Abstract
Dipeptidyl peptidase 4 (DPP-4) inhibitors have recently been reported to exhibit additional cardioprotective effects; however, their effect in atrial remodeling, such as in atrial fibrillation (AF), remains unclear. In this study, the effect of linagliptin on atrial electrical and structural remodeling was evaluated in a canine AF model. Sixteen beagle dogs with 3-week atrial rapid stimulation were divided into the linagliptin group (9 mg/kg/day, n = 8) and pacing control group (n = 8). Three additional dogs without rapid pacing were assigned into non-pacing group, which was used as sham in this study. In the dogs with rapid pacing, the atrial effective refractory period (AERP), conduction velocity (CV), and AF inducibility were evaluated and blood was sampled every week. After the entire protocol, atrial tissue was sampled for histological examinations using HE, Azan, and dihydroethidium (DHE) staining to evaluate any tissue damage or oxidative stress. The pacing control group exhibited a gradual AERP shortening and CV decrease along the time course as previously reported. In the linagliptin group, the AERP shortening was not affected, but the CV decrease was suppressed in comparison to the control group (p < 0.05). The AF inducibility was increased in the control group and suppressed in the linagliptin group (p < 0.05). The control group exhibited tissue fibrosis, the degree of which was suppressed in the linagliptin group. DHE staining exhibited suppression of the reactive oxygen species expression in the linagliptin group in comparison to the pacing control group. Linagliptin, a DPP-4-inhibitor, suppressed the AF inducibility, CV decrease, and overexpression of oxidative stress in the canine AF model. Such suppressive effects of linagliptin on AF in the canine model may possibly be related to the anti-oxidative effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dipeptidyl peptidase-4 (DPP-4) inhibitor is a member of protease inhibitors used clinically as antidiabetic agents [1]. In addition to the antidiabetic effects, recent reports have exhibited the cardioprotective and/or anti-fibrotic effects of DPP-4 inhibitors [2, 3]. Although the mechanisms of such additional effects are unclear, an increase in the glucagon like protein-1 (GLP-1) is considered to play a role. GLP-1 is one of the target substrates of DPP-4 and the availability of intrinsic GLP-1 is increased under the action of DPP-4 inhibitors. Because GLP-1 is considered to suppress the inflammatory process through unknown mechanisms, various cardiovascular injuries can also be suppressed through the action of GLP-1 [4,5,6,7].
In the process of the formation of an arrhythmogenic substrate for atrial fibrillation (AF), the inflammatory process is considered to play an important role. We recently reported the suppression of AF inducibility using several drugs with anti-inflammatory and/or anti-oxidative effects in a canine AF model [8,9,10]. Therefore, the production of an AF substrate can also be suppressed by DPP-4 inhibitors in the same AF model. In the present study, we evaluated the effect of linagliptin, a DPP-4 inhibitor, on the atrial electrical and structural remodeling in a canine AF model with special focus on its anti-oxidative effect.
Methods
Initial surgery
The canine AF model was set up as previously described [8,9,10]. Nineteen adult female beagle dogs (10.9 ± 1.2 kg body weight) were anesthetized with pentobarbital (Nembtal® 25 mg/kg intravenous), and followed by an additional dose of 2 mg/kg at the end of each hour. Mechanical ventilation was maintained through an endotracheal tube with a mechanical ventilator (Model SN-480-5; Shinano Manufacturing, Tokyo, Japan) with 100% oxygen. Two pairs of electrodes were sutured onto the left atrial appendage and right atrial free wall for monitoring of the atrial electrograms. The other ends of the electrode wires were tunneled subcutaneously and exposed on the back of the neck. For continuous rapid atrial pacing, a unipolar screw-in lead (CapSureFix 5568; Medtronic Inc. Minneapolis, MN, USA) was inserted through the right external jugular vein and the distal end of the lead was screwed into the endocardial side of the right atrial appendage. The proximal end of the pacing lead was connected to a rapid pulse generator (Soletra, Medtronic Inc. Minneapolis, MN, USA), which was implanted into a subcutaneous pocket on the back of the neck. No atrioventricular block was produced in this study in order to mimic the hemodynamic situation in clinical cases of AF [8,9,10].
