Abstract
Background
Self-expandable metallic stents (SEMS) are now regarded as an effective and safe intervention for malignant colorectal obstruction (MCO). However, manipulation of the tumor might lead to the spillage of tumor cells and result in distant metastases. We aimed to compare the long-term oncologic outcomes of SEMS as a bridge to surgery with those of emergency surgery for MCO.
Methods
Between June 2005 and December 2011, 60 patients who underwent elective curative resection after endoscopic SEMS insertion were included in the “SEMS group”. The SEMS group was matched to 180 patients who underwent emergency curative surgery for MCO during the same period [“Emergency surgery (ES) group”]. The clinicopathologic characteristics, recurrence-free survival (RFS), and overall survival (OS) were compared between the two groups.
Results
There were no significant differences in demographics, tumor stage, location, and histology between the SEMS group and the ES group. The median follow-up times were 41.4 months (IQR, 22.2–60.0 months) for the SEMS group and 45.0 months (IQR, 20.9–68.1 months) for the ES group. The proportions of patients who received postoperative adjuvant chemotherapy were comparable (SEMS group vs. ES group, 68.3 % vs. 77.8 %; P = 0.210). The long-term prognosis did not significantly differ between two groups in either the 5-year RFS rate (79.6 % vs. 70.2 %; P = 0.218) or the 5-year OS rate (97.8 % vs. 94.3 %; P = 0.469).
Conclusions
Long-term oncologic outcomes of SEMS insertion as a bridge to surgery were comparable to those of primary curative surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer is the third most commonly diagnosed malignancy in the world and the fourth leading cause of cancer related mortality in Korea [1, 2]. The incidence and mortality rate of colorectal cancer are still increasing in Korea. In 2010, there were 20,711 new cases of colorectal cancer and approximately 7,700 deaths attributable to this disease [2]. It has been reported that approximately 8–29 % of the patients with primary colorectal cancer present with symptoms and signs of obstruction at the time of diagnosis [3]. In the past, the standard treatment for acute colonic obstruction was emergency surgical decompression. However, an emergency colectomy or colostomy is associated with a postoperative mortality rate of 15–20 % and a morbidity rate of 40–50 % due to poor general conditions and unprepared bowels [4–6]. Moreover, many patients may have to keep the colostomy temporarily or permanently.
Since the early 1990s, when self-expandable metallic stents (SEMS) for the treatment of malignant colonic obstruction (MCO) were introduced for the first time, SEMS have been used as an alternative to emergency surgery [7]. When used as a bridge to surgery, SEMS can decompress the obstructed bowel, provide an opportunity to clean the colon, and allow physicians more time to perform a precise preoperative evaluation [8, 9]. It has been reported that SEMS enable primary anastomosis with a single-stage operation, reduce mortality and morbidity, and lower the rate of stoma formation [10, 11]. Despite these advantages, SEMS placement can cause complications, such as bleeding, perforation, stent displacement, and restenosis [12]. Furthermore, there is mechanical stress of tumor through advancing the scope, insufflation of air, and expanding the stent during procedure. These manipulations of the tumor might lead to the spillage of tumor cells into systemic circulation; consequently, there is a potential to worsen the development of a potentially curable disease into a non-curable state [13]. A few studies have reported on the long-term outcomes of SEMS, but they included small numbers of patients and relatively short follow-up periods [14–16]. Research on oncologic outcomes, such as recurrence and survival after SEMS insertion, is still lacking.
The objectives of this study were to compare the group who underwent SEMS placement followed by curative resection with the group who underwent emergency surgery, with respect to the recurrence and long-term survival rates.
Materials and methods
Patients and study design
The MCO was defined by the presence of clinical symptoms or signs of bowel obstruction, and patients with MCO were enrolled in the study. Clinical symptoms of MCO were defined as constipation, vomiting, or abdominal pain, and the patients who had at least two of the three symptoms were enrolled. The signs of obstruction were defined as (1) distended proximal bowel, transitional zone or collapsed distal bowel on abdominal CT scans or (2) impossibility to pass through the stenotic area in the colonoscopic evaluation.
