Abstract
Mandibular advancement device (MAD) is an alternative therapeutic option for CPAP to treat obstructive sleep apnea (OSA). While MAD showed the better adherence, patients with over moderate OSA have been treated more frequently with CPAP despite increasing positive evidence on the cardiovascular outcome with MAD, even in severe patients. Thus, more information is needed regarding the cardiovascular and symptomatic outcome of MAD treatment objectively compared to CPAP. Forty-five supine-dependent OSA patients (apnea–hypopnea index 20–40/h) were randomized to either CPAP or MAD and treated for 8 weeks and switched to another for 8 weeks. The primary endpoint was improvement in the endothelial function, indexed by the flow-mediated dilatation (FMD), and the secondary endpoint was the sleep-time blood pressure (BP). The duration of MAD use was evaluated objectively by an implanted adherence monitor. Treatment efficacy was also evaluated by home sleep monitor and a questionnaire about the symptoms. The adherence was not significantly different (CPAP vs. MAD: 274.5 ± 108.9 min/night vs. 314.8 ± 127.0 min/night, p = 0.095). FMD and sleep-time mean BP were not markedly changed from the baseline with either approach (CPAP vs. MAD: FMD, + 0.47% ± 3.1% vs. + 0.85% ± 2.6%, p = 0.64; BP, − 1.5 ± 5.7 mmHg vs. − 1.2 ± 7.5 mmHg, p = 0.48), although sleepiness, nocturia, and sleep-related parameters were similarly improved and more patients preferred MAD. As MAD and CPAP showed similar effects on cardiovascular outcome and symptomatic relief even with a comparable length of usage, we might expect MAD as an alternative treatment option for CPAP in this range of OSA group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is characterized by the recurrent collapses of upper airway, worsening of the sleep quality and inducing excessive daytime sleepiness [1]. Furthermore, hypoxia, negative intrathoracic pressure, and the frequent arousal caused by OSA activate the sympathetic nervous system and deteriorate the endothelial function, eventually leading to elevation of the nocturnal as well as daytime blood pressure (BP) and atherosclerotic changes in the cerebral, cardiac, and systemic arteries [2,3,4]. OSA, therefore, constitutes a strong risk of hypertension, coronary artery disease, heart failure, and cerebrovascular disease [5, 6]. A recent population-based report indicated surprisingly high prevalence (49.7% of men and 23.4% of women) of OSA with an apnea–hypopnea index (AHI) over 15/h in the general population, suggesting that OSA might have a greater impact on general healthcare than previously estimated [7].
For over 30 years, continuous positive airway pressure (CPAP) has been the first-line therapeutic option for treating moderate to severe OSA, improving the subjective symptoms and preventing the new onset of hypertension, cardiovascular and cerebrovascular diseases and eventually reducing the rate of all-cause mortality [8]. A mandibular advancement device (MAD) is an alternative and well-established device for treating OSA that keeps the lower jaw at an advanced position to prevent the upper airway from collapsing, and its effectiveness in improving OSA has been reported in patients with a wide range of severity, especially in those with supine-dependent OSA, a low body mass index, a female sex, or a relatively young age [9,10,11,12]. While CPAP can effectively treat even very severe OSA patients and numerous studies have shown its efficacy for improving the symptomatic and cardiovascular outcomes, MAD has been used more in those with mild to moderate symptoms or in patients for whom CPAP therapy failed for various reasons. However, since recent studies have reported the promising efficacy of MAD even in severe OSA patients, the recent guidelines for MAD do not restrict its use to only patients with mild to moderate symptoms [11, 13,14,15,16]. Furthermore, the acceptance and adherence to MAD have been repeatedly reported to be better than those with CPAP, which is an advantage of MAD. However, a problem in such studies is that the adherence was mainly assessed with a self-reported time of use. Some recent studies have used an implantable adherence monitor to evaluate the duration of use precisely [13, 17]. Expanding the therapeutic options among patients with moderate to severe OSA will, therefore, require evidence that MAD and CPAP are similarly effective in improving the vascular function or reducing the blood pressure (BP) in patients who are suitable for MAD therapy, with objectively precise adherence.
To this end, we evaluated the changes in symptoms, sleep-related parameters, and major cardiovascular system indices, such as the endothelial function and sleep-time BP, after crossover treatment with both CPAP and a MAD using precise adherence monitoring in moderate to severe OSA patients, particularly those in whom MAD was expected to be comparably effective with CPAP, such as those with supine-dependent OSA.
