Abstract
Giant cavernous hemangiomas occur very rarely, and little has been reported about their behavior. In this case report three cavernous hemangiomas with a diametric measure between 6 cm and 7 cm and distinct features will be described. A 36-year-old female patient presented with headache and nausea. A CT scan disclosed a large circumscribed tumor with strong contrast enhancement in the temporo-parieto-occipital region of the right cerebral hemisphere and extension into the right cerebellar hemisphere. A 35-year-old woman was admitted to our emergency ward with a generalized seizure and a dilated pupil. The CT scan showed an extensive left frontal lesion containing a substantial hyperintense part, suspicious for hemorrhage. A 3-year-old girl was admitted with generalized seizure and progressively declining consciousness. A large left frontotemporal paraventricular multi-cystic lesion was encountered on the CT scan. All three patients were operated on. Two recovered very well. In the case of the 3-year-old girl stable disease was reached. Giant cavernomas do not differ from average-sized cavernomas in clinical, surgical or histopathological presentation but may differ radiologically. However, the possible diagnosis of cavernoma can be overlooked, due to their size and possible differential diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a result of improved diagnostic methods, cavernous hemangiomas (CHs) are identified more often nowadays. Reported incidences range from 0.4% to 0.9% in MRI studies. The first symptoms usually include, in order of decreasing frequency, seizures, neurological deficits and headaches. Sometimes they are found by coincidence. Though evidence of microscopic hemorrhage is found regularly through histopathological examination, the incidence of hemorrhages leading to clinically significant symptoms tends to be low. After surgical intervention most patients have a good outcome. CH consist of sinusoidal spaces, with low-flow, lined by a single layer of endothelial cells and often surrounded by gliotic neuronal tissue and hemosiderin staining. The sinusoidal spaces may contain thromboses in varying degrees. Normally, there is no neuronal tissue intervening in the spaces. All these aspects result in the mulberry-like configuration as well as in the reticulated appearance on MRI.
Few data can be found about the size or volume of these malformations or the range in which they appear. In the study of Clatterbuck et al., who used a volumetric measurement, the mean volume was 2,779±560 mm3 and the range was from 0.5 mm3 to 46.533 mm3. Another study reports sizes between 1 mm and 75 mm, with a mean size of 14.2 mm [19], applying diametric measuring. We could not find detailed studies about very large cavernomas. Although sizes up till 140 mm have been mentioned, these very large CHs are incidentally reported as part of a larger general study, without being high-lighted, and only a few isolated case reports on giant cavernomas have been published [2, 5, 8, 13–18, 21, 26, 31, 35, 38, 41].
Because giant CHs seem to be very rare and, thus, usually not considered in the differential diagnosis of large tumors, we would like to present three cases of cerebral giant CH with a diametric measurement between 6 cm and 7 cm, in which the diagnosis of cavernoma was not considered initially.
Case A
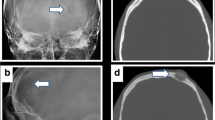
A 36-year-old woman presented with headache and nausea, which she had suffered for a year and a half. The symptoms were aggravated during pregnancy with her third child and, after delivery, by vomiting, dizziness, difficulties in writing and an unsteady gait. Family history was unremarkable. On neurological examination the patient seemed to be distracted and indifferent to the things happening around her. Clinically, ataxia and positive Romberg test results were found. CT and MRI showed a large circumscribed temporo-parietal lesion in the right hemisphere, with transtentorial extension in the right cerebellar hemisphere of 6.5 cm×6.5 cm×6 cm, with mixed intensities, peri-focal edema, midline shift and compression of the fourth ventricle, and occlusive hydrocephalus, as well as strong contrast enhancement (Fig. 1a–c). Our primary differential diagnosis was of meningioma or sarcoma.
