Abstract
Purpose
Split cord malformation (SCM) presenting concomitant with spinal teratoma without any open spinal dysraphism has rarely been reported in the literature. We aimed to make a systematic review and qualitative analysis of the literature about the topic and present the first case of SCM concomitant with spinal teratoma harboring papillary thyroid carcinoma (PTC) component.
Methods
Two big search tools (Pubmed/MEDLINE) and Scopus were used. The search strategy was compatible to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). An exemplary case of ours was also presented.
Results
There were 30 patients (15 pediatric and 15 adult). Female and male distribution was even. Median age of the patients was 18 years (range = 0–66 years). The most common presenting symptoms were back pain and lower limb weakness. Spinal teratoma and SCM mostly presented at thoracic/thoracolumbar region in children and lumbar region in adults. Surgical outcome was better in the children compared to the adults.
Conclusion
Thoracolumbar region is the most common location for such entity in children, whereas lumbar region for the adults. Surgical resection should be done as much as possible under neuromonitorization. The resected material should be evaluated thoroughly not to miss any malign pathology. Surgical outcome is better when it is done at an early age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Split cord malformations (SCMs) are congenital spinal cord malformations that bear cleft within the spinal cord together with bony or soft tissue spicules arising from the spine [1]. There are two types of SCMs: type I, divided hemicords via an osteocartilaginous septum; type II, divided hemicords via fibrous septum. Hemicords reside in two separate dura and one uniform dura in type I and type II SCMs, respectively [2].
Split cord malformations are rare and have been reported in 1 of every 5000 live births [2,3,4]. They have commonly been reported together with thickened filum terminale, low lying spinal cord, syringomyelia, and kyphoscoliosis. Rarely, SCMs were observed also with lipoma, neuroenteric cyst, dermoid cyst, and dermal sinus tract [2, 5, 6]. Concomitant presentation of SCMs with spinal teratomas without open spinal dysraphism has rarely been reported in the literature [7]. However, there is no one paper dealing with large case series including both pediatric and adult patients giving descriptive statistics of the pathology. We presented an exemplary case of papillary thyroid carcinoma (PTC) residing within spinal teratoma concomitant with SCM. To the best of our knowledge, this phenomenon has never been reported in the literature. We aimed to grab attention of neurosurgeons, dealing with congenital malformations of spine and spinal cord, to such a rare entity with a systematic review and qualitative analysis of the literature.
Materials and methods
The present study is a systematic review and qualitative analysis of the literature. We used two big search tools (Pubmed/MEDLINE) and Scopus. The search was conducted through a timeline including inception of the relevant databases and up to April 2022. The search strategy was compatible to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Fig. 1) [8]. The search protocol included the following terms: “split cord malformation” OR “diastematomyelia” and “spinal teratoma.”
A total of 643 citations were reached up following database search. Of those 643 citations, 310 were eliminated as they were duplicate ones. In the present systematic review, we aimed to work on isolated SCM cases presented concomitant with spinal teratoma without any associated open spinal dysraphism (myelomeningocele, meningocele, lipomyelomeningocele, lipomeningocele). We excluded any cases of SCM presented with other kinds of spinal tumors (epidermod, dermoid, etc.) if there was no concomitant spinal teratoma. References of relevant citations were screened for any relevant, yet missed, citation through database search.
There were 21 citations about SCM presented concomitant with spinal teratoma, yet without open spinal dyraphism. Of those 21 citations, 9 (41%) were about pediatric, 11 (50%) were about adult, and 1 (9%) was about both pediatric and adult patients. We included one adult case of ours to the final qualitative analysis (Tables 1 and 2). Continuous and dichotomous variables were presented as median (with range) and absolute (with percentage) values. Fisher’s exact test was used to make comparisons between dichotomous variables. Excel 2021 (for Mac, Microsoft, Redmond, WA, USA) was used for descriptive and qualitative statistics. An alpha value of < 0.05 was accepted as statistically significant.
Results
There were 15 (50%) pediatric and 15 (50%) adult patients in the final analysis. Age and gender were unclear for one pediatric patient. Median age of all the patients, pediatric, and adult patients were 18 years (range = 0–66 years), 8.5 years (range = 0–17 years), and 34 years (range = 18–66 years), respectively. There were 7 pediatric male (50%) and 7 pediatric female (50%) patients. There were 8 adult male (53%) and 7 adult female (47%) patients. Back pain, lower limb weakness and/or paresthesia, back mass, kyphosis, scoliosis, lower limb deformity, constipation, fecal incontinence, urinary problems, hypertrichosis at back, radiculopathy, neurogenic claudication, and sexual dysfunction were the main signs and symptoms reported in those patients with SCM concomitant with spinal teratoma. Back pain and lower limb weakness were the most common presenting findings.