Methods
Study design and participants
This study was an open label, prospective, randomized, crossover trial to test the non-inferiority of MAD to CPAP in improving cardiovascular markers. Eligible participants were those over 20 years of age who had been diagnosed with OSA with an overall apnea–hypopnea index (AHI) of 20–40/h and supine dependency (defined as the AHI in the supine position being more than twice that in other positions, according to the definition of Joosten et al.) based on overnight polysomnography (PSG) at Kyushu University Hospital or Saiseikai Futsukaichi Hospital [18].
The reason why we selected 20–40/h for the severity of our patients was that though we would like to prove the cardiovascular effectiveness of MAD among those with as severe as possible OSA patient group, MAD treatment would not be ethically allowed in more severe patients because CPAP treatment had been established as effective treatment in this range of patients.
Other inclusion criteria were as follows: two or more symptoms of OSA among nighttime dyspnea, fragmented sleep, non-restorative sleep, and excessive daytime sleepiness (EDS).
We excluded patients with a history of OSA treatment, those who needed or wanted immediate treatment for OSA, those with central sleep apnea (central apnea index > 5/h), those with insomnia and hypersomnia from diseases other than OSA such as psychophysiological insomnia, primary hypersomnia, narcolepsy, recurrent hypersomnia, or idiopathic hypersomnia, those with chronic obstructive pulmonary disease or psychological diseases, those contraindicated for MAD use due to problems of the teeth or jaw, those with symptomatic coronary artery disease or a history of cerebrovascular disease, those on hemodialysis, and those deemed inappropriate for this study such as patients who are difficult to visit regularly or who were judged as not to be cooperative to this study by the attending doctors (S.A, M.N, H.T). Suitable patients in both facilities were referred to the outpatient clinic at the Sleep Apnea Center of Kyushu University Hospital from August 2014 to September 2016.
This study was approved by ethics committee of Kyushu University Hospital, and written informed consent was obtained from all participants. This trial was registered as UMIN000014723.
Sleep studies and analyses
Patients who visited each hospital with OSA-related symptoms underwent PSG (Neurofax, Nihon Kohden, Tokyo, Japan; or Alice 5, Philips-Respironics, Murrysville, PA, USA) with electroencephalogram, electro-oculogram, submental electromyogram, thoracic and abdominal respiratory effort, airflow (nasal-oral thermocouple and nasal pressure), pulse oximetry and postures in each hospital. The recorded data were scored by registered PSG technicians, and respiratory events were defined using the 2007 American Association of Sleep Medicine guidelines [19]. In brief, an apneic event was defined as any reduction in nasal–oral thermistor ≥ 90% for over 10 s, and a hypopneic event was defined as any reduction in nasal pressure ≥ 50% for over 10 s associated with arousal or desaturation ≥ 3%. The AHI and 3% oxygen desaturation index (3%ODI) were defined as the hourly number of apneic and hypopneic events and the hourly number of desaturation ≥ 3% events, respectively. We also calculated the cumulative oxygen desaturation using the time desaturation summation index (TDS) = (100% − averaged arterial oxygen saturation during sleep) × total sleep-time hours; which we had defined for representing the cumulative hypoxic insult during whole sleeping time judged from self-reports of activity and sensors that recorded the body movement and illuminance (Actiwatch®; Phillips Respironics, Murrysville, PA, USA) [3].
At the end of CPAP and MAD treatment, we evaluated the severity of OSA using a home sleep apnea monitor (LS-120S; Fukuda Lifetec, Tokyo, Japan) equipped with an SpO2 sensor, a nasal flow sensor, and an abdominal movement sensor. We obtained the respiratory event index (REI), which is the total number of apneic and hypopneic events per hour of recording time, the 3% ODI, the minimum SpO2, and the mean SpO2. We analyzed these parameters only during sleep by judgement using self-report as same as when TDS was calculated.
Ambulatory blood pressure measurement (ABPM)
When the patients were invited to participate in the study according to the results of their PSG testing and consented to participate, the office BP and heart rate were measured twice and averaged, and the ambulatory BP was measured using a FB-270 (Fukuda Denshi, Tokyo, Japan) every 30 min between 6 a.m. and 9 p.m. and every 60 min otherwise. The ABPM was also obtained after the end of treatment with CPAP and MAD. Whether a patient was awake or asleep was determined by self-reporting.
Measurement of the endothelial function using flow-mediated vasodilatation (FMD)
The forearm endothelial function was evaluated based on the FMD response according to the recommendations using a cross-sectional ultrasound probe in the forearm (UNEX EFR; UNEX, Nagoya, Japan) [20]. FMD was defined as the % change in the arterial diameter after arterial occlusion for 5 min. We used the FMD method to assess the endothelial function because we have used this method to analyze the endothelial function in our previous studies, and FMD measurement had showed little variation among observers or institutes, since the analysis is performed using a software program [21]. The FMD were obtained before the randomization and after the end of each treatment with CPAP and MAD.
