Abstract
Purpose
Since colorectal endoscopic submucosal dissection (ESD) requires higher-level skills than endoscopic mucosal resection (EMR), it is recommended to acquire sufficient experience in gastric ESD prior to attempting colorectal ESD. We evaluated the ability of experienced endoscopists with limited experience in gastric ESD to perform colorectal ESD.
Methods
We retrospectively reviewed 120 colorectal ESDs performed by two endoscopists who had expertise in colonoscopy and colorectal EMR but experience of fewer than five gastric ESDs. Main outcomes were the en bloc resection rate with tumor-free margins (R0 resection rate) and adverse events rate. Using only clinical characteristics prior to ESD, we also identified factors affecting outcomes.
Results
A total of 113 patients (94.2 %) received en bloc resection, and the R0 resection rate was 80.0 % (96/120). Perforation and postoperative hemorrhage occurred in eight (6.7 %) and two (1.7 %) patients, respectively. Dividing the 120 cases into three learning phases, R0 resection and perforation rates improved from 77.5 % (31/40) and 12.5 % (5/40) in phase 1 to 85.0 % (34/40) and 2.5 % (1/40) in phase 3, respectively. Multivariate analysis revealed that lesions at junctions (dentate line, sigmoid-descending junction, splenic flexure, hepatic flexure, ileocecal valve) and lesions with factors reflecting fibrosis in the submucosal layer (based on endoscopic findings before ESD) were significantly correlated with R0 resection failure, with adjusted odds ratios of 10.5 (95 % CI 2.1–67.6) and 10.4 (2.7–48.6), respectively.
Conclusions
Colorectal ESD is feasible for experienced endoscopists with limited experience in gastric ESD. Novices should avoid lesions at junctions or those with factors reflecting fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The efficacy of endoscopic submucosal dissection (ESD) for early gastric neoplasia has been established both in Japan and Western countries. However, ESD for colorectal neoplasia has yet to be recognized as a standard therapy. Colorectal ESD requires a higher level of endoscopic skill and is associated with a higher risk of adverse events than endoscopic mucosal resection (EMR) [1]. Several studies reported that the rate of perforation during colorectal ESD is higher than during EMR, even in referral centers [2, 3]. Therefore, sufficient experience in gastric ESD has been required prior to attempting colorectal ESD [4–7]. In fact, in previous reports on the efficacy of colorectal ESD [8–13], ESD procedures were probably performed by endoscopists with sufficient experience in gastric ESD. A step-up approach has also been recommended, starting with lesions presenting in the rectum or distal stomach, then colon, proximal stomach, and finally in the esophagus [14, 15]. However, since there are fewer early gastric neoplasias in Western countries compared to Japan [16], endoscopists not based in Japan may require a longer time to gain sufficient experience in gastric ESD.
In our previous study, we presented the feasibility and safety of colorectal ESD performed by novices in ESD [17]. Without sufficient experience in gastric ESD, colorectal ESD might still be introduced safely. Of course, in order to introduce it safely, endoscopists should have expertise in total colonoscopy and therapeutic endoscopy, such as colorectal EMR or endoscopic piecemeal mucosal resection (EPMR). They should also have acquired sufficient knowledge of the ESD procedure, served as assistants to senior endoscopists, and undergone training using animal models. Moreover, the colorectal ESD procedures should be supervised by senior endoscopists with sufficient experience in ESD.
Our previous study had some limitations. Firstly, the number of 20 cases for each endoscopist was too small to adequately elucidate the feasibility of ESD novices performing colorectal ESD. Secondly, we excluded lesions with fibrosis due to biopsy or peristalsis, sporadic localized lesions in chronic inflammation, and local residual carcinoma after EMR. Those lesions often show fibrosis in the submucosal layer, which may have affected the main outcome measurements. In fact, fibrosis in the submucosal layer has been reported to be a major factor preventing en bloc resection and correlated with perforation during colorectal ESD [18–21]. In addition, the grade of fibrosis in the submucosal layer was generally evaluated based on endoscopic findings obtained during ESD, because it is difficult to accurately predict the existence of fibrosis before ESD. However, in order to identify factors that can effectively predict treatment outcomes, we should include only factors available before the ESD procedure.