Spinal teratomas presented mostly at thoracic region or thoracolumbar junction in pediatric patients (n = 12, 80%), whereas at lumbar region (n = 10, 66%) in adult patients. The difference between pediatric and adult patients in respect of teratoma location within the spine was statistically significant (p = 0.03). Intramedullary location was the most prevalent location for spinal teratomas (n = 17, 57%). Split cord malformation was mostly diagnosed at thoracic or thoracolumbar region (n = 7, 70%) in pediatric patients, whereas at lumbar region (n = 10, 91%) in adult patients. The difference in location of SCM between pediatric and adult patients was statistically significant (p = 0.008).
Treatment modality was unclear in 3 (10%) patients. One (3%) patient denied surgery. Outcome was unknown for 12 patients (40%). Three pediatric patients (20% of pediatric and 10% of all patients) died due to concomitant systematic diseases. Complete recovery was possible in all remaining pediatric patients (n = 4/7, 57%), whereas only 4/11 (27%) of the adult patients could manage to recover fully following the surgeries (p = 0.63) (Tables 1 and 2).
Exemplary case
A 34-year-old woman applied to our outpatient clinic with recent complaints of left hip pain and right foot weakness for the last 8 months. She began to feel weakness in her left foot, too. She had no urinary or defecation problem. She had motor deficits in her lower extremity most pronounced at her right side. Left S1 dermatomal region was hypoesthetic. Her lower extremity deep tendon reflexes were bilaterally hyperactive. There were no skin stigmata on the patient back.
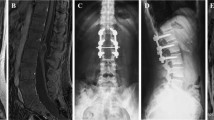
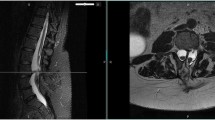
On radiological examination, we observed SCM beginning at lower thoracic level. There was a bony septum at the L2–L3 levels. There was intradural lipoma infiltrating right hemicord, extending down to the back between the L3 and S1 levels. A homogenously enhanced isolated mass lesion with a dimension of 2 × 3.5 cm was observed within the defined area of interest (Fig. 2).
The patient was operated under surveillance of neuromonitorization. Laminectomies of L2 and L3 vertebrae were accomplished. Two hemicords splitted with a bony spur located between the L2 and L3 levels were observed. The bony spur was resected and durotomy of both hemicords was done to revise the dura as a whole piece. Fibrous bands tethering the spinal cord were released. Lipoma was observed as infiltrating posterior and medial aspects of the right hemicord. It was resected as much as possible leaving a thin remnant tissue, under guidance of neuromonitorization. Another mass lesion was arising from the right hemicord and filling whole spinal canal and pressing over the neural tissues. It was a paste-like, sandy colored mass and it was resected totally. The dura was closed primarily in water-tight fashion. There was no abnormal signal or lost signal on neuromonitorization. She had no additional deficit following the surgery and she partly recovered under supervision of physical therapy sessions in 1 year.
Pathology confirmed the intradural intramedullary and extradural lipomatous mass lesion as benign fiprolipomatous tissue. The second biopsied, intramedullary lesion was observed to be a teratomatous lesion consisting of mature adipose tissue, fibrous tissue, and some glandular tissue resembling thyroid follicles, some of which lined with non-specific columnar epithelium. Morphological and immunohistochemistry analysis confirmed the second mass lesion as papillary thyroid carcinoma developed in teratoma (Figs. 3, 4, 5 and 6). The patient was scanned with positron emission tomography (PET), and no primary or metastatic lesion of PTC was found. The patient was consulted to oncology and endocrinology departments and put on close surveillance for any recurrence of the disease. The patient was free of cancer at her 1st year control.
