Abstract
Purpose
The aim of this study is for the surgical treatment and outcome of the endoscopic fenestration of the arachnoid cyst located in the ventricular body to trigone in the pediatric population. Special concern was paid for the developmental origin of the intraventricular cysts estimated from the postoperative follow-up neuroimagings.
Patients and methods
Between July 2002 and June 2015, we performed endoscopic and partly CT/MRI navigated fenestrations of intraventricular arachnoid cysts located at the body to trigone of the lateral ventricle in ten pediatric patients aged 2 months to 5 years. Based on the long axis of the cyst, we have opted for two surgical approaches: anterior approach via burr hole at Kocher’s point and posterior approach via burr hole at the posterior occipital region. Fenestration was performed based on the intraoperative findings, either ventriculocystostomy, ventriculocystoventriculostomy, or ventriculocystocisternostomy.
Results
Intraventricular arachnoid cysts located in the body-trigone region showed a favorable outcome after endoscopic fenestration. All of the cysts shrank postoperatively. Follow-up neuroimagings taken between 6 and 126 months after surgery strongly suggested its relationship with the midline cisterns. Of our ten cases, eight were suggestive for originating from the velum interpositum cistern while two seemed to root from the quadrigeminal cistern.
Conclusion
In the present study, we found that endoscopic fenestration of intraventricular arachnoid cysts in the body to trigone is a safe procedure with a satisfactory outcome. In our limited experience, there are two anatomic backgrounds; velum interpositum cistern and quadrigeminal cistern. Differentiation can be possible by neuroimagings, especially those obtained after surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital arachnoid cysts are a central nervous system malformation that accounts for about 1 % of all intracranial masses in newborns. They are benign accumulation of clear fluid within the arachnoid membrane, and most of them do not communicate with the subarachnoid space. Cerebral arachnoid cysts are mainly located in the middle and posterior fossa while the ventricular region is a relative rare location [2]. Pediatric patients with an intracranial arachnoid cyst are more likely to undergo surgery if the cyst is large, compresses a narrow CSF flow pathway to cause hydrocephalus, or has ruptured/hemorrhaged [3, 13]. Surgical procedures include cystoperitoneal shunt placement [18, 20], stereotactic aspiration [25], excision [5, 24], and open [19, 27] or endoscopic fenestration [9, 12, 14, 15, 16, 21, 26]. Due to continuous advances in neurosurgical techniques and neuroendoscopy, neuroendoscopic fenestration tends to be the treatment of choice for initial cyst decompression [23]. In a retrospective study by Tamburrini et al., of 26 patients (21 children) who underwent endoscopic management of intra and paraventricular cysts, control of clinical symptoms was obtained in around 80 % of the patients, while radiological evidence of cyst size reduction occurred in more than 95 % of them [28]. However, Cinalli et al. reported in 32 out of 231 cases (13.8 %) complications recorded in a prospectively collected database of pediatric patients undergoing neuroendoscopic procedures [8].
In the present study, we report about the surgical outcome of endoscopic fenestration of intraventricular arachnoid cysts in the ventricular body to trigone. Furthermore, we investigated the origin of intraventricular arachnoid cysts in this particular location based on long-term postoperative MRI follow-up.
Patients and methods
Between July 2002 and July 2015, the senior author (NM) performed 363 pediatric neuroendoscopic surgeries in 284 children. Among 66 fenestrations, there were ten children with intraventricular arachnoid cysts located at the body to trigone of the lateral ventricle. Our study group consists of eight boys and two girls aged 2 months to 5 years (median age was 4 months). Eight patients in our series were harboring a velum interpositum cyst. In these eight cases, we performed a posterior approach. The other two cases had a quadrigeminal cistern cyst and were operated from an anterior approach. Since 2011, we were using CT/MRI navigation, which means the last six surgeries have been navigation-assisted. Ethics board approval for the study was given by the Tokyo Metropolitan Children’s Medical Center committee. The clinical and surgical data of our study group are summarized in Table 1.
We used a flexible neuroendoscope with a diameter of the scope of 4.8 mm and a working channel of 2.0 mm. It was equipped with a CCD at the tip (Olympus VEF type V, Tokyo, Japan). This flexible neuroendoscope provided a high-quality visual image competitive with that of a rigid scope.
