF in patients with OSA was four times higher than that in patients without OSA [27]. However, data on the association between OSA and AF are not always consistent. For example, the MrOS study showed that, after adjustment for confounding factors, AF was associated with CSA, which is more common in heart failure patients, but not with OSA [11, 30].
The association and dependency of atrial arrhythmias and SDB are of great interest, as both influence the progression of HF. Moreover, there is growing evidence for a link between SDB and heart failure (HF) [31–33] and long lasting AF can lead to HF [34, 35]. SDB is an under-recognized but highly prevalent comorbidity in patients with heart failure, who often develop AF, and has an important impact on patients’ prognosis [32, 36, 37]. Hospitalizations and acute heart failure are common in patients with atrial arrhythmias and are predictive of reduced quality of life and mortality [38]. However, few data are available and no prospective randomized trials have been conducted to date.
This study investigated the relationship between restoration of sinus rhythm and SDB in patients with AF or atrial flutter immediately after CV. The hypothesis was that restoring sinus rhythm would decrease the total number of respiratory events, mainly as a result of decreases in the number of central apnoeas and hypopnoeas.
Methods
A total of 138 consecutive patients recruited at the Department of Cardiology, Heart- and Diabetes Center, Academic Medical Center of Ruhr-University in Bad Oeynhausen, Germany, were included in this prospective, observational trial. Inclusion criteria were atrial dysrhythmia and planned cardioversion, patient age >18 years and all patients had to be willing to take part in the trial. All patients with AF had persistent AF. Exclusion criteria were known atrial thrombus or other reasons that would prevent the patient from cardioversion and patients were excluded from the study if cardioversion was unsuccessful or cardioversion was not performed for newly discovered atrial thrombus, and no sustained sinus rhythm was restorable. Other exclusion criteria were acute coronary syndrome within 3 months prior to enrollment, active myocarditis, complex congenital heart disease, constrictive pericarditis, clinical evidence of digoxin toxicity, need for mechanical hemodynamic support, chronic hypoxemia as evidenced by sustained saturation ≤85 % (to exclude severely underling lung disease or oxygenation dysfunctions), transient ischemic attack (TIA) or stroke within 3 months prior to enrollment, status post-heart transplant or LVAD, prescribed inotrope therapy, known amyloidosis, arteriovenous fistulas, primary hemodynamically significant uncorrected valvular heart disease, pregnancy, and participation in pharmaceutical or treatment-related clinical study within 6 months of study enrollment. None of the patients had valvular AF. Successful cardioversion was defined as sustained restoration of sinus rhythm and cardioversion was exclusively performed as external, electrical cardioversion with paddles and Propofol i.v. anaesthesia. The study was approved by the institutional ethical committee and was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent before being screened for SDB by multichannel cardiorespiratory polygraphy (PG). The day after SDB screening, patients underwent cardioversion treatment for their atrial arrhythmia followed by a second PG on the next night. All PG sleep studies were performed by in-hospital unattended overnight cardiorespiratory polygraphy. Nasal airflow measured by nasal pressure, chest and abdominal effort, pulse oximetry, snoring and body position were continuously recorded. More than 85 % of total recording time had to be of good quality to be included in this study. The temporary loss of not more than one channel (except nasal air flow) was accepted. Analyses were performed by a physician specially trained in SDB, who was not involved in the clinical treatment of patients. Standard definitions were used to describe and score SDB as follows. An apnoea was scored if the breathing signal had a reduction in flow of ≥90 % for at least 10 s, in case of CSA without any abdominal or thoracic breathing efforts, in case of OSA with visible ribcage and abdominal respiratory impedance signals [36]. Hypopnoea was defined as decrease to ≥30 % reduction in flow, lasting ≥10 s and accompanied by a ≥3 % drop in oxygen saturation. Patients were classified to have either predominately CSA or OSA. Obstructive hypopnea events were scored if snoring during the event, “flattening” of nasal pressure during inspiration and/or paradox (antiparallel) thoracoabdominal excursions during the event was present. Hypopnoeas were scored central, when no criterion matched. The apnoea–hypopnoea index (AHI) is an established maker of SDB severity. AHI describes the number of episodes of apnoea and hypopnoea per hour of sleep. SDB severity was graded according to guideline recommendations [39] and our clinical routine as mild (AHI >5–<15/h), moderate (≥15–<30/h) and severe (AHI ≥30/h). Patients with an AHI ≤5/h were considered to have no relevant SDB.
