Abstract
Purpose
Pediatric primary high-grade spinal glioma (p-HGSG) is an extremely rare disease process, with little data within the current literature. Akin to primary high-grade gliomas, this cancer has been exemplified by dismal prognosis and poor response to modern treatment paradigms. This study seeks to investigate the current trends affecting overall survival using the National Cancer Database (NCDB).
Methods
The NCDB was queried for p-HGSG between 2004 and 2016, by utilizing the designated diagnosis codes. Kaplan-Meier curves were generated, and log-rank testing was performed to analyze factors affecting overall survival. In addition, a Cox proportional-hazards model was used to perform multivariate regression analysis of survival outcomes.
Results
A cohort of 97 patients was identified with a histologically confirmed p-HGSG. The overall incidence of p-HGSG in all pediatric spinal cord tumors is 7.5%, with a mean survival time of 25.3 months (SD, 21.0) and 5-year overall survival of 17.0%. The majority of patients underwent surgery (n = 87, 89.7%), radiotherapy (n = 73, 75.3%), and chemotherapy (n = 60, 61.9%). Univariate, multivariate, and Kaplan-Meier log-rank testing failed to demonstrate an association between performing surgery, extent of resection, radiotherapy, or chemotherapy with improved survival outcomes.
Conclusions
The current study constitutes the largest retrospective analysis of p-HGSGs to date, finding that current treatment options of surgery, radiotherapy, and chemotherapy have unclear benefit. This disease process has a poor prognosis without a current modality of treatment that conclusively alters survival. The risks and side effects of these treatment modalities must be carefully considered in such a highly aggressive disease process, especially given potentially limited survival benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary spinal cord tumors are a relatively rare neoplasm of the central nervous system (CNS) in the pediatric population, occurring as little as 0.30 per 100,000 person-years [1]. These uncommon tumors constitute less than 5% of all pediatric CNS tumors [1, 2]. The intramedullary subtypes, within all spinal cord tumors, occur more frequently in the pediatric population (35%), relative to that of the adult population (20%) [3]. In regard to histology, astrocytic tumors account for majority (60%) of cases in children, whereas, ependymomas encompass the most common pathology found in adults [4]. Due to the rare nature of primary high-grade spinal gliomas (p-HGSGs), there is a paucity of data within the literature that is mainly limited to case reports and small case series. The prognosis of children with high-grade astrocytic tumors is estimated to be as little 7 to 12 months, and p-HGSG follows a similar disease course [5]. Further study of p-HGSG is vital in understanding this disease pathology in hopes of modifying its dismal outcome.
Overall, little is known about the incidence, prognosis, treatment paradigm, and utility of various treatments in survival of children with p-HGSGs. The necessary population-based analysis for p-HGSG that can guide clinical decision-making is currently lacking, due to the low frequency of this disease process. This gap in prospective data leaves the clinicians to extrapolate a combination of surgical, radio-, and chemotherapy options from the more established neoplastic treatment paradigms of similar etiology. The current study of p-HGSG is the largest to date, utilizing the National Cancer Database (NCDB), to elucidate epidemiology, survival risk factors, and effect of current treatment patterns on outcome.
Methods
Study cohort
This study employed the use of the NCDB, a prospectively collected cancer registry maintained jointly by the American College of Surgeons and the American Cancer Society. This database is sourced from over 1500 cancer centers and represents more than 70% of newly diagnosed cancer cases and more than 34 million historical records.
