Abstract
Objective
The aim of this study was to analyze the role of endoscopic third ventriculostomy (ETV) in the treatment of pediatric chronic communicating congenital hydrocephalus (CCCH).
Material and methods
This retrospective study comprised a series of 11 children with CCCH treated with ETV. Data were recorded on gender, history, presenting symptoms, age at surgery, complications during surgery, clinical evolution, ETV survival, and follow-up period. Radiological variables including ventricular and cephalic diameters were also recorded to determine a series of ventricular indexes in magnetic resonance imaging (MRI) before and after the ETV procedure. The procedure was considered to be successful when there was clinical stability or improvement accompanied by a reduction in the radiological indexes in the postoperative control images, such that there was no need to place an extrathecal cerebrospinal fluid shunt.
Results
Over a mean follow-up period of 35.8 months (range: 6–108 months) from the ETV procedure, three patients required shunt placement; one of these was due to early failure in an 8-month old girl, the only patient younger than 12 months in our series. The radiological indexes were reduced in all patients except for one of the cases of ETV failure. The mean ETV survival among the successful cases was 32.1 months (range: 6–108 months), whilst that of the failed cases was 16 months (range: 6–108 months).
Conclusion
Although studies with larger sample sizes are needed, ETV appears to be a promising option for the treatment of this type of patient with CCCH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endoscopic third ventriculostomy (ETV) is considered the treatment of choice for patients with non-communicating hypertensive hydrocephalus [1,2,3], though controversy exists for its use in other indications, in particular chronic communicating hydrocephalus [4,5,6,7,8,9,10,11].
Two problems exist concerning the use ETV in chronic hydrocephalus. The first relates to the prediction of success, given that the prediction scales developed to date are only for use in hypertensive hydrocephalus [7]. The second problem is the assessment of success or failure of the procedure, as most authors consider ETV to be successful if there is then no need to place a shunt, though this criterion can be disregarded in chronic or “long standing” cases with procedure failure but with symptoms that are tolerated by the patient. On the other hand, patients with long-term signs or symptoms like macrocephaly, mild headache, psychomotor retardation, or poor sphincter control for example, may not improve obviously or significantly in the immediate post-operative period and require radiological studies to confirm the patency of the ventriculostomy [12]. Other problems also surround the use of ETV in communicating hydrocephalus due to the varying concepts reported about this particular type [8, 13,14,15]. The aim, therefore, of this study was to analyze our results in a series of children with CCCH.
Methods and patients
Inclusion criteria and radiological findings
This retrospective study involved 11 patients with CCCH. The data were extracted from the endoscopy database of our center, which is constantly updated and records all cases. This report covers the period January 2003 to October 2020. Communicating hydrocephalus was defined radiologically as ventricular dilatation that on MRI shows aqueductal flow artifact, no block in the foramen magnum or in the IV ventricle outflow, and patency of the interpeduncular and suprasellar “key cisterns”; that is, the flow artifact should extend from the prepontine cistern to the interpeduncular and suprasellar cisterns [16,17,18].
The communicating hydrocephalus was considered to be clinically chronic if it was characterized by lack of symptoms or the presence of long-term symptoms (months), like headache but with no intracranial hypertension (nausea, vomiting, papillary edema, diplopia), macrocephaly, or alterations in cognitive development, gait, or sphincter control [19, 20].
Patients with a previous history of head injury, CSF infection, or hemorrhage or previous interventions for hydrocephalus were excluded. Thus, the MRI datasets of 11 patients who met the inclusion criteria for this study were analyzed for the data described below.
Demographic and clinical variables
The following variables were included: gender, background, age at surgery, clinical symptoms before and after surgery, perioperative complications, clinical outcome, and follow-up time.
Concerning prognosis, improvement was defined as a combination of clinical and radiological data. This group included both patients with significant sustained improvement as well as clinically stable patients provided there was improvement in the ventricular indexes.
Radiological variables
Radiological variables were measured and recorded in both pre- and post-ETV MRI control studies and compared. MRI measurements were obtained using the most recent MRI control before surgical intervention as the pre-ETV control, and the latest MRI as the post-ETV control. Heavily T2-weighted sequences (Philips Intera 1.5 T MRI scanner) and 3D T2 high spatial resolution (sampling perfection with application-optimized contrasts using different flip angle evolution: SPACE) (Siemens Aera 1.5 T MRI scanner) were obtained in midline in sagittal planes, and TSE T2 sequences of the whole brain in the axial plane. The following measurements were made, and classified into four groups according to their characteristics: [21,22,23].