a T2-weighted axial MR image showing a large right temporal intra-axial tumor with calcifications. b Gadolinium-enhanced T1-weighted sagittal MR image showing a contrast-enhanced right temporodorsal/suboccipital supra- and infratentorial tumor. c Gadolinium-enhanced T1-weighted coronal MR image showing infra- and supratentorial tumor extension. d Postoperative T2-weighted axial MR image showing total cavernoma removal
A combined subtemporal/suboccipital retrosigmoid craniotomy was performed, with preservation of transverse and sigmoid sinus. During the operation the tumor was well delineated, firm, with highly vascularized parts as well as avascular parts, and appeared to contain old hemorrhages but did not have the aspect of a typical cavernoma. Vascularization seemed to come from the tentorium. Macroscopically, a radical resection with resection of the involved part of the tentorium was achieved. Histological examination revealed a (dural) cavernoma.
After surgery the patient had a quick and uncomplicated recovery, without any neurological deficits. MRI performed 6 month afterwards demonstrated total tumor resection, and, at 4 years’ follow-up, no recurrence was observed (Fig. 1d).
Case B
This 35-year-old female patient had had headaches apathy and bradyphrenia for a few months. Seven days before admission she started vomiting and developed paresis of her right leg. On the emergency ward of a local hospital she had a generalized seizure, with a non-reactive and dilated right pupil. A CT scan without contrast disclosed a left frontobasal lesion of 6 cm×6 cm×5 cm with a hyperintense part, measuring 3 cm×3 cm×3 cm, suspicious of an (sub) acute hemorrhage, causing perifocal edema, midline shift and hydrocephalus (Fig. 2a). She was transferred to our institution immediately. During transfer she regained consciousness, opening her eyes and performing tasks but not speaking. After mannitol and dexamethasone had been administered the paresis improved and the right pupil became reactive again. On arrival she opened her eyes when addressed and performed some tasks, but, on physical examination, aphasia and slight right-sided hemiparesis were found.
a Non-enhanced CT scan showing a large left frontal space-occupying lesion with an intra-lesional hemorrhage at the level of the foramen of Monro. b Gadolinium-enhanced T1-weighted axial MR image showing an irregular contrast enhancement of a left frontobasal intra-axial tumor. c Postoperative T2-weighted axial MR image showing complete removal of the cavernoma
A T1-weighted axial MR image, with gadolinium, taken according to a neuronavigation protocol without any other MR sequences, showed the same lesion with a lobulated appearance and mixed intensities and unsharp demarcation (Fig. 2b). Our primary differential diagnosis was malignant primary intra-cerebral tumor with intra-tumoral hemorrhage. A cavernoma was never considered. In the night following the admission more generalized seizures occurred and anti-epileptic medication was started. The next day a left frontolateral craniotomy was performed, and a macroscopic gross total resection of blood clots and a large reddish-brown solid tumor was accomplished. After surgery the patient still suffered from bradyphrenia, short-term memory loss and partial left facial nerve palsy. The latter could not be explained by the course of the operation. However, these neurological deficits showed improvement, which were still ongoing at the time the patient was transferred back to the local hospital for further recovery. At the latest follow-up some bradyphrenia persisted, but the patient was living independently and taking up most of her former activities. In retrospect, her family history was negative. Histological examination disclosed a cavernoma. Follow-up MRI showed postoperative defects but no residual cavernoma (Fig. 2c).
Case C
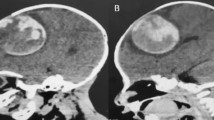
The patient, a 3-year-old girl with a negative family history started vomiting after she fell from her bicycle. Her consciousness progressively declined. At first she was admitted to a local hospital, where she suffered a generalized seizure. A CT scan disclosed a multi-cystic lesion of 6.5 cm×4 cm×6 cm with mixed densities in the left frontotemporoparietal-paraventricular region. The child was transferred to our hospital and showed no focal neurological deficits on admission. MRI demonstrated the same multi-cystic lesion of mixed intensities in the left fronto-temporo-parietal and insular regions, surrounded by a hypo-intense rim, visible on the T1-weighted scan and even more clearly on the T2-weighted scan (Fig. 3a,b). No enhancement was detected after gadolinium administration. MR angiography showed no abnormalities.