All studies were performed in accordance with the guidelines specified by the Animal Experimentation and Ethics Committee of the Kitasato University School of Medicine. The investigation conformed with the Guide for the Care and Use of Laboratory Animals, US National Institutes of Health (NIH Publication No. 85-23, revised 2011).
Study protocol
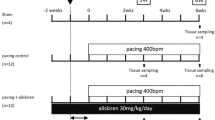
To obtain a stable baseline condition, each dog was allowed to recover after the initial surgical procedure (day 7) for at least 1 week without pacing. Atrial rapid pacing at a rate of 400 beats per minute (bpm) was performed in 16 of the 19 dogs starting at day 7, and the remaining 3 dogs without pacing were assigned into the non-pacing group. The 16 dogs with atrial rapid pacing were divided into two groups: the pacing control group (n = 8), i.e., dogs without any oral administration, and the linagliptin group (n = 8), i.e., dogs with the oral administration of linagliptin (9 mg/kg/day) starting 1 week before the rapid pacing (Fig. 1). The left atrial tissue of the non-pacing group was used as the sham data for the histological evaluation.
Schematic of the study protocol. Each dog underwent an initial surgery and was allowed to recover for 1 week without pacing before the start of the atrial rapid pacing (day 0). Atrial rapid pacing (400 bpm) was performed for 3 weeks in the pacing control and linagliptin groups. In the linagliptin group, linagliptin (9 mg/kg/day) was orally administered starting from day 7 until day 21. Atrial tissue was sampled in each dog at the end of the protocol. See the text for the details
Electrophysiological studies
Electrophysiological studies were performed every week to evaluate the AF inducibility, atrial effective refractory period (AERP), and atrial conduction velocity (CV) between both atria along the time course. For the electrophysiological evaluation, the rapid pacing was temporarily stopped and all measurements were performed under a pharmacological block of the autonomic nervous system (infusing atropine 0.05 mg/kg and propranolol 0.2 mg/kg) to exclude any influence from the autonomic nervous tone [8,9,10]. To evaluate the AF inducibility, the incidence of AF induction was evaluated with atrial burst pacing for 3 s at the minimal pacing cycle length that achieved 1:1 atrial capture at the left atrial pacing site. This pacing was delivered at fourfold the diastolic threshold with a pulse width of 2 ms. We defined AF as a spontaneous irregular atrial rhythm lasting longer than 5 s. Atrial burst pacing for AF induction was delivered 5 times at the left atrial pacing sites. At each evaluation time point, the AERP was measured with a basic drive cycle length of 300 ms at the left atrial site where the electrodes were sutured. The pacing energy output was set at twice the diastolic threshold during each evaluation at the left atrial pacing site. The coupling interval of the premature stimulus was shortened by 2-ms steps. The longest coupling interval of the premature beat that failed to capture the atrium was determined as the local AERP. Because the AERP data varied among the individual dogs, the change in AERP (∆AERP) was calculated by subtracting the AERP on day 0 [8,9,10]. The CV was calculated as the reciprocal of the conduction time between the left and right atrial sites during right atrial appendage pacing at a drive cycle length of 300 ms. Because the distance between the right and left atrial electrodes varied among the individual dogs, the change in the CV was calculated as the %CV by setting the data on day 0 as 100% [8,9,10].
Hemodynamic evaluation
To exclude the hemodynamic difference caused by linagliptin administration and/or rapid atrial pacing, the hemodynamic parameters, including the systemic blood pressure, pulmonary arterial pressure, pulmonary arterial wedge pressure, and cardiac output, were evaluated using a thermo-dilution catheter in the pacing control and linagliptin groups at the end of the 3-week protocol.
Histological analysis of the atrial tissue
At the end of the protocol, small portions of the left atrial free wall were excised for histological and biochemical analyses. The histology of the atrial tissue was evaluated by hematoxylin–eosin (HE) and Azan staining. For the analysis of the in situ localization of the reactive oxygen species (ROS), we incubated frozen sections (25 μm) of the left atrial tissue with fluorophores sensitive to O2−, dihydroethidium (DHE, 10 μmol/L; Sigma). DHE specifically reacted with intracellular O2− and was converted to the green fluorescent compound ethidium, which then bound irreversibly to double-stranded DNA and appeared as punctuate nuclear staining. The specificity of DHE for O2− was confirmed by preincubation with polyethylene glycolsuperoxide dismutase (PEG-SOD; 500 U/mL, Sigma). The stained sections were then observed with a fluorescence microscope (LSM710; Carl Zeiss MicroImaging, USA). To localize the nuclei of cardiomyocytes, 4′,6-diamidino-2-phenylindole (DAPI) staining was used.