-
1.
Measurements of the cerebral ventricles and cranium: internal bifrontal diameter (IFD), bifrontal horn width (BFHW), frontal horn width (FHW), internal biparietal diameter (IPD), bioccipital horn width (BOHW), occipital horn width (OHW), and third ventricle width (TVW)
-
2.
Measurements of the third ventricle: third ventricle floor distance (VFD), third ventricle floor bowing (VFB), lamina terminalis distance (LTD), lamina terminalis bowing (LTB), anterior commissure-tuber cinereum distance (ACTC), mamillary body-lamina terminalis distance (MBLT)
-
3.
Cerebral ventricle relative indices: Evan’s index (EI), frontal horn index (FHI), frontal occipital horn index (FOHI), third ventricular morphological index (TVMI)
-
4.
Anatomical landmarks: aqueductal artifact (AA), prepontine membrane (PPM), patency of ventriculostomy (PV)
Table 1 gives the definitions of these radiological parameters.
Image analysis and measurement were done by an experienced pediatric radiologist (MIML). For the data analysis, radiological success was considered to be when there was improvement in the radiological parameters relating to the relative indexes (EI, FHI, FOHI, TVMI) (Figs. 1 and 2).
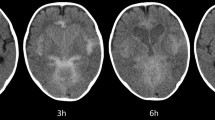
Patient no. 10, 23 months old. Pre-surgery MRI. a, b Transverse TSE T2 sequence. Generalized ventriculomegaly, large subarachnoid frontal space (asterisk), open aqueduct (black arrow). Measurements of the lateral and third ventricles, and the skull vault (in millimeter): internal bifrontal diameter (IFD, blue line), bifrontal horn width (BFHW; grey line), frontal horn width (FHW; black line), internal biparietal diameter (IPD; green line), bioccipital horn width (BOHW; red line), occipital horn width (OHW; brown line), third ventricle width (TVW; orange line). Evan’s index (EI): 0.50; frontal horn index (FHI): 0.56; frontal occipital horn ratio (FOHR): 1.01; third ventricle morphological index (TVMI): 0.24. c. d Sagittal 3D T2-SPACE sequence. Generalized ventriculomegaly, open aqueduct (black arrow), megacisterna magna (black asterisk), free communication seen as a flow void towards the spinal canal (white asterisk), there is no prepontine membrane (blue arrow). Third ventricle floor distance (VFD, green arrow): 11.7; third ventricle floor bowing (VFB, black arrow): 0.6; lamina terminalis distance (LTD, purple arrow): 13.6; lamina terminalis bowing (LTB, yellow arrow): 2.9; anterior commissure-tuber cinereum distance (ACTC, red arrow): 13.5; mammillary body-lamina terminalis distance (MBLT, blue arrow): 19
Patient no. 10. MRI control study one month after ETV. a, b Transversal TSE T2 sequence. Aqueduct is open (black arrow), subarachnoid frontal space is smaller (black asterisk). After ETV, all ventricular measures have decreased: frontal horn width (FHW, black line): 31.2; bifrontal horn width (BFHW, grey line): 60.2; occipital horn width (OHW, brown line): 28.3; bioccipital horn width (BOHW, red line): 65.7, third ventricle width (TVW, orange line): 11.3. The skull vault increased in size as the patient is older: internal bifrontal diameter (IFD, blue line): 122, internal biparietal diameter (IPD, green line): 137. The data showed a decrease in all the relative indices: Evan´s index (EI): 0.43, frontal horn index (FHI): 0.49, frontal occipital horn ratio (FOHR): 0.91, and third ventricle morphological index (TVMI): 0.21. c. d Sagittal 3D T2-SPACE sequence. ETV is open (white arrows); aqueduct is also open (black arrow). Radiological demonstration of flat ventricular walls after successful management, most of the previous third ventricular measures and all the indexes have decreased after ETV: third ventricle floor distance (VFD, green line): 13.7; third ventricle floor bowing (VFB not measureable, ETV localization), lamina terminalis distance (LTD, purple line): 13.8, lamina terminalis bowing (LTB, yellow line): 1.9), anterior commissure-tuber cinereum distance (ACTC, red line): 12.3; mammillary body-lamina terminalis distance (MBLT, blue line): 17.6)
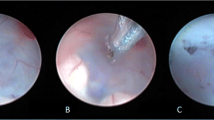
A descriptive qualitative analysis was also done for the presence in the pre- and post-surgical MRI images of the changes in the parameters as well as the visualization of the depth of the convexity sulci, the corpus callosum thickness, the presence or otherwise of periventricular edema, and the morphological change in the third ventricle from “omega” (pre-ETV) to “w” (post-ETV) (Fig. 3) [24]. The existence of benign enlargement of the subarachnoid space (BESS) pre- and post-ETV was also documented.