a Gadolinium-enhanced T1-weighted sagittal MR image shows a multi-cystic left frontotemporoparietal lesion. b T2-weighted axial MR image with multiple cysts of different content in the left basal ganglia, hemosiderin staining and microcalcifications. c Postoperative T2-weighted axial MR image with some cystic remnants of cavernoma periventricularly
A left temporal craniotomy was performed, during which multiple cysts, containing various liquids, were partially removed, but evidence of recent hemorrhage was not encountered. After the operation the patient made a fast recovery without any neurological deficits. The pathohistological findings were not conclusive, due to lack of material, and the differential diagnosis of dermoid cysts was made; a cavernoma was not suspected. In the first year the seizures remained, which required the anti-epileptic medication to be adjusted, but there were no seizures afterwards. However, the patient was slowly and progressively declining in motor, speech and language development and she developed a progressive gait disturbance due to right-sided spastic hemiparesis and slight central facial nerve palsy. A CT scan taken 2 years after surgery revealed a slight growth of the lesion.
Four years after the surgery the child developed loss of appetite and frequently vomited. MRI showed an image comparable to the previous scan. When the size of the tumor and the progressive symptoms were considered, it was decided to try and remove the tumor and establish a conclusive histological diagnosis. Because of the size and localization of the tumor a two-stage procedure was planned.
At the first operation a left pterional craniotomy was performed. During the surgery mulberry-like cysts with clear, yellow and brown contents (as were found during the preceding operation) were encountered as well as reactive gliosis and deposition of hemosiderin. Guided by neuronavigation, a partial debulking of the anterior part of the tumor was performed. Pathohistological examination clearly disclosed a cavernoma.
Recovery was uncomplicated, with no new neurological dysfunctions. The second-stage operation was planned 3 months later. In the meantime the gait improved.
At the second stage, from a left parietal transcortical/transventricular approach, further debulking of the remaining cavernoma was achieved, leaving some cysts in the corpus callosum, the thalamus and amygdalo-hippocampal gyrus. After surgery, the paresis of the right arm increased initially, but the arm regained its preoperative function during the following months. The function of the right leg slightly improved, with improvement of gait as well. Nausea and vomiting did not occur again. The last MRI showed some residual cysts in eloquent areas, which are not considered amenable to further surgical resection (Fig. 3c). Four years of MRI follow-up showed no evidence of growth of the lesion.
Discussion
Cavernomas are benign vascular malformations. Their occurrence in newborn infants is reason to believe that these lesions arise secondary to failure of normal embryonic vascular development. Most of these cavernomas are parenchymal lesions, although extra-axial locations have been frequently reported. The gradual enlargement of these lesions may be secondary to a number of factors, including thrombosis, engorgement, recruitment of adjacent feeding vessels, episodes of peri-lesional hemorrhage and hormonal influences [34], but also growth [29, 39]. The majority of cavernomas is small, but cavernomas may reach a significant size. The definition of a giant cavernoma is arbitrary, but, in accordance with Lawton et al., we define a giant cavernoma as a cavernoma with a diameter ≥6 cm [21]. In Table 1 we include details of all 17 cavernomas >6 cm that we could retrieve from the literature, including our own cases. However, those giant cavernomas that were part of a larger series of cavernomas, and that have not been described in detail as separate entities, have not been included.
With increasing size the radiological appearance of cavernomas may become less typical, and, therefore, a cavernoma is often not considered in the differential diagnosis of a patient with a very large solitary intra-cranial mass. Different aspects of giant cavernomas will therefore be highlighted.