DPP-4 and GLP-1 activities
For the analysis of the DPP-4 and GLP-1 activity, blood was sampled every week during the 3-week pacing protocol. The plasma DPP4 activity was determined as the rate of cleavage of 7-amino-4-methylcoumarin (AMC) from the synthetic substrate H-glycyl-prolyl-AMC (H-Gly-Pro-AMC; BioVision, Milpitas, CA, USA). In brief, the method used involved preparing duplicate test samples (one for background control) up to 50 µL/well, adjusting that to a 50 µL volume into a 96-well plate using a DPP4 Assay Buffer (5 μL of plasma were mixed with 45 μL of assay buffer). Then, 10 µL of the DPP4 Assay Buffer was added to one sample replicate and 10 µL of the DPP4 inhibitor (sitagliptin) to another sample as a sample background control. That was mixed well and incubated for 10 min at 37 °C. Then, 40 µL of the Reaction Mix (38 µL DPP4 Assay Buffer and 2 µL DPP4 Substrate H-Gly-Pro-AMC) was added into each well for each sample. That was mixed well and incubated for 30 min at 37 °C. The liberation of AMC was monitored at an excitation of 360 nm and emission of 460 nm before and after the incubation. The DPP4 activity was expressed as the amount of cleaved AMC (nmol/min/mL or unit/L). One unit was defined as the amount of DPP4 that hydrolyzed the DPP4 substrate to yield AMC at 1.0 µmol/min at 37 °C. The active plasma GLP-1 level was measured by an ELISA (Immuno-Biological Laboratories, Gunma, Japan; catalog number 27784). The plasma DPP-4 activity was also measured using a commercial assay (Enzo Life Sciences, Farmingdale, NY, USA; catalog number BML-AK498).
Statistical analysis
All statistical analyses were performed using JMP statistical software (SAS Institute Inc., Cary, NC, USA). The values are presented as the mean (standard deviation). The basic comparative statistics were analyzed with a nonparametric test (Friedman test), or Mann–Whitney U test. A p value of < 0.05 was considered to indicate statistical significance.
Results
DPP-4 activity and GLP-1 activities
Figure 2 shows the DPP-4 and GLP-1 activity along the time course of the rapid pacing in the pacing control and linagliptin groups. The DPP-4 and GLP-1 activity did not exhibit any significant changes in the pacing control group; however, the DPP-4 activity tended to increase at the end of 3-week pacing protocol. In contrast, in the linagliptin group, the DPP-4 activity was strongly suppressed from the beginning of the rapid pacing (day 0) by starting linagliptin from day 7. Conversely, the GLP-1 activity was enhanced from day 0 and such enhancement continued until the end of the 3-week pacing protocol. That indicated that the dose of the linagliptin (9 mg/kg/day) achieved a sufficient suppression of the DPP-4 activity in this study protocol.
DPP4 and GLP-1 activity along the time course of rapid pacing. After starting the administration of linagliptin (9 mg/kg/day), the DPP-4 activity was clearly suppressed in the linagliptin group. In contrast, the GLP-1 activity was enhanced during the entire pacing protocol. See the text for the details
Parameters in the electrophysiological studies
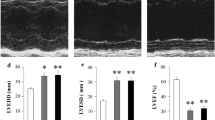
Figure 3 summarizes the parameters of the electrophysiological studies in the pacing control and linagliptin groups. As previously reported, the pacing control group exhibited a gradual shortening of the AERP, gradual decrease in the CV, as well as a gradual increase in AF inducibility along the time course of the 3-week rapid atrial pacing protocol [10]. In the linagliptin group, the change in the AERP did not exhibit any difference in comparison to the control. However, the decrease in the CV tended to be suppressed and the difference between the 2 groups became significant on day 21. AF inducibility was also suppressed in the linagliptin group and the difference was significant at least on days 7 and 21.