a Pre-surgery MRI. b Post-surgery MRI. Incomplete prepontine membrane (blue arrow) without upward bulging, visualized in patient number 4 (patient data in Table 2). “Omega” shape previous to ETV surgery (red circle), and “W” shape after ETV surgery (green circle). Open ETV with a week signal artifact (purple arrow)
Surgical technique
All procedures were performed by two pediatric neurosurgeons, BRL and SIM. Patients underwent ETV with the standard procedure through a right precoronal burr hole. A rigid endoscope AESCULAP MINOP ® with a 0° scope was used. The third ventricular floor was fenestrated between the mammillary bodies and the tuber cinereum to establish a CSF flow between the third ventricle and the basal cisterns. The opening was created by perforating the floor membrane with a blunt instrument, usually a monopolar probe without cautery, followed by enlargement of the opening with a Fogarty balloon catheter to a diameter of approximately 6 mm past the membrane with the endoscope. When the opening was considered insufficient, another contiguous fenestration was performed to ensure CSF flow. Both the ependymal layer and the so-called internal membrane were pierced. The arachnoid of the interpeduncular cistern was also opened. Soon after the fenestration, pulsations of the third ventricle floor were observed intraoperatively in most cases.
Follow-up studies
The first outpatient clinical evaluation after the ETV took place 1 week after hospital discharge. Subsequent revisions were at 1 month and 3 months. The first MRI after the ETV was done during the first week prior to discharge when possible; otherwise, it was done on an outpatient basis during the first month. The minimum follow-up period was 6 months. All surgical interventions after ETV were evaluated during follow-up. If shunt implantation became necessary during follow-up, ETV was rated as a failure.
Results
Baseline characteristics of the population
The study included 11 patients (8 male, 3 female) who matched the inclusion/exclusion criteria. The clinical presentation in eight patients was macrocephaly (head circumference at 98th percentile or greater than two standard deviations above the mean); four showed psychomotor retardation (age matched). Two patients presented headache (mild and chronic), and another two had gait disturbance. One patient experienced urinary incontinence. The patient characteristics of our cohort are shown in Table 2.
The first ETV procedure was performed in 2006 and the rest during the period 2013–2020. The mean age of the patients at the time of their ETV was 77 months (approximately 6.5 years) with a median of 39 months (approximately 3 years) and a range of 8–229 months. In this study, to evaluate psychomotor retardation, gait disturbance, and clinical changes after the ETV, we considered the observations of the children’s parents and teachers. Post-ETV improvement was considered to be when there was a cause-effect relation between the ETV and clinical improvement, i.e., when the parents noted an important sustained improvement during the first post-operative weeks.
MRI characteristics
Analysis of the pre- and post-ETV radiological measurements showed a generalized relative improvement in the ventricular indexes in the successful ETV procedures (Figs. 1 and 2). Tables 3, 4 and 5 give the results of the radiological measurements. An improvement was also noted in the qualitative parameters in three patients after the procedure (Fig. 3) [24].
Assessment of ETV success or failure
Patients were closely monitored for re-occurrence of their clinical symptoms. The mean follow-up period of the patients from the ETV was 35.8 months, with a median of 27.7 months (range 6–108 months). The clinical outcome was stable or showed an improvement immediately after ETV in all patients. Three cases experienced clinical worsening in further follow-up and required shunt implantation. Two of these three also experienced neurological deterioration at three years of follow-up. The third had an early risk of a leak due to a recurring tension pseudomeningocele after the ETV. All patients experienced improvement in at least one of the ventricular indexes. The combination of the clinical and radiological data showed ETV success in eight patients, with three failures, two late and one early. All the patients in this cohort were aged ≥ 23 months, except for the patient who experienced early failure, who was 6 months of age.