All recordings were performed by Embletta™ polygraph (Embla, Rotterdam, The Netherlands). The definition used in this study for the oxygen desaturation index (ODI) was ≥3 % arterial oxygen desaturations per hour [39].
The Epworth Sleepiness Scale (ESS) was used to determine daytime sleepiness assessed at study inclusion [40].
None of the patients had known SDB at the time point of study inclusion. This is a natural history study without any ventilation therapy treatment, none of the patients was treated with ventilation therapy and all patients were not pretreated with ventilation therapy to demonstrate pristine and natural SDB dynamics when cardiac rhythm changes.
Statistics
Statistical analysis was performed using IBM SPSS 22, IBM Corporation, Armonk, NY, USA, for Mac, Apple Inc., Cupertino, CA, USA. A p value of <0.05 was defined as statistically significant. Data are expressed as percentages for discrete variables and as mean ± standard deviation for continuous variables. Continuous variables were compared by ANOVA. Categorical comparisons were compared using Chi-square analysis and non-parametric inferential statistical analyses were performed using Mann–Whitney U, Wilcoxon rank sum test and Spearman’s rank correlation coefficient.
Results
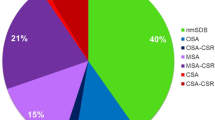
A total of 300 patients were screened, and 138 patients were finally eligible to be enrolled in the study (Fig. 1). Patient demographic and clinical characteristics are shown in Table 1. The majority of patients (119/138 [86.2 %]) had AF, and the remainder had atrial flutter. At baseline, the prevalence of SDB (defined as AHI ≥5/h) was 92 %. AHI was <5/h in 8 % of patients, ≥5 but <15/h in 37 % and ≥15/h in 55.1 % (Fig. 3). The proportion of patients with OSA, CSA or no SDB at baseline and the night after cardioversion is shown in Fig. 2.
Successful cardioversion was achieved in 84.1 % of patients. Reasons for unsuccessful cardioversion included newly discovered atrial thrombi (cardioversion not performed for newly discovered atrial thrombi), insufficient anticoagulation, giant left atrial size (n = 22 patients, anteroposterior measurement) or no restoration of a sustained sinus rhythm possible. Analysing the 22 patients with non-successful cardioversion, including cardioversion not performed for newly discovered atrial thrombus, no factors, including the degree of sleep apnoea (AHI 22.3/h vs 26.2/h, ODI 28/h vs 20.5/h, T <90 % 31.1 min vs 29.4 min, SaO2 92.1 vs 93.2 %) were identified as being responsible for non-successful cardioversion (Table 2).
Both the characteristics and severity of SDB improved markedly within 24 h of cardioversion (Table 2). For patients with CSA (n = 53), central apnoea index (cAI) was 5.0 ± 8.5/h before cardioversion and 1.9 ± 5/h after successful cardioversion (p < 0.001). Cardioversion was associated with a reduction in mean apnoea duration from 20.4 ± 8.7 to 18.8 ± 5.9 s (p < 0.05). This was a result of a reduction in central apnoea duration (17.1 ± 4.7–15.4 ± 4.4 s) rather than obstructive apnoea duration (22.1 ± 10.3–21.2 ± 6.5 s).
After successful cardioversion, 16 patients no longer had CSA, but were classified as having OSA. No previously classified OSA patient developed CSA after cardioversion. Three patients (3/116 = 2.6 %) with OSA had complete resolution of SDB after cardioversion. In our cohort, no echocardiographic parameter was identified to influence SDB severity or success of cardioversion. Post hoc power calculation revealed a 98.4 % power of our study.
Discussion
The results of this study showed that prevalence of SDB in general and central sleep apnoea in particular are high in patients with AF or atrial flutter admitted for electrical cardioversion. Successful cardioversion into sinus rhythm seems to substantially reduce total numbers of apnoeas and hypopnoeas (AHI) immediately and especially reduces the proportion of central respiratory events. Thus, restoring of sinus rhythm might just unmask underlying obstructive sleep apnoea in some cases.
The prevalence of moderate-to-severe SDB in our cohort was 55 %, comparable to previously reported data [5, 12, 13, 30]. First studies on SDB and cardiac arrhythmias were presented by Guilleminault et al. They studied 400 patients using 24-h Holter electrocardiography and polygraphy recording in parallel. A total of 193 patients (all had SDB, AHI ≥5/h), 48 % had cardiac arrhythmias during sleep [6]. In other studies, approximately half of all patients presenting with AF had been identified to have SDB, primarily OSA, although different AHI definitions for SDB have been used. The prevalence of AF was even higher (up to 75 %) in patients with frequent episodes of AF [5, 12].