Benign seromucinous glandular structures (arrows) and mature adipose cells (A) (A, H&E × 40 magnification), partially squamous cell-like structured epithelial cell lining of the benign cyst (B, H&E × 100 magnification), benign squamous glands (multiple thin arrows) and squamous epithelial cell clusters (thick arrow) of the cyst wall (C, H&E × 100 magnification), and papillary tumor (PCa) showing growing pattern within the cyst (D, H&E × 40 magnification)
Papillary tumor proliferation supported by fibrovascular stroma (A, H&E × 200 magnification), tumor part showing cystic-solid growing pattern within the cyst wall (B, H&E × 200 magnification), atypical epithelial cells with vesicular nucleus showing overlapping during tumoral proliferation (C, H&E × 400 magnification), and cytokeratin 19 positivity of the tumor (D, IHC × 100 magnification)
Discussion
Split cord malformation or diastematomyelia is a congenital spinal cord malformation with two hemicords, splitted into two by a bony or fibrous tissue. The SCMs are classified as SCM-I or SCM-II depending on what type of tissue splitted the spinal cord: bony spur in SCM-I and fibrous band in SCM-II [2]. Split cord malformation presents early in human life, yet some rare cases presented in adults, even in elderly [29]. Traction on the conus determines the age of onset of symptoms in patients with SCM as a consequence of repetitive movements due to acute trauma or the aging process [29, 30].
Spinal cord lipomas are progressive congenital disorders of the spinal cord [31]. Asymptomatic lipomas have a likelihood of 33–40% to deteriorate over 9–10 years [32, 33]. Surgery, even in asymptomatic subjects, should be aimed to resect spinal cord lipomas as much as possible [31]. There are subtypes of spinal cord lipomas depending on how they develop during embryogenesis: dorsal lipoma, transitional lipoma, terminal lipoma, and chaotic lipoma [31]. Details of each lipoma type are beyond the scope of the present study. Nevertheless, the one presented in the present case was dorsal lipoma, which develops if a dorsal defect develops in the dura and neural tube during normal ascent of spinal cord in embryogenesis phase. The surrounding mesenchyme invades the defect and forms a fibrofatty stalk that attaches to the sliding neural tube and entraps it [31]. It is a mistimed disjunction happening during primary neurulation [34]. This pathogenesis would also explain concomitant teratoma development in the present case. The ependymal lining of the neural tube might induce the invading mesenchyme to form fat, muscles, collagen, cartilage, and bone [31]. Another possible theory for pathogenesis of spinal lipoma will be explained in the forthcoming paragraphs.
Teratomas are neoplasms of multipotential cells of all three germ cell layers with power of autonomous growth [35]. Spinal teratoma, except sacrococcygeal one, is very rare (0.2–0.5% of all spinal cord tumors and 2% of all central nervous system teratomas) [35]. They mostly present at thoracolumbar segment of the spine. Adult-onset presentation is rare and mostly observed in men in their 4th and 5th decades of life [35]. The most common presentation is back pain, limb weakness, and bowel/bladder disturbance with gradual symptom onset. Back pain and lower limb weakness were the two most common presenting symptoms in the present series. They could present intramedullary, extramedullary, or extradurally [17, 36,37,38,39]. Intramedullary area was the most pronounced location of the spinal teratomas presented concomitant with SCM without any open spinal dysraphism.
Spinal teratomas have diverse imaging patterns on MRI due to heterogenous cell content. Generally, spinal teratomas appear as hyperintense lesions on T1-weighted MRI sequences because of their fat content. However, lesions with hypointense or isointense T1-weighted imaging properties have also been reported [35]. Concomitant congenital anomalies have been reported together with spinal teratomas [40]. Those dysraphic spinal lesions could be spina bifida, myelomeningocele, dermal sinus, tethered cord syndrome, and split cord malformation (9.6% of all spinal teratomas) [35, 41, 42]. Concomitant presentation of SCM with spinal teratoma without any open spinal dysraphism is rare, there have been thirty cases including the present case [7, 9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Split cord malformations are more pronounced in female gender (specifically for SCM type I) [18]. Female and male distribution was even in the present case series. Spinal teratomas were observed at any spine level, yet with an important difference between pediatric and adult patients. They presented at thoracic or thoracolumbar region in pediatric patients, whereas at lumbar region in adult patients, same as SCM location in those patients. Lumbar location of SCM with spinal teratoma might have caused delay in diagnosis of those adult cases.