The mean ETV survival in the successful cases was 32.1 months (range 6–108 months), and in the failed cases, it was 16 months (range 6–108 months).
Discussion
Although ETV is considered the treatment of choice in non-communicating hypertensive hydrocephalus [1,2,3, 38], several articles have reported its use in other types of hydrocephalus, such as chronic communicating hydrocephalus [4,5,6,7,8,9,10,11]. However, two problems exist concerning the use of ETV in chronic hydrocephalus. The first concerns the prediction of success, as the prediction scales developed are only for use with hypertensive hydrocephalus, as for example the Endoscopic Third Ventriculostomy Success Score (ETVSS) of Kulkarni et al. [7], constructed from a series where all the patients had symptomatic high-pressure hydrocephalus. These authors defined ETV success as the absence of ETV failure during the 6 months after the procedure, and ETV failure as the need for any later procedure for CSF shunt or death related with the hydrocephalus within 6 months of the procedure [7].
The second problem concerning the use of ETV in chronic hydrocephalus relates to the evaluation of success or failure. Most authors consider ETV success to be the lack of requirement to place a shunt [25,26,27,28,29,30,31,32], though this can be disregarded in chronic or “long standing” cases with procedure failure but with symptoms that are tolerated by the patients. On the other hand, patients with long-term signs or symptoms like macrocephaly, mild headache, psychomotor retardation, or poor sphincter control for example may not improve obviously or significantly in the immediate post-operative period and require radiological studies to confirm the patency of the ventriculostomy. This problem has been studied in some works that propose the combination of clinical and radiological criteria for the reliable definition of success in all types of hydrocephalus, and particularly in chronic hydrocephalus [12].
Concerning the chronic nature of hydrocephalus, hydrocephalus is considered chronic taking as a reference the “Multi-categorical Hydrocephalus Classification (McHC),” proposed by Shizuo Oi in the seventh category, where hydrocephalus is classified based on its chronology as acute, chronic, or long-standing, and from the point of view of its progression as progressive or detained [20, 39]. The concept “long standing” was described by Shizuo Oi in 2000 in the work on “long-standing overt ventriculomegaly in adults” (LOVA) and considers that from a clinical viewpoint, this group of patients is characterized by being able to have macrocephaly with or without an intellectual quotient below normal, headache, dementia, gait disturbance, urinary incontinence, vegetative state, akinetic mutism, apathy, or parkinsonism [33].
From the clinical viewpoint in children, the concept of chronic or normal-pressure hydrocephalus in childhood and adolescence was defined by Bret and Chazal, where most patients present at least two elements of the adult triad: psychomotor retardation, gait abnormality, or poor sphincter control. In this series, a shunt was implanted or revised in 16 persons, in 5 of whom the symptoms resolved completely, 7 improved, and 4 failed to improve [19]. The presence of alterations associated with the cerebral parenchyma seemed to play an important role in treatment failure. Psychomotor retardation in this study was considered to be subtle worsening in psychomotor activity, involving difficulty for the families and the physicians when trying to distinguish between clinical changes, as well as highlighting the difficulty to distinguish active dynamic changes in the CSF from an arrested hydrocephalus [19]. In our series, we included patients with no symptoms (patient 9) or with just macrocephaly (patients 10 and 11). The indication for ETV in these cases was based on the conviction that the severe ventriculomegaly, if left untreated, could lead in the pediatric age to a slow but progressive alteration in psychomotor development that might remain silent until it manifested clinically years later and was then irreversible. In these patients, therefore, ETV would have a certain preventive nature. Postoperative “stability” would mean the absence of clinical worsening. It is thus so important to consider radiological variables as well in order to determine the possible success of the endoscopic procedure. The future development and application of standardized tools to evaluate the symptoms and CSF dynamics (ICP monitoring, infusion studies) would be of great help in decision making variables. In the case of patient no. 6, we consider the fact that the FHW measurements experienced an isolated decrease could be related with the long time interval between the pre- and post-ETV MRI studies, undertaken when the patient was 9 months and 19 days and again at the age of 3 years and 6 months, respectively. This idea is reinforced by the fact that both the radiological indexes and the clinical situation improved after the ETV.