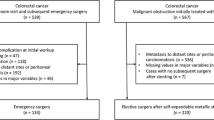
Between June 2005 and December 2011, 1009 patients with acute MCO from colorectal cancer were admitted to Seoul National University Hospital. We excluded 129 patients of inoperable disease. Among the remaining patients, emergency surgery was done for 789 patients (control group), and SEMS were placed as a bridge to surgery in 91 patients. From these patients, we additionally excluded 31 patients who underwent fluoroscopy-guided stent insertion. We retrospectively reviewed the medical records of patients in the SEMS group and control group, including age, gender, Charlson comorbidity score, tumor stage, site of obstruction, tumor size, surgical procedure, adjuvant chemotherapy after surgery, and time to progression or death.
We used a case–control design with 3 controls individually matched to each case. The 60 patients in the SEMS group were matched to the subjects in control group based on TNM stage according to the 7th American Joint Committee on Cancer, age (±5 years), and sex, as possible. The study was approved by the ethics committee of the Seoul National University Hospital (IRB no. H-1202-043-398) and was conducted in accordance with the Declaration of Helsinki.
SEMS insertion
Self-expandable metallic stents insertions were performed as previous reported [17]. Before stent insertion, the site, length, and degree of obstruction were assessed by colonoscopy, conventional CT, three-dimensional CT colonography, and/or water-soluble contrast enemas. The stent size (diameter, 18–24 mm) and length (80–170 mm) were chosen according to the measured length of the obstruction, and uncovered SEMS were used. The length of the stent was at least 3 cm longer than the stenosis at both sides to allow for adequate margins. Generally, patients underwent cleansing enemas for bowel preparation and were maintained under conscious sedation with 0.05 mg/kg intravenous midazolam administration. SEMS insertions were performed using a conventional endoscope (CF-H260, Olympus, Tokyo, Japan) by two experienced, qualified endoscopists (S.G. Kim and J.P. Im). Once the stent had been inserted along the guidewire across the obstruction by endoscopy, the stent was deployed through direct endoscopic guidance. After placement, the correct position and expansion of the stent were confirmed by simple abdominal films.
Emergency surgery
All surgeries were performed by five experienced surgeons who were familiar with colorectal procedures. The type and extent of surgery were determined by the treating surgeon according to the tumor location, stage, and the general condition of the patient. The surgeon attempted a single-stage resection with a primary anastomosis; a Hartmann’s operation was performed, if primary anastomosis was not possible.
Clinical outcomes
Long-term outcomes were compared between the two groups. Recurrence-free survival (RFS) was defined as the time interval from the operation or SEMS insertion until cancer recurrence or the last follow-up. The last follow-up was based on review of hospital records. The patients who did not attend a scheduled visit within 1 year of the previous visit were considered loss to follow-up and were censored. Overall survival (OS) was defined as the time interval from the date of operation or SEMS insertion to either death or the last follow-up visit [14].
Statistical analysis
The data were analyzed using SPSS software (version 19.0, SPSS, Chicago, IL, USA) and MedCalc software (version 13.0.0.0, MedCalc software, Mariakerke, Belgium). Patient demographics and clinical characteristics were expressed as the means and standard deviations or as numbers (percentages). The means of continuous variables were analyzed between two groups using Student’s t test, and categorical variables were analyzed by the Chi square test or Fisher’s exact test. The means of the variables that were not distributed normally were compared with the Mann–Whitney U test. Survival analysis was performed using Kaplan–Meier method, and the findings were compared using a log-rank test. P < 0.05 was considered statistically significant.
Results
Baseline characteristics
The mean ages of the SEMS group and the ES group were 65.2 ± 11.1 and 64.8 ± 10.6 years, respectively. The groups were well balanced with regard to clinical variables, such as age, gender, and adjuvant chemotherapy status (P = 0.792, P = 0.175, and P = 0.210, respectively) (Table 1). The Charlson comorbidity index was higher for SEMS group, but it was statistically insignificant (P = 0.090).