To evaluate the feasibility of colorectal ESD during the clinical learning curve, we retrospectively reviewed the results of all colorectal ESDs performed by endoscopists who had expertise in colonoscopy and colorectal EMR but had experience of fewer than five gastric ESDs. Using only clinical characteristics obtained before the ESD procedure, we also identified factors influencing treatment outcomes.
Materials and Methods
Colorectal ESD was performed by two endoscopists with experience of fewer than five gastric ESDs. A total of 120 cases, comprising each endoscopist’s first 60 ESDs, were retrospectively investigated. Details of the indication criteria, procedure, and training system for colorectal ESD, as well as study subjects, are presented below.
Indication criteria for colorectal ESD
The indications for colorectal ESD are as follows: (1) lesions difficult to remove en bloc with snare EMR, such as nongranular laterally spreading tumors (LSTs, particularly the pseudo-depressed type), lesions with a type VI pit pattern, and large lesions of the protruding type suspected to be carcinoma; (2) lesions with fibrosis due to biopsy or peristalsis; (3) sporadic localized lesions involving chronic inflammation such as ulcerative colitis; and (4) local residual carcinoma after EMR [4]. In our previous report [17], we excluded (2), (3), and (4) because of the high rate of fibrosis and risk of perforation, especially when colorectal ESD was performed by novices. However, in the present study, endoscopists performed all types of colorectal ESD after the initial 20 cases.
Procedure of colorectal ESD
Procedural details of colorectal ESD were previously described [8–13]. We described our methods of colorectal ESD and histological analysis in our previous report such as bowel preparation, endoscopes, knife type used, high-frequency generator, and fixing of resected specimens [17].
If the tumor was resected in one single mass, it was endoscopically judged as an en bloc resection. In the histological analysis, the grade of resection was also evaluated. If the tumor was resected en bloc endoscopically and the lateral and basal margins were free of tumor cells, it was defined as R0 (complete) resection. Other cases were defined as R1 (incomplete) or Rx (not evaluable) resection [8].
Training system of colorectal ESD
Both endoscopists had expertise in therapeutic endoscopy, performing a total of more than 2000 colonoscopies and more than 300 colorectal EMRs or endoscopic piecemeal mucosal resections (EPMRs). Prior to performing colorectal ESD, they had acquired sufficient knowledge of the ESD procedure, served as assistants to senior endoscopists in more than 20 ESDs, and had undergone training using animal models. During the same period, they had performed fewer than five gastric ESDs.
The colorectal ESD procedures were supervised by senior endoscopists with experience of more than 50 ESDs. The senior endoscopists assumed only verbal control of the procedure except for in cases of perforation. Therefore, the self-completion rate was almost the same as the rate without perforation.
Study subjects
Both endoscopists performed colorectal ESD at Tohoku University Hospital or Iwate Prefectural Isawa Hospital between July 2009 and November 2013. The first 60 consecutive ESDs of each endoscopist were evaluated retrospectively. In the same period, a total of 284 colorectal ESDs were performed in both hospitals. Written informed consent for the ESD procedure was obtained from all participants based on a protocol approved by the Committee for Clinical Investigation at Tohoku University Hospital or Iwate Prefectural Isawa Hospital.
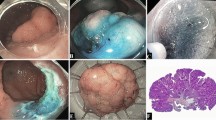
All tumors were more than 20 mm in diameter. The sites of tumors were divided into four groups: rectum, left colon (sigmoid colon and descending colon), right colon (transverse colon, ascending colon, and cecum), and junction. Tumors ranging over the dentate line, sigmoid-descending junction, splenic flexure, hepatic flexure, or ileocecal valve were defined as tumors at junctions. According to the Paris endoscopic classification [22], macroscopic types of tumors were classified as protruding (0-I) or nonprotruding and excavated (0-II). Type 0-II lesions were subdivided into slightly elevated (IIa) or depressed (IIc, IIa + IIc). IIa lesions greater than 20 mm in diameter were defined as “LSTs,” and these were then divided into the granular (LST-G) or nongranular (LST-NG) type [8–13, 23]. We defined lesions with factors reflecting fibrosis in the submucosal layer as lesions with a definite scar due to biopsy, lesions with three or more folds, sporadic localized lesions with ulcerative colitis, and local residual tumors after EMR are often accompanied by fibrosis in the submucosal layer (Fig. 1). In the present study, an endoscopist other than those who performed colorectal ESD independently judged the existence of factors reflecting fibrosis in the submucosal layer.