The immediate effect of cardioversion on SDB has not previously been studied. Successful restoration of sinus rhythm decreased the number of central respiratory events, while it had no immediate impact on pre-existing obstructive events, which may be explained through the fluid-shift theory, as recently reported [41]. This theory hypothesizes that severity of OSA is related to overnight rostral leg fluid displacement and increase in neck circumference and severity of CSA is related to overnight rostral fluid displacement and to sleep pCO2 and fluid accumulation may be promoted in AF and reduced in sinus rhythm. The theory indicates that in CSA overnight fluid is displaced into the lungs, where irritant receptors may contribute to CSA [41]. We hypothesize that in patients with pre-existing CSA, after cardioversion and sinus rhythm restoration, less fluid accumulates in the legs, for hemodynamic improvements through re-established atrial contraction and therefore less overnight fluid shift into the lungs occurs, which might explain the fewer presence of CSA through cardioversion in our study. While pre-existing or underlying OSA, based on anatomic upper airway narrowness is not influenced by cardioversion, but OSA seems to be unmasked by disappearance of CSA through cardioversion. Furthermore, surrounding cardioversion patients are fasting and for the procedure and for anaesthesia reclined in bed, which results in fluid-shift omittance and therefore no impact on obstructive events, but a reduction of central events occurs, as recently elucidated by Yumino et al. [41] through hemodynamic improvements in restored sinus rhythm.
But in contrast to baseline and during AF and atrial flutter, more events turned obstructive from central before CV. Thus, improvement in hemodynamics, as achieved with CV into sinus rhythm, might reduce central respiratory events [41], but unmasks underlying obstructive sleep apnoea, as shown before [42].
Although strong epidemiological associations between SDB and AF have been reported [11–14], the mechanisms by which SDB causes AF, or vice versa, are not well understood. There are multiple factors that may cause or promote AF in SDB patients [43]. Mechanical stretch of the atria and the walls of the pulmonary vein creating large changes in transmural pressure, as well as high sympathetic activity, sudden blood pressure surges, hypoxaemia, hypercarbia, acidosis, and systemic inflammation can stimulate atrial dilation and predispose to AF [44]. In addition, arrhythmogenic changes of the atrial myocardium including atrial size enlargement, scarring and fibroses can serve as a substrate for AF [45]. SDB has been shown to have a direct effect on electrical and mechanical remodelling of the heart [45]. A key factor, therefore, seems to be the renin–angiotensin–aldosterone system; previous studies have documented elevated levels of angiotensin II and aldosterone in OSA patients [46, 47]. Increased aldosterone serum levels stimulate collagen synthesis by myocardial fibroblasts and may also play a role in myocyte death via their effect on electrolyte balance [48, 49]. In addition, SDB is a common comorbidity in patients with structural heart disease secondary to cardiac remodelling, such as systolic or diastolic heart failure or cardiomyopathies [36, 50, 51].
The results of this study confirmed previous data showing that typical SDB symptoms, such as daytime sleepiness, as documented by ESS [40] in this study, do not correlate with the presence of SDB in these patients [14]. Patients with AF have been shown to have reduced sleep time and impaired sleep quality [52]. Patients may not complain about typical SDB symptoms and are therefore not aware that they may have SDB, highlighting the importance of screening for SDB in patients with AF even in the absence of indicative symptoms [52].
This study also documents changes in the nature of SDB after cardioversion, with obstructive events becoming more frequent after CV. It is likely that OSA was always present, but was not detected because of the high prevalence of central apnoea events, which diminish according to fluid-shift theory [41]. Upper airway narrowing and occlusion during the course of CSA have previously been documented [41, 53, 54]. Therefore, the upper airway becomes vulnerable to closure, in the nadir of the ventilatory cycle of periodic breathing, resulting in mixed apnoeas, according to our findings [55, 56]. Furthermore, patients with heart failure who are prone to upper airway occlusion (e.g. obese subjects) will develop obstructive or mixed apnoeas in the setting of background periodic breathing [55]. Improvements in CSA after cardioversion therefore may have resulted in an “unmasking” of underling OSA. Given that pulmonary congestion in the setting of heart failure is redistributed by overnight rostral fluid displacement to the lungs [41], lowering carbon dioxide pressure and predisposing to more CSA events [41], another possibility is that hemodynamic improvements secondary to restoration of sinus rhythm alleviate fluid congestion resulting in less impulse to maintain or trigger CSA events [55]. Our study result hereby encourages further research and future studies on this important topic.