There were also no differences between the groups in terms of the location, stage, and differentiation of tumors (Table 2). The tumors were mainly located in the sigmoid colon and rectum, but the overall distribution did not differ between the two groups. Eight patients (2 patients in the SEMS group and 6 patients in the ES group) had resectable liver or lung metastases at the time of initial presentation and underwent metastasectomy synchronously with colectomy.
For the SEMS group, only uncovered stents were used; the median time interval from the stent placement to the surgery was 15 (range 2–115) days. Two patients experienced a longer wait (111 and 115 days) before surgery for SEMS insertion because of neo-adjuvant chemotherapy prior to the surgery.
Recurrence-free survival
Mostly, follow-up visit was conducted with imaging study (CT, MRI, PET, or abdominal sonography) or endoscopic study and in case of positive finding of these studies, biopsies were taken to confirm the recurrence. The median follow-up durations were 41.4 months (IQR, 22.2–60.0 months) in the SEMS group and 45.0 months (IQR, 20.9–68.1 months) in the ES group. The follow-up duration was longer for the ES group than the SEMS group, but this difference did not reach statistical significance (Mann–Whitney U test, P = 0.419). In the SEMS group, 10 (16.7 %) of the 60 patients had recurrence during follow-up, with local recurrences in 2 (3.3 %) cases and distant metastases in 8 (13.3 %) cases, respectively. In the ES group, 46 (25.6 %) of the 180 patients had recurrence during follow-up, with local recurrences in 6 (3.3 %) cases and distant metastases in 41 (22.8 %) cases. 40 of the 60 patients who underwent SEMS insertion (66.7 %) and 124 of the 180 patients in ES group (68.9 %) were included in the analysis of 3-year survival without recurrence. Across the 5-year assessments of RFS, 23 patients (38.3 %) were continued to follow-up in SEMS group, 97 patients (53.9 %) did in ES group. The 3- and 5-year RFS rates were 82.7 and 79.6 % for the SEMS group patients, compared with 73.4 and 70.2 % for the ES group patients; however, there were no statistically significant differences between the two groups (log-rank test, P = 0.218; Fig. 1). The RFS did not differ statistically by stage according to American Joint Committee on Cancer (AJCC) classification (Fig. 2).
Kaplan–Meier curve of recurrence-free survival for the SEMS and ES groups. The 3- and 5-year RFS rates were 82.7 and 79.6 % for the SEMS group, compared with 73.4 and 70.2 % for the ES group. There were no statistically significant differences between the two groups (log-rank test, P = 0.218). Number of patients at risk is the number of patients who remain recurrence-free at any time point and whose follow-up extends at least that far into the curve
Overall survival
During follow-up, 1 (1.7 %) patient in the SEMS group and 7 (3.9 %) patients in the ES group were died. Of the 8 patients who died, 7 (87.5 %) died of cancer, and 1 (12.5 %) in the ES group died of unknown causes. According to the OS rate, follow-up observation was continued for 3 years in 31 patients of SEMS group (51.7 %) and 104 patients of ES group (57.8 %), 13 subjects (21.7 %) and 69 subjects (38.3 %) for 5 years, respectively. The 3- and 5-year OS rates were both 97.8 % for the SEMS group and 96.5 and 94.3 % for the ES group, respectively. The Kaplan–Meier curve showed no difference in the OS between the two groups (log-rank test, P = 0.469; Fig. 3). In addition, the subgroup analysis of OS yielded comparable results regarding AJCC tumor staging (Fig. 4).
Kaplan–Meier curve of overall survival for the SEMS and ES groups. The 3- and 5-year OS rates were both 97.8 % for the SEMS group vs. 96.5 and 94.3 % for the ES group. These results were comparable between the two groups (log-rank test, P = 0.469). Number of patients at risk is the number of individuals who are still alive at any time point and whose follow-up extends beyond the time in the curve
Discussion
This study consisted of a large number of patients and provided a relatively longer duration of follow-up. Our study confirms that SEMS insertion followed by curative resection for MCO did not have unfavorable effects on long-term prognosis.