Parameters in the electrophysiological studies. Along the time course of the rapid pacing, a gradual AERP shortening, CV decrease, and increase in the AF inducibility were observed in the pacing control group. In contrast in the linagliptin group, although the change in the AERP did not exhibit a significant difference, the CV decrease and increase in the AF inducibility were suppressed in comparison to that in the pacing control group (p < 0.05). See the text for the details
Hemodynamic parameters
Table 1 shows the hemodynamic parameters evaluated at the end of the 3-week pacing protocol using a thermo-dilution catheter. The pacing control and linagliptin groups did not show any significant difference in those hemodynamic parameters.
Histopathology
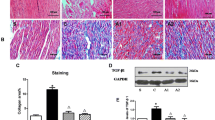
Figure 4 shows representative examples of the microscopic findings of the left atrial tissue in the 3 groups, including the non-pacing group. Compared with the non-pacing group, the pacing control group exhibited histological changes including an irregularity of the cardiomyocytes and interstitial edema and/or fibrosis. In contrast in the linagliptin group, the degree of those changes tended to be suppressed compared to that in the pacing control group.
Histological findings in the left atrial tissue. In comparison to the non-pacing group, the pacing control group exhibited advanced histological changes, such as an irregularity of the cardiomyocytes and interstitial fibrosis. In contrast in the linagliptin group, the degree of tissue fibrosis tended to be suppressed in comparison to that in the pacing control group. See the text for the details. HE hematoxylin–eosin staining
Expression of reactive oxygen species
Figure 5 shows representative examples of the immune-fluorescent staining with DHE. DHE clearly stained the myocardial cells and could be localized by merging the image of the DHE and DAPI staining, in the pacing control group. Because this expression was negated by PEG-SOD, it was considered to reflect an enhanced ROS expression. In the linagliptin group, this DHE expression was suppressed.
Immuno-fluorescent staining of DHE in the left atrial tissue. In comparison to the non-pacing group, DHE clearly stained the myocardial cells in the pacing control group and it was negated by PEG-SOD. In accordance with the merged image of the DHE and DAPI nuclei staining, the expression of ROS was enhanced in the myocardial cells in the pacing control group, but was suppressed in the linagliptin group. See the text for the details
Discussion
In the present study, we documented several important findings. First, the 3-week rapid atrial pacing produced a canine AF model in which the electrical remodeling was characterized by an AERP shortening, CV decrease, and increase in the AF inducibility as previously reported [8,9,10]. Second, atrial structural remodeling, i.e., interstitial fibrosis, was observed in parallel with the overexpression of the oxidative stress. Finally, such electrical and structural remodeling was suppressed by the oral administration of linagliptin, a DPP-4 inhibitor.
Mechanism of the suppressive effect of linagliptin on the canine AF model
As discussed in our previous reports [8,9,10], oxidative stress was considered to play a key role in the process of the construction of an arrhythmogenic substrate in this canine AF model. Although the degree of atrial electrical and structural remodeling was more prominent in the 6-week than 3-week pacing model [10, 11], the ROS expression had already advanced by the 3-week time point and such oxidative stress seemed to precede the appearance of various mediators of interstitial proliferation, such as connective tissue growth factor, periostin, and fibronectine [10]. Those factors are considered to produce later interstitial fibrosis in atrial tissue and then result in the construction of an arrhythmogenic substrate of AF. Zheng et al. also reported in an ischemic heart disease model that hyper-synthesis of ROS is observed to be associated with cardiovascular damage [12]. Practically, medications with an anti-oxidative effect, such as atorvastatin and carvedilol, suppress the oxidative stress and AF inducibility, as well as the CV decrease, but not the AERP shortening as in our canine AF model [8, 9]. Those results suggest the key role of oxidative stress in the structural remodeling observed in this canine AF model.
