Abstract
Aliskiren, a direct renin inhibitor is expected to achieve sufficient suppression of renin–angiotensin system. We evaluated the effect of aliskiren on the electrical and structural remodeling in a canine atrial fibrillation (AF) model. Twenty-eight dogs were divided into three groups: (1) pacing control group (n = 12), with continuous atrial rapid pacing for 3 or 6 weeks, (2) pacing + aliskiren group (n = 12), with oral aliskiren (30 mg/kg/day), and (3) sham group (n = 4), no pacing nor drug administration. Electrophysiological properties and AF inducibility were evaluated every week. After the protocol, the left atrial tissue was sampled for the further histological and mRNA analysis. The electrical remodeling, AF inducibility, the left atrial enlargement and interstitial fibrosis were observed in pacing control group and were more prominent in the 6-week protocol (vs. 3 week, p < 0.05). The mRNA expressions of matricellular proteins exhibited upregulation in 3-week pacing control, but these upregulations became insignificant in 6 weeks. In contrast, collagen type 3 exhibited significant upregulation in 6 week but not in 3-week protocol. These changes were suppressed in the pacing + aliskiren group. Aliskiren suppressed the atrial remodeling in a canine AF model. This effect was accompanied by the suppression of tissue fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice, and it occasionally leads to serious cerebral infarction [1–5]. Several basic studies demonstrate the potential importance of the renin–angiotensin system (RAS) on the occurrence of AF through a mechanism that promotes electrical and structural atrial remodeling, although it is suggested that angiotensin II receptor blockers (ARBs) could be used to suppress such atrial remodeling [6–10]. However, several large clinical trials failed to demonstrate the efficacy of ARBs on suppression of AF (i.e., “up-stream therapy” for AF) [11–13]. Although the precise mechanism of this discrepancy between basic researches and clinical trials is unclear, several possible explanations have been proposed.
ARBs just block the receptor of the most downstream component of the RAS without suppressing other parts of the RAS, resulting in an increase in aldosterone level (i.e., the so-called “aldosterone breakthrough phenomenon”) [14–17]. Moreover, recent reports have revealed an important role of intracellular RAS in fibroblast proliferation and interstitial fibrosis [18–20]. For example, ARBs and/or angiotensin converting enzyme inhibitors (ACEIs) have been shown to have negligible influence on suppressing intracellular RAS, resulting in apoptosis of myocytes and tissue fibrosis [18–20].
In contrast to traditional ARBs and/or ACEI, aliskiren is a direct renin inhibitor that is used for antihypertensive therapy. Aliskiren blocks the action of most upstream components of the RAS [21]. In this context, we suggest that aliskiren should be effective in suppressing atrial remodeling associated with AF. In this study, we evaluated the effects of aliskiren on the progression of atrial remodeling as an arrhythmogenic substrate in a canine model of AF.
Materials and methods
Objectives and initial surgery
Twenty-eight adult female beagle dogs (10.5 ± 1.1 kg) were anesthetized using pentobarbital (30 mg/kg IV), and butorphanol (0.3 mg/kg) was additionally administered for analgesia. Anesthesia levels were monitored by eyelash reflex activity every 15 min and pentobarbital (5 mg/kg IV) was added if necessary. Mechanical ventilation was maintained via an endotracheal tube using a mechanical ventilator (Model SN-480-5, Shinano Manufacturing, Tokyo, Japan) with 100 % oxygen. End-tidal capnometry was monitored throughout surgical procedures (CAPNOGARD1265, Fukuda Denshi Co. Ltd., Tokyo, Japan). Body temperatures were maintained at >37.0 °C throughout the surgery (THARMO-FINER type N-1, Terumo, Tokyo, Japan). Heart rate and rhythm were monitored using an electrocardiogram (ECG). A catheter was inserted in the right femoral artery to monitor the arterial blood pressure and arterial oxygen pressure. Two pairs of stainless steel wire electrodes were sutured against the epicardial surfaces of the free wall of the right atrium (RA) in the pectinate muscle area and left atrial appendage (LAA). The other ends of the wire electrodes were tunneled subcutaneously and exposed at the back of the neck. For continuous rapid atrial pacing, a unipolar screw-in pacing lead (CapSureFix 5568, Medtronic Inc., Minneapolis, MN, USA) was inserted through the right external jugular vein, and the distal end of the lead was screwed into the endocardial side of the right atrial appendage (RAA). The proximal end of the pacing lead was connected to a rapid pulse generator (Soletra®, Medtronic Inc.), which was implanted in a subcutaneous pocket in the neck. In this study, atrioventricular block was not produced to mimic the hemodynamic characteristics of clinical cases of AF [22–25]. All studies were performed in accordance with the guidelines specified by the Animal Experimentation and Ethics Committee of the Kitasato University School of Medicine, which were based on the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Study protocol
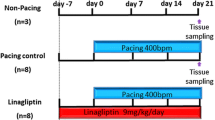
Study protocol is schematically summarized in Fig. 1. All dogs underwent echocardiography before initial surgery. To obtain stable baseline conditions, each dog was allowed to recover after the initial surgical procedure for at least 1 week without pacing. The 28 dogs were divided into the following three groups: (1) pacing control group (n = 12), (2) pacing + aliskiren (n = 12), and (3) sham (n = 4). In the pacing control group, rapid atrial pacing (400 beats/min) was initiated after 1-week recovery and continued for 3 (n = 4) or 6 weeks (n = 8). In the pacing + aliskiren group, rapid pacing was similarly performed as in the pacing control, i.e., for 3 (n = 4) or 6 weeks (n = 8), with the addition of oral administration of aliskiren (30 mg/kg/day). In the sham group, similar to other groups, initial surgery was performed in the absence of pacing and/or drug administration, and the dogs were put down after 6 weeks of observation.
Schematic of the study protocol. Observations started 1 week after the initial surgery, and atrial rapid pacing was delivered 3 or 6 weeks in the pacing control and pacing + aliskiren groups. Aliskiren was administered starting 1 week preceding the initial surgery in the pacing + aliskiren group. The left atrial tissue was sampled at 3- or 6-week time points
The 3-week protocol was used to specifically evaluate histological and molecular changes of the atria. Rapid atrial pacing was performed at an output of fourfold the diastolic threshold and with a pulse width of 2 ms. Electrophysiological properties were evaluated every week. At the end of the 6-week protocol, the echocardiogram was recorded and hemodynamic parameters were evaluated via catheterization under anesthesia with pentobarbital (30 mg/kg IV) before final killing.
Evaluation of the electrophysiological properties
In the 24 dogs with continuous rapid pacing, the atrial effective refractory period (AERP), the conduction time (CT) substituted for the conduction velocity as the reflection of the conduction property, inducibility of AF by burst pacing, and duration of induced AF were evaluated every week as previously described [22–25]. All ECGs were recorded using a polygraph system (Bioelectric AMPL, NEC, Tokyo, Japan). Analog signals were converted to digital signals and stored on a computer hard disk (Power Lab, ADInstrument, CO, USA) for data analyses [22–25]. During electrophysiological studies, rapid pacing was stopped temporarily, and all measurements were performed after pharmacological block of the autonomic nervous system had been produced by infusing atropine 0.05 mg/kg and propranolol 0.2 mg/kg [22–25].
At each evaluation time point, ERP was measured at the LA-free wall with basic drive cycle lengths (BCL) of 300, 200, and 150 ms. Pacing output was set at twice the diastolic threshold during each evaluation. The coupling interval of the premature stimulus was shortened by 2-ms steps. The longest coupling interval of the premature beat that failed to capture the atrium was determined as the local AERP. Conduction time between the LA and RA was measured during LA pacing at cycle lengths of 300, 200, and 150 ms. Because conduction velocity could not be directly calculated in our study design, the conduction property was expressed as the reciprocal of conduction time (1/CT), and the values were expressed in percentages as the %1/CT by dividing each piece of data by the data for day 0 to exclude the influence of the difference in the actual distance of the electrodes in each dog. To evaluate AF inducibility, AF was induced with atrial burst pacing for 3 s at the minimal pacing cycle length that achieved 1:1 atrial capture at the LA. This pacing was delivered at fourfold the diastolic threshold with a pulse width of 2 ms. When AF was induced, its duration was measured. We defined AF as a spontaneous irregular atrial rhythm lasting longer than 1 s, and the duration of the induced AF was subclassified as long (>10 s), medium (5–10 s), or short (1–5 s). Atrial burst pacing for AF induction was delivered five times at each evaluation time point during the entire protocol.
Evaluation in echocardiography
To evaluate structural and functional cardiac differences amongst the three groups, LA dimension (LAD), LA volume (LAV), left ventricular diastolic dimension (LVDd), and left ventricular ejection fraction (LVEF) were evaluated using echocardiography (EUB-6500 and EUP-S50, Hitachi Medical Corporation, Tokyo, Japan) at initial surgery and at the end of protocol for dogs in the CTL and pacing + aliskiren groups. All dogs were not anesthetized during the echocardiography. Changes in parameters were calculated as ΔLAD, ΔLAV, ΔLVDd, and ΔLVEF by subtracting the data at the time of initial surgery from the data after 6-week pacing.
Administration of aliskiren
Aliskiren (30 mg/kg/day) was orally administered in 12 out of 28 dogs starting 1 week preceding the initial surgery, and they were assigned as the pacing + aliskiren group. This protocol was designed to evaluate the effect of aliskiren on atrial electrical remