Abstract
Background
Shunt insertion for hydrocephalus is a common paediatric neurosurgery procedure. Shunt complications are frequent with an estimated 20–40% failure rate within the first year, and 4.5% per year subsequently. We have an open-door ‘possible shunt malfunction’ pathway for children treated with a shunt or endoscopic third ventriculostomy, providing direct ward access to ensure rapid assessment and timely management of children.
Objective
To audit the ‘possible shunt malfunction’ pathway in terms of clinical outcomes (percentage-confirmed shunt dysfunction and number of re-attendances) and costs.
Methods
Clinical data for patients attending the triage service were prospectively recorded over 7 months—including the number of attendances, previous shunt revisions, shunt type, investigations performed (CT, x-rays), and outcome. Costings (e.g. costs of physician, inpatient stay, investigations) were obtained from the hospital’s procurement department.
Results
In the study period, there were 81 attendances by 62 patients and only 16% of attendances resulted in surgical management (either shunt revision or ETV). Approximately 17% of patients re-attended at least once. The average cost per attendance in our pathway was £765.57 ($969.63; €858.73). The total expenditure for the pathway over 7 months was £62,011.03 ($78,540.07; €69,556.81), with inpatient stay making up the biggest percentage of cost (49.2%).
Conclusion
Only 16% (13 attendances) of those attending through our pathway required neurosurgical intervention. Investigations for possible blocked shunt come at significant health, social, and financial cost. High rates of shunt failure, re-attendance, investigations, and inpatient stays incur a sizable financial burden to the healthcare system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus remains the most common condition treated by paediatric neurosurgeons [4]. Annually, 1660 paediatric shunt procedures are undertaken in the UK (33.5% primary insertions, 66.5% revisions) [12]. Shunt complications are frequent with an estimated 20–40% failure rate within the first year, and a 4.5% failure rate subsequently [9]. We previously reported a 30-day failure rate of 8.8% for primary shunts and 23.4% for shunt revision in children [2]. Investigation for possible shunt malfunction is frequently required and complicated by the fact that symptoms are commonly mimicked by other paediatric ailments [7].
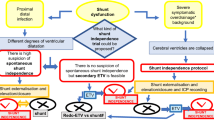
Our neurosurgical department offers an open-door ‘possible shunt malfunction” pathway for patients with possible shunt failure (Fig. 1). This facilitates direct ward access without needing additional referral, enabling rapid assessment and management. The pathway is also open to children after endoscopic third ventriculostomy (ETV) and those under surveillance for hydrocephalus but in whom the threshold for treatment has not been reached.
Previous studies [6,7,8, 10, 13, 17, 23, 24, 26, 29] describe that, out of overall attendances with possible shunt malfunction, the actual proportion with true shunt dysfunction is low (9.8–30%). A high rate of re-attendance has also been noted in these studies [10, 26, 28, 29]. This results in financial burdens on families and healthcare systems.
To our knowledge, there are no European studies evaluating this problem and there is a paucity of literature assessing the associated costs. We therefore audited our ‘possible shunt malfunction’ pathway for clinical outcomes and costs.
Methods
‘Possible shunt malfunction’ pathway (Fig. 1)
Access to this pathway is given to children with hydrocephalus treated with a shunt or ETV or children requiring active monitoring for possible hydrocephalus. In our region, we have > 350 children aged 0–16 years with this access. Patients/parents/carers have direct access to contact the ward for same-day assessment without referral from other healthcare providers. Patients are initially assessed by a hydrocephalus nurse specialist or neurosurgical trainee. Patients with a high suspicion of shunt dysfunction and/or who are clinically unwell, CT imaging is organized urgently (using a ‘low-dose’ protocol). In selected cases, following discussion with the on-call consultant, additional shunt series x-rays or shunt tap may be performed. If the clinical assessment or imaging is inconclusive, patients may be admitted for observation and/or intracranial pressure (ICP) monitoring. If shunt dysfunction is excluded but symptoms continue, a review by a neurologist or general paediatrician will be requested on an individual basis.