Factors reflecting fibrosis in the submucosal layer. Prior to performing colorectal ESD, it is very difficult to accurately predict whether or not lesions are accompanied by fibrosis in the submucosal layer. However, a lesions with a definite scar due to biopsy, b lesions with three or more folds, c sporadic localized lesions with ulcerative colitis (surrounded with many scars), and d local residual tumors after EMR are often accompanied by fibrosis in the submucosal layer
The main outcome of this study was the en bloc resection rate with tumor-free margins (R0 resection rate). To identify factors that can be used to predict the treatment outcome, we reviewed only clinical characteristics obtained before the ESD procedure, such as the site (rectum, left colon, right colon, or junction), macroscopic type (LST-G, LST-NG, depressed, or protruding), factors reflecting fibrosis, tumor size, and learning phase. The histological type (adenocarcinoma or adenoma) and existence of actual fibrosis in the submucosal layer were excluded. We also evaluated the rate of adverse events: perforation, postoperative hemorrhage, and others. Delayed postoperative hemorrhage was defined as clinical evidence of bleeding manifested by melena or hematochezia requiring endoscopic hemostasis within 0 to 14 days post-procedure [11].
Statistical analysis
The quantitative data are presented as the mean ± standard deviation (SD). All statistical analyses were performed using JMP version 11 (SAS Institute Inc., Cary, NC, USA). Differences among groups were evaluated using the chi-squared test or Fisher’s exact probability test, as appropriate. Among the clinical characteristics, factors influencing the main outcome were identified using a multiple logistic regression method. The level of significance was set at P < 0.05.
Results
Clinical characteristics
The 120 cases surveyed comprised 79 male (65.8 %) and 41 female (34.2 %) patients. The mean overall age was 68.4 ± 11.5 years. Tumors were located in the rectum, left colon, right colon, and junction (dentate line, SD junction, splenic flexure, hepatic flexure, and ileocecal valve) in 22 (18.3 %), 19 (15.8 %), 51 (42.6 %), and 28 (23.3 %) patients, respectively. The macroscopic type was divided into LST-G, LST-NG, depressed, and protruding in 56 (46.7 %), 52 (43.3 %), 4 (3.3 %), and 8 (6.7 %) patients, respectively. Based on endoscopic findings before ESD, 20 of the 120 patients showed factors reflecting fibrosis in the submucosal layer (Table 1).
Performance of colorectal ESD
Sufficient bowel preparation was performed in all cases before colorectal ESD. The Dual Knife and SB Knife Jr were used in 106 (88.3 %) and 14 (11.7 %) patients, respectively. The selection of knives was left to the judgments of endoscopists. Because of technical difficulty or fibrosis in the submucosal layer, 16 patients (13.3 %) required the use of another knife or snare.
After ESD, tumors were endoscopically resected en bloc in 113 patients. Therefore, the endoscopic en bloc resection rate was 94.2 % (113/120). The mean procedural time was 101.7 ± 65.9 min. Of the 120 patients, the procedural time was 90 min or longer in 60 (50.0 %). The mean tumor diameter was 38.5 ± 18.3 mm. Among the 120 patients, the tumor diameter was ≥40 mm in 45 (37.5 %) and <40 mm in the remaining 75 (62.5 %) (Table 2).
Histological analysis
Histological analysis showed that 59 (49.2 %) and 61 (50.8 %) patients had adenocarcinomas and adenomas, respectively. In the 59 with adenocarcinomas, 49 had intramucosal carcinomas and 10 had submucosal invasions. Of the ten patients with submucosal invasive adenocarcinomas, surgical resection with lymph node dissection was recommended in four because of the high risk of lymph node metastasis (lymphatic or venous invasion, massive submucosal invasion of more than 1000 μm). In these four patients, two underwent surgical resection. Remaining two patients declined surgical resection due to an advanced age (Table 2).