There were two surgical approaches we used based on the long axis of the cyst or the shortest distance from the cortex to the cyst for opening a burr hole. Hereby, damage to the eloquent cortex must be avoided. The anterior approach was done in the typical way via burr hole at Kocher’s point. The patient’s head was placed on a horseshoe rest or a Sugita head frame. In the posterior approach, the patient was placed in prone position in the same way. When approached from posterior, we did a high parietal to occipital approach aiming to take the shortest tract to the ventricle towards the anterior horn. In this case, the axis of the trajectory pointed to the anterior horn and the fenestration was done from trigone to cyst or from trigone to cyst to anterior horn. This was the preferred approach in large ventricular cyst occupying the body to trigone of the lateral ventricle (Fig. 1). If the cyst had a thick wall, it was opened by a dot-to-dot fenestration, which means several small holes were opened to form a circle, and the central part of the circle was removed using a biopsy forceps. In some cases, we performed a balloon widening followed by electrical cutting and/or removal of the cyst wall by a biopsy forceps. Carefully, hemostasis was performed, and hematoma if present, was aspirated from the working channel as much as possible. At the end of the endoscopic procedure, the pathway was packed with absorbable hemostatic materials to shut down the communication to the subdural space [6].
Surgical procedure (posterior approach): Prone position with fixation in Sugita head frame, MRI/CT navigation (since 2011), and burr hole is placed high parietal to occipital, the shortest trajectory to the ventricle towards the anterior horn was chosen on preoperative MRI. White arrow: surgical approach, white broken arrow: direction of cyst fenestration into the ventricle
Results
The neuroendoscopic fenestration involved, based on the location and size of the cyst, ventriculocystostomy (VC) in four cases, ventriculocystoventriculostomy (VCV) (Fig. 2) in five cases, and ventriculocystocisternostomy (VCC) in one case. The indication for surgery was seizure onset in two patients, hydrocephalus in one patient, and mass effect or slow-growing clinical course in seven patients. The surgical outcome was excellent as all cysts markedly shrank postoperatively, which was confirmed on postoperative images.
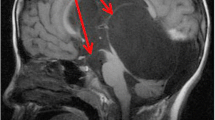
During follow-up of the patients which was between six and 126 months, we observed no recurrence so far. No surgical complications such as hemorrhage, meningitis, CSF leak, or subdural fluid collection have been noted. In two patients, temporary seizures occurred perioperatively but no special medication was needed. In the postoperative follow-up CT/MRI, we found that in eight of our ten patients, the cyst shrank towards the velum interpositum cistern (Fig. 3), while in two cases it retracted towards the quadrigeminal cistern (Fig. 4).
Pre and postoperative MRI suggesting the intraventricular arachnoid cyst originating from the velum interpositum cistern. Upper: MR images of 3-year-old boy (case 1): coronal MRI images of pre (left) and postop 9 years (right) demonstrating the cyst has markedly shrunk. Only a remnant of the cyst stays in the velum interpositum cistern where it originated from. Lower: MR images of 5-year-old boy (case 7): pre (left) and postop. One and a half years (right) also showed the cyst had retreated into the velum interpositum cistern. Note that the internal cerebral veins locate ventrocaudal side of the cyst in both cases. Black star: intraventricular cyst, black arrow: internal cerebral veins, white star: retreated cyst in the cavum interpositum
Pre and postoperative CT suggesting the intraventricular arachnoid cyst (case 8) originating from the quadrigeminal cistern. Preoperative contrast enhanced CT a demonstrated the cyst extending to the left side while compressing the internal cerebral veins (white arrow) contralateral superolateral direction. Postoperative MRI b taken 10 days after surgery showed shrinkage of the cyst which retreated towards the quadrigeminal cistern
Discussion
Intracerebral arachnoid cysts are benign accumulation of clear fluid within the arachnoid membrane. The origin of arachnoid cysts as well as the cause of cyst enlargement are discussed controversially in the literature. A ball valve mechanism, an osmotic gradient between the intra and extracystic medium, primary malformation of the arachnoid membrane or cerebral lobe agenesis, and fluid hypersecretion by the lining cells of the cyst wall have been proposed [4]. Intraventricular cysts can be classified as of endodermal origin (neurenteric cyst, colloid cyst), of exodermal origin (ependymal cyst, choroid plexus cyst, interhemispheric cyst, Rathke’s cyst, pineal cyst, and cyst of septum pellucidum) or of mesenchymal origin which are arachnoid cysts [29]. Their treatment by neuroendoscopy has been arising as the latest surgical tactics. Because of a usually wide working space which is secured by the cyst itself or enlarged ventricle neuroendoscopic surgery is regarded as the best treatment for symptomatic arachnoid cysts. A better endoscopic surgical outcome was found in arachnoid cysts in the suprasellar and quadrigeminal region with up to 90 % and in the posterior cranial fossa with over 80 % success rate compared with sylvian (70 %) and cortical and interhemispheric (75 %) regions [14]. It should be reminded that treatment of arachnoid cysts from the quadrigeminal cistern could be not straightforward and may require repeated surgeries [9]. A review of previous cases published of intraventricular arachnoid cysts is summarized in Table 2.