Data collection
Clinical data for pathway attendances were prospectively recorded from 1 December 2017 to 31 July 2018. During the study period, 10 patients were referred from other routes (other hospitals, inpatient transfer, emergency department (ED), community nurse specialists) and are not included in the pathway. Data collected included the number of attendances, number of previous shunt revisions, type of shunt, investigations performed during the admission, number of overnight stays, and outcome. We grouped patients attending our pathway into group A (ventriculo-atrial or ventriculo-peritoneal shunt) and group B (no shunt; either active monitoring for hydrocephalus or previous ETV). One or more of the following outcomes was noted:
-
Reassured: patient discharged, no illness/shunt dysfunction
-
Mild illness: illness not requiring inpatient treatment and patient discharged (e.g. viral illnesses)
-
Seizures: not requiring admission and unrelated to shunt
-
Inpatient treatment: of illnesses unrelated to shunt function
-
Surgical intervention: shunt revision or ETV
-
Outpatient follow-up: No shunt dysfunction; but close follow-up, within 2 weeks, by hydrocephalus nurse specialist or neurosurgeon, in clinic or by phone call.
Costings were obtained from the hospital’s procurement department. The breakdown available for each attendance was:
-
Cost of pathway assessment: each attendance charged as one assessment episode at one tariff, irrespective of the number of clinical reviews; with exception of Ophthalmology which can be additionally charged.
-
Cost of investigations: CT scans, shunt series, other radiology or laboratory tests
-
Out-of-hours additional tariffs for radiology investigations: a set fee covering this service
-
Inpatient costs: for all admissions, irrespective of outcome; a set fee for overnight stay in the ward, comprising:
-
Nursing staff
-
Administrative staff
-
Clinical supplies/services (e.g. pharmaceutical supplies, equipment)
-
Other non-pay costs (e.g. gowns, laundry, cleaning)
-
Student’s t test was used to compare continuous variables, and a p value of < 0.05 was considered significant.
Results
Clinical outcomes (Fig. 2)
In total, there were 81 attendances by 62 patients. Fifty-five patients had shunted hydrocephalus (group A) and attended 72 times (88.9% attendances). Twelve attendances (16.7%) resulted in shunt revision surgery for shunt dysfunction. The ratio of overall attendance to confirmed shunt dysfunction was 6.75:1. Two attendances led to admission for intravenous antibiotics for other infections—1 urinary tract infection and 1 mastoiditis. One patient was admitted for ICP monitoring, which was unremarkable. Seven patients without shunts also accessed the pathway (group B). They attended 9 times (11.1%). One attendance resulted in ETV. Overall, 16% of pathway attendances resulted in surgery. The ratio of overall attendance to those requiring surgery was 6.2:1.
CT brain scans were undertaken in 38/81 attendances (group A 36/72, 50%; group B 2/9, 22.2%). The diagnosis of shunt dysfunction was confirmed by CT in nine patients who went on to have surgery. Two patients had CT scans on outpatient appointments which identified shunt dysfunction, leading to surgery. Two patients undergoing surgery had recent MRIs and hence did not have pre-op CT scans. Shunt series x-rays were performed in 9 attendances (12.5%) in group A, of which 2 were done in patients who had surgery. One shunt series was done post-shunt revision to check the distal catheter position. The other showed a disconnection. Hence, 1 out of 9 shunt series changed management and was potentially unnecessary investigation in the others—this underlines the decision in our department to move away from routine shunt series.
Re-attendances
Over the study period, 21.8% group A and 28.6% group B attended more than once (Fig. 3). Almost 60% group A attenders had at least one previous shunt revision (Table 1). The average attendance number was slightly higher in patients with previous revisions, compared with those with previous shunt revisions (1.38 vs 1.22), but not statistically significant. Patients with 2 previous revisions had the highest average attendance number at 2.
Clinical outcomes were assessed for re-attenders in both groups A and B (Table 2)—12 group A and 2 group B.
Costs incurred by ‘possible shunt malfunction’ pathway
Pathway cost figures were sourced from the procurement department (Table 3). We calculated the costs incurred in total and subdivided by group (Table 4).
The total expenditure in the ‘possible shunt malfunction’ pathway in the study period was £62,011.03 ($78,540.07; €69,556.81). The largest expenditure was on inpatient stay (49.2%), followed by triage assessment (27%), then investigation costs (23.7%). Group A incurred a total cost of £54,960.03 ($69,609.63; €61,647.81), averaging £763.33 ($966.80; €856.22) per attendance. Group B incurred a total cost of £7051 ($9123.96; €8053.47), averaging £783.44 ($1013.77; €894.83) per attendance.