Although the lateral margins were tumor cell-free in all slices, the tumor was present in the first or end slice in 17 of the 113 patients resected en bloc endoscopically. In these patients, we could not judge perfectly whether lateral margins were negative. Therefore, the histological R0 resection rate was 80.0 % (96/120) (Table 2).
Adverse events
Perforation and postoperative hemorrhage occurred in eight (6.7 %) and two (1.7 %) patients, respectively. One patient with a past history of abdominal surgery showed symptoms of bowel obstruction 2 days after ESD. The rate of all ESD-induced adverse events was 9.2 % (11/120). All eight patients with perforation had colonic lesions (left colon, 1; right colon, 4; and junction, 3). Perforation was managed by endoscopic closure and conservative medical treatments with bowel rest and intravenous antibiotics. Both patients with delayed postoperative hemorrhage had rectal lesions and were treated by endoscopic hemostasis. Bowel obstruction was managed solely with bowel rest. No patient underwent surgery due to adverse events.
Clinical learning curve
We divided the total of 120 cases into three learning phases: phase 1, consisting of the first 40 cases; phase 2, consisting of the middle 40 cases; and phase 3, consisting of the last 40 cases. The en bloc resection rate was 92.5 % (37/40), 92.5 % (37/40), and 97.5 % (39/40) in phases 1, 2, and 3, respectively. Similarly, the R0 resection rate was 77.5 % (31/40), 77.5 % (31/40), and 85.0 % (34/40), respectively. The average speed of dissection was 11.0 ± 8.2, 10.1 ± 10.9, and 6.3 ± 3.7 min/cm2, respectively. The dissection speed in phase 3 was significantly faster than in the other two phases. On the other hand, the rate of perforation decreased from 12.5 % (5/40) in phase 1 to 5.0 % (2/40) in phase 2 and 2.5 % (1/40) in phase 3 (Fig. 2).
Clinical learning curve. We divided the total of 120 cases into three learning phases: phase 1, consisting of the first 40 cases; phase 2, consisting of the middle 40 cases; and phase 3, consisting of the last 40 cases. The en bloc resection and R0 resection rates in phase 3 have improved. The dissection speed in phase 3 was significantly faster than in the other two phases
Clinical characteristics affecting the main outcome
As above-mentioned, the endoscopic en bloc and histological R0 resection rates were 94.2 % (113/120) and 80.0 % (96/120), respectively. On univariate analyses using the clinical characteristics obtained before the ESD procedure (disease site, macroscopic type, factors reflecting fibrosis, tumor size, and learning phase), the R0 resection rate of lesions at junctions was significantly lower than at other sites. Lesions with factors reflecting fibrosis in the submucosal layer also showed a significantly lower R0 resection rate. There was no significant difference in the macroscopic type, tumor size, or learning phase.
Multivariate analysis revealed that lesions at junctions and those with factors reflecting fibrosis were significantly correlated with R0 resection failure, with adjusted odds ratios (ORs) of 10.5 (95 % CI 2.1 to 67.6) and 10.4 (2.7 to 48.6), respectively. No other factors had a significant influence on the R0 resection rate (Table 3).
Discussion
Differing from gastric ESD, colorectal ESD has yet to be recognized as a standard therapy both in Japan and Western countries. In endoscopic treatments for colorectal tumors, EMR has been established as a standard technique. On the other hand, colorectal ESD is associated with a higher risk of adverse events than EMR. Therefore, prior to attempting colorectal ESD, it is recommended to gain sufficient experience in gastric ESD. In the present study involving novices in colorectal ESD with limited experience in gastric ESD, the rates of perforation and postoperative hemorrhage (6.7 and 1.7 %, respectively) were not so high, even when compared with those in previous reports involving experts in gastric ESD [8–13]. In addition, the en bloc resection rate (94.2 %) was satisfactory. The R0 resection rate (80.0 %) will become more satisfactory and approach the en bloc resection rate by planning slightly larger lateral margins.