The ventricular region is a relatively rare location for arachnoid cysts. Al-Holou et al. reported only 0.3 % located in the ventricle among 309 cases of intracranial arachnoid cysts [2]. The ventricles though are not containing arachnoid membranes but they are lined with ependyma. It has remained an unanswered question from where those intraventricular arachnoid cysts originate. In the present study, we evaluated two anatomical backgrounds that may contribute to the development of intraventricular arachnoid cysts by analyzing follow-up neuroimages after neuroendoscopic cyst fenestration. Our findings suggest that the intraventricular cysts were either originating from the velum interpositum cistern or from the quadrigeminal cistern.
The different origins of cysts in and around the lateral ventricle are illustrated in Fig. 5.
Illustration of proposed anatomical origins of lateral ventricle arachnoid cysts, velum interpositum arachnoid cysts and choroid plexus cyst. The lateral ventricle arachnoid cyst (7) is originating from the velum interpositum cistern (1) and growing laterally in to the lateral ventricle (8). The velum interpositum arachnoid cyst is growing in the midline (9). The choroid plexus cyst is also growing laterally with no extension into the velum interpositum cistern (10, 11)
The velum interpositum cistern is the space between the two layers of the tela choroidea of the third ventricle. It extends from the habenular commissure to the foramen of Monro. The roof is formed by the splenium of the corpus callosum and the floor is formed by the roof of the third ventricle. The anterior wall of the velum interpositum cistern converges below the fornix to a point at the foramen of Monro, and the posterior wall has no clear distinction from the quadrigeminal cistern [1]. Some authors previously proposed the tela choroidea, which develops as fusion of two layers of “pia-arachnoid”, as a possible origin of the velum interpositum arachnoid cyst [11]. Funaki et al. suggested that velum interpositum cysts are originating from the paramedian wall of the velum interpositum [11]. Based on this theory an arachnoid cyst that has its origin laterally may extent through the choroidal fissure to the lateral ventricle when growing. As the lateral ventricle is larger than the velum interpositum cistern, this cyst becomes more prominent.
A velum interpositum cistern cyst has to be distinguished from an arachnoid cyst originating from the quadrigeminal cistern, which can mimic that of the velum interpositum if it extends in the superoanterior direction [22]. Cinalli et al. have proposed a classification of quadrigeminal cistern cysts as follows: Type I, cysts with supratentorial and infratentorial extension; Type II, cysts with infratentorial extension (supracerebellar or supraretrocerebellar); and Type III, cysts with lateral extension towards the temporal lobe [9]. A velum interpositum cyst never extends to the infratentorial space but is directed mainly onto the lateral ventricular body.
We suggest that based on the analysis of neuroimagings (CT/MRI), it would be possible to describe the origin of an arachnoid cyst beforehand. We differentiated cysts originating from velum interpositum cistern from those originating from the quadrigeminal cistern by the long axis of the cyst. A cyst originating from the velum interpositum cistern is usually located parietal at the long axis of the ventricle. A quadrigeminal cyst extends from the midbrain in a parietal direction towards the corpus callosum. During its extension it can grow laterally into the lateral ventricle, which would correspond to a quadrigeminal cistern cyst type III of the Cinalli-Classification, and thus mimicking a velum interpositum cyst.
A velum interpositum cistern cyst is estimated to originate from the upper layer of the tela choroidea. Histologically, the cyst wall is arachnoid origin but can be covered with the ependymal layer when it protrudes into the lateral ventricle through the choroidal fissure. The internal cerebral veins are shifted inferiorly due to compression by the cyst. In contrast to this, a cyst of the quadrigeminal cistern displaces the internal cerebral veins upward because the arachnoid membrane in the quadrigeminal cistern is topographically below the internal cerebral veins (Fig. 6).
Arachnoid cysts of the quadrigeminal cistern have a close relationship to the posterior midbrain, which causes distortion or compression of the aqueduct leading to hydrocephalus, whereas in cysts deriving from the velum interpositum cistern hydrocephalus is a rare finding [7, 9]. This is the reason why arachnoid cyst of the quadrigeminal cistern is usually operated via anterior approach while that of the velum interpositum via posterior approach [9]. In our study group we had one patient with hydrocephalus, where we favored an anterior approach, and in the follow-up images we estimated the quadrigeminal cistern as origin of the cyst. The other patient that was treated by an anterior approach had no signs of hydrocephalus except a mild ventriculomegaly and had also a quadrigeminal cistern cyst.