Unsurprisingly, > 30% overall pathway cost was incurred by the 16% who required surgery. The 13 patients who required surgery incurred a total cost of £18,921.91 ($23,965.55; €21,224.41), representing 30.5% of total pathway expenditure.
Outcome associated with referral source
The outcome in relation to referral source was assessed (Table 5). Most patients self-referred (92.6% attendances) with others referred from outpatient clinic (6.2%). One child re-attended for a ‘planned’ review following an abnormal shunt tap and required shunt revision. Only 14.7% of self-referrals needed surgery compared with 20% from outpatient referral. Cost per attendance was the highest for the planned review (£1648; $2150; €1941) due to the number of investigations undertaken and 2 overnight stays.
Discussion
Clinical outcomes
Out of all attendances through our pathway, only a small proportion requires surgical management. This is consistent with other studies recognizing the ‘low yield’ of a paediatric shunt malfunction pathway (Tables 6 and 7) [5, 7, 8, 10, 14, 17, 23, 24, 26, 29]. Chern et al. [7] implemented a triage protocol (algorithm classifying urgency of patient’s symptoms triggering early assessment/scans) in their ED for assessing shunt malfunction. They found that the shunt malfunction diagnosis rate pre-protocol was 17%, rising to 28% post-protocol. Cohen et al. [8] found that 17.5% of children with CT scans had shunt dysfunction requiring surgical management. Mater et al. [17] reported that 30% of children who had a shunt series with/without CT scan had shunt dysfunction. Razmara et al. [24] studied a cohort of 74,552 ED attendances, reporting 12.8% shunt malfunction.
The assessment of children with possible shunt malfunction remains a diagnostic challenge since symptoms such as headache or vomiting also occur with common childhood illnesses [10]. The variability of symptoms also means that clear guidelines to rule out shunt malfunction are challenging [6, 13, 15, 24].
Re-attendance rates
Like other studies [10, 17, 26, 29], we found a high re-attendance rate with almost 17% returning at least once (Table 6). Sribnick et al. [29] reported almost 20% of patients sent home re-presented within the next month. The reasons for this could include the non-specific nature of ongoing symptoms, other comorbidities, and parental anxiety. Having prior shunt revisions is associated with a higher shunt failure rate and may translate into increased anxiety and high re-attendance rates. Our study did not show statistically significant higher rates of attendance in children with previous revisions. However, this is in the context of a small study cohort.
Two patients with possible shunt malfunctions were kept under observation after their first pathway attendance and were seen on a number of occasions by a hydrocephalus nurse and consultant before being revised. Both had outpatient investigations and eventually had their shunt revised 4 weeks later. The first patient presented with increased head circumference but otherwise well, and the second patient initially presented with irritability and a family history of gastroenteritis. Their eventual surgery confirmed blocked proximal catheters (for both patients). These two cases demonstrate possible diagnostic delays despite the open-door policy. However, this probably reflects the complex nature of hydrocephalus management and it was possible to closely monitor both patients through this pathway. In these two instances, we only included costs incurred during their index pathway attendance and listed their outcome as a shunt malfunction. In addition to the pathway’s usefulness for rapid access and close follow-up, patients and carers have provided feedback that they value the sense of security this direct access offers.
Investigations
We found a high rate of investigations undertaken in our pathway. Shunted patients attending for assessment had an average of 1 CT scan for every 2 attendances. This is similar to Florin et al. [10], where children with shunts received a head CT in almost 50% ED attendances. Shunt series x-rays were less frequently used in our department due to the low diagnostic yield (only 1/9 shunt series altered management). Overall, 60/81 (74.1%) attendances had at least one investigation (radiological or laboratory). This highlights the diagnostic uncertainty around shunt malfunctions and a high reliance on additional investigations, especially head CT scans.