Clinical presentation
Of our three cases, patient A presented with headache and neurological deficits, which became aggravated during her pregnancy. Hypothetically, this could be attributed to hormonal influence on the behavior of the CH, because women are noted to have more acute clinical symptoms and aggravation of an already existing CH during, particularly, the first trimester of their pregnancies [7, 12, 25, 28]. This phenomenon suggests a connection between a cavernous malformation and, possibly, the hormonal status and the cardiovascular condition of the patient. No research in this realm has yet been performed [22].
Patient A had a dural cavernoma originating from the tentorium. Dural cavernomas are extremely rare entities and known to mimic other tumors [16, 20]. They also have a varied clinical presentation, predominantly based on their location [34]. The time course of symptomatology of such a cavernoma is usual insidious, but exacerbation during pregnancy has previously been reported [34].
Patient B had already had headaches for a few months before being suddenly admitted to hospital owing to fast deterioration with generalized seizure and neurological deficits. The latter could very well be attributable to the intra-lesional hemorrhage, disclosed later by CT and MRI. Women tend to present more frequently with overt hemorrhage leading to symptoms. Although it does not occur often, the percentages still amount to 6.5% to 37% [10–23, 28, 31, 37, 38]. A life-threatening hemorrhage from a cavernoma is considered rare, and this bleeding is never as immediately devastating as a hemorrhage originating from a high-flow, high-pressure arteriovenous malformation (AVM) [15]. It should be mentioned that no correlation has been found between the size of the lesion and the risk of hemorrhage.
In case C the child was admitted because of generalized seizure. After anti-epileptic medication had been administered, she showed no neurological deficits at initial presentation. Afterwards, however, she developed neurological deficits in the later course of the disease, due to the growth of the cavernoma.
CHs are less frequently reported in children [22]. Nevertheless, the majority of giant CHs in the literature has occurred in children, with the youngest one being 3.5 months of age [5, 8, 14, 15, 17, 18, 35]. The oldest patient was 77 years of age [16]. Whereas those case reports tend to show a preference for the occurrence of giant CHs in young children, Table 1 shows that these entities can occur in the adult population just as well. Overall, the average size of a cavernoma seems to be larger in children than in adults, averaging 6.7 cm in a pediatric cases series of 36 patients, with the largest being 14 cm [8, 24].
Whereas average-sized cerebral CHs are believed to grow by recurrent internal microhemorrhage and organization (in up to 38% of cases), giant CHs seem more to resemble extra-axial CHs [22, 41]. Those lesions do not exhibit evidence of prior hemorrhage, and their growth seems to be the result of capillary budding, vascular ectasia or vascular thrombosis and organization [41].
All our three cases presented with the usual symptoms of a cavernoma: seizures (37–\100%) [6, 9, 19, 22, 23, 27, 28, 37, 38], clinical deficits (8.2–46.6%) [9, 19, 22, 23, 27, 28, 37, 38] and headaches (6–65%) [19, 23, 25, 27, 28]. A substantial number of CHs is asymptomatic and found by coincidence. In clinical studies a percentage of 4.1–44% [1, 22, 23, 25, 33, 37, 42] is mentioned. The clinical presentation of our large CHs did not differ from the presentation of the smaller CHs, although in cases A and B the symptoms seemed to be more serious than in smaller CHs. Especially in case B the hemorrhage had caused a life-threatening situation. All three cases had in common that a CH was not considered in the differential diagnosis by their radiologists, neurologists and neurosurgeons. The presentation of the other giant CHs in the literature, however, did differ somewhat from that of smaller CHs, by presenting as a large intra-cranial mass with signs of raised ICP in children (Table 1). Also, one giant CH presented as a large lump of the calvarium [41].
Hemorrhage of the CH is reported to be 8% to 37% in adults and 36% to 78% in children, but in this reported group of giant CHs, true hemorrhage occurred only twice [21, 22, 24].
The incidence of familial CH has been estimated to be close to 20% [24]. In our listing of giant CHs no familial occurrence was reported. Lesion multiplicity occurs frequently in CHs in about one-third of sporadic cases and up to 73% in familial cases, but it was not seen in any of the giant CHs listed in Table 1 [22].