Of course, it goes without saying that not everyone can perform colorectal ESD. Endoscopists included in the present study had expertise in total colonoscopy and therapeutic endoscopy. They had also acquired sufficient knowledge of the ESD procedure, served as assistants to senior endoscopists, and undergone training using animal models. With these steps and expertise in total colonoscopy and colorectal EMR, they were able to perform colorectal ESD safely without sufficient experience in gastric ESD. Since there are fewer early gastric tumor patients in Western countries compared to Japan [16], endoscopists in Western countries may require a longer time to gain sufficient experience in gastric ESD. In such situation, the present study provides useful information. However, many experts recommend that novices accumulate experience of performing 20–50 supervised gastric ESDs prior to beginning colorectal ESD [6, 7, 24]. For novices in colorectal ESD, if a training system from gastric ESD to colorectal ESD has been established in their hospitals or universities, it may be desirable to gain some experience in gastric ESD.
With regard to the clinical learning curve, both the en bloc and R0 resection rates increased from phases 1 to 3. From phase 2, endoscopists performed colorectal ESD of all types including local residual tumors. That is why there were no differences in either rate between phases one and two. Conversely, the perforation rate gradually decreased and reached a satisfactory rate (2.5 %) in phase 3. All perforation cases were treated endoscopically, revealing the feasibility and safety of colorectal ESD during the clinical learning curve. However, in order to effectively and safely perform colorectal ESD, experience of about 40 cases may be required. On the other hand, the rate of delayed postoperative hemorrhage was low through all phases. Terasaki reported that lesions located in the rectum were significantly related to delayed bleeding after colorectal ESD [25].
To identify factors that accurately predict the treatment outcomes, we included only factors able to be obtained before the ESD procedure. As mentioned in “Introduction,” it is difficult to precisely predict the existence of fibrosis before ESD. Similarly, before the ESD procedure, we are not able to accurately predict the histological type, depth of submucosal invasion, or presence of lymphatic or venous invasion. Therefore, we excluded these factors in the analysis of factors influencing treatment outcomes.
Concerning the clinical characteristics (disease site, macroscopic type, factors associated with fibrosis, tumor size, and learning phase), multivariate analysis revealed that lesions at junctions were significantly correlated with R0 resection failure. Poor endoscopic operability is reported to be an important factor which influences treatment outcomes in many studies. However, operability is a very subjective factor and relates to various factors (disease site, paradoxical movement of the endoscope, adhesion, movement due to heart beat or deep breathing, etc.). In many factors, disease site is one of the most objective factors. In terms of disease site, compared with rectal lesions, colonic lesions are associated with poorer operability. Therefore, a training system for colorectal ESD from the rectum to colon has been recommended [7]. For the same reason, colorectal ESD for lesions in the right colon were also reported to be more difficult than in the left colon [12]. Lesions at junctions were also expected to exhibit poorer operability due to the narrow and angulated lumen [18]. In order to analyze disease site more in detail, we divided the site into four groups: rectum, left colon (sigmoid colon and descending colon), right colon (transverse colon, ascending colon, and cecum), and junction (dentate line, sigmoid-descending junction, splenic flexure, hepatic flexure, and ileocecal valve). However, disease sites judged in endoscopes might slightly differ from precise sites.
Lesions with factors reflecting fibrosis were also significantly correlated with R0 resection failure. Prior to performing colorectal ESD, it is very difficult to accurately predict whether or not lesions are accompanied by fibrosis in the submucosal layer. Therefore, we defined lesions with factors reflecting fibrosis as lesions accompanied by a definite scar due to biopsy, lesions with three or more folds, sporadic localized lesions with ulcerative colitis, and local residual tumors after EMR. Those lesions usually show moderate to severe fibrosis in the submucosal layer. However, against expectations, they could sometimes be resected easily because of only mild fibrosis. In the present study, we attached greater importance to the macroscopic view before ESD than to the actual grade of fibrosis in the submucosal layer.