Interestingly, linagliptin, a DPP-4 inhibitor, suppressed AF and its electrophysiological basis in the same canine model in the present study. Because the oxidative stress was also suppressed in this study, this suppressive effect of linagliptin on AF inducibility was possibly considered to be related to the anti-oxidative effect. To the best of our knowledge, this is the first report that documented the suppressive effect of the DPP-4 inhibitor on AF in an in vivo model. When considering this result, the cardioprotective effect of the DPP-4 inhibitor in humans might be understandable [2, 3]. The precise mechanism of such a suppressive effect of linagliptin on the production of an AF substrate is unclear, but it might be partly explained by the possible relationship with the anti-oxidative effect. In the present study, DHE staining exhibited ROS overexpression in the atrial myocardium, which was almost fully negated by treatment with linagliptin. DPP-4 inhibitors inhibit the degradation of GLP-1 by suppressing the plasma DPP-4 enzyme and it results in an increase in the intrinsic GLP-1 level. In the present study, this effect of linagliptin was evident in Fig. 2, which demonstrates the time course of the plasm GLP-1 activity in this model. GLP-1 suppresses the ROS production by suppressing the NADPH-oxidase activity in various organs and it may result in the suppression of the inflammatory process [13, 14]. GLP-1 also facilitates the adenylyl cyclase activity and causes an increase in cyclic adenosine monophosphate (cAMP). This cAMP facilitates protein kinase A (PKA) activity and upregulates heme oxygenase-1 expression (HO-1), which is known as one of the stress-induced proteins and protects various cells against oxidative stress [15, 16]. This HO-1 upregulation may also be expressed as attenuation of the tissue injury caused by hyper-oxidative stress. Additionally, DPP-4 is a ligand for membrane-bound adenosine deaminase (ADA), which scavenges adenosine from the cellular environment [17]. Because ADA binds to the cellular membrane through DPP-4 [18], DPP-4 inhibitors may prevent a lack of energy by inhibiting the degradation of adenosine, especially in an ischemic condition. Adenosine suppresses the superoxide production by signaling through adenosine A1 receptors and the decrease in the inosine level suppresses the superoxide production by decreasing the formation of xanthine oxidase substrates [19, 20]. Besides these anti-oxidant mechanisms, a direct effect of DPP-4 inhibitors may be suspected. Because it is reported that there is enhanced activity of DPP-4 in patients with permanent AF [21], AF itself, i.e., rapid myocardial excitement, or its secondary effects such as increased atrial wall stress and/or neurohumoral stimulation, may promote DPP-4 activity. In such an environment, DPP4- inhibitors might directly affect the AF substrate even in humans.
Limitations
Our study had several limitations. Because a His-bundle ablation was not performed in our animal model, progression to tachycardia-induced heart failure cannot be ruled out. We previously confirmed that the influence of this potential progression was small [8,9,10, 12]. As we found no significant changes in the hemodynamic parameters, we consider that this model was suitable for evaluating the possible clinical effects of the drugs, as it closely mimicked clinical AF. Second, because there was only a single dose of linagliptin used in this study, the dose response could not be determined. Additionally, it was not clear whether the clinical dose of linagliptin would cause similar anti-remodeling and/or anti-oxidative effects as in humans. Third, although the suppression of atrial remodeling and ROS overexpression by linagliptin treatment was observed in this canine AF model, those were just parallel phenomenon and we did not exhibit any evidence of a relationship between them. Therefore, the anti-oxidative mechanism of linagliptin involved in the anti-remodeling effect should be understood as one possible speculation. Finally, because the expression of ionic channels was not examined, the effect of linagliptin on the cellular electrical remodeling could not be discussed.
Conclusions
In the canine AF model with 3-week rapid atrial pacing, linagliptin suppressed the AF inducibility and CV decrease, as well as the overexpression of ROS in atrial tissue. Because the AF suppression paralleled the suppression of interstitial fibrosis and ROS overexpression, there might have been a possible relationship between the suppressive effect of linagliptin on AF and the anti-oxidative effect of linagliptin.