There may be also some transitional forms that are growing from quadrigeminal cistern to velum interpositum cistern as the posterior wall of the velum interpositum cistern has no clear distinction from the quadrigeminal cistern [1].
Beyond the extension of the cyst, it is actually considered relevant in the diagnostic workout of intraventricular arachnoid cysts to complete MR studies with an angio-MR in order to directly verify the deep veins in relation to the cyst. However, even without an angio-MRI, T2 coronal views would demonstrate detailed anatomical relationship between the cyst and internal cerebral veins, which helps to differentiate each cyst.
The indication for surgery in our study group included the presence of neurological symptoms, mass effects, presence of hydrocephalus/ventriculomegaly, and chronological growth of cyst size. Size of a cyst matters. Guissani et al. presented a case of a pediatric patient with a large velum interpositum cistern arachnoid cyst experiencing episodes of hypertonic loss of consciousness due to intermittent occlusions of the foramen of Monro by the cyst. Endoscopic fenestration of the cyst resolved the symptoms [17]. Although D’Addario et al. reported about a series of five fetuses with velum interpositum cistern arachnoid cyst who demonstrated no postnatal growth of the cyst, our five among ten cases showed substantial growth of the cyst as a large mass extending from the ventricle to periventricular parencyme after birth [10]. It should be reminded that three of five growing cysts were diagnosed at fetal period. It seems that the intraventricular cyst can grow its size and careful follow up by neuroimaging studies are strongly recommended even if the child is asymptomatic.
Conclusion
Intraventricular arachnoid cysts located in the body-trigone region showed a favorable outcome after endoscopic fenestration, especially when combined with CT/MRI navigation. There has been no difference in the surgical outcome of the different fenestration techniques (cyst to ventricle, ventricle to cyst to ventricle or ventricle to cyst to cistern). On long-term follow-up neuroimagings, we found that most intraventricular arachnoid cysts seemed to originate from the velum interpositum cistern and some seemed to extend from the quadrigeminal cistern. Since clinical presentation, course and surgical outcome are different, preoperative differentiation of each arachnoid cyst would be strongly recommended.
References
Adeeb N, Deep A, Griessenauer CJ, Mortazavi MM, Watanabe K, Loukas M, Tubbs RS, Cohen-Gadol AA (2013) The intracranial arachnoid mater: a comprehensive review of its history, anatomy, imaging, and pathology. Childs Nerv Syst 29:17
Al-Holou WN, Yew AY, Boomsaad ZE, Garton HJ, Muraszko KM, Maher CO (2010) Prevalence and natural history of arachnoid cysts in children. J Neurosurg Pediatrics 5:578–585
Ali M, Bennardo M, Almenawer SA, Zagzoog N, Smith AA, Dao D, BHSc, Ajani O, Farrokhyar F, Singh SK (2015) Exploring predictors of surgery and comparing operative treatment approaches for pediatric intracranial arachnoid cysts: a case series of 83 patients. J Neurosurg Pediatr 12:1–8
Basaldella L, Orvieto E, Dei Tos AP, Della Barbera M, Valente M, Longatti P (2007) Causes of arachnoid cyst development and expansion. Neurosurg Focus 22(2):E4
Boockvar JA, Shafa R, Forman MS, O’Rourke DM (2000) Symptomatic lateral ventricular ependymal cysts: criteria for distinguishing these rare cysts from other symptomatic cysts of the ventricles: case report. Neurosurgery 46:1229–1233
Cappabianca P, Cappabianca P, Cinalli G, Gangemi M, Brunori A, Cavallo LM, de Divitiis E, Decq P, Delitala A, Di Rocco F, Frazee J, Godano U, Grotenhuis A, Longatti P, Mascari C, Nishihara T, Oi S, Rekate H, Schroeder HW, Souweidane MM, Spennato P, Tamburrini G, Teo C, Warf B, Zymberg ST (2008) Application of neuroendoscopy to intraventricular lesions. Neurosurgery 62(Suppl 2):575–598
Cinalli G, Spennato P, Cianciulli E, D’Armiento M (2004) Hydrocephalus and aqueductal stenosis. In: Cinalli G, Maixner WJ (eds) Saint-Rose C Pediatric hydrocephalus. Springer, Milan, pp. 279–293