It has been known that acute bowel obstruction occurs in 8–29 % of patients with primary colorectal cancer. Colorectal cancer with MCO tends to occur at more advanced stages, with an increased risk of potential for local extension and distant metastasis than non-obstructive cancer [3, 18]. Traditionally, patients with MCO have been managed by emergency surgical decompression. However, as these patients were usually in poor general condition and because the surgery was performed on an unprepared bowel, emergency surgical intervention was associated with high mortality and morbidity [19, 20].
Since 1991, when Dohmoto et al. reported the first clinical experience of endoscopic stenting for rectal cancer as a palliative measure, there has been a heightened interest in the use of SEMS as an alternative to emergency surgery, particularly in patients with potentially curable colorectal cancer [7]. Through the use of SEMS as a bridge to elective surgical resection, perioperative morbidity and mortality were reduced, and the rate of stoma formation was also significantly decreased [14, 21, 22]. In contrast of those favored short-term outcome, there was a concern of long-term oncologic outcome of stenting, because of the manipulation of the tumor leading to the increased risk of tumor cell dissemination [13]. Until now, there have been only a few studies on the long-term outcomes of colonic SEMS as a bridge to curative surgery. A recent meta-analysis by Zhang et al. demonstrated that the OS was not different between SEMS and ES groups at 1 year [risk ratio (RR), 1.07; 95 % confidence interval (CI), 0.87–1.31; P = 0.510], 2 years (RR, 1.14; 95 % CI, 0.98–1.34; P = 0.100), or 3 years (RR, 1.08; 95 % CI, 0.90–1.31; P = 0.390) [22]. But, Kim et al. showed that SEMS had a deleterious effect on the long-term outcome of colon cancer, the 5-year progression-free survival rate was 48.3 % in SEMS group and 75.5 % in surgery group (P = 0.024), and 5-year OS rate was 38.4 and 65.6 %, respectively (P = 0.025) [23]. In Kim’s study, SEMS group enrolled patients who had left-sided CRC with obstruction, and the control group enrolled patients with non-obstructing left sided CRC based on stage according to AJCC criteria in ratio of 1:10. This discrepancy may be a possible selection bias, because the presence of MCO has been known as a poor prognostic factor. In the former study, it is unclear that this worsened prognosis of SEMS group was affected by the indwelling stent or by the bowel obstruction itself [13, 24–27]. On the other hand, Gianotti et al. recently conducted a prospective study and showed the longer survival in the SEMS group (hazard ratio, 0.412; 95 % CI, 0.217–0.785; P = 0.007) [28]. In this study, the control group was the patient who underwent immediate surgery for MCO. Considering these discrepancies, the long-term oncological consequences of colorectal SEMS for MCO have not yet been well established.
In our study, the long-term outcomes of SEMS as a bridge to surgery were comparable with those of emergency surgery. The 3- and 5-year RFS rates were 82.7 and 79.6 % for the SEMS group and 73.4 and 70.2 % for the ES group (P = 0.218; Fig. 1), while the OS rates were both 97.8 % for the SEMS group and 96.5 % and 94.3 % for the ES group (P = 0.469; Fig. 3). The RFS and OS rates were not significantly different, even at advanced stages (Figs. 2, 4).
Bowel perforation is one of the associated risks of potential tumor dissemination, but no stent-related perforation or micro-perforation cases occurred in this study [27, 29]. In the recent meta-analysis, the clinical perforation rate was 6.9 % (8 of 116), and the silent perforation rate was 14 % (11 of 77), but there was no perforation case reported in our study and only one perforation (1.2 %) in recent study [5, 17, 22]. This finding might relate to the high volume of our hospital and the experience of endoscopists. A volume-outcome relationship has been well established for various surgical procedure and also endoscopic procedures [30–32]. In case of SEMS, more large studies will be needed to confirm a volume-outcome relationship.