References
Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, Maeda H, Fujisue K, Yamamoto E, Kaikita K, Hokimoto S, Jinnouchi H, Ogawa H (2013) Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J 77(5):1337–1344
Monami M, Ahrén B, Dicembrini I, Mannucci E (2013) Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 15(2):112–120
Avogaro A, de Kreutzenberg S, Fadini G (2014) Dipeptidyl-peptidase 4 inhibition: linking metabolic control to cardiovascular protection. Curr Pharm Des 20(14):2387–2394
Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, Sjöholm A (2004) Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 287:E1209–E1215
Zeisberg M, Zeisberg EM (2015) Kidney Evidence for antifibrotic incretin-independent effects of the DPP-4 inhibitor linagliptin. Kidney Int 88(3):429–431
Inthachai T, Lekawanvijit S, Kumfu S, Apaijai N, Pongkan W, Chattipakorn SC, Chattipakorn N (2015) Dipeptidyl peptidase-4 inhibitor improves cardiac function by attenuating adverse cardiac remodelling in rats with chronic myocardial infarction. Exp Physiol 100(6):667–679
Murase H, Kuno A, Miki T, Tanno M, Yano T, Kouzu H, Ishikawa S, Tobisawa T, Ogasawara M, Nishizawa K, Miura T (2015) Inhibition of DPP-4 reduces acute mortality after myocardial infarction with restoration of autophagic response in type 2 diabetic rats. Cardiovasc Diabetol 14:103
Kiryu M, Niwano S, Niwano H, Kishihara J, Aoyama Y, Fukaya H, Masaki Y, Izumi T (2012) Angiotensin II mediated up-regulation of connective tissue growth factor promotes atrial tissue fibrosis in the canine atrial fibrillation model. Europace 14:1206–1214
Kishihara J, Niwano S, Niwano H, Aoyama Y, Satoh A, Oikawa J, Kiryu M, Fukaya H, Masaki Y, Tamaki H, Izumi T, Ako J (2014) Effect of carvedilol on atrial remodeling in canine model of atrial fibrillation. Cardiovasc Diagn Ther 4:28–35
Satoh A, Niwano S, Niwano H, Kishihara J, Aoyama Y, Oikawa J, Fukaya H, Tamaki H, Ako J (2017) Aliskiren suppresses atrial electrical and structural remodeling in a canine model of atrial fibrillation. Heart Vessels 32(1):90–100
Fukaya H, Niwano S, Satoh D, Masaki Y, Niwano H, Kojima J, Moriguchi M, Izumi T (2008) Inhomogenic effect of bepridil on atrial electrical remodeling in a canine rapid atrial stimulation model. Circ J 72:318–326
Zheng MQ, Tang K, Zimmerman MC, Liu L, Xie B, Rozanski GJ (2009) Role of gamma-glutamyl transpeptidase in redox regulation of K+ channel remodeling in postmyocardial infarction rat hearts. Am J Physiol Cell Physiol 297:C253–C262
Hendarto H, Inoguchi T, Maeda Y, Ikeda N, Zheng J, Takei R, Yokomizo H, Hirata E, Sonoda N, Takayanagi R (2012) GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(p)H oxidase. Metabolism 61:1422–1434
Wang X, Ding Z, Yang F, Dai Y, Chen P, Theus S, Singh S, Budhiraja M, Mehta JL (2016) Modulation of myocardial injury and collagen deposition following ischemia-reperfusion by linagliptin and liraglutide, and both together. Clin Sci 130:1353–1362
Alam J, Cook JL (2003) Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des 9(30):2499–2511
Issan Y, Kornowski R, Aravot D, Shainberg A, Laniado-Schwartzman M, Sodhi K, Abraham NG, Hochhauser E (2014) Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS One 9(3):e92246
Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C (1993) Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science 261(5120):466–469
Zhong J, Rao X, Rajagopalan S (2013) An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis 226(2):305–314
Narayan P, Mentzer RM Jr, Lasley RD (2001) Adenosine A1 receptor activation reduced reactive oxygen species and attenuates stunning in ventricular myocytes. J Mol Cell Cardiol 33:121–129
Xiang F, Huang Y, Zhang D, Chu ZG, Zhang JP, Zhang Q (2010) Adenosine A1 receptor activation reduces opening of mitochondrial permeability transition pores in hypoxic cardiomyocytes. Clin Exp Pharmacol Physiol 37(3):343–349
Lendeckel U, Arndt M, Wrenger S, Nepple K, Huth C, Ansorge S, Klein HU, Goette A (2001) Expression and activity of ectopeptidases in fibrillating human atria. J Mol Cell Cardiol 33(6):1273–1281
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was partly supported by a grant for scientific research from the Ministry of Education Science and Culture of Japan (No. 26461085). The compound of linagliptin was offered from Boehringer-Ingelheim GmbH, Germany. J.A. received a speaker honorarium and research grant from Boehringer-Ingelheim. The other authors report no conflict of interest.
Rights and permissions
About this article
Cite this article
Igarashi, T., Niwano, S., Niwano, H. et al. Linagliptin prevents atrial electrical and structural remodeling in a canine model of atrial fibrillation. Heart Vessels 33, 1258–1265 (2018). https://doi.org/10.1007/s00380-018-1170-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-018-1170-0