Subjective symptoms and others
Responses to a questionnaire for evaluating EDS were obtained using the Japanese version of the Epworth Sleepiness Scale (JESS) (Table 1) [22]. Blood samples were drawn to determine the cardiovascular risk factors. The subjective symptoms, including nocturia and comfort, were obtained using an original questionnaire at the beginning and end of the first study (Table 5). The patients’ preference for devices was also inquired about at the end of all therapeutic regimens (Table 4).
Treatment with CPAP and MAD
For CPAP, we used a Sleepmate S9 (Resmed, San Diego, CA, USA) or REMstar Pro System One 60 series (Phillips Respironics, Murrysville, PA, USA) in automatic pressure mode initially set between 4 and 12 cmH2O by referring the analysis of the pressure in our institute with a humidifier when needed. We raised the maximum pressure up to 15 cmH2O in 5 patients at the end of the 4 weeks treatment according to the results of the pressure analysis during this period. As a MAD, we used a Somnodent® (Somnomed Inc., Sydney, Australia) with a recordable temperature sensor (Dentitrac®; Braebon Ltd., Ontario, Canada) to objectively record the time of use [23]. MADs were custom-made products for each patient and titrated with consideration of patient’s comfort and the results of the SpO2 monitoring.
A dentist at Kyushu University Hospital (HT) took the impression and bite registration of the patients and sent it to a central laboratory where all the MAD of the manufacturer in Japan were made. The maximal advancement was set as 75% of maximum and vertical opening was decided as minimum of each patient before sending the lab. After the MAD being sent back to our institute, the patient was asked about the comfortability of jaw and, if there is not any problem, the patient start to use it. It took about four weeks as titrated period and jaw positions were titrated in reference to the patient’s comfort and the data of SpO2 monitoring which performed two weeks after starting of treatment at home. We finally evaluated the effects of MAD at the end of the MAD-treated period (7–9 weeks after treatment) by a home sleep apnea monitor.
Procedures
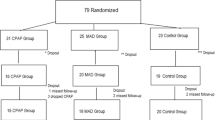
Participants were randomly assigned to either of two groups using computer-generated blocked randomization: CPAP first and then switched to MAD (CPAP-MAD group) or the opposite order (MAD–CPAP group) (Fig. 1).
After a test period of 4 (± 1) weeks of each device, we checked the time of use objectively using the adherence monitors equipped to both devices; this 4 (± 1) week period was not included in the later analysis. We defined a patient who used the device for > 4 h/night for more than 70% of the treatment period as a “well-adherent user” for the device. After another 4 weeks of treatment (after 7–9 weeks of treatment in total with each device), we evaluated the objective time of use of each device, sleepiness, office BP, ambulatory BP, severity of OSA, and subjective symptoms.
Endpoints
The primary endpoint was the change in FMD after treatment. The secondary endpoint was the change in the mean sleep-time BP and the correlations between FMD and the sleep-related parameters (3% ODI, minimum SpO2, mean SpO2, and TDS).
Statistical analyses
To prove the non-inferiority of the MAD, we hypothesized that the change in FMD would be 4% with a standard deviation of 1% for differences in the rate of change, a 2-sided 5% significance level, and a power of 80%. We also hypothesized the non-inferiority margin to be 1% [24]. With these assumptions and an expected dropout rate of 10%, we considered a sample size of nearly 40 participants to be necessary.
Among the 45 patients who were enrolled in the study, we could not evaluate the adherence of MAD of 4 patients due to possible mechanical problem of sensor and one patient due to intolerance for treatment with CPAP and analyzed the results of 40 patients (Fig. 1). We then analyzed 17 “well-adherent users” as described above (10 patients in the CPAP-MAD arm and 7 in the MAD–CPAP arm) for a sub-analysis.
Statistical analyses were performed using the JMP 12 software program (SAS Institute Inc., Cary, NC, USA). The data are presented as the mean ± standard deviation. A comparison between groups was performed using Wilcoxon’s test, the χ2 test, and the Steel–Dwass test. p < 0.05 was considered statistically significant.
Results
Among the 108 eligible participants, 63 were excluded for various reasons (Fig. 1). Of the remaining 45 participants, 23 were allocated to the CPAP–MAD group and 22 to the MAD–CPAP group, and 40 participants completed treatments using both devices. Their baseline characteristics are shown in Table 1.