There is a growing concern regarding radiation exposure for children with ventricular shunts [3, 8, 10, 28]. Florin et al. [10] undertook a 10-year longitudinal study of children with ventricular shunts, finding that 5.8% had 10 or more head CT scans. Cohen et al. [8] found a median of 2.6 CT scans per person per year in their ED pathway. Antonucci et al. [3] reported that children with ventricular shunts underwent a median of 8.5 CTs, 3 shunt series x-rays, 1 skull x-ray, and 1 brain MRI in the 10 years following shunt placement. This translates to a potentially significant radiation dosage over the child’s lifetime.
Recent literature has suggested that ionizing radiation from imaging studies may influence the development of future malignancies [28]. Children are felt to be particularly vulnerable due to the increased dose volume and increased lifetime risk from early exposure [28]. Determining the total lifetime dose of radiation is challenging [10, 28], but it has been estimated that the future malignancy risk for a child from a CT head scan is 24% higher than for non-exposed children [18]. Another study estimated that the risk of brain tumour development following a CT head scan in a child aged 10 years or younger is approximately 1:10,000 in the first 2 decades following the CT [22].
Even considering the above risks, brain imaging is still required for many children attending shunt malfunction pathways, due to the serious consequences of misdiagnosis and the lack of current alternatives. However, we can work to reduce the amount of radiation received by children through pathway developments and innovations.
One opinion could be the development of better guidelines as to when to arrange a scan or whether to consider observation instead. This comes with the benefit of fewer CT scans but potentially increases cost through increasing admission rates. Limited-protocol CT with reduced slice number is another strategy reported [1, 20]. Park et al. [20] report a limited 4-slice CT protocol, resulting in 87% radiation dose reduction. Alhilali et al. [1] describe a 3-slice CT protocol with a 90% dose reduction and adequate diagnostic accuracy. However, this has only been evaluated in theoretical and feasibility studies. An alternative strategy would be to reduce radiation dose per scan with a ‘low-dose’ protocol.
Rapid sequence magnetic resonance imaging (MRI) is reported by some centres as an alternative to CT [3, 5, 8, 10, 14, 19, 28]. This has been reported as non-inferior to CT for detecting shunt malfunction [5, 14]. The benefits include a lack of ionizing radiation, high-resolution ventricular system imaging, and reduced sedation requirement due high-speed sequence acquisition [3, 28]. However, disadvantages include difficulty visualizing catheter position with small ventricles [19, 28], higher costs [3, 19], and patients with programmable shunts will usually require shunt reprogramming after MRI [19].
Another important strategy is to reduce the use of shunt series x-rays. In general, these are insensitive as a primary assessment for possible shunt malfunction [11, 16, 17, 23], although targeted use may be helpful [23]. We previously did a formal audit of shunt series x-rays in children (and adults) at our institution [25] which demonstrated that they typically did not alter management decisions. This finding is in line with other publications [11, 16, 17, 23]. We have since moved away from routine pre-operative shunt series and instead use targeted single body-region x-rays where indicated.
Costs
Through this study, we found sustained high expenditure through the ‘possible shunt malfunction’ pathway, mainly due to re-attendances, the number of investigations, and number of inpatient bed days. The average cost of each attendance was £765.57 ($969.63; €858.73). However, there are additional costs not quantified here, including psychological stresses for parents and children from hospital appointments and financial burdens of time out of work, costs of transport, and costs associated with care of siblings [27].
Some financial data regarding shunt malfunction and revision is available from North America. However, this does not include acute triage services or EDs. In 2000, Padwardhan et al. [21] examined the US Nationwide Inpatient Sample database (representing 20% community hospitals, including paediatric and public hospitals). They found a total of 5574 shunt-related admissions, where the 2 most common procedures performed were ventriculo-peritoneal shunt placement (43.4%) and ventricular shunt replacement (42.8%). The average cost per admission was $35,816 (£27,506; €32,185).
The availability of implantable telemetric ICP monitoring devices may have a role to play in stream-lining the investigation and management of this cohort of patients—although to impact significantly, this will depend on systems being developed which are affordable and which remain accurate for years rather than months.
Limitations of this study include the short study period of 7 months, resulting in small cohort numbers and may not identify seasonal variability. There were also difficulties obtaining accurate costing of the pathway, given the complexity of NHS cost calculations. Some costings were generalized, rather than patient-specific, and the breakdown was limited. There may also be some inaccuracy because, in line with NHS cost rules, only one nurse/physician cost was included for each attendance even though a patient may have been reviewed several times.
We believe that this is the first study assessing both clinical outcomes and costs of a UK-based acute assessment pathway for shunt malfunction. Despite the limitations above, we feel that it provides valuable insight into pathway costs and represents a starting point for further studies.
Conclusion
Shunt failure is a neurosurgical emergency which necessitates timely diagnosis and treatment. Like us, many institutions have an open-door pathway for hydrocephalus patients. In this study, only a minority of attendees actually had shunt dysfunction requiring surgical intervention (16%). The remainder (84%) still underwent investigations to exclude shunt dysfunction, but this comes at significant health, social, and financial costs. The average cost of each attendance was £765.57 ($969.63; €858.73), with a significant proportion (49.2%) spent on inpatient stay. However, it is necessary to accept the ‘low yield’ to encourage early detection of true shunt malfunctions. Nonetheless, more research is warranted into methods to streamline investigations, to prevent unnecessary radiation and increase cost-effectiveness. Implantable telemetric ICP devices may play a role in the future but this will require a significant improvement in implant ‘survival’.
References
Alhilali LM, Dohatcu AC, Fakhran S (2013) Evaluation of a limited three-slice head CT protocol for monitoring patients with ventriculoperitoneal shunts. Am J Roentgenol 201(2):400–405
Anderson IA, Saukila LF, Robins JMW, Akhunbay-Fudge CY, Goodden JR, Tyagi AK, Puillips N, Chumas PD (2018) Factors associated with 30-day ventriculoperitoneal shunt failure in pediatric and adult patients. J Neurosurg 130(1):1–9
Antonucci MC, Zuckerbraun NS, Tyler-Kabara EC, Furtado AD, Murphy ME, Marin JR (2017) The burden of ionizing radiation studies in children with ventricular shunts. J Pediatr 182:210–216
Badhiwala JH, Kulkarni AV, Nassiri F (2018) Quality of life in childhood hydrocephalus. In: Cinalli G, Ozek M, Sainte-Rose C (eds) Pediatric hydrocephalus, 2nd edn. Springer, New York, pp 1–15
Boyle TP, Paldino MJ, Kimia AA, Fitz BM, Madsen JR, Monuteaux MC, Nigrovic LE (2014) Comparison of rapid cranial MRI to CT for ventricular shunt malfunction. Pediatrics 134(1):e47–e54
Boyle TP, Kimia AA, Nigrovic LE (2018) Validating a clinical prediction rule for ventricular shunt malfunction. Pediatr Emer Care 34(11):751–756
Chern JJ, Macias CG, Jea A, Curry DJ, Luerssen TG, Whitehead WE (2010) Effectiveness of a clinical pathway for patients with cerebrospinal fluid shunt malfunction. J Neurosurg Pediatr 6(4):318–324
Cohen JS, Jamal N, Dawes C, Chamberlain JM, Atabaki SM (2014) Cranial computed tomography utilization for suspected ventriculoperitoneal shunt malfunction in a pediatric emergency department. J Emerg Med 46(4):449–455
Drake JM, Kestle JR, Milner R, Cinalli G, Boop F, Piatt J Jr, Haines S, Schiff SJ, Cochrane DD, Steinbok P, MacNeil N (1998) Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurg 43(2):294–303
Florin TA, Aronson PL, Hall M, Kharbanda AB, Shah SS, Freedman SB, Alpern ER, Mistry RD, Simon HK, Berry J, Coley BD, Neuman MI (2015) Emergency department use of computed tomography for children with ventricular shunts. J Pediatr 167(6):1382–1388
Griffey RT, Ledbetter S, Khorasani R (2007) Yield and utility of radiographic ‘shunt series’ in the evaluation of ventriculoperitoneal shunt malfunction in adult emergency patients. Emerg Radiol 13(6):307–311
Joannides A, Mendez RF, Pickard J, Richards H, Seeley H (2017) UK Shunt Registry Draft Report 2017. In: UK Shunt Registry. The Society of British Neurological Surgeons; 2017. Available via https://www.sbns.org.uk/index.php/audit/shunt-registry/. Accessed 27 Apr 2020.
Kim TY, Stewart G, Voth M, Moynihan JA, Brown L (2006) Signs and symptoms of cerebrospinal fluid shunt malfunction in the pediatric emergency department. Pediatr Emer Care 22(1):28–34
Kim I, Torrey SB, Milla SS, Torch MC, Tunik MG, Foltin JC (2015) Benefits of brain magnetic resonance imaging over computer tomography in children requiring emergency evaluation of ventriculoperitoneal shunt malfunction. Pediatr Emer Care 31(4):239–242
Lee RP, Ajmera S, Thomas F, Dave P, Lillard JC, Wallace D, Broussard A, Motiwala M, Norrdahl SP, Venable GT, Khan NR, Harrell C, Jones TL, Vaughn BN, Gooldy T, Hersh DS, Kilmo P (2019) Shunt failure- the first 30 days. Neurosurg 0(0):1–7
Lehnert BE, Rahbar H, Relyea-Chew A, Lewis DH, Richardson ML, Fink JR (2011) Detection of ventricular shunt malfunction in the ED: relative utility of radiography, CT and nuclear imaging. Emerg Radiol 18(4):299–305
Mater A, Shroff M, Al-Farsi S, Drake J, Goldman RD (2008) Test characteristics of neuroimaging in the emergency department evaluation of children for cerebrospinal fluid shunt malfunction. CJEM 10(2):131–135
Matthews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, Giles GG, Wallace AB, Anderson PR, Guiver TA, McGale P, Cain TM, Dowty JG, Bickerstaffe AC, Darby SC (2013) Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 346:f2360
O’Neill BR, Pruthi S, Bains H, Robison R, Weir K, Ojemann J, Ellenbogen R, Avellino A, Browd SR (2013) Rapid sequence magnetic resonance imaging in the assessment of children with hydrocephalus. World Neurosurg 80(6):e307–e312
Park DB, Hill JG, Thacker PG, Rumboldt Z, Huda W, Ashley B, Hulsey T, Russell WS (2016) The role of limited head computed tomography in the evaluation of pediatric ventriculoperitoneal shunt malfunction. Pediatr Emerg Care 32(9):585–589
Patwardhan RV, Nanda A (2005) Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurg 56(1):139–145
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 280(9840):499–505
Pitetti R (2007) Emergency department evaluation of ventricular shunt malfunction: is the shunt series really necessary? Pediatr Emer Care 23(3):137–141
Razmara A, Jackson EM (2019) Clinical indicators of pediatric shunt malfunction: a population-based study from the nationwide emergency department sample. Pediatr Emer Care 00(00):1–3
Robins JMW, Sivakumar G, Tyagi AK, Chumas PD, Goodden JR (2017) Shunt-series x-rays in pediatric shunt revision. How useful are they? Childs Nerv Syst 33:1828–1853
Sarda S, Simon HK, Hirsh DA, Wang A, Tubbs RS, Chern JJ (2016) Return to the emergency department after ventricular shunt evaluation. J Neurosurg Pediatr 17(4):397–402
Shannon CN, Simon TD, Reed GT, Franklin FA, Kirby RS, Kilgore ML, Wellons JC (2011) The economic impact of ventriculoperitoneal shunt failure. J Neurosurg Pediatr 8(6):593–599
Smyth MD, Narayan P, Tubbs RS, Leonard JR, Park TS, Loukas M, Grabb PA (2008) Cummulative diagnostic radiation exposure in children with ventriculoperitoneal shunts: a review. Child Nerv Syst 24(4):493–497
Sribnick E, Sarda S, Moore M, Capasse M, Tubbs RS, Wrubel D, Chern JJ (2015) Clinical outcome of children with suspected shunt malfunction evaluated in the emergency department. Neurosurg 76(6):695–699
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. The authors declare no conflict of interests.
Ethics
The Leeds (East) Research Ethics Committee has previously confirmed that ‘ethics committee approval is not needed for the collation and analysis of patient data collected over a period of time on a routine basis during normal clinical care, on the understanding that the data is pooled and therefore non-identifiable.’
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tong, L., Higgins, L., Sivakumar, G. et al. ‘Possible shunt malfunction’ pathway for paediatric hydrocephalus—a study of clinical outcomes and cost implications. Childs Nerv Syst 37, 499–509 (2021). https://doi.org/10.1007/s00381-020-04878-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04878-y