Limitations
One of the limitations of this study was the use of multichannel PG, rather than multichannel polysomnography (PSG), meaning that sleep and sleep quantity of the study population could not be determined. The duration of continuous AF pre-cardioversion is not known for the patients, but can be assumed as long time as the average left atrium is big.
Conclusions
In conclusion, SDB is a highly prevalent comorbidity in patients with atrial arrhythmias. Through cardioversion, an immediate reduction of SDB can be detected especially in a significant reduction in central respiratory events.
References
Digby GC, Baranchuk A (2012) Sleep apnea and atrial fibrillation; 2012 update. Curr Cardiol Rev 8(4):265–272
Sabouret P, Depret-Bixio L, Cotte FE, Marie P, Bedira N, Blin P (2014) Sex differences in stroke prevention in atrial fibrillation in French primary care. Results of the AFIGP (atrial fibrillation in general practice) database. Clin Res Cardiol 103(11):887–893. doi:10.1007/s00392-014-0726-y
Gitt AK, Smolka W, Michailov G, Bernhardt A, Pittrow D, Lewalter T (2013) Types and outcomes of cardioversion in patients admitted to hospital for atrial fibrillation: results of the German RHYTHM-AF Study. Clin Res Cardiol 102(10):713–723. doi:10.1007/s00392-013-0586-x
Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V (2005) Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353(19):2034–2041. doi:10.1056/NEJMoa043104
Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK (2004) Association of atrial fibrillation and obstructive sleep apnea. Circulation 110(4):364–367. doi:10.1161/01.CIR.0000136587.68725.8E
Guilleminault C, Connolly SJ, Winkle RA (1983) Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol 52(5):490–494
Mooe T, Gullsby S, Rabben T, Eriksson P (1996) Sleep-disordered breathing: a novel predictor of atrial fibrillation after coronary artery bypass surgery. Coron Artery Dis 7(6):475–478
Tanigawa T, Yamagishi K, Sakurai S, Muraki I, Noda H, Shimamoto T, Iso H (2006) Arterial oxygen desaturation during sleep and atrial fibrillation. Heart 92(12):1854–1855. doi:10.1136/hrt.2005.081257
Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK (2007) Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 49(5):565–571. doi:10.1016/j.jacc.2006.08.060
Monahan K, Storfer-Isser A, Mehra R, Shahar E, Mittleman M, Rottman J, Punjabi N, Sanders M, Quan SF, Resnick H, Redline S (2009) Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 54(19):1797–1804. doi:10.1016/j.jacc.2009.06.038
Mehra R, Stone KL, Varosy PD, Hoffman AR, Marcus GM, Blackwell T, Ibrahim OA, Salem R, Redline S (2009) Nocturnal Arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 169(12):1147–1155. doi:10.1001/archinternmed.2009.138
Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM (2008) Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J 29(13):1662–1669. doi:10.1093/eurheartj/ehn214
Braga B, Poyares D, Cintra F, Guilleminault C, Cirenza C, Horbach S, Macedo D, Silva R, Tufik S, De Paola AA (2009) Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med 10(2):212–216. doi:10.1016/j.sleep.2007.12.007
Albuquerque FN, Calvin AD, Sert Kuniyoshi FH, Konecny T, Lopez-Jimenez F, Pressman GS, Kara T, Friedman P, Ammash N, Somers VK, Caples SM (2012) Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest 141(4):967–973. doi:10.1378/chest.11-0975
Leung RS, Huber MA, Rogge T, Maimon N, Chiu KL, Bradley TD (2005) Association between atrial fibrillation and central sleep apnea. Sleep 28(12):1543–1546
Schaefer CA, Adam L, Weisser-Thomas J, Pingel S, Vogel G, Klarmann-Schulz U, Nickenig G, Pizarro C, Skowasch D (2015) High prevalence of peripheral arterial disease in patients with obstructive sleep apnoea. Clin Res Cardiol. doi:10.1007/s00392-015-0834-3
Punjabi NM (2008) The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5(2):136–143. doi:10.1513/pats.200709-155MG
Stradling JR, Davies RJ (2004) Sleep. 1: obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax 59(1):73–78
Partinen M, Palomaki H (1985) Snoring and cerebral infarction. Lancet 2(8468):1325–1326