On-treatment analysis
Endpoints
Compared with the initial value, FMD showed no significant changes after each therapy (CPAP vs. MAD: + 0.47% ± 3.1% vs. + 0.85% ± 2.6%, p = 0.64). This result was within the margin of non-inferiority. The changes in the mean sleep-time BP after each therapy were also not significant and comparable (CPAP vs. MAD: − 1.5 ± 5.7 mmHg vs. − 1.2 ± 7.5 mmHg, p = 0.48). There was no significant correlation between the changes in the FMD and sleep-related parameters (3% ODI, minimum SpO2, mean SpO2, or TDS) at the end of each therapy.
Effects of treatments on symptoms, other BP parameters, sleep-related parameters, and usage time (Table 2)
Daytime sleepiness evaluated using JESS was significantly and comparatively improved, and the frequency of nocturia changed favorably after each therapy (baseline vs. CPAP vs. MAD: 1.4 ± 1.2 vs. 0.71 ± 0.93 vs. 0.78 ± 0.94 times per night). All sleep-related parameters (3%ODI, minimum SpO2, mean SpO2, and TDS) were significantly improved by both therapies, and the severity of OSA measured by the REI after treatment remained low (REI < 10/h) with both treatments, although CPAP was able to reduce the number of apneic or hypopneic events (CPAP vs. MAD 4.5 ± 4.9 vs. 8.9 ± 7.7, p = 0.001), the mean SpO2, and the TDS more effectively than the MAD. The MAD tended to be used longer than CPAP (CPAP vs. MAD: 274.5 ± 108.9 min vs. 314.8 ± 127.0 min, p = 0.095), but the rate of using each device > 4 h/night was similar (CPAP vs. MAD: 62.7% ± 29.3% vs. 70.8% ± 27.4%, p = 0.14). No BP parameters were significantly changed by either therapy.
We also analyzed the time in the supine position while asleep and the proportion of time spent in the supine position at the end of each therapy. These values were comparable at the end of each therapy (time in the supine position while asleep each night: CPAP vs. MAD 159.7 ± 119.9 min vs. 186.5 ± 115.8 min, p = 0.29, the proportion of times spent in the supine position at the end of each therapy: CPAP vs. MAD 43.4% ± 30.8% vs. 45.0% ± 25.9%, p = 0.61).
Analysis in “well-adherent users” (Table 3)
We conducted a sub-analysis in only “well-adherent users”, excluding 13 of 23 participants in the CPAP–MAD arm and 15 of 22 participants in the MAD–CPAP arm. Even in this analysis, the changes in FMD remained insignificant after each therapy, and there were no correlations between FMD and any sleep-related parameters, such as the minimum SpO2, mean SpO2, and TDS. In addition, the BP parameters were also not significantly improved by either device. In contrast, the daytime sleepiness, 3% ODI, mean SpO2, and TDS were improved by both therapies from the baseline, and the severity of OSA measured by the REI after treatment remained < 10/h. Nocturia was favorably affected by both treatments.
Similar to the results of the on-treatment analysis, the mean duration of device use per night for the MAD was longer than that for CPAP, but the rate of using each device > 4 h/night was comparable between the two therapies.
The treatment preference and the subjective impression of the treatment effects (Table 4 and Table 5)
We compared the preference for devices in several aspects using a questionnaire as shown in Table 4. Ultimately, patients preferred the MAD to CPAP in all aspects. We also evaluated the subjective impression of the effectiveness of treatment with each device as shown in Table 5. Patients felt that all aspects of their disease were improved similarly by both therapies.
Discussion
In this randomized crossover study among moderate to severe supine-dependent OSA (AHI: 20–40/h) patients, we showed that (1) the endothelial function measured by FMD and the ambulatory sleep-time BP were unchanged by treatment with either CPAP or MAD; (2) OSA-related parameters, such as the 3% ODI, minimum SpO2, mean SpO2, and TDS, were significantly improved, and the severity of OSA measured by the REI remained low after treatment with both therapies; (3) the subjective sleepiness measured by JESS was improved, and the average frequency of nocturia was reduced by both therapies; (4) the rate of using the device for > 4 h/night was comparable, but the objective time of use per night of the MAD tended to be longer than that of the CPAP; and (5) the patients preferred the MAD to CPAP in all aspects as a treatment device but were similarly satisfied with both therapies.