This study had some limitations, however, mostly stemming from its retrospective design. First, we were unable to fully evaluate possible confounders, such as the performance status and duration of symptoms at the time of initial diagnosis, owing to the retrospective nature of this study. In the present study, the mean Charlson comorbidity index tended to be lower in the ES group, although the differences did not reach statistical significance (0.67 vs. 0.43, respectively; P = 0.090). This could lead to a selection bias, because emergency resection is indicated in patients in good general condition, and this consideration may have influenced the results obtained to some extent. The second limitation of this study was the low incidence of recurrence or death events in both groups due to insufficiently long follow-up times (median follow-up 41.4 months in the SEMS group and 45.0 months in the ES group). However, as the disease recurrence of colorectal cancer typically occurs within 3.5 years after curative resection, we might have been able to detect most of the recurrence cases [33]. In addition, further follow-up studies are required to more accurately define the long-term outcomes of SEMS.
In conclusion, the SEMS placement as a bridge to surgery in the patients with MCO was safe and feasible, and the long-term oncologic outcomes of SEMS were comparable to those of primary curative surgery, in case of successful placement without perforation, when the procedure was conducted by an experienced endoscopist in high-volume hospital. The oncologic outcome of SEMS for MCO should be studied further in randomized controlled trials at high-volume centers.
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
http://www.index.go.kr. Accessed 29 May 2013
Deans GT, Krukowski ZH, Irwin ST (1994) Malignant obstruction of the left colon. Br J Surg 81:1270–1276
Bokey EL, Chapuis PH, Fung C, Hughes WJ, Koorey SG, Brewer D, Newland RC (1995) Postoperative morbidity and mortality following resection of the colon and rectum for cancer. Dis Colon Rectum 38:480–486; discussion 486–487
Tan CJ, Dasari BV, Gardiner K (2012) Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg 99:469–476
Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD (2004) The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg 240:76–81
Dohmoto M (1991) New method: endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig 3:1507–1512
Mainar A, De Gregorio Ariza MA, Tejero E, Tobio R, Alfonso E, Pinto I, Herrera M, Fernandez JA (1999) Acute colorectal obstruction: treatment with self-expandable metallic stents before scheduled surgery—results of a multicenter study. Radiology 210:65–69
Tejero E, Mainar A, Fernandez L, Tieso A, Cuezva JF, San Jose A (1995) New procedure for relief of malignant obstruction of the left colon. Br J Surg 82:34–35
Baron TH (2010) Colonic stenting: a palliative measure only or a bridge to surgery? Endoscopy 42:163–168
Cennamo V, Luigiano C, Manes G, Zagari RM, Ansaloni L, Fabbri C, Ceroni L, Catena F, Pinna AD, Fuccio L, Mussetto A, Casetti T, Coccolini F, D’Imperio N, Bazzoli F (2012) Colorectal stenting as a bridge to surgery reduces morbidity and mortality in left-sided malignant obstruction: a predictive risk score-based comparative study. Dig Liver Dis 44:508–514
Khot UP, Lang AW, Murali K, Parker MC (2002) Systematic review of the efficacy and safety of colorectal stents. Br J Surg 89:1096–1102
Koch M, Kienle P, Sauer P, Willeke F, Buhl K, Benner A, Lehnert T, Herfarth C, von Knebel Doeberitz M, Weitz J (2004) Hematogenous tumor cell dissemination during colonoscopy for colorectal cancer. Surg Endosc 18:587–591
Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC (2008) Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 12:51–55
Pessione S, Petruzzelli L, Gentilli S, Mioli P (2007) Treatment of neoplastic stenosis of the left colon: presurgical expandable metal stent vs emergency surgery. Comparison of results and survival rates. Chir Ital 59:661–669
