Abstract
Introduction
Giant cavernous malformation (GCM) in children is a rare vascular anomaly, and its natural history is unclear. Despite their giant size, intraparenchymal GCMs are low-flow vascular malformations. Herein, we report a case of hyper-vascular intraparenchymal GCM with an AV shunt in a child.
Case
A 3-year-old boy had had an enlarged head since infancy. Magnetic resonance (MR) images on admission showed a strikingly enhanced mass lesion, 6 cm in size. A 4-vessel CAG demonstrated a hyper-vascular mass with an AV shunt. After transarterial embolization, the patient underwent total excision of the mass. The tumor bled easily, during surgery the patient lost 400 cm3 in blood. Histopathological examination confirmed the diagnosis of cavernous hemangioma.
Conclusion
The differential diagnosis of intraparenchymal, strikingly-enhanced tumors with an AV shunt include hyper-vascular GCMs. Consideration of potential for bleeding during the operation is also important.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cavernous malformations (CMs), also termed cavernomas and cavernous angiomas, have an incidence of 0.4–0.9 % [1]. Thirty-seven percent of CM patients present with seizures, 36 % with hemorrhage, 23 % with headaches, 22 % with focal neurological deficits, while 10 % are asymptomatic. Magnetic resonance imaging (MRI) on T2 weighted images (T2WI) show a complicated central core and a peripheral rim of decreased signal intensity due to hemosiderin deposition in the surrounding parenchyma [1]. Giant cavernous malformations (GCMs) are defined as a lesion larger than 4 cm [2, 3]. GCM in children is a rare vascular anomaly, and its natural history is unclear. Dural GCMs were reported to be a hyper-vascular lesion [4, 5]. However, despite their giant size, intraparenchymal GCMs are low-flow vascular malformations [2, 3, 6]. GCMs are rarely investigated with an angiogram or undergo preoperative embolization, and to our knowledge, there are no reports regarding a hyper-vascular intraparenchymal GCM with an arteriovenous shunt (AV shunt). Herein, we report a case of hyper-vascular intraparenchymal GCM with an AV shunt in a child and review the relevant literature.
Case report
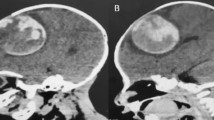
A 3-year-old right-handed boy had had an enlarged head since infancy. His head circumference at 3 years old was 54.3 cm (more than 97th percentile). He was referred to our hospital for macrocephaly. On examination, he had no neurologic symptoms or visual disturbances. His family history was unremarkable. Computed tomography (CT) images and magnetic resonance (MR) images (Fig. 1) on admission showed a strikingly enhanced mass lesion, 6 cm in size, accompanied by a posterior giant cyst. T2WI showed plural flow voids and a dilated drainage vein from the tumor. An astrocytoma, hemangioblastoma, and choroid plexus papilloma was our initial diagnosis. We decided to treat the lesion as it was symptomatic macrocephaly, and we considered that it would interfere with his growth and development. And the lesions location was not difficult to approach.
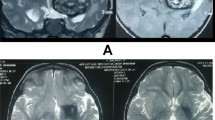
a CT images showed a large, isodense tumor in the trigone of the lateral ventricle with punctate calcification. b, c T2 weighted (b), contrast enhanced T1 weighted (c), and MR 3D images showing the cut vessels on the right side. d A–P view. e R–L view. MR images showed a strikingly enhanced mass lesion, 6 cm in size, beside the trigone of the lateral ventricle, accompanied by a posterior giant cyst. The tumor had flow voids, and a dilated drainage vein (blue, arrow) and artery (red, arrow head) in 3D images. f, g Left ICA angiography showed a tumor stain and an arteriovenous shunt (AV shunt) (arrow). The tumor fed by the branches from the middle cerebral artery (MCA). f A-P view. g Lateral view. h, i Left VA angiography, early (h) and late (i) arterial phase, showed a mass with an AV shunt (arrow). The tumor was fed by the branches from the posterior cerebral artery (PCA), which drained into the superior sagittal sinus (SSS). j After TAE, the tumor stain disappeared almost entirely. Right vertebral artery (VA) angiography faintly revealed a residual branch (arrow) from the PCA
A 4-vessel CAG (Fig. 1) demonstrated a hyper-vascular mass with an AV shunt. The tumor was fed by the branches from the middle cerebral artery (MCA) and posterior cerebral artery (PCA), which drain into the superior sagittal sinus (SSS). We performed transarterial embolization (TAE) to reduce intraoperative blood loss (Fig. 1). A Marathon microcatheter (Covidien, Irvine, CA, USA) was navigated selectively over a Traxcess microwire (Terumo, Tokyo, Japan) into the distal aspect of the PCA branch. Superselective angiography demonstrated tumor stain and an AV shunt. N-butyl cyanoacrylate (NBCA) (B. Braun GmbH Spangenberg, Germany) was infused from this catheter position. TAE was performed a total of four times, after which the tumor stain disappeared almost entirely. Right vertebral artery (VA) angiography faintly revealed a residual branch from the PCA supply to the GCM. However, we did not perform TAE as we could approach it at the beginning of the resection operation.