Another controversial aspect concerns the indication for ETV in communicating hydrocephalus. The very concept of communication can cause confusion. Dandy’s definition of communicating or non-communicating hydrocephalus refers to the presence of CSF passing between the lateral ventricle and the lumbar subarachnoid space, confirmed by injection of contrast in a lateral ventricle and its detection in a lumbar puncture [34]. Once the CSF is found in the subarachnoid space, Russell proposed the concept of obstructive/non-obstructive. According to this, all hydrocephalus are obstructive except those caused by hyperproduction of CSF in a plexus papilloma/carcinoma or in venous sinus thrombosis [9]. All these concepts have recently been recorded by Oi and Di Rocco [35].
Concerning CSF circulation in the subarachnoid space, Dandy had already defined in 1938 the concept of “key cisterns” (interpeduncular and chiasmatic) that distribute the CSF to the interhemispheric and the Sylvian fissures, and where an obstruction anterior to these key cisterns could be treated by opening the third ventricle floor unlike an obstruction posterior to the key cisterns [16].
Al-Hakim et al. recently reported on a cohort of 21 patients with idiopathic hydrocephalus treated by ETV. The MRI showed a pressure gradient in the third ventricle and obstructions of the Sylvian aqueduct, fourth ventricle, or the foramen magnum were ruled out [4]. These authors assumed the presence of a “midline prepontine membrane,” that is an extraventricular intracisternal obstruction in the prepontine arachnoid septa, clearly visualized or suspected in 38.1% and 47.6% of the patients, respectively [4]. In our study cohort, we identified one case where an incomplete prepontine membrane was seen, with no signs of “upward bulging” (Fig. 3), which we therefore considered non-obstructive. These membranes were not clearly seen in the other study subjects. The cases reported by Al Hakim could correspond to Dandy’s concept of pre-key cistern obstruction, in a type of communicating hydrocephalus (according to Dandy), but at the same time obstructive (according to Russell) [9, 34].
The literature contains many definitions of communication or obstruction, ranging from functional aqueduct stenosis to traumatic or post-infectious hydrocephalus and even those occurring after resection of posterior tumors [8, 13,14,15]. Other studies, however, like that of Kulkarni et al., define a subtype called “congenital communicating hydrocephalus” for this type of hydrocephalus [17].
Concerning developmental hydrocephalus, Tully et al. studied 236 children, classifying the cases in five groups, one of which comprised communicating hydrocephalus. A subgroup of this latter group, called “severe idiopathic communicating hydrocephalus,” was formed by those cases with marked ventriculomegaly, and characteristically in some of these, there was an excessive accumulation of extra-axial fluid in the posterior fossa, seen as the presence of extra-axial fluid collections on MRI [18]. The hydrocephalus in our patients were communicating, chronic, and idiopathic, presenting many similarities to this subgroup. Three patients (pts 6, 10, and 11) had excessive fluid accumulation in the posterior fossa on pre-ETV MRI. A notable extra-axial space may be present in these patients, thus underlying the communicating nature of the hydrocephalus and not contraindicative of surgery. The existence of BESS in these patients is information that is difficult to interpret, as only one of them had macrocephaly. Although all three were younger than 29 months, patient no. 9 (also in this age range) did not have either macrocephaly or BESS. Finally, patient no. 11 continued to have BESS after the ETV (still younger than 24 months and still with macrocephaly). Further studies are required to establish any relation between the so called “benign subdural effusion of infancy” and a possible evolution towards CCCH in some cases.
CSF absorption shows a specific dynamics in the immature brain, predominating the minor pathway of CSF absorption and lack of development of the major pathway, as proposed in 2006 by Oi and Di Rocco in the evolution theory in CSF dynamics [35, 39]. Concerning the case in our cohort mentioned above with early failure, the patient was 8 months of age at the time of ETV, and was the only case in our series in which ETV was performed before the age of 12 months. This failure can be assumed to be due to the predominance of the minor CSF absorption pathway.