Regarding the treatment effect of CPAP for improving the endothelial function in sleep-disordered breathing patients, CPAP has shown constantly favorable effects [25, 26]. In contrast, findings regarding the effect of a MAD on the endothelial function have been controversial. Although Itzhaki et al. and Lin et al. reported a positive effect of a MAD on the endothelial function, in a recent large-scale study comparing the effect of a MAD with that of a sham MAD, the endothelial function measured by peripheral arterial tonometry (Endo PAT) was not improved by MAD [13, 27, 28]. We chose another method (FMD) to evaluate the endothelial function that has been also widely used due to its established reliability. Under this slightly different measurement condition, we also failed to note any significant beneficial effect on the endothelial function after either CPAP or MAD treatment. One possible reason for this negative result might be the relatively low severity of OSA in our patients. The reason why we recruited the patients with 20–40/h of AHI was that we would like to examine the effectiveness of MAD on the symptoms and cardiovascular system among the patients who are moderate to marginally severe OSA patients and chose 30 ± 10/h of AHI. It would have been better if we could prove the cardiovascular effectiveness of MAD among more severe OSA patient group, but it was not allowed ethically when CPAP had been believed to be gold standard therapy in severe OSA patients.
Kallianos et al. reported that the endothelial function measured by FMD improved from 3.13 ± 3.15 to 5.40 ± 2.91% after treatment with CPAP for 3 months [29]. Considering their result and the already slightly impaired FMD (3.9% ± 1.9%) at baseline in our study, the effect of treatment by both devices in our study might have been hidden by a ceiling effect. As the endothelial function is known to be more deteriorated in patients with more severe OSA, a study conducted in patients with more severe OSA could have shown significant improvement in the FMD after treatment with both devices [3, 29].
While many previous studies have reported a reduction in the BP, especially by CPAP, and a meta-analysis by Bratton revealed a reduction in the BP by both CPAP and MAD (CPAP: SBP/DBP − 2.5/2.0 mmHg, MAD: SBP/DBP − 2.1/1.9 mmHg), we observed no significant reduction in the BP after OSA treatment with either device [30, 31]. One reason for these discrepant findings may have been the differences in the OSA severity between the patient groups (mean baseline AHI in the previous BP meta-analysis = 37.9 ± 14.4/h). Indeed, another meta-analysis suggested that the severity of OSA might affect the degree of BP reduction after CPAP [32]. Furthermore, another previous report found that the severity of hypertension before treatment was associated with the positive effect of BP reduction [33]. As we did not selectively recruit hypertensive patients, only 37.5% of the participants had hypertension, and the average BP at baseline of our participants was already within the normal range. Here as well, a ceiling effect due to their successful medical treatment for hypertension might have resulted in our lack of an anti-hypertensive effect of OSA treatment. Further studies in patients with an higher BP or worse endothelial function may be needed to further evaluate the effects of CPAP and MAD. And it also should be extremely important to evaluate the effectiveness for improving endothelial function and blood pressure in future study including more severe OSA patients.
In the present study, we recruited patients with supine dependency, since some previous studies have indicated that a MAD can more reduce the AHI among supine-dependent patients and we intended to evaluate the effectiveness of a MAD in patients for whom we can expect an obvious improvement in OSA-related parameters [10, 12, 18]. However, this might have led to an unexpected bias toward the selection of leaner, younger, and ultimately healthier individuals, which may have unintentionally led to the negative results in showing the favorable effects on the FMD and BP.
The degree of hypoxemia has been recently considered to be more closely related to the progression of various cardiovascular diseases than the AHI in OSA patients [34, 35]. We, therefore, tried to analyze the relationship between the parameters representing the degree of desaturation, such as the minimal SpO2, mean SpO2, or TDS (an index we developed to summate the hypoxic burden throughout the night), and the change in the BP or FMD [3]. Although the hypoxic parameters were significantly improved by both therapies, especially by CPAP, we noted no relationship between the improvement in the hypoxic parameters and the changes in the BP or FMD. This result may also be due to the relatively low-severity desaturation among our patients. Further examinations including patients with more severe hypoxia may clarify the relationship between hypoxia and the BP or endothelial function.
While CPAP has been established as an effective treatment for improving subjective symptoms, its efficacy in preventing cardiovascular disease is unclear [36]. McEvoy et al. evaluated the effectiveness of CPAP on the cardiovascular system among patients with almost the same severity of OSA as in our study and reported a lack of effectiveness of CPAP for the secondary prevention of cardiovascular events [37]. Another meta-analysis on the effect of treatment including CPAP for OSA and central sleep apnea also failed to show a positive impact for the prognostic improvement [38]. However, as implied in the other studies in the meta-analysis, a larger-scale study including patients with more severe OSA in terms of desaturation and followed for a longer duration with higher objective adherence might yield different results.