After 3 days, the patient underwent left temporoparietal craniotomy and total excision of the mass. The cyst contained a yellowish fluid indicating previous hemorrhage. The solid components were dark red, like mulberries (Fig. 2). The lesion was fairly well demarcated from the adjacent gliotic brain and was excised circumferentially. As there were numerous small arterial feeders, the tumor bled easily, and we could not remove the tumor piecewise because of massive blood loss. During surgery the patient lost 400 cm3 in blood, equal to 44 % of the blood in his body, and received 280 cm3 in blood transfusions. The surgery was successful. Histological examination (Fig. 2) of the excised lesion showed multiple dilated vascular spaces containing red blood cells. Histopathology was reported as a cavernous hemangioma. A postoperative MR scan (Fig. 3) showed complete excision of the lesion. The patient had an uneventful postoperative recovery and was discharged on the 12th postoperative day. In 2 years of follow-up, the patient’s course has been uneventful.
Discussion
GCMs are very rare, and there are limited reports on their clinical characteristics. GCMs, especially intraparenchymal GCMs, were previously proposed to be low-flow vascular malformations and typically angiographically occult [2, 3, 6]. We found 34 previously reported pediatric (under 15 years) and surgical cases of GCM (Table 1). Most GCMs were not enhanced, although five cases had slight enhancement on CT or MR images [2, 6, 8]. Three cases had heterogeneous enhancement [5, 9, 10], one had a developmental venous anomaly (DVA) [9], and the other case of dural or tentorial GCM involved the transverse-sigmoid sinus [5]. CAG was performed for five cases [5, 8, 10, 11, 12]. Two cases were enhanced in the artery phase [5, 8]. The remaining cases showed no obvious findings. In our case, the tumor was strikingly enhanced on MRI, and CAG demonstrated a hyper-vascular mass with an AV shunt, which is very rare for intraparenchymal GCMs. The size criteria for GCM are not well defined. The minimum limit used in the literature varies between 4 and 6 cm. We adopted the definition of a lesion larger than 4 cm [2, 3].
The radiological characteristics of GCMs in the brain show a wide range of variation, so that accurate preoperative diagnosis is often difficult. Ozgen et al. reported radiological features of childhood GCMs [3]. A GCM should be considered in cases with a very large hemorrhagic intra-axial mass with a “bubbles of blood” multicystic appearance, accompanied by a hemosiderin ring, fluid-fluid levels, and an accompanying edema-mass effect. However, our case had neither of those characteristics nor atypical imaging features, and we misdiagnosed it preoperatively as a neoplasm such as an astrocytoma, hemangioblastoma, or choroid plexus papilloma. The differential diagnosis of intraparenchymal, strikingly enhanced tumors with an AV shunt include GCMs.
A number of studies have reported that surgical extirpation of GCMs is safe and possible without significant blood loss despite their giant size, as GCMs are low-flow vascular malformations [8, 13, 14]. CAG is typically of minimal value in assessing CMs, which show no vascular blush and no feeding arteries or draining veins [11]. However, Dashti et al. reported a case of a tentorial GCM involving the dural sinuses [5]. The patient had undergone two unsuccessful attempts at resection. Both surgeries were complicated by massive blood loss and were aborted. After preoperative TAE with Onyx, the surgery was successful. Our operation was delayed because of bleeding despite adequate preoperative TAE with NBCA, although we avoided fatal intraoperative blood loss. If we had not performed preoperative TAE, the surgery would have been unsuccessful. Preoperative TAE was important even if the tumor was an intraparenchymal tumor, and the possibility of intraoperative blood loss should be considered even if TAE is performed in cases where the mass is heavily enhanced on MRI or CAG and especially associated with an AV shunt.
The prevalence of CMs is equal in male and female patients [22], while Van et al. reported a higher prevalence of GCM in females [19]. By contrast, in our literature search of 33 pediatric GCMs, there were 20 males and 13 females, suggesting a male predominance for GCM in children. Almost all GCMs become symptomatic because of their giant size, while asymptomatic GCMs are rare. Seizure and headache or vomiting are common symptoms of GCMs [3]. However, the symptom of macrocephaly in our case was atypical. GCMs can occur anywhere and can almost be considered intraparenchymal lesions. We found only a few reports of tentorial GCMs. The CMs in children tended to present most frequently in the frontal regions [19]. However, the GCM in our case was located in the parietal lobe. An affinity for periventricular locations was also observed in childhood GCMs, as observed in our case [2, 6, 8, 19].