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328(17):1230–1235. doi:10.1056/NEJM199304293281704
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM (2013) Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177(9):1006–1014. doi:10.1093/aje/kws342
Dimitriadis Z, Wiemer M, Scholtz W, Faber L, Piper C, Bitter T, Messaritakis I, Bullert K, Boergermann J, Kleikamp G, Prinz C, Horstkotte D, Oldenburg O (2013) Sleep-disordered breathing in patients undergoing transfemoral aortic valve implantation for severe aortic stenosis. Clin Res Cardiol 102(12):895–903. doi:10.1007/s00392-013-0603-0
Fox H, Nolker G, Gutleben KJ, Bitter T, Horstkotte D, Oldenburg O (2014) Reliability and accuracy of sleep apnea scans in novel cardiac resynchronization therapy devices: an independent report of two cases. Herzschrittmachertherapie Elektrophysiologie 25(1):53–55. doi:10.1007/s00399-014-0298-6
Ponikowski P, Javaheri S, Michalkiewicz D, Bart BA, Czarnecka D, Jastrzebski M, Kusiak A, Augostini R, Jagielski D, Witkowski T, Khayat RN, Oldenburg O, Gutleben KJ, Bitter T, Karim R, Iber C, Hasan A, Hibler K, Germany R, Abraham WT (2012) Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 33(7):889–894. doi:10.1093/eurheartj/ehr298
Fox H, Oldenburg O, Nolker G, Horstkotte D, Gutleben KJ (2014) Detection and therapy of respiratory dysfunction by implantable (cardiac) devices. Herz 39(1):32–36. doi:10.1007/s00059-014-4062-9
Strollo PJ Jr, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, Hanson RD, Padhya TA, Steward DL, Gillespie MB, Woodson BT, Van de Heyning PH, Goetting MG, Vanderveken OM, Feldman N, Knaack L, Strohl KP, Group ST (2014) Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 370(2):139–149. doi:10.1056/NEJMoa1308659
Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S, Sleep Heart Health S (2006) Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 173(8):910–916. doi:10.1164/rccm.200509-1442OC
Malhotra A, White DP (2002) Obstructive sleep apnoea. Lancet 360(9328):237–245. doi:10.1016/S0140-6736(02)09464-3
Davies RJ, Ali NJ, Stradling JR (1992) Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax 47(2):101–105
Linz D, Woehrle H, Bitter T, Fox H, Cowie MR, Bohm M, Oldenburg O (2015) The importance of sleep-disordered breathing in cardiovascular disease. Clin Res Cardiol. doi:10.1007/s00392-015-0859-7
Jelic S, Le Jemtel TH (2009) Sleep-disordered breathing in acute decompensated heart failure. Curr Heart Fail Rep 6(3):169–175
Oldenburg O, Bitter T, Fox H, Horstkotte D (2014) Sleep-related breathing disorders and (resulting) cardiovascular diseases. Herz 39(1):37–44. doi:10.1007/s00059-013-4050-5
Nakamura S, Asai K, Kubota Y, Murai K, Takano H, Tsukada YT, Shimizu W (2015) Impact of sleep-disordered breathing and efficacy of positive airway pressure on mortality in patients with chronic heart failure and sleep-disordered breathing: a meta-analysis. Clin Res Cardiol 104(3):208–216. doi:10.1007/s00392-014-0774-3
von Scheidt W, Zugck C, Pauschinger M, Hambrecht R, Bruder O, Hartmann A, Rauchhaus M, Zahn R, Brachmann J, Tebbe U, Neumann T, Strasser RH, Bohm M, Stork S, Hochadel M, Heidemann P, Senges J (2014) Characteristics, management modalities and outcome in chronic systolic heart failure patients treated in tertiary care centers: results from the EVIdence based TreAtment in Heart Failure (EVITA-HF) registry. Clin Res Cardiol 103(12):1006–1014. doi:10.1007/s00392-014-0743-x
Dolliner P, Brammen L, Graf S, Huelsmann M, Stiebellehner L, Gleiss A, Ubl P, Steurer G (2013) Portable recording for detecting sleep disorder breathing in patients under the care of a heart failure clinic. Clin Res Cardiol 102(7):535–542. doi:10.1007/s00392-013-0563-4
Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V (2007) Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 9(3):251–257. doi:10.1016/j.ejheart.2006.08.003
Javaheri S, Shukla R, Zeigler H, Wexler L (2007) Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 49(20):2028–2034. doi:10.1016/j.jacc.2007.01.084
Jilek C, Krenn M, Sebah D, Obermeier R, Braune A, Kehl V, Schroll S, Montalvan S, Riegger GA, Pfeifer M, Arzt M (2011) Prognostic impact of sleep disordered breathing and its treatment in heart failure: an observational study. Eur J Heart Fail 13(1):68–75. doi:10.1093/eurjhf/hfq183