The OSA-related parameters of 3% ODI, mean SpO2, and minimum SpO2 in our participants showed significant improvement, and the severity of OSA as measured by the REI remained low after treatment by both CPAP and the MAD. Although CPAP resulted in greater improvement in OSA-related parameters than the MAD, we believe that the improvement achieved with MAD might still be satisfactory, as this device reduced OSA-related parameters to the mild OSA range with only mild residual desaturation, and the sleepiness significantly improved to a degree similar to that achieved with CPAP in the present and previous studies [39].
The participants preferred MAD to CPAP in this study which was slightly different from the results in previous studies. The difference might have occurred due to the differences in various factors, such as the personality or economic situation of the attending patients [11, 40,41,42]. These results suggest that MAD might be used for a longer duration with higher adherence than CPAP, thereby restoring satisfactory sleep, even if not completely normal, leading to improvement of the subjective symptoms. However, as our study suggested, attention should be paid to the fact that subjective improvement might not guarantee cardiovascular improvement.
Nocturia is considered an important symptom of OSA, and the effectiveness of CPAP on relieving nocturia among patients with sleep-disordered breathing has been reported [43]. Although we had not precisely identified the comorbidities which might cause nocturia, such as benign prostate hypertrophy and overactive bladder in the questionnaire for our patients, we did confirm that MAD improved nocturia in our cases similar to the findings of a previous study on CPAP treatment.
The strength of the current study is that we used a MAD equipped with an implanted temperature monitor to objectively record the duration of use and were thereby able to compare the efficacy of treatment precisely with CPAP. Several previous reports comparing the adherence of CPAP and a MAD found that the adherence to a MAD tended to be better than that to CPAP, although the adherence to a MAD was evaluated based only on a questionnaire in many studies. As the adherence of MAD in this study was similar to the time reported in the previous literatures [11, 41, 44], we believe that our results would be applicable even for other type of MAD. To our knowledge, this is the first study to compare the outcome of treatment by CPAP and MAD using an objective adherence monitor.
In this study, we adopted a 4-week treatment period after a 3- to 5-week test use period. It might be rather short but we consider that our study period of 3–5 + 4 weeks was appropriate for affecting of cardiovascular system and for washing out the effect of the previous treatment as well, since previous studies evaluated the endothelial function after 2–24 weeks treatment with CPAP or a MAD, and another study showed that the BP and heart rate as well as FMD began to deteriorate as early as two weeks after the removal of OSA treatment [2, 9, 26]. However, while we chose the treatment periods based on the findings of previous studies, this period might not have been completely sufficient to fully evaluate the effects of these devices on the FMD and BP in some patients.
Several limitations associated with the present study should be mentioned. First, we were unable to precisely compare the degree of OSA before and after each treatment because we used polysomnography for the baseline evaluation and a home sleep apnea test for the evaluation after each treatment. However, although we used different devices at baseline and after the treatments, we used the same devices at the end of each therapy, since the main purpose of this study was to compare the efficacy of each device. We, therefore, believe that our results are reliable in terms of the comparison of the influence of the devices. Although this might have affected the comparisons of the parameters to some extent, we believe that our results were never widely skewed for this reason, as the clinical accuracy of home sleep apnea tests has been repeatedly shown [45, 46]. Second, our lack of observing any significant changes in this patient size might mean that the therapeutic effects of MAD and CPAP are practically identical. Although there were no significant effects on cardiovascular parameters, such as the FMD and BP, in our study, a larger study might be required to confirm our results. In this study, we did not set the washout period between the treatments because we considered the first four weeks of each treatment as test period would work as a washout period. However, there remains a possibility that carry-over effect of each treatment might have affected to the result of latter treatment due to the relatively short test period.
In conclusion, MAD and CPAP device used for objectively similar durations of treatment showed similar efficacies in improving the subjective symptoms and OSA-related parameters in moderate to slightly severe OSA patients, although neither improved the cardiovascular parameters, such as the endothelial function nor ambulatory BP. We, therefore, feel that we can confidently recommend a MAD as an alternative treatment option for CPAP especially for improvement of subjective symptoms and QOL, as we do not have to expect favorable effects on cardiovascular parameters with either treatment modality in patients with this severity of OSA and more patients preferred MAD to CPAP in our study.