Lellouch-Tubiana et al. [43] analyzed pathology specimens of 234 patients with former diagnosis of intraspinal lipoma. They found an interesting fact that those lipomas had contained foci of endodermal, mesodermal, and ectodermal origin, depicting a teratomatous component. This could explain concomitant presentation of teratoma within lipoma bed in the exemplary case. Spinal teratomas emerging concomitant with spinal dysraphic lesions have been presumed to have a dysembryogenic origin as other tumors (such as medulloblastoma) that occur in the midline [12]. This dysembryogenic theory is interrelated with pathogenesis of SCM: SCMs occur secondary to ontogenetic error happening during closure of primitive neuroenteric canal. An accessory neuroenteric canal emerges through trilaminar embryonic disc. It connects the amniotic cavity and the yolk sac. This neuroenteric canal ends up with split notochord and split neural plate. Endodermal and mesenchymal cells line up inside the neuroenteric canal. If endodermal cells survive up to the birth, then neuroenteric cysts would emerge. If mesenchymal cells survive, which have pluripotential capacity, then spinal teratomas would appear. If the neuroenteric canal has ectodermal connection, then congenital dermal sinus would be present at birth [44,45,46]. It should be kept in mind that spinal teratomas could present at remote side from the congenital spinal malformation and all spine should be scanned in such scenario [35]. Complete resection of spinal teratoma is warranted with attention given to possible intimate relation with spinal cord parenchyma [47]. Malignant transformation of intramedullary teratoma concomitant with SCM has rarely been reported in the literature that would necessitate complete resection of the tumor as much as possible under intraoperative electrophysiologic monitoring [9, 24].
Papillary thyroid carcinoma is one of the subtypes of differentiated thyroid carcinoma [48]. It composes the most common variant (> 80%) of all thyroid malignancies [49]. Surgery is the main treatment body with adjuvant therapies depending on histological and genetic properties of the tumor [48]. Metastasis of PTC is mostly local to the regional lymph nodes, with rare occurrence of metastasis to distal sites (5–7% of the cases) [50]. In the exemplary case, no primary or any other metastatic site for PTC was depicted on PET scan. The tumor was a teratocarcinoma rather than a metastatic one, similar to the case of Wang et al. [42], who presented carcinoid tumor in a lumbar teratoma associated with tethered cord syndrome in an adult. Presence of PTC within teratoma was formerly reported in an ovarian mass lesion of a 34-year-old woman [51]. They analyzed her thyroid gland and detected some nodules, which turned to be benign thyroid nodule following fine needle aspiration. This is the second case of PTC presenting in a teratoma, and the first case presented within a spinal teratoma. Thyroid gland and full body surveillance should be conducted whenever such or similar case is diagnosed, before acceptance of the lesion is a primary component of teratoma. Endocrine tumors arising within teratomas, such as in the present case, are presumed to originate from neuroendocrine cells of the respiratory or gastrointestinal epithelium that are parts of teratomatous neoplasms [42].
Conclusion
Concomitant presentation of SCM with spinal cord lipoma and teratoma including papillary thyroid carcinoma has not been reported until now, to the best of our knowledge. Such variable presentation of congenital spinal malformations is rare but not absent in the literature. Thoracolumbar region is the most common location for such entity in children, whereas lumbar region for the adults. Surgical resection should be done as much as possible under neuromonitorization. The resected material should be evaluated thoroughly not to miss any malign pathology. Surgical outcome is better when it is done at an early age.