Concerning the patency of the ventriculostomy, in all our patients, it was possible to identify an artifact in the stoma, even in the cases of clinical ETV failure. In one case, the patient experienced clinical improvement for 18 months, after which persistent headache developed with a recording of pathological intracranial pressure. Another case involved a scarce fine artifact, with little improvement in the radiological indexes but with data suggesting clinical improvement during 30 months, after which there was an enlargement of the cranial perimeter, psychomotor deterioration, tremor, and occasional headache. The third failure in the series concerned an early failure in an 8-month-old girl, with early development of a pseudomeningocele with the risk of a leak. All three cases required shunt placement. The presence of an artifact in these cases of failed ETV suggests the need for a combination of clinical and radiological criteria to assess ETV success.
The question therefore remains of whether it is possible to perform ETV in CCCH with no radiological data of non-communication or obstruction of the subarachnoid space (at least until Dandy’s key cisterns). Greitz’s hydrodynamic theory and the more modern pulsatile vector theory of Preuss justify the possible performance of ETV in pure communicating hydrocephalus via attenuation of the reflection wave produced after opening a second communication, this time in the third ventricle floor [36, 37]. The improvement in the transmission of the pulse wave in the subarachnoid space could, in part, account for the improvement in the radiological parameters in these patients.
Conclusion
Our data show that ETV is associated with a good result in the treatment of children with CCCH. A combination of radiological and clinical criteria increases the reliability in the definition of ETV success. Pending further studies with larger sample sizes, ETV appears to be a valid alternative for the treatment of these patients.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Di Rocco C, Massimi L, Tamburrini G (2006) Shunts vs endoscopic third ventriculostomy in infants: are there different types and/or rates of complications? A review Childs Nerv Syst 22(12):1573–1589. https://doi.org/10.1007/s00381-006-0194-4
Iantosca MR, Hader WJ, Drake JM (2004) Results of endoscopic third ventriculostomy. Neurosurg Clin N Am 15(1):67–75. https://doi.org/10.1016/S1042-3680(03)00067-6
Limbrick DD Jr, Baird LC, Klimo P Jr, Riva-Cambrin J, Flannery AM (2014) Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 4: cerebrospinal fluid shunt or endoscopic third ventriculostomy for the treatment of hydrocephalus in children. J Neurosurg Pediatr 14(Suppl 1):30–34. https://doi.org/10.3171/2014.7.PEDS14324
Al-Hakim S, Schaumann A, Tietze A, Schulz M, Thomale UW (2019) Endoscopic third ventriculostomy in children with third ventricular pressure gradient and open ventricular outlets on MRI. Childs Nerv Syst 35(12):2319–2326. https://doi.org/10.1007/s00381-019-04383-x
Gangemi M, Maiuri F, Naddeo M, Godano U, Mascari C, Broggi G, Ferroli P (2008) Endoscopic third ventriculostomy in idiopathic normal pressure hydrocephalus: an Italian multicenter study. Neurosurgery 63(1):62–7; discussion 67–9. https://doi.org/10.1227/01.NEU.0000335071.37943.40
Hailong F, Guangfu H, Haibin T, Hong P, Yong C, Weidong L, Dongdong Z (2008) Endoscopic third ventriculostomy in the management of communicating hydrocephalus: a preliminary study. J Neurosurg 109(5):923–930. https://doi.org/10.3171/JNS/2008/109/11/0923
Kandasamy J, Yousaf J, Mallucci C (2013) Third ventriculostomy in normal pressure hydrocephalus. World Neurosurg 79(2 Suppl):S22.e1–7. https://doi.org/10.1016/j.wneu.2012.02.008. Epub 2012 Feb 10
Rangel-Castilla L, Barber S, Zhang YJ (2012) The role of endoscopic third ventriculostomy in the treatment of communicating hydrocephalus. World Neurosurg 77(3–4):555–560. https://doi.org/10.1016/j.wneu.2011.06.038.Epub2011Nov7