Conclusion
To our knowledge, this is the first report describing a hyper-vascular GCM with an AV shunt. The differential diagnosis of intraparenchymal, strikingly-enhanced tumors with an AV shunt include hyper-vascular GCMs. Consideration of potential for bleeding during the operation is also important.
References
Bradley AG, Ning L, Rose D, Arthur LD (2011) The natural history of intracranial cavernous malformations. Neurosurg Focus 30(6):E24
Kan P, Tubay M, Osborn A, Blaser S, Couldwell WT (2008) Radiographic features of tumefactive giant cavernous angiomas. Acta Neurochir 150:49–55
Ozgen B, Senocak E, Oguz KK, Soylemezoglu F, Akalan N (2011) Radiological features of childhood giant cavernous malformations. Neuroradiology 53:283–289
Yoshimura J, Tsukamoto Y, Sano M, Hasegawa H, Nishino K, Saito A, Fukuda M, Okamoto K, Fujii Y (2014) Successful removal of a huge hyper-vascular tentorial cavernous angioma after preoperative endovascular embolization. J Neurosurg Pediatrics 14:43–47
Dashti SR, Fiorella D, Spetzler RF, Beres E, McDougall CG, Albuquerque FC (2009) Preoperative Onyx embolization of a giant cavernous malformation involving the dural sinuses. J Neurosurg Pediatr 3:302–306
Kim YJ, Kim JE, Kim NR, Kim HS (2007) Imaging findings of giant cavernous malformation with a focal infiltrative pattern. Pediatr Radiol 37:1039–1042
Khosla VK, Banerjee AK, Mathuriya SN, Mehta S (1984) Giant cystic cavernoma in a child. Case report J Neurosurg 60:1297–1299
Kawagishi J, Suzuki M, Kayama T, Yoshimoto T (1993) Huge multilobular cavernous angioma in an infant: case report. Neurosurgery 32:1028–1031
Alvarez H, Perry V, Solle M, Castillo M (2012) De novo cerebral arteriovenous malformation in a child with previous cavernous malformation and developmental venous anomaly. J Neurosurg Pediatr 9:327–330
Hayashi T, Fukui M, Shoyima K, Utsonomiya H, Kawasaki K (1985) Giant cerebellar hemangioma in an infant. Childs Nerv Syst 1:230–233
Chicani CF, Miller NR, Tamargo RJ (2003) Giant cavernous malformation of the occipital lobe. J Neuroophthalmol 23:151–153
Lew SM (2010) Giant posterior fossa cavernous malformations in 2 infants with familial cerebral cavernomatosis: the case for early screening. Neurosurg Focus 29:E18
Lawton MT, Vates GE, Quinones-Hinojosa A, McDonald W, Marchuk D (2004) Giant infiltrative cavernous malformation: clinical presentation, intervention, and genetic analysis: case report. Neurosurgery 55:988–995
Thakar S, Furtado SV, Ghosal N, Hegde AS (2010) A peri-trigonal giant tumefactive cavernous malformation: case report and review of literature. Childs Nerv Syst 26:1819–1823
de Andrade GC, Prandini MN, Brage FM (2002) Giant cavernous angioma: report of two cases. Arq Neuropsiquiatr 60:481–486
Avci E, Ozturk A, Baba F, Karabag H, Cakir A (2007) Huge cavernoma with massive intracerebral hemorrhage in a child. Turk Neurosurg 17:23–26
Agrawal A, Banode P, Shukla S (2012) Giant cavernous hemangiomas of the brain. Asian J Neurosurg 7:220–222
Houtteville JP (1997) Brain cavernoma: a dynamic lesion. Surg Neurol 48:610–614
Van Lindert EJ, Tan TC, Grotenhuis JA, Wesseling P (2007) Giant cavernous hemangiomas: report of three cases. Neurosurg Rev 30:83–92
Kasliwal MK, Gupta A, Sharma BS (2010) Rare case of giant temporal cavernous angioma in a child. Pediatr Neurosurg 46:324–325
Mohindra S, Sodhi HS, Rane S (2013) Tumefactive presentation of a supratentorial cavernous hemangioma: a report of two cases. J Pediatr Neurosci 8:232–234
Maraire JN, Awad IA (1995) Intracranial cavernous malformations: lesion behavior and management strategies. Neurosurgery 37:591–605
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
This paper has not been published before and has not been submitted for publication to any other journal in part or full. The authors report no conflict of interest concerning the materials or methods used in this study or the findingsspecified in this paper.
Rights and permissions
About this article
Cite this article
Hirata, K., Ihara, S., Sato, M. et al. Hyper-vascular giant cavernous malformation in a child: a case report and review. Childs Nerv Syst 33, 375–379 (2017). https://doi.org/10.1007/s00381-016-3234-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-016-3234-8