Cinalli G, Spennato P, Ruggiero C, Aliberti F, Trischitta V, Buonocore MC, et al. (2007) Complications following endoscopic intracranial procedures in children. Childs Nerv Syst 23(6):633–644
Cinalli G, Spennato P, Columbano L, Ruggiero C, Aliberti F, Trischitta V, et al. (2010) Neuroendoscopic treatment of arachnoid cysts of the quadrigeminal cistern: a series of 14 cases. J Neurosurg Pediatrics 6:489–497
D’Addario V, Pinto V, Rossi AC, Pintucci A, Di Cagno L (2009) Cavum veli interpositi cyst: prenatal diagnosis and postnatal outcome. Ultrasound Ostet Gynecol 34:52–54
Funaki T, Makino Y, Arakawa Y, Hojo M, Kunieda T, Takagi Y, et al. (2012) Arachnoid cyst of the velum interpositum originating from tela choroidea. Surg Neurol Int 3:120
Gaab MR, Schroeder HW (1999) Neuroendoscopic approach to intraventricular lesions. Neurosurg Focus 6:E5
Gallassi E, Tognetti F, Gaist G, Fagioli L, Frank F, Frank G (1982) CT scan and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: classification and pathophysiological aspects. Surg Neurol 17:363
Gangemi M, Seneca V, Colella G, Cioffi V, Imperato A, Maiuri F (2011) Endoscopy versus microsurgical cyst excision and shunting for treating intracranial arachnoid cysts. J Neurosurg Pediatr 8(2):158–164
Gangemi M, Maiuri F, Colella G, Sardo L (1999) Endoscopic surgery for intracranial cerebrospinal fluid cyst malformations. Neurosurg Focus 6(4):E6
Greenfield JP, Souweidane MM (2005) Endoscopic management of intracranial cysts. Neurosurg Focus 19:E7
Giussani C, Fiori L, Trezza A, Riva M, Sganzerla EP (2011) Cavum veli interpositi: just an anatomical variant or a potentially symptomatic CSF compartmentalization? Pediatr Neurosurg 47:364–368
Hervey-Jumper SL, Ziewacz JE, Heth JA, Sullivan SE (2010) Frontal- to-temporal horn shunt as treatment for temporal horn entrapment. J Neurosurg 112:410–413
Levy ML, Wang M, Aryan HE, Yoo K, Meltzer H (2003) Microsurgical keyhole approach for middle fossa arachnoid cyst fenestration. Neurosurgery 53:1138–1144
Maurice-Williams RS, Choksey M (1986) Entrapment of the temporal horn: a form of focal obstructive hydrocephalus. J Neurol Neurosurg Psychiatry 49:238–242
Oertel JM, Wagner W, Mondorf Y, Baldauf J, Schroeder HW, Gaab MR (2010) Endoscopic treatment of arachnoid cysts: a detailed account of surgical techniques and results. Neurosurgery 67:824–836
Park SW, Yoon SH, Cho KH, Shin YS (2006) A large arachnoid cyst of the lateral ventricle extending from the supracerebellar cistern - case report. Surg Neurol 65:611–614
Pradilla G, Jallo G (2007) Arachnoid cysts: case series and review of the literature. Neurosurg Focus 22:E7
Pawar SJ, Sharma RR, Mahapatra AK, Dev EJ (2001) Giant ependymal cyst of the temporal horn - an unusual presentation. Case report with review of the literature. Pediatr Neurosurg 34:306–310
Ross DA, Muraszko K, Dauser R (1994) A special cyst puncture catheter for use in thick-walled or mobile intracranial cysts. Neurosurgery 34:191–192
Schulz M, Bohner G, Knaus H, Haberl H, Thomale UW (2010) Navigated endoscopic surgery for multiloculated hydrocephalus in children. J Neurosurg Pediatr 5:434–442
Silav G, Sarı R, Bölükbaşı FH, Altaş M, Işık N, Elmacı İ (2015) Microsurgical fenestration and cystoperitoneal shunt through preauricular subtemporal keyhole craniotomy for the treatment of symptomatic middle fossa arachnoid cysts in children. Childs Nerv Syst 31:87–93
Tamburrini G, D’Angelo L, Paternoster G, Massimi L, Caldarelli M, Di Rocco C (2007) Endoscopic management of intra and paraventricular CSF cysts. Childs Nerv Syst 23:645–651
Yoshioka S (2014) Intracranial cystic disease: a review. Nerv Syst Children 37:398–408 Japanese
Acknowledgements
The clinical fellowship of BK at TMCMC was supported by the Carl Zeiss scholarship from the German Society of Neurosurgery.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest declared by the authors.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Knie, B., Morota, N., Ihara, S. et al. Pediatric intraventricular arachnoid cysts in the body of lateral ventricle: surgical outcome and its embryologic background. Childs Nerv Syst 32, 2197–2204 (2016). https://doi.org/10.1007/s00381-016-3203-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-016-3203-2