Russell DS (1949) Observation on the pathology of hydrocephalus. Medical research council Special Report Series No. 265. His Majesty’s Stationery Office, London. pp. 112–113
Tasiou A, Brotis AG, Esposito F, Paterakis KN (2016) Endoscopic third ventriculostomy in the treatment of idiopathic normal pressure hydrocephalus: a review study. Neurosurg Rev 39(4):557–563. https://doi.org/10.1007/s10143-015-0685-4.Epub2015Dec10
Warf BC (2013) Congenital idiopathic hydrocephalus of infancy: the results of treatment by endoscopic third ventriculostomy with or without choroid plexus cauterization and suggestions for how it works. Childs Nerv Syst 29(6):935–940. https://doi.org/10.1007/s00381-013-2072-1 (Epub 2013 Mar 13)
Ros B, Romero L, Ibáñez G, Iglesias S, Rius F, Pérez S, Arráez MA (2012) Success criteria in pediatric neuroendoscopic procedures. Proposal for classification of results after 67 operations. Childs Nerv Syst 28(5):691–7. https://doi.org/10.1007/s00381-012-1689-9. Epub 2012 Mar 14
Grand W, Leonardo J, Chamczuk AJ, Korus AJ (2016) Endoscopic third ventriculostomy in 250 adults with hydrocephalus: patient selection, outcomes, and complications. Neurosurgery 78(1):109–119. https://doi.org/10.1227/NEU.0000000000000994
Rekate HL (2007) Longstanding overt ventriculomegaly in adults: pitfalls in treatment with endoscopic third ventriculostomy. Neurosurg Focus 15;22(4):E6ç
Singh I, Haris M, Husain M, Husain N, Rastogi M, Gupta RK (2008) Role of endoscopic third ventriculostomy in patients with communicating hydrocephalus: an evaluation with MR ventriculography. Neurosurg Rev 31(3):319–325. https://doi.org/10.1007/s10143-008-0137-5 (Epub 2008 May 10)
Goodrich, JT (2000) Reprint of The operative treatment of communicating hydrocephalus by Walter E Dandy, MD. 1938. Childs Nerv Syst 16(9):545–50. https://doi.org/10.1007/s003810000254
Kulkarni AV, Riva-Cambrin J, Holubkov R, Browd SR, Cochrane DD, Drake JM, Limbrick DD, Rozzelle CJ, Simon TD, Tamber MS, Wellons JC 3rd, Whitehead WE, Kestle JR, Hydrocephalus Clinical Research Network (2016) Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr 18(4):423–429. https://doi.org/10.3171/2016.4.PEDS163 (Epub 2016 Jun 3)
Tully HM, Ishak GE, Rue TC, Dempsey JC, Browd SR, Millen KJ, Doherty D, Dobyns WB (2016) Two hundred thirty-six children with developmental hydrocephalus: causes and clinical consequences. Child Neurol 31(3):309–320. https://doi.org/10.1177/0883073815592222 (Epub 2015 Jul 16)
Bret P, Chazal J (1995) Chronic (“normal pressure”) hydrocephalus in childhood and adolescence. A review of 16 cases and reappraisal of the syndrome. Child’s Nerv Syst 11:687–691. https://doi.org/10.1007/BF00262232
Oi S (2011) Classification of hydrocephalus: critical analysis of classification categories and advantages of “Multi-categorical Hydrocephalus Classification” (Mc HC). Childs Nerv Syst 27:1523–1533. https://doi.org/10.1007/s00381-011-1542-6.Epub2011Sep17
Börcek AO, Uçar M, Karaaslan B (2017) Simplest radiological measurement related to clinical success in endoscopic third ventriculostomy. Clin Neurol Neurosurg 152:16–22. https://doi.org/10.1016/j.clineuro.2016.11.006
Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S, Canadian Pediatric Neurosurgery Study Group (2010) Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J Neurosurg Pediatr 6(4):310–315. https://doi.org/10.3171/2010.8.PEDS103
Kolsur N, Radhika PM, Shetty S, Kumar A (2018) Morphometric study of ventricular indices in human brain using computed tomography scans in Indian Population. Int J Anat Res 6(3.2):5574–5580. https://doi.org/10.16965/ijar.2018.286
Foroughi M, Wong A, Steinbok P, Singhal A, Sargent MA, Cochrane DD (2011) Third ventricular shape: a predictor of endoscopic third ventriculostomy success in pediatric patients. J Neurosurg Pediatr 7:389–396. https://doi.org/10.3171/2011.1.PEDS10461