The prevalence of CH is equal in male and female patients in the majority of the studies, but, in giant CHs, there seems to be a preponderance in female patients (Table 1) [22].
Radiological findings
All three patients had different radiological presentations. The typical presentation of a CH on a CT scan discloses a well-circumscribed lesion incorporating various densities, attributable to hemorrhage (subacute or chronic) and variable degrees of thrombosis including calcifications, as well as cystic components [7, 22, 25, 30, 32, 36–38, 42]. In most cases of intra-parenchymal CH only a faint enhancement appears when contrast agent is used [36], although delayed images may show intense contrast enhancement [40].
However, in our case A, strong contrast enhancement was seen. There was no hemosiderin staining in the MRI. This case was special, because it was a dural CH with strong vascularization from the dura. Strong contrast enhancement in T1-weighted sequences, absence of subacute and chronic hemorrhage both centrally and peripherally, T2-weighted hyperintensity to brain (due to slow flow in vascular spaces) and, sometimes, tumor calcifications are typical of a dural CH [34]. The T2-weighted hyperintensity may help one to differentiate a dural CH from a meningioma. Our case A was, therefore, typical of a dural CH. Nevertheless, owing to its rarity, a dural CH was not considered in the differential diagnosis preoperatively.
In case B an overt hemorrhage was partially obliterating the lesion, which has been described more often as one of the obscuring components in diagnosis through overlapping, and because of rapid deterioration and need for emergency surgery, no further diagnostic investigations were conducted in this patient, except a MRI in accordance with our neuronavigation protocol.
In case C the MRI was typical for a cavernoma, disclosing a well-defined multi-cystic lesion, which incorporated the frontal, temporal and parietal lobe as well as the corpus callosum and the basal ganglia, with a lobulated appearance and containing a reticulated core, giving mixed signal intensity surrounded by a hypo-intense rim on the T1-weighted image and an outer hyperintense rim on the T2-weighted MR image.
Penfield described, in 1948, a voluminous and extensively calcified cavernoma localized in the temporal lobe, with a size of 6 cm, which he named “haemangioma calcificans” [26]. Several others reported giant CHs that were extensively calcified as well [15, 16]. In our three cases no significant calcification was observed, and it was not a main feature of most other reported giant CHs.
Although Lawton et al. posit that giant CHs have the same MRI characteristics as smaller CHs, two of our three cases were not typical for CH in their radiological presentation, and, in conjunction with the untypical size of the lesion, a CH was, therefore, not considered in the differential diagnosis [21]. This has been the case in most other reported giant CHs as well [5, 8, 14, 18, 35].
Gradient-echo T2-weighted MR sequences are the most sensitive for the depiction of smaller lesions, owing to their sensitivity to the magnetic susceptibility artifacts induced by hemosiderin and are, therefore, ideal for screening for multiple intra-cerebral lesions. These sequences, however, have not been part of our MRI protocols for these larger lesions or during follow-up investigations but will be added to a revised future protocol.
Treatment and outcome
All three patients were operated on, and, in two cases, complete removal was accomplished. In case A the CH seemed to originate from the tentorium, which was also resected. In case C only incomplete debulking could be achieved, owing to the extension of the CH into the corpus callosum, thalamus and amygdalo-hippocampal gyrus. No new symptoms were evident after the operations. The outcomes were good, with full recovery in case A, and a good recovery with minor persisting deficits in case B. Only in case C did neurological symptoms not improve significantly. However, preoperative progressive worsening seems to have been halted during the 5 years of follow-up. This outcome concurs with the findings in other studies on CHs, which mention a very low morbidity rate in cases of supratentorial CH removal [1, 4, 6, 17, 22, 23, 25, 33, 37]. Also in the other reports on giant CHs, good surgical outcome has been reported (Table 1). In cases A and B no recurrence was observed during a 4-year follow-up period. The condition of Case C is still under control, because of the remnants of the cavernoma, but, so far in the last 5 years, no growth has been observed.