The present study had some limitations. Firstly, we could not exclude selection bias in terms of the disease site or factors reflecting fibrosis. At the beginning of the learning curve, we may have avoided colorectal ESD for lesions with poor operability, and recommended surgery. Secondly, the grade of fibrosis predicted before the ESD procedure may have been different from the actual grade. It might be better for the presence or absence of fibrosis to be validated histologically. Thirdly, only two endoscopists were involved in the present study, and the supervisory and teaching skills of the senior endoscopists might have affected the treatment outcomes.
Without sufficient experience in gastric ESD, experts in total colonoscopy and colorectal EMR may still be able to safely perform colorectal ESD. We hope that the present study will be useful for endoscopists who have expertise in total colonoscopy and colorectal EMR, but belong to institutions without well-established training systems from gastric to colorectal ESD.
References
Repici A, Hassan C, De Paula PD, Pagano N, Arezzo A, Zullo A, Lorenzetti R, Marmo R (2012) Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy 44(2):137–150. doi:10.1055/s-0031-1291448
Taku K, Sano Y, Fu KI, Saito Y, Matsuda T, Uraoka T, Yoshino T, Yamaguchi Y, Fujita M, Hattori S, Ishikawa T, Saito D, Fujii T, Kaneko E, Yoshida S (2007) Iatrogenic perforation associated with therapeutic colonoscopy: a multicenter study in Japan. J Gastroenterol Hepatol 22(9):1409–1414. doi:10.1111/j.1440-1746.2007.05022.x
Oka S, Tanaka S, Kanao H, Ishikawa H, Watanabe T, Igarashi M, Saito Y, Ikematsu H, Kobayashi K, Inoue Y, Yahagi N, Tsuda S, Simizu S, Iishi H, Yamano H, Kudo SE, Tsuruta O, Tamura S, Saito Y, Cho E, Fujii T, Sano Y, Nakamura H, Sugihara K, Muto T (2010) Current status in the occurrence of postoperative bleeding, perforation and residual/local recurrence during colonoscopic treatment in Japan. Dig Endosc: Official journal of the Japan Gastroenterological Endoscopy Society 22(4):376–380. doi:10.1111/j.1443-1661.2010.01016.x
Tanaka S, Oka S, Chayama K (2008) Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol 43(9):641–651. doi:10.1007/s00535-008-2223-4
Yoshida N, Wakabayashi N, Kanemasa K, Sumida Y, Hasegawa D, Inoue K, Morimoto Y, Kashiwa A, Konishi H, Yagi N, Naito Y, Yanagisawa A, Yoshikawa T (2009) Endoscopic submucosal dissection for colorectal tumors: technical difficulties and rate of perforation. Endoscopy 41(9):758–761. doi:10.1055/s-0029-1215028
Hotta K, Oyama T, Shinohara T, Miyata Y, Takahashi A, Kitamura Y, Tomori A (2010) Learning curve for endoscopic submucosal dissection of large colorectal tumors. Dig Endoscopy: official journal of the Japan Gastroenterological Endoscopy Society 22(4):302–306. doi:10.1111/j.1443-1661.2010.01005.x
Niimi K, Fujishiro M, Goto O, Kodashima S, Koike K (2012) Safety and efficacy of colorectal endoscopic submucosal dissection by the trainee endoscopists. Dig Endosc: official journal of the Japan Gastroenterological Endoscopy Society 24(Suppl 1):154–158. doi:10.1111/j.1443-1661.2012.01251.x
Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M (2007) Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol: the official clinical practice journal of the American Gastroenterological Association 5(6):678–683. doi:10.1016/j.cgh.2007.01.006, quiz 645
Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, Kogure E, Liu Y, Uemura N, Saito K (2007) Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy 39(5):418–422. doi:10.1055/s-2007-966427
Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K (2007) Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointestinal endoscopy 66(1):100–107. doi:10.1016/j.gie.2007.02.032
Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D (2007) Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointestinal endoscopy 66(5):966–973. doi:10.1016/j.gie.2007.02.053
Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S (2009) Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 41(8):679–683. doi:10.1055/s-0029-1214979
Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, Yoshida S, Ikehara H, Otake Y, Nakajima T, Matsuda T, Saito D (2010) A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 72(6):1217–1225. doi:10.1016/j.gie.2010.08.004
Deprez PH, Bergman JJ, Meisner S, Ponchon T, Repici A, Dinis-Ribeiro M, Haringsma J (2010) Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy 42(10):853–858. doi:10.1055/s-0030-1255563
Iacopini F, Bella A, Costamagna G, Gotoda T, Saito Y, Elisei W, Grossi C, Rigato P, Scozzarro A (2012) Stepwise training in rectal and colonic endoscopic submucosal dissection with differentiated learning curves. Gastrointest Endosc 76(6):1188–1196. doi:10.1016/j.gie.2012.08.024
Suzuki H, Gotoda T, Sasako M, Saito D (2006) Detection of early gastric cancer: misunderstanding the role of mass screening. Gastric Cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 9(4):315–319. doi:10.1007/s10120-006-0399-y
Shiga H, Endo K, Kuroha M, Kakuta Y, Takahashi S, Kinouchi Y, Shimosegawa T (2014) Endoscopic submucosal dissection for colorectal neoplasia during the clinical learning curve. Surg Endosc 28(7):2120–2128. doi:10.1007/s00464-014-3443-8
Hori K, Uraoka T, Harada K, Higashi R, Kawahara Y, Okada H, Ramberan H, Yahagi N, Yamamoto K (2014) Predictive factors for technically difficult endoscopic submucosal dissection in the colorectum. Endoscopy 46(10):862–870. doi:10.1055/s-0034-1377205
Hayashi N, Tanaka S, Nishiyama S, Terasaki M, Nakadoi K, Oka S, Yoshihara M, Chayama K (2014) Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 79(3):427–435. doi:10.1016/j.gie.2013.09.014
Mizushima T, Kato M, Iwanaga I, Sato F, Kubo K, Ehira N, Uebayashi M, Ono S, Nakagawa M, Mabe K, Shimizu Y, Sakamoto N (2014) Technical difficulty according to location, and risk factors for perforation, in endoscopic submucosal dissection of colorectal tumors. Surgical endoscopy. doi:10.1007/s00464-014-3665-9
Sato K, Ito S, Kitagawa T, Kato M, Tominaga K, Suzuki T, Maetani I (2014) Factors affecting the technical difficulty and clinical outcome of endoscopic submucosal dissection for colorectal tumors. Surgical endoscopy. doi:10.1007/s00464-014-3558-y
Workshop. PitP (2003) The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon. Gastrointest Endosc 58(6 Suppl):S3–43
Tanaka S, Tamegai Y, Tsuda S, Saito Y, Yahagi N, Yamano HO (2010) Multicenter questionnaire survey on the current situation of colorectal endoscopic submucosal dissection in Japan. Dig Endosc: official journal of the Japan Gastroenterological Endoscopy Society 22(Suppl 1):S2–8. doi:10.1111/j.1443-1661.2010.00952.x
Probst A, Golger D, Anthuber M, Markl B, Messmann H (2012) Endoscopic submucosal dissection in large sessile lesions of the rectosigmoid: learning curve in a European center. Endoscopy 44(7):660–667. doi:10.1055/s-0032-1309403
Terasaki M, Tanaka S, Shigita K, Asayama N, Nishiyama S, Hayashi N, Nakadoi K, Oka S, Chayama K (2014) Risk factors for delayed bleeding after endoscopic submucosal dissection for colorectal neoplasms. Int J Colorectal Dis 29(7):877–882. doi:10.1007/s00384-014-1901-3
Conflict of interests
Hisashi Shiga, Masatake Kuroha, Katsuya Endo, Tomoya Kimura, Yoichi Kakuta, Yoshitaka Kinouchi, Shoichi Kayaba, and Tooru Shimosegawa have no conflicts of interest or financial relationships to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiga, H., Kuroha, M., Endo, K. et al. Colorectal endoscopic submucosal dissection (ESD) performed by experienced endoscopists with limited experience in gastric ESD. Int J Colorectal Dis 30, 1645–1652 (2015). https://doi.org/10.1007/s00384-015-2334-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-015-2334-3