References
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Kohler M, Stoewhas AC, Ayers L, Senn O, Bloch KE, Russi EW, Stradling JR (2011) Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea a randomized controlled trial. Am J Respir Crit Care Med 184:1192–1199
Sawatari H, Chishaki A, Nishizaka M, Tokunou T, Adachi S, Yoshimura C, Ohkusa T, Ando SI (2016) Cumulative hypoxemia during sleep predicts vascular endothelial dysfunction in patients with sleep-disordered breathing. Am J Hypertens 29:458–463
Nakabayashi K, Jujo K, Saito K, Oka T, Hagiwara N (2017) Evaluation of the association between sleep apnea and polyunsaturated fatty acids profiles in patients after percutaneous coronary intervention. Heart Vessels 32:1296–1303
Shamsuzzaman ASM, Gersh BJ, Somers VK (2003) Obstructive sleep apnea: implications for cardiac and vascular disease. J Am Med Assoc 290:1906–1914
Akita K, Maekawa Y, Kohno T, Tsuruta H, Murata M, Fukuda K (2017) Ameliorating the severity of sleep-disordered breathing concomitant with heart failure status after percutaneous transluminal septal myocardial ablation for drug-refractory hypertrophic obstructive cardiomyopathy. Heart Vessels 32:1320–1326
Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J (2015) Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 3:310–318
Marin JM, Carrizo SJ, Vicente E, Agusti AGN (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–1053
Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, Marks GB, Cistulli PA (2013) Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 187:879–887
Marklund M, Stenlund H, Franklin KA (2004) Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest 125:1270–1278
Gagnadoux F, Fleury B, Vielle B, Pételle B, Meslier N, N’Guyen XL, Trzepizur W, Racineux JL (2009) Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur Respir J 34:914–920
Chan ASL, Cistulli PA (2009) Oral appliance treatment of obstructive sleep apnea: an update. Curr Opin Pulm Med 15:591–596
Gagnadoux F, Pépin JL, Vielle B, Bironneau V, Chouet-Girard F, Launois S, Meslier N, Meurice JC, Nguyen XL, Paris A, Priou P, Tamisier R, Trzepizur W, Goupil F, Fleury B (2017) Impact of mandibular advancement therapy on endothelial function in severe obstructive sleep apnea. Am J Respir Crit Care Med 195:1244–1252
Trzepizur W, Gagnadoux F, Abraham P, Rousseau P, Meslier N, Saumet JL, Racineux JL (2009) Microvascular endothelial function in obstructive sleep apnea: impact of continuous positive airway pressure and mandibular advancement. Sleep Med 10:746–752
Hoekema A, Stegenga B, Wijkstra PJ, Van Der Hoeven JH, Meinesz AF, De Bont LGM (2008) Obstructive sleep apnea therapy. J Dent Res 87:882–887
Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, Chervin RD (2015) Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med 11:773–828
Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van De Heyning PH, Braem MJ (2013) Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax 68:91–96
Joosten SA, O’Driscoll DM, Berger PJ, Hamilton GS (2014) Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev 18:7–17
Taylor F, Pembrey MS, Beddard AP, French H (1907) Observations upon two cases of Cheyne-stokes’ respiration. Med Chir Trans 90:83–98
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the international brachial artery reactivity task force. J Am Coll Cardiol 39:257–265
Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D (2001) Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91:929–937
Takegami M, Suzukamo Y, Wakita T, Noguchi H, Chin K, Kadotani H, Inoue Y, Oka Y, Nakamura T, Green J, Johns MW, Fukuhara S (2009) Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on Item Response Theory. Sleep Med 10:556–565
Berry SJ, Coffey DS, Walsh PC, Ewing LL (1984) The development of human benign prostatic hyperplasia with age. J Urol 132:474–479
Kohler M, Craig S, Pepperell JCT, Nicoll D, Bratton DJ, Nunn AJ, Leeson P, Stradling JR (2013) CPAP improves endothelial function in patients with minimally symptomatic OSA: results from a subset study of the MOSAIC trial. Chest 144:896–902
Ip MSM, Tse HF, Lam B, Tsang KWT, Lam WK (2004) Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 169:348–353
Schwarz EI, Puhan MA, Schlatzer C, Stradling JR, Kohler M (2015) Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: a systematic review and meta-analysis. Respirology 20:889–895
Itzhaki S, Dorchin H, Clark G, Lavie L, Lavie P, Pillar G (2007) The effects of 1-year treatment with a herbst mandibular advancement splint on obstructive sleep apnea, oxidative stress, and endothelial function. Chest 131:740–749
Lin CC, Wang HY, Chiu CH, Liaw SF (2015) Effect of oral appliance on endothelial function in sleep apnea. Clin Oral Investig 19:437–444