Histopathology and biology of CHs
In all three cases the histopathological findings did not differ from the morphology of an average-sized CH [3, 23, 25, 32, 33, 37]. In all three cases vascular lumina of variable sizes were found in the tissues, resembling caverns, with signs of old and new hemorrhages and thrombosis in different stages of organization. All the tissue samples showed hemosiderin staining and reactive gliosis. No signs of neoplasm were present.
Recently, studies have been conducted in search of the role of angiogenic growth factors and their receptors in the pathogenesis of cerebral cavernomas, especially Flk-1, Flt-1 (both receptors of the vascular endothelial growth factor, responsible for proliferation, migration, adhesion of the endothelial cell and forming of tubes), CD31 (marker of the endothelial cell, mediating in endothelial cell–cell interaction and angiogenesis) and Tie-1 and Tie-2 (conducting interaction between endothelial cell and matrix as well as mature development of the vessels). A positive immune expression of Flt-1, Flk-1 and Tie-2 were found in the CH samples, in contrast to normal brain tissue. CD31 was down-regulated in comparison with normal brain. The cause of the expression and down-regulation has not yet been determined. One hypothesis suggests a non-specific pathological reaction to hemorrhage, flow or ischemia. Another suggests a primary misbalance of the growth factors and their receptors as the pathogenesis of the cavernous malformations. It is clear, however, that CHs are dynamic and acquired lesions.
Further discussion of the biological activity of giant CHs is beyond the scope of this article, but the interested reader is referred to recent overviews [29, 39]. The proliferative activity and the neo-angiogenic capacity of any of the reported giant CHs has not been studied, except for one case in which the CCM1 (KRIT1) and CCM2 (malcavernin) genes did not reveal any abnormal mutations [21]. Lacking any relevance for the individual patient, because there was no positive family history, these investigations have not been performed on our own patients.
Conclusion
Giant CHs seem not to differ clinically, surgically or histopathologically from small CHs other than by their size and increased prevalence in children. However, radiologically, they can be atypical. Although they are rare, the possibility of a CH should also be considered in the case of very large intra-cranial intra-axial tumors. Complete surgical removal should be attempted, since good recovery is very possible and morbidity is low.
References
Aiba T, Tanaka R, Koike T, Kameyama S, Takeda N, Komata T (1995) Natural history of intracranial cavernous malformations. J Neurosurg 83:56–59
Anderson RC, Connolly ES Jr, Ozduman K, Laurans MSH, Gunel M, Khandji A, Faust PL, Sisti MB (2003) Clinicopathological review: giant intraventricular cavernous malformation. Neurosurgery 53:374–379
Awad IA, Robinson JR Jr, Mohanty S, Estes ML (1993) Mixed vascular malformations of the brain: clinical and pathogenetic considerations. Neurosurgery 33:179–188
Bertalanffy H, Benes L, Miyazawa T, Alberti O, Siegel AM, Sure U (2002) Cerebral cavernomas in the adult. Review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev 25:1–53
Chicani CF, Miller NR, Tamargo RJ (2003) Giant cavernous malformation of the occipital lobe. J Neuroophthalmol 23:151–153
Churchyard A, Khangure M, Grainger K (1992) Cerebral cavernous angioma: a potentially benign condition? Successful treatment in 16 cases. J Neurol Neurosurg Psychiatry 55:1040–1045