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep M (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med (JCSM) 8(5):597–619. doi:10.5664/jcsm.2172
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14(6):540–545
Yumino D, Redolfi S, Ruttanaumpawan P, Su MC, Smith S, Newton GE, Mak S, Bradley TD (2010) Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 121(14):1598–1605. doi:10.1161/CIRCULATIONAHA.109.902452
Oldenburg O, Faber L, Vogt J, Dorszewski A, Szabados F, Horstkotte D, Lamp B (2007) Influence of cardiac resynchronisation therapy on different types of sleep disordered breathing. Eur J Heart Fail 9(8):820–826. doi:10.1016/j.ejheart.2007.03.009
Linz D, Linz B, Hohl M, Bohm M (2015) Atrial arrhythmogenesis in obstructive sleep apnea: therapeutic implications. Sleep Med Rev. doi:10.1016/j.smrv.2015.03.003
Rajagopalan N (2011) Obstructive sleep apnea: not just a sleep disorder. J Postgrad Med 57(2):168–175. doi:10.4103/0022-3859.81866
Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB, Kalman JM (2003) Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation 108(12):1461–1468. doi:10.1161/01.CIR.0000090688.49283.67
Calhoun DA, Nishizaka MK, Zaman MA, Harding SM (2004) Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest 125(1):112–117
Moller DS, Lind P, Strunge B, Pedersen EB (2003) Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens 16(4):274–280
Brilla CG, Zhou G, Matsubara L, Weber KT (1994) Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol 26(7):809–820. doi:10.1006/jmcc.1994.1098
Sheehan JP, Seelig MS (1984) Interactions of magnesium and potassium in the pathogenesis of cardiovascular disease. Magnesium 3(4–6):301–314
Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O (2009) Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 11(6):602–608. doi:10.1093/eurjhf/hfp057
Prinz C, Bitter T, Oldenburg O, Horstkotte D, Faber L (2011) Incidence of sleep-disordered breathing in patients with hypertrophic cardiomyopathy. Congest Heart Fail 17(1):19–24. doi:10.1111/j.1751-7133.2010.00196.x
Kayrak M, Gul EE, Aribas A, Akilli H, Alibasic H, Abdulhalikov T, Yildirim O, Yazici M, Ozdemir K (2013) Self-reported sleep quality of patients with atrial fibrillation and the effects of cardioversion on sleep quality. Pacing Clin Electrophysiol (PACE) 36(7):823–829. doi:10.1111/pace.12115
Guilleminault C, Hill MH, Simmons FB, Powell N, Riley R, Stoohs R (1997) Passive constriction of the upper airway during central apneas: fiberoptic and EMG investigations. Respir Physiol 108(1):11–22
Badr MS, Toiber F, Skatrud JB, Dempsey J (1995) Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 78(5):1806–1815
Javaheri S (2005) Central sleep apnea in congestive heart failure: prevalence, mechanisms, impact, and therapeutic options. Semin Respir Crit Care Med 26(1):44–55. doi:10.1055/s-2005-864206
Dowdell WT, Javaheri S, McGinnis W (1990) Cheyne-stokes respiration presenting as sleep apnea syndrome. Clinical and polysomnographic features. Am Rev Respir Dis 141(4 Pt 1):871–879. doi:10.1164/ajrccm/141.4_Pt_1.871
Acknowledgments
English language editorial assistance was provided by Nicola Ryan, independent medical writer, on behalf of ResMed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was conducted as a non-commercial trial with capital resources of the Department of Cardiology at the Heart and Diabetes Center North Rhine-Westphalia, Ruhr University Bochum, Georgstrasse 11, D-32545 Bad Oeynhausen, Germany.
Conflict of interest
The authors state that there are no disclosures to be declared related to this study.
Additional information
Henrik Fox and Thomas Bitter have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fox, H., Bitter, T., Horstkotte, D. et al. Cardioversion of atrial fibrillation or atrial flutter into sinus rhythm reduces nocturnal central respiratory events and unmasks obstructive sleep apnoea. Clin Res Cardiol 105, 451–459 (2016). https://doi.org/10.1007/s00392-015-0940-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0940-2