References
Mishra A, Nadeem M, Prabhuraj AR, Paul P, Bhat D (2021) Tetrad of split cord malformation I with neuroenteric cyst, dermoid cyst, and thickened filum terminale in a 2-year-old child: a case report. Pediatr Neurosurg 56:448–454
Pang D, Dias MS, Ahab-Barmada M (1992) Split cord malformation: part I: a unified theory of embryogenesis for double spinal cord malformations. Neurosurgery 31:451–480
Bademci G, Saygun M, Batay F, Cakmak A, Basar H, Anbarci H, Unal B (2006) Prevalence of primary tethered cord syndrome associated with occult spinal dysraphism in primary school children in Turkey. Pediatr Neurosurg 42:4–13
Rajpal S, Salamat MS, Tubbs RS, Kelly DR, Oakes WJ, Iskandar BJ (2007) Tethering tracts in spina bifida occulta: revisiting an established nomenclature. J Neurosurg Spine 7:315–322
Mahapatra AK, Gupta DK (2005) Split cord malformations: a clinical study of 254 patients and a proposal for a new clinical-imaging classification. J Neurosurg 103:531–536
Sinha S, Agarwal D, Mahapatra AK (2006) Split cord malformations: an experience of 203 cases. Childs Nerv Syst 22:3–7
Babu R, Reynolds R, Moreno JR, Cummings TJ, Bagley CA (2014) Concurrent split cord malformation and teratoma: dysembryology, presentation, and treatment. J Clin Neurosci 21:212–216
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Lemmen LJ, Wilson CM (1951) Intramedullary malignant teratoma of the spinal cord; report of a case. AMA Arch Neurol Psychiatry 66:61–68
Ugarte N, Gonzalez-Crussi F, Sotelo-Avila C (1980) Diastematomyelia associated with teratomas. Report of two cases. J Neurosurg 53:720–725
Ersahin Y, Mutluer S, Kocaman S, Demirtas E (1998) Split spinal cord malformations in children. J Neurosurg 88:57–65
Koen JL, McLendon RE, George TM (1998) Intradural spinal teratoma: evidence for a dysembryogenic origin. Report of four cases. J Neurosurg 89:844–851
Jarmundowicz W, Tabakow P, Markowska-Woyciechowska A (2004) Composite split cord malformation coexisting with spinal cord teratoma—case report and review of the literature. Folia Neuropathol 42:55–57
Uzum N, Dursun A, Baykaner K, Kurt G (2005) Split-cord malformation and tethered cord associated with immature teratoma. Childs Nerv Syst 21:77–80
Suri A, Ahmad FU, Mahapatra AK, Mehta VS, Sharma MC, Gupta V (2006) Mediastinal extension of an intradural teratoma in a patient with split cord malformation: case report and review of literature. Childs Nerv Syst 22:444–449
Ye G, Li D (2008) Congenital scoliosis associated with coexistent split cord malformation (SCM) and intramedullary teratoma: report of a case. J Musculoskelet Res 11:15–19
Sharma MC, Jain D, Sarkar C, Suri V, Garg A, Singh M, Mahapatra AK, Sharma BS (2009) Spinal teratomas: a clinico-pathological study of 27 patients. Acta Neurochir (Wien) 151:245–252; discussion 252
Ersahin Y (2013) Split cord malformation types I and II: a personal series of 131 patients. Childs Nerv Syst 29:1515–1526
Rosenbaum TJ, Soule EH, Onofrio BM (1978) Teratomatous cyst of the spinal canal. Case report J Neurosurg 49:292–297
Garza-Mercado R (1983) Diastematomyelia and intramedullary epidermoid spinal cord tumor combined with extradural teratoma in an adult. Case report J Neurosurg 58:954–958
Conti P, Conti R, De Luca G (1984) Observations on some rare cases of vertebro-medullar malformations associated with tumors. J Neurosurg Sci 28:81–87
Elmaci I, Dagcinar A, Ozgen S, Ekinci G, Pamir MN (2001) Diastematomyelia and spinal teratoma in an adult. Case report. Neurosurg Focus 10:ecp2
Tsitsopoulos P, Rizos C, Isaakidis D, Liapi G, Zymaris S (2006) Coexistence of spinal intramedullary teratoma and diastematomyelia in an adult. Spinal Cord 44:632–635
Mut M, Shaffrey ME, Bourne TD, Jagannathan J, Shaffrey CI (2007) Unusual presentation of an adult intramedullary spinal teratoma with diplomyelia. Surg Neurol 67:190–194
Conti P, Tenenbaum R, Capozza M, Mouchaty H, Conti R (2010) Diastematomyelia and tumor in adults: report of two cases and literature review. Spine (Phila Pa 1976) 35:E1438–1443
Maiti TK, Bhat DI, Devi BI, Sampath S, Mahadevan A, Shankar SK (2010) Teratoma in split cord malformation: an unusual association: a report of two cases with a review of the literature. Pediatr Neurosurg 46:238–241
Kafadar C, Incedayi M, Sildiroglu O, Sonmez G (2016) Intradural extramedullary teratoma coexisting with multiple spinal anomalies in an adult. Spine J 16:e389-390