Baldauf J, Oertel J, Gaab MR, Schroeder HW (2007) Endoscopic third ventriculostomy in children younger than 2 years of age. Childs Nerv Syst 23(6):623–626. https://doi.org/10.1007/s00381-007-0335-4 (Epub 2007 Apr 6)
Bognar L, Markia B, Novak L (2005) Retrospective analysis of 400 neuroendoscopic interventions: the Hungarian experience. Neurosurg Focus 15;19(6):E10. https://doi.org/10.3171/foc.2005.19.6.11
Boschert J, Hellwig D, Krauss JK (2003) Endoscopic third ventriculostomy for shunt dysfunction in occlusive hydrocephalus: long-term follow up and review. J Neurosurg 98(5):1032–1039. https://doi.org/10.3171/jns.2003.98.5.1032
Fukuhara T, Vorster SJ, Luciano MG (2000) Risk factors for failure of endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurgery 46(5):1100–9; discussion 1109–11. https://doi.org/10.1097/00006123-200005000-00015
Greenfield JP, Hoffman C, Kuo E, Christos PJ, Souweidane MM (2008) Intraoperative assessment of endoscopic third ventriculostomy success. J Neurosurg Pediatr 2(5):298–303. https://doi.org/10.3171/PED.2008.2.11.298
Navarro R, Gil-Parra R, Reitman AJ, Olavarria G, Grant JA, Tomita T (2006) Endoscopic third ventriculostomy in children: early and late complications and their avoidance. Childs Nerv Syst 22(5):506–513. https://doi.org/10.1007/s00381-005-0031-1
Ogiwara H, Dipatri AJ Jr, Alden TD, Bowman RM, Tomita T (2010) Endoscopic third ventriculostomy for obstructive hydrocephalus in children younger than 6 months of age. Childs Nerv Syst 26(3):343–347. https://doi.org/10.1007/s00381-009-1019-z
Ray P, Jallo GI, Kim RY, Kim BS, Wilson S, Kothbauer K, Abbott R (2005) Endoscopic third ventriculostomy for tumor-related hydrocephalus in a pediatric population. Neurosurg Focus 15;19(6):E8. https://doi.org/10.3171/foc.2005.19.6.9
Oi S, Shimoda M, Shibata M, Honda Y, Togo K, Shinoda M, Tsugane R, Sato O (2000) Pathophysiology of long-standing overt ventriculomegaly in adults. J Neurosurg 92(6):933–940. https://doi.org/10.3171/jns.2000.92.6.0933
Dandy WE (1919) Experimental hydrocephalus. Ann Surg 70(2):129–142. https://doi.org/10.1097/00000658-191908000-00001
Oi S, Di Rocco C (2006) Proposal of “evolution theory in cerebrospinal fluid dynamics” and minor pathway hydrocephalus in developing immature brain. Childs Nerv Syst 22(7):662–669. https://doi.org/10.1007/s00381-005-0020-4
Greitz D (2007) Paradigm shift in hydrocephalus research in legacy of Dandy’s pioneering work: rationale for third ventriculostomy in communicating hydrocephalus. Childs Nerv Syst 23(5):487–489. https://doi.org/10.1007/s00381-007-0303-z
Preuss M, Hoffmann KT, Reiss-Zimmermann M, Hirsch W, Merkenschlager A, Meixensberger J, Dengl M (2013) Updated physiology and pathophysiology of CSF circulation–the pulsatile vector theory. Childs Nerv Syst 29(10):1811–1825. https://doi.org/10.1007/s00381-013-2219-0.Epub2013Jul7
Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S (2009) Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr 155(2):254–9.e1. https://doi.org/10.1016/j.jpeds.2009.02.048.Epub2009May15
Oi S, Inagaki T, Shinoda M, Takahashi S, Ono S, Date I, Nomura S, Miwa T, Araki T, Ito S, Uchikado H, Takemoto O, Shirane R, Nishimoto H, Tashiro Y, Matsumura A, COE-Fetal and Congenital Hydrocephalus Top 10 Japan Study Group (2011) Guideline for management and treatment of fetal and congenital hydrocephalus: Center Of Excellence-Fetal and Congenital Hydrocephalus Top 10 Japan Guideline 2011. Childs Nerv Syst 27(10):1563–1570. https://doi.org/10.1007/s00381-011-1541-7 (Epub 2011 Sep 17)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baeza-Antón, L., Martínez-León, M.I., Ros-López, B. et al. Endoscopic third ventriculostomy in children with chronic communicating congenital hydrocephalus: a single-center cohort retrospective analysis. Childs Nerv Syst 38, 319–331 (2022). https://doi.org/10.1007/s00381-021-05380-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05380-9