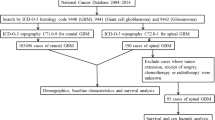
For the purposes of this study, the database was queries for all pediatric patients (age < 18), diagnosed with p-HGSG, between the years 2004 and 2016, by using the International Classification of Disease (ICD) codes. All patients with tumors originating from the spinal cord (primary site code C72.0) were first queried. The histologic subtypes that satisfied p-HGSG, as classified by the ICD for Oncology Third Edition (ICD-O-3) were further delineated. These histologic subtypes included all patients with malignant glioma (9380/3), anaplastic oligoastrocytoma (9382/3), anaplastic ependymoma (9392/3), anaplastic astrocytoma (9401/3), astroblastoma (9430/3), glioblastoma (9440/3), giant cell glioblastoma (9441/3), gliosarcoma (9442/3), and anaplastic oligodendroglioma (9451/3). This study included only pediatric patients for which p-HGSG was recorded as their first and primary tumor. Subsequent tumors, recurrences, and cases diagnosed at autopsy were excluded. Furthermore, all cases that lacked histopathological confirmation were also excluded (Fig. 1).
NCDB data are publicly available and de-identified, and thus did not require review from our Institutional Review Board or patient consent.
Statistical analysis
Descriptive analyses were performed to evaluate patient, tumor, and treatment characteristics. Survival status was the variable employed to assess outcomes in this study. This outcome variable was defined as either alive or not alive (i.e., all-cause mortality). This value was determined as the interval in months between the time of diagnosis and death or last follow-up as reported by NCDB. All available demographic and treatment data were analyzed with respect to survival status.
The student’s t test was used for comparison of all continuous variables, while the Fisher exact test (or c2 test when appropriate) was used for all categorical variables. Kaplan-Meier curves were generated for comparative visualization of various demographic and treatment variables. All-inclusive multivariate Cox proportional hazard regression was used to analyze survival and adjust for confounding variables, as a means of reducing bias. Coefficients in the model were converted to hazard ratios (HR) for analysis and survival rates were calculated for 1-, 3-, and 5-year intervals. All p values were reported as two sided, with statistical significance defined as p < 0.05. Statistical analysis was performed using R statistical software (version 3.4.0, 2017; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline demographics
Over the 13-year period evaluated, 1333 pediatric patients with spinal cord tumors were identified from the NCDB. Amongst this population, 100 patients were identified as having first and primary malignancy, documented as high-grade glioma diagnosis. From this subsequent sub-population, 97 patients were reported to have a histology-confirmed diagnosis (Fig. 1).
The results of this cohort analysis revealed the overall incidence of p-HGSG amongst all pediatric spinal cord tumors to be 7.5%. The most common age groups affected by p-HGSG consisted of patients between 6 and 11 years (n = 42, 43.3%), followed by 12 and 17 years (n = 40, 41.2%). There was a slight male predominance (n = 52, 53.6%), with majority of the population being white (n = 77, 79.4%), followed by black (n = 14, 14.4%), and other (n = 6, 6.2%). Most patients in the cohort (n = 83, 85.6%) were healthy with a Charlson-Deyo score of 0 at the time of diagnosis. While most tumors lacked information regarding size, 1–25 mm was the most common (n = 19, 19.6%) followed by greater than 50 mm (n = 18, 18.6%). Baseline characteristics of the entire population are reported in Table 1.
Treatment characteristics
Surgery was performed in the majority of cases (n = 87, 89.7%) (Table 2). When surgery was not performed, it was either not recommended (n = 9, 9.3%) or not feasible due to comorbidities (n = 1, 1.0%). The majority of the cohort underwent partial resection (n = 44, 45.4%), while gross-total resection occurred in only three cases (3.1%). In a significant portion of patients in which surgery was performed, the extent of resection was unknown (n = 40, 41.2%). On average, surgery was performed 7.1 days (SD, 34.9) after diagnosis, and patients had 12.1 days (SD, 14.9) in the hospital length of stay.
Most patients underwent radiation therapy of various modalities, duration, and fractionation (n = 73, 75.3%) (Table 3). The most common treatment modality was conventional variants of external beam radiation therapy (EBRT) (n = 43, 58.9%), followed by newer variants such as intensity-modulated radiation treatment (IMRT) (n = 16, 21.9%). A small number of patients underwent proton therapy (n = 6, 8.2%). Radiotherapy was started an average of 33.0 days (SD, 22.4) after diagnosis, performed for an average of 19.0 volumes (SD, 15.9), and for an average treatment duration of 30.4 days (SD, 20.9). The mean regional dose used was 3164.4 cGy (SD, 2156.2). A boost treatment was employed in 31 patients, for an average dose of 378.3 cGy (SD. 811.4).
Majority of patients received chemotherapy (n = 60, 61.9%) (Table 3). Single-agent therapy was most common (n = 30, 30.9%), followed by multiple agent therapy (n = 27, 27.8%). Treatment was not administered due to being declined in two cases (2.1%). Chemotherapy was started an average of 37.6 days (SD, 29.9) after diagnosis.
Survival analysis
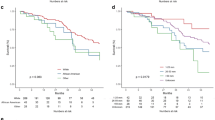
The mean survival time for all patients was 25.3 months (SD, 21.0), and 5-year overall survival was 17.0% (Table 4). On univariate regression, no variables demonstrated a statistically significant association with survival outcomes (p < 0.05). After adjustment for the confounding effects of each variable, multivariate analysis also failed to reveal a significant association of any variable with overall survival (p < 0.05). Most notably, treatment variables such as extent of resection, radiotherapy, and chemotherapy failed to demonstrate an association with overall survival (p < 0.05).
Kaplan-Meier log-rank testing demonstrated similar findings across demographic and treatment variables (Table 5). Log-rank testing did not demonstrate an association between age, gender, race, or tumor size with survival (Fig. 2a–d). The findings of our multivariate analysis were confirmed on log-rank testing in regard to treatment variables, failing to demonstrate and association of extent of resection, radiotherapy, or chemotherapy with overall survival (Fig. 3a–c).
Discussion
Pediatric-HGSG is a rare neoplasm with sparse literature pertaining to its epidemiology. Previous studies have estimated the incidence of various sub-groups of all primary spinal cord tumors, including astrocytic tumors and ependymal tumors [6,7,8]. The results of our analysis suggest that the overall incidence of p-HGSG in all pediatric spinal tumors is approximately 7.5%; this constitutes a significantly higher rate than that found in adult (1.5%) of all adult spinal cord tumors [9]. These unfortunate children represent some of the most challenging cases in oncologic care and suffer a dismal prognosis. In this largest cohort analysis to date, the mean survival of patients with this pathology is estimated to be 25.3 months (SD, 21.0). Due to the rarity in presentation, along with an aggressive natural course, the existing medical literature has demonstrated unclear evidence to the effectiveness of currently available treatment strategies, both in single and multimodal treatments.
In a series of 28 patients by Wolff et al., astrocytic lineage tumors were found to have a predilection for the cervical and thoracic segments of the spine [10]. Investigators reported an average of 87.2 days (SD, 18.2) from the time of first symptom to diagnosis and a median survival time of 2.5 years (SD, 1.6). Analysis of this series suggests that gross-total resection was associated with improved overall survival (p = 0.012), although the event rate of such was relatively low amongst the cohort. These findings are generally more positive and in contrast with a population-based study of the surveillance, epidemiology, and end results (SEER) conducted by Lam et al. that found that neither extent of resection nor radiotherapy to be associated with improved survival outcomes in high-grade spinal astrocytomas [5]. Median survival time in the 48 patient cohort was only 10 months, and younger age identified as a positive prognostic factor. Similar to our epidemiologic findings that most cases were aged between 6 and 18 years (84.5%) in all HGSG, most patients in the SEER study were between 7 and 18 years (81.2%), as well. A systematic review comprised of 53 children diagnosed with primary spinal glioblastoma multiforme by Konar et al. reported that gross total resection with radiotherapy was associated with improved survival outcomes in comparison with subtotal resection and radiotherapy by log-rank testing (p = 0.04) [11]. In addition, the investigators found that the presence of adjuvant therapy (either radiotherapy or chemotherapy) was associated with improvements in survival outcome by the same methodology (p = 0.01).
Overall, the existing literature has been unclear on adjuvant therapy in regard to specific regimens and evidence of effectiveness. Allen et al. reported a series of 18 children that were trialed with multi-agent therapy consisting of 8 chemotherapeutic agents in conjunction with postoperative radiotherapy to limited benefit [12]. Tendulkar et al. reported on the relatively poor prognosis found in patients receiving postoperative radiotherapy, noting a propensity of neuraxis metastasis as a component of progression after radiotherapy [13]. These investigators employed generally higher radiation doses (median = 5220 cGy) that is more in line with cranial high grade glioma treatment regimen, compared with the results of this analysis (mean = 3164.4 cGy). Of note, craniospinal radiation was also used in several patients, while this detail was unable to be assessed from the NCDB dataset. Guss et al. demonstrated similar findings in the treatment of five high-grade astrocytic lesions, reporting one patient surviving at last follow-up at 17 months and a median progression-free survival of 14 months [14]. These researchers noted that the side-effect profile was generally mild with only low-grade acute toxicities. This analysis also showed six cases of proton therapy utilized in the p-HGSG; while this has been unreported in the literature, it has been investigated in pediatric cranial glioma [15].
The current study constitutes the largest retrospective analysis of pediatric high-grade spinal glial neoplasm of any histology type, with several significant findings. Furthermore, there is great generalizability of the findings given that the NCDB data utilizes patients treated at cancer centers around the USA capable of providing access to modern treatment paradigms in regard to microsurgical resection and adjuvant therapy. Our investigation suggests that p-HGSG in children may not be a disease process that is amenable to effective surgical resection that correlates with significantly prolonged overall survival. Univariate, multivariate, and log-rank analysis of performing surgery and extent of resection were not associated with improved survival outcomes. Surgical intervention beyond a biopsy should be cautiously considered in light of the likelihood of recurrence, infiltrative process, poor margins, potential for functional neurological compromise, and limited survival benefit. Further, resection may impair a child’s remaining quality of life in a terminal disease. Constantini et al. reported in a large series of 164 pediatric patients with intramedullary spinal cord tumors a 23.8% rate of neurologic deterioration after surgical resection, notably finding elevated risk of post-operative deficits in patients with high-grade lesions (p = 0.005) [16]. Interestingly, however, extent of resection was not associated with increased incidence of post-operative deficits in this series, suggesting that other tumor-specific factors such as local invasiveness may play a significant role.
Our investigation also failed to show a demonstrable impact on survival with radiotherapy. Our results indicate a wide gamut of radiotherapy regimens employed by providers in regard to the treatment dose, duration, fractions, and modality. This finding underscores the lack of evidence and guidelines in utilizing radiotherapy for treating this pathology, within the pediatric population. Similarly, adjuvant therapy with chemotherapy did not demonstrate a significant impact on survival. Notably, these options, as it relates to adjuvant therapy, can sometimes have significant side effect profiles and should be carefully weighed in light of potentially limited benefits.
There exist a number of limitations that governs the current study. The use of NCDB, a large registry-based dataset, renders our analysis vulnerable to factors that are not routinely collected. For instance, a large number of patients did not have information regarding tumor size, limiting the power of the assessment of tumor size on overall survival. The NCDB data also does not provide information regarding the specific location of tumor in the spine, rendering analysis of such clinically and therapeutically relevant information impossible. In addition, specific coding patterns in regard to extent of resection are unavailable and may differ significantly between cases. For example, the subtotal resection group is assumed to be rather heterogenous in regard to exact extent of resection, combining tumors that are nearly completely resected (yet not coded as gross total resection) with tumors that undergo minimal resection. Thus, the possibility that some patients may benefit from surgery cannot be ruled out. Moreover, the database used lacks independent validation with data such as imaging studies and pathologic diagnoses. Similarly missing is information regarding the decision-making process on adjuvant therapy.
The role of selection bias is also an important consideration in our study. For instance, patients with lesions that are more amenable to successful resection due to location, invasiveness, and margins might actually demonstrate improved outcomes due to these lesion-specific factors. This potential effect of selection bias should also be mentioned in interpreting the previously published literature in this population. While these studies have attempted an analysis of the effect of surgical resection or other treatment strategies, the data presented is generally not granular enough for more in-depth analysis on important tumor-specific factors—especially in regard to difficult-to-define characteristics such as invasiveness. For the results of this analysis, all high-grade glial neoplasms were amalgamated to allow for greater generalizability of our analysis. However, a more specific approach in either histology or WHO grading may offer additional insight, with the simultaneous drawback of more limited cohort size. Finally, we have employed a non-parametric Kaplan-Meier estimator (product limit approach) to generate survival curves. This statistical methodology is limited by varying and incomplete follow-up times and data in a portion of the patients. Thus, significant data censoring can result in curves that are most effective in comparing parameters but not necessarily in deriving exact survival percentages.
Despite these drawbacks, our study provides a description of the largest sample of pediatric primary HGSG patients to date. The usage of a national cancer registry affords significant power to analysis of epidemiology and current treatment patterns and associated effects on survival; however, the usage of such large database does not substitute the need for robust, prospective clinical trials in comparing treatment strategies. From this analysis, current treatment modalities offer unclear impact on overall survival. Consequently, other experimental therapies such as novel drug targeting techniques or immunotherapy models may hold the key in this challenging disease process [17, 18]. Further advances in our understanding of the complex pathobiology of glioma and its proteogenomic characterization will allow for molecular diagnostics, in addition to emerging next-generation molecular therapeutic strategies [19, 20].
Conclusion
The current study constitutes the largest retrospective analysis of pediatric high-grade spinal glial neoplasm of any histology-type finding that current treatment options of surgery, radiotherapy, and chemotherapy have unclear benefit. This disease process has a poor prognosis without a current modality of treatment that conclusively alters survival. The risks and side effects of these treatment modalities must be carefully considered in such a highly aggressive disease process, especially given potentially limited survival benefits.
References
Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS (2019) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro-Oncol 21:v1–v100. https://doi.org/10.1093/neuonc/noz150
DeSousa AL, Kalsbeck JE, Mealey J et al (1979) Intraspinal tumors in children. A review of 81 cases. J Neurosurg 51:437–445. https://doi.org/10.3171/jns.1979.51.4.0437
Constantini S, Houten J, Miller DC, Freed D, Ozek MM, Rorke LB, Allen JC, Epstein FJ (1996) Intramedullary spinal cord tumors in children under the age of 3 years. J Neurosurg 85:1036–1043. https://doi.org/10.3171/jns.1996.85.6.1036
Reimer R, Onofrio BM (1985) Astrocytomas of the spinal cord in children and adolescents. J Neurosurg 63:669–675. https://doi.org/10.3171/jns.1985.63.5.0669
Lam S, Lin Y, Melkonian S (2012) Analysis of risk factors and survival in pediatric high-grade spinal cord astrocytoma: a population-based study. Pediatr Neurosurg 48:299–305. https://doi.org/10.1159/000353135
Benesch M, Frappaz D, Massimino M (2012) Spinal cord ependymomas in children and adolescents. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 28:2017–2028. https://doi.org/10.1007/s00381-012-1908-4
Kutluk T, Varan A, Kafalı C, Hayran M, Söylemezoğlu F, Zorlu F, Aydın B, Yalçın B, Akyüz C, Büyükpamukçu M (2015) Pediatric intramedullary spinal cord tumors: a single center experience. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc 19:41–47. https://doi.org/10.1016/j.ejpn.2014.09.007
Rossitch E, Zeidman SM, Burger PC et al (1990) Clinical and pathological analysis of spinal cord astrocytomas in children. Neurosurgery 27:193–196. https://doi.org/10.1097/00006123-199008000-00003
Alvi MA, Ida CM, Paolini MA, Kerezoudis P, Meyer J, Barr Fritcher EG, Goncalves S, Meyer FB, Bydon M, Raghunathan A (2019) Spinal cord high-grade infiltrating gliomas in adults: clinico-pathological and molecular evaluation. Mod Pathol Off J U S Can Acad Pathol Inc 32:1236–1243. https://doi.org/10.1038/s41379-019-0271-3
Wolff B, Ng A, Roth D, Parthey K, Warmuth-Metz M, Eyrich M, Kordes U, Kortmann R, Pietsch T, Kramm C, Wolff JEA (2012) Pediatric high grade glioma of the spinal cord: results of the HIT-GBM database. J Neuro-Oncol 107:139–146. https://doi.org/10.1007/s11060-011-0718-y
Konar SK, Bir SC, Maiti TK, Nanda A (2017) A systematic review of overall survival in pediatric primary glioblastoma multiforme of the spinal cord. J Neurosurg Pediatr 19:239–248. https://doi.org/10.3171/2016.8.PEDS1631
Allen JC, Aviner S, Yates AJ, Boyett JM, Cherlow JM, Turski PA, Epstein F, Finlay JL, _ _ (1998) Treatment of high-grade spinal cord astrocytoma of childhood with “8-in-1” chemotherapy and radiotherapy: a pilot study of CCG-945. Children’s Cancer Group. J Neurosurg 88:215–220. https://doi.org/10.3171/jns.1998.88.2.0215
Tendulkar RD, Pai Panandiker AS, Wu S, Kun LE, Broniscer A, Sanford RA, Merchant TE (2010) Irradiation of pediatric high-grade spinal cord tumors. Int J Radiat Oncol Biol Phys 78:1451–1456. https://doi.org/10.1016/j.ijrobp.2009.09.071
Guss ZD, Moningi S, Jallo GI, Cohen KJ, Wharam MD, Terezakis SA (2013) Management of pediatric spinal cord astrocytomas: outcomes with adjuvant radiation. Int J Radiat Oncol Biol Phys 85:1307–1311. https://doi.org/10.1016/j.ijrobp.2012.11.022
Indelicato DJ, Rotondo RL, Uezono H, Sandler ES, Aldana PR, Ranalli NJ, Beier AD, Morris CG, Bradley JA (2019) Outcomes following proton therapy for pediatric low-grade Glioma. Int J Radiat Oncol Biol Phys 104:149–156. https://doi.org/10.1016/j.ijrobp.2019.01.078
Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ (2000) Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg 93:183–193. https://doi.org/10.3171/spi.2000.93.2.0183
Kheirkhah P, Denyer S, Bhimani AD, Arnone GD, Esfahani DR, Aguilar T, Zakrzewski J, Venugopal I, Habib N, Gallia GL, Linninger A, Charbel FT, Mehta AI (2018) Magnetic drug targeting: a novel treatment for intramedullary spinal cord tumors. Sci Rep 8:11417. https://doi.org/10.1038/s41598-018-29736-5
Wang SS, Bandopadhayay P, Jenkins MR (2019) Towards immunotherapy for pediatric brain tumors. Trends Immunol 40:748–761. https://doi.org/10.1016/j.it.2019.05.009
Rajesh Y, Pal I, Banik P, Chakraborty S, Borkar SA, Dey G, Mukherjee A, Mandal M (2017) Insights into molecular therapy of glioma: current challenges and next generation blueprint. Acta Pharmacol Sin 38:591–613. https://doi.org/10.1038/aps.2016.167
Alinezhad A, Jafari F (2019) Novel management of glioma by molecular therapies, a review article. Eur J Transl Myol 29. https://doi.org/10.4081/ejtm.2019.8209
Funding
The grant support from the AOSPine Innovation Grant (awarded to senior author) was utilized for funding this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nunna, R.S., Khalid, S., Behbahani, M. et al. Pediatric primary high-grade spinal glioma: a National Cancer Database analysis of current patterns in treatment and outcomes. Childs Nerv Syst 37, 185–193 (2021). https://doi.org/10.1007/s00381-020-04722-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04722-3