Ge CY, Hao DJ, Shan LQ (2020) Rare bony diastematomyelia associated with intraspinal teratoma. World Neurosurg 133:185–187
Maebe H, Viaene A, De Muynck M (2018) Diastematomyelia and late onset presentation: a case report of a 72-year-old woman. Eur J Phys Rehabil Med 54:618–621
Prasad VS, Sengar RL, Sahu BP, Immaneni D (1995) Diastematomyelia in adults. Modern imaging and operative treatment. Clin Imaging 19:270–274
Pang D (2019) Surgical management of complex spinal cord lipomas: how, why, and when to operate. A review J Neurosurg Pediatr 23:537–556
Kulkarni AV, Pierre-Kahn A, Zerah M (2004) Conservative management of asymptomatic spinal lipomas of the conus. Neurosurgery 54:868–873; discussion 873–865
Wykes V, Desai D, Thompson DN (2012) Asymptomatic lumbosacral lipomas—a natural history study. Childs Nerv Syst 28:1731–1739
Hillman J, Bynke O (1992) Description of two informative cases of occult spinal dysraphism with remarks on possible traits in the embryogenesis. Childs Nerv Syst 8:211–214
Prasad GL, Divya S (2020) A comprehensive review of adult onset spinal teratomas: analysis of factors related to outcomes and recurrences. Eur Spine J 29:221–237
Li Y, Yang B, Song L, Yan D (2013) Mature teratoma of the spinal cord in adults: an unusual case. Oncol Lett 6:942–946
Poeze M, Herpers MJ, Tjandra B, Freling G, Beuls EA (1999) Intramedullary spinal teratoma presenting with urinary retention: case report and review of the literature. Neurosurgery 45:379–385
Kalani MY, Iyer S, Coons SW, Smith KA (2012) Spinal intradural teratomas: developmental programs gone awry? Neurosurg Focus 33:E1
Park SC, Kim KJ, Wang KC, Choe G, Kim HJ (2010) Spinal epidural teratoma: review of spinal teratoma with consideration on the pathogenesis: case report. Neurosurgery 67:E1818-1825
Makary R, Wolfson D, Dasilva V, Mohammadi A, Shuja S (2007) Intramedullary mature teratoma of the cervical spinal cord at C1–2 associated with occult spinal dysraphism in an adult. Case report and review of the literature. J Neurosurg Spine 6:579–584
Ebner FH, Roser F, Acioly MA, Schoeber W, Tatagiba M (2009) Intramedullary lesions of the conus medullaris: differential diagnosis and surgical management. Neurosurg Rev 32:287–300; discussion 300–281
Wang H, Yang Y, Liao S, Zheng J, McNutt MA, Yu T, Wang S, Liu C (2009) Carcinoid tumour in a lumbar teratoma associated with tethered cord syndrome in an adult. Br J Neurosurg 23:199–202
Lellouch-Tubiana A, Zerah M, Catala M, Brousse N, Kahn AP (1999) Congenital intraspinal lipomas: histological analysis of 234 cases and review of the literature. Pediatr Dev Pathol 2:346–352
Muthukumar N (2003) Split cord malformation and cystic teratoma masquerading as lipomeningomyelocele. Childs Nerv Syst 19:46–49
Muthukumar N, Arunthathi J, Sundar V (2000) Split cord malformation and neurenteric cyst—case report and a theory of embryogenesis. Br J Neurosurg 14:488–492
Munoz Montoya JE, Jara MA, Vargas Osorio MP, Franco FR (2020) Dermal sinus tract associated with type I and type II split cord malformation. Asian J Neurosurg 15:172–175
Wan W, Yang C, Yan W, Liu T, Yang X, Song D, Xiao J (2017) Adult-onset intradural spinal teratoma: report of 18 consecutive cases and outcomes in a single center. Eur Spine J 26:1917–1928
Cipriani NA (2019) Prognostic parameters in differentiated thyroid carcinomas. Surg Pathol Clin 12:883–900
Giordano TJ (2018) Genomic hallmarks of thyroid neoplasia. Annu Rev Pathol 13:141–162
Cho M, Acosta-Gonzalez G, Brandler TC, Basu A, Wei XJ, Simms A (2019) Papillary thyroid carcinoma metastatic to the pancreas: case report. Diagn Cytopathol 47:214–217
Pineyro MM, Pereda J, Schou P, de Los SK, de la Pena S, Caserta B, Pisabarro R (2017) Papillary thyroid microcarcinoma arising within a mature ovarian teratoma: case report and review of the literature. Clin Med Insights Endocrinol Diabetes 10:1179551417712521
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest related with the current study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hazneci, J., Bastacı, F., Börekci, A. et al. Split cord malformation concomitant with spinal teratoma without open spinal dysraphism. Childs Nerv Syst 38, 1977–1986 (2022). https://doi.org/10.1007/s00381-022-05578-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-022-05578-5