Kallianos A, Panoutsopoulos A, Mermigkis C, Kostopoulos K, Papamichail C, Kokkonouzis I, Kostopoulos C, Nikolopoulos I, Papaiwannou A, Lampaki S, Organtzis J, Pitsiou G, Zarogoulidis P, Trakada G (2015) Sex differences of continuous positive airway pressure treatment on flow-mediated dilation in patients with obstructive sleep apnea syndrome. Clin Interv Aging 10:1361–1367
Andrén A, Hedberg P, Walker-Engström ML, Wahlén P, Tegelberg A (2013) Effects of treatment with oral appliance on 24-h blood pressure in patients with obstructive sleep apnea and hypertension: a randomized clinical trial. Sleep Breath 17:705–712
Bratton DJ, Gaisl T, Wons AM, Kohler M (2015) CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea a systematic review and meta-analysis. JAMA J Am Med Assoc 314:2280–2293
Bazzano LA, Khan Z, Reynolds K, He J (2007) Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 50:417–423
Baguet JP, Barone-Rochette G, Pépin JL (2009) Hypertension and obstructive sleep apnoea syndrome: current perspectives. J Hum Hypertens 23:431–443
Yamaguchi T, Takata Y, Usui Y, Asanuma R, Nishihata Y, Kato K, Shiina K, Yamashina A (2016) Nocturnal intermittent hypoxia is associated with left ventricular hypertrophy in middle-aged men with hypertension and obstructive sleep apnea. Am J Hypertens 29:372–378
Oldenburg O, Wellmann B, Buchholz A, Bitter T, Fox H, Thiem U, Horstkotte D, Wegscheider K (2016) Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J 37:1695–1703
Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S, Initiative I (2017) Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation 136:1840–1850
McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS (2016) CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 375:919–931
Yu J, Zhou Z, McEvoy R, Anderson CS, Rodgers A, Perkovic V, Neal B (2017) Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA 318:156–166
Sharples LD, Clutterbuck-James AL, Glover MJ, Bennett MS, Chadwick R, Pittman MA, Quinnell TG (2016) Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med Rev 27:108–124
Engleman HM, McDonald JP, Graham D, Lello GE, Kingshott RN, Coleman EL, Mackay TW, Douglas NJ (2002) Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med 166:855–859
Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, Pierce RJ (2004) Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med 170:656–664
Almeida FR, Henrich N, Marra C, Lynd LD, Lowe AA, Tsuda H, Fleetham JA, Pliska B, Ayas N (2013) Patient preferences and experiences of CPAP and oral appliances for the treatment of obstructive sleep apnea: a qualitative analysis. Sleep Breath 17:659–666
Hajduk IA, Jasani RR, Strollo PJ Jr, Atwood CW Jr, Sanders MH (2000) Nocturia in sleep disordered breathing. Sleep Med 1:263–271
El-Solh AA, Homish GG, Ditursi G, Lazarus J, Rao N, Adamo D, Kufel T (2017) A randomized crossover trial evaluating continuous positive airway pressure versus mandibular advancement device on health outcomes in veterans with posttraumatic stress disorder. J Clin Sleep Med 13:1327–1335
Masa JF, Corral J, Pereira R, Duran-Cantolla J, Cabello M, Hernández-Blasco L, Monasterio C, Alonso A, Chiner E, Rubio M, Garcia-Ledesma E, Cacelo L, Carpizo R, Sacristan L, Salord N, Carrera M, Sancho-Chust JN, Embid C, Vázquez-Polo FJ, Negrín MA, Montserrat JM (2011) Effectiveness of home respiratory polygraphy for the diagnosis of sleep apnoea and hypopnoea syndrome. Thorax 66:567–573
Durán-Cantolla J, Zamora Almeida G, de Vegas Diaz Guereñu O, Saracho Rotaeche L, Hamdan Alkhraisat M, Durán Carro J, Egea Santaolalla C, Anitua E, on Behalf of the Spanish Sleep N (2017) Validation of a new domiciliary diagnosis device for automatic diagnosis of patients with clinical suspicion of OSA. Respirology 22:378–385
Acknowledgements
We appreciate the help of Chikara Yoshimura, MD, PhD and Sakiko Handa, MD, PhD, in the conduct of the study as well as that of Hiroyuki Sawatari, RN, Sayaka Soda, CT, and Yumiko Kubota, RPSGT, in collecting the data for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This work was supported by JSPS KAKENHI Grant Number JP17K09555. In this trial, the MAD was provided by SomnoMed, Ltd., without any monetary support, and this study was performed independently of this company in its design, the collection and analysis of the data, and the preparation of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamamoto, U., Nishizaka, M., Tsuda, H. et al. Crossover comparison between CPAP and mandibular advancement device with adherence monitor about the effects on endothelial function, blood pressure and symptoms in patients with obstructive sleep apnea. Heart Vessels 34, 1692–1702 (2019). https://doi.org/10.1007/s00380-019-01392-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-019-01392-3