Saida Y, Sumiyama Y, Nagao J, Uramatsu M (2003) Long-term prognosis of preoperative “bridge to surgery” expandable metallic stent insertion for obstructive colorectal cancer: comparison with emergency operation. Dis Colon Rectum 46:S44–S49
Im JP, Kim SG, Kang HW, Kim JS, Jung HC, Song IS (2008) Clinical outcomes and patency of self-expanding metal stents in patients with malignant colorectal obstruction: a prospective single center study. Int J Colorectal Dis 23:789–794
Willett C, Tepper JE, Cohen A, Orlow E, Welch C (1985) Obstructive and perforative colonic carcinoma: patterns of failure. J Clin Oncol 3:379–384
Ohman U (1982) Prognosis in patients with obstructing colorectal carcinoma. Am J Surg 143:742–747
Runkel NS, Schlag P, Schwarz V, Herfarth C (1991) Outcome after emergency surgery for cancer of the large intestine. Br J Surg 78:183–188
Varadarajulu S, Roy A, Lopes T, Drelichman ER, Kim M (2011) Endoscopic stenting versus surgical colostomy for the management of malignant colonic obstruction: comparison of hospital costs and clinical outcomes. Surg Endosc 25:2203–2209
Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX (2012) Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc 26:110–119
Kim JS, Hur H, Min BS, Sohn SK, Cho CH, Kim NK (2009) Oncologic outcomes of self-expanding metallic stent insertion as a bridge to surgery in the management of left-sided colon cancer obstruction: comparison with nonobstructing elective surgery. World J Surg 33:1281–1286
Crucitti F, Sofo L, Doglietto GB, Bellantone R, Ratto C, Bossola M, Crucitti A (1991) Prognostic factors in colorectal cancer: current status and new trends. J Surg Oncol Suppl 2:76–82
Griffin MR, Bergstralh EJ, Coffey RJ, Beart RW Jr, Melton LJ 3rd (1987) Predictors of survival after curative resection of carcinoma of the colon and rectum. Cancer 60:2318–2324
Ratto C, Sofo L, Ippoliti M, Merico M, Doglietto GB, Crucitti F (1998) Prognostic factors in colorectal cancer. Literature review for clinical application. Dis Colon Rectum 41:1033–1049
Maruthachalam K, Lash GE, Shenton BK, Horgan AF (2007) Tumour cell dissemination following endoscopic stent insertion. Br J Surg 94:1151–1154
Gianotti L, Tamini N, Nespoli L, Rota M, Bolzonaro E, Frego R, Redaelli A, Antolini L, Ardito A, Nespoli A, Dinelli M (2013) A prospective evaluation of short-term and long-term results from colonic stenting for palliation or as a bridge to elective operation versus immediate surgery for large-bowel obstruction. Surg Endosc 27:832–842
McArdle CS, McMillan DC, Hole DJ (2006) The impact of blood loss, obstruction and perforation on survival in patients undergoing curative resection for colon cancer. Br J Surg 93:483–488
Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL (2003) Surgeon volume and operative mortality in the United States. N Engl J Med 349:2117–2127
Kim CG, Kwak EK, Lee SI (2011) The relationship between hospital volume and outcome of gastrointestinal cancer surgery in Korea. J Surg Oncol 104:116–123
Varadarajulu S, Kilgore ML, Wilcox CM, Eloubeidi MA (2006) Relationship among hospital ERCP volume, length of stay, and technical outcomes. Gastrointest Endosc 64:338–347
Walter CJ, Al-Allak A, Borley N, Goodman A, Wheeler JM (2013) Fifth-year surveillance computed tomography scanning after potentially curative resections for colorectal cancer. Surgeon 11:25–29
Disclosures
Drs. Ji Min Choi, Changhyun Lee, Yoo Min Han, Minjong Lee, Young Hoon Choi, Dong Kee Jang, Jong Pil Im, Sang Gyun Kim, Joo Sung Kim, and Hyun Chae Jung have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, J.M., Lee, C., Han, Y.M. et al. Long-term oncologic outcomes of endoscopic stenting as a bridge to surgery for malignant colonic obstruction: comparison with emergency surgery. Surg Endosc 28, 2649–2655 (2014). https://doi.org/10.1007/s00464-014-3517-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3517-7