Clatterbuck RE, Moriarity JL, Elmaci I, Lee RR, Breiter SN, Rigamonti D (2000) Dynamic nature of cavernous malformations: a prospective magnetic resonance imaging study with volumetric analysis. J Neurosurg 93:981–986
de Andrade GC, Prandini MN, Braga FM (2002) [Giant cavernous angioma: report of two cases]. Arq Neuropsiquiatr 60:481–486
Del CO Jr, Kelly DL Jr, Elster AD, Craven TE (1991) An analysis of the natural history of cavernous angiomas. J Neurosurg 75:702–708
Detwiler PW, Porter RW, Zabramski JM, Spetzler RF (1997) De novo formation of a central nervous system cavernous malformation: implications for predicting risk of hemorrhage. Case report and review of the literature. J Neurosurg 87:629–632
Duffau H, Capelle L, Sichez JP, Faillot T, Bitar A, Arthuis F, Van ER, Fohanno D (1997) Early radiologically proven rebleeding from intracranial cavernous angiomas: report of 6 cases and review of the literature. Acta Neurochir (Wien) 139:914–922
Flemming KD, Goodman BP, Meyer FB (2003) Successful brainstem cavernous malformation resection after repeated hemorrhages during pregnancy. Surg Neurol 60:545–547
Gelal F, Feran H, Rezanko T, Dirim Vidinli B (2005) Giant cavernous angioma of the temporal lobe: a case report and review of the literature. Acta Radiol 46:310–313
Hayashi T, Fukui M, Shoyima K, Utsonomiya H, Kawasaki K (1985) Giant cerebellar hemangioma in an infant. Childs Nerv Syst 1:230–233
Houtteville JP (1997) Brain cavernoma: a dynamic lesion. Surg Neurol 48:610–614
Hyodo A, Yanaka K, Higuchi O, Tomono Y, Nose T (2000) Giant interdural cavernous hemangioma at the convexity. Case illustration. J Neurosurg 92:503
Kawagishi J, Suzuki M, Kayama T, Yoshimoto T (1993) Huge multilobular cavernous angioma in an infant: case report. Neurosurgery 32:1028–1031
Khosla VK, Banerjee AK, Mathuriya SN, Mehta S (1984) Giant cystic cavernoma in a child. Case report. J Neurosurg 60:1297–1299
Kim DS, Park YG, Choi JU, Chung SS, Lee KC (1997) An analysis of the natural history of cavernous malformations. Surg Neurol 48:9–17
Kocak A, Cayli SR, Onal SC, Kutlu R, Aydin N (2002) Dural cavernous hemangioma originating from superior petrosal sinus. J Neurosurg Sci 46:143–146
Lawton MT, Vates GE, Quinones-Hinojosa A, McDonald W, Marchuk D (2004) Giant infiltrative cavernous malformation: clinical presentation, intervention, and genetic analysis: case report. Neurosurgery 55:988–995
Maraire JN, Awad IA (1995) Intracranial cavernous malformations: lesion behavior and management strategies. Neurosurgery 37:591–605
Moriarity JL, Wetzel M, Clatterbuck RE, Javedan S, Sheppard JM, Hoenig-Rigamonti K, Crone NE, Breiter SN, Lee RR, Rigamonti D (1999) The natural history of cavernous malformations: a prospective study of 68 patients. Neurosurgery 44:1166–1171
Mottolese C, Hermier M, Stan H, Jouvet A, Saint-Pierre G, Froment JC, Bret P, Lapras C (2001) Central nervous system cavernomas in the pediatric age group. Neurosurg Rev 24:55–71
Ojemann RG, Crowell RM, Ogilvy CS (1995) Cranial and spinal cavernous malformations. In: Ojemann RG, Ogilvy CS, Crowell RM, Heros RC (eds) Surgical management of neurovascular disease. Williams & Wilkins, Baltimore, pp 538–557
Penfield W, Ward A (1948) Calcifying epileptogenic lesions. Haemangioma calcificans. Report of a case. Arch Neurol Psychiatry 60:20–36
Porter PJ, Willinsky RA, Harper W, Wallace MC (1997) Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg 87:190–197
Pozzati E, Acciarri N, Tognetti F, Marliani F, Giangaspero F (1996) Growth, subsequent bleeding, and de novo appearance of cerebral cavernous angiomas. Neurosurgery 38:662–669
Raychaudhuri R, Batjer HH, Awad IA (2005) Intracranial cavernous angioma: a practical review of clinical and biological aspects. Surg Neurol 63:319–328
Requena I, Arias M, Lopez-Ibor L, Pereiro I, Barba A, Alonso A, Monton E (1991) Cavernomas of the central nervous system: clinical and neuroimaging manifestations in 47 patients. J Neurol Neurosurg Psychiatry 54:590–594
Reyns N, Assaker R, Louis E, Lejeune JP (1999) Intraventricular cavernomas: three cases and review of the literature. Neurosurgery 44:648–654
Rigamonti D, Drayer BP, Johnson PC, Hadley MN, Zabramski J, Spetzler RF (1987) The MRI appearance of cavernous malformations (angiomas). J Neurosurg 67:518–524
Robinson JR, Awad IA, Little JR (1991) Natural history of the cavernous angioma. J Neurosurg 75:709–714
Rosso D, Lee DH, Ferguson GG, Tailor C, Iskander S, Hammond RR (2003) Dural cavernous angioma: a preoperative diagnostic challenge. Can J Neurol Sci 30:272–277
Sansone ME, Liwnicz BH, Mandybur TI (1980) Giant pituitary cavernous hemangioma: case report. J Neurosurg 53:124–126
Savoiardo M, Strada L, Passerini A (1983) Intracranial cavernous hemangiomas: neuroradiologic review of 36 operated cases. Am J Neuroradiol 4:945–950
Scott RM, Barnes P, Kupsky W, Adelman LS (1992) Cavernous angiomas of the central nervous system in children. J Neurosurg 76:38–46
Steiger HJ, Markwalder TM, Reulen HJ (1987) Clinicopathological relations of cerebral cavernous angiomas: observations in eleven cases. Neurosurgery 21:879–884
Sure U, Freman S, Bozinov O, Benes L, Siegel AM, Bertalanffy H (2005) Biological activity of adult cavernous malformations: a study of 56 patients. J Neurosurg 102:342–347
Thiex R, Kruger R, Friese S, Gronewaller E, Kuker W (2003) Giant cavernoma of the brain stem: value of delayed MR imaging after contrast injection. Eur Radiol 13 [Suppl 4]:L219–L225
Voelker JL, Stewart DH, Schochet SS Jr (1998) Giant intracranial and extracranial cavernous malformation. Case report. J Neurosurg 89:465–469
Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP, Brown B, Rigamonti D, Brown G (1994) The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 80:422–432
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Ulrich Sure, Wuttipong Tirakotai, Marburg
With their article “Giant cavernous hemangiomas. Report of three cases”, Dr. van Lindert et al. add three more cases of unusually large cavernomas to the neurosurgical literature. The authors eloquently describe the clinical course of their patients and show high-quality neuroradiological figures of the pathology. Giant cavernomas are interesting lesions to look at, if one is interested in the biology of cavernomas. Although these lesions do not differ in routine histopathological aspects from those of “normal sized” cavernomas as stated by the authors, it is likely that they harbor a somewhat increased potential for growth. Therefore, it would be very interesting to study such lesions for their proliferative and neo-angiogenic ability. It is of particular interest that one of the authors’ patients became symptomatic during pregnancy, which, once again, focuses our interest on the long-lasting discussion on whether the genesis of cavernomas might be related to a specific hormone excess. Finally, the information that all three patients lacked a family history is very interesting, because sporadic cavernomas as in the present study are usually considered to be biologically more static than familial cavernomas that are well known for a notable growth potential over time.
Rights and permissions
About this article
Cite this article
van Lindert, E.J., Tan, T.C., Grotenhuis, J.A. et al. Giant cavernous hemangiomas: report of three cases. Neurosurg Rev 30, 83–92 (2007). https://doi.org/10.1007/s10143-006-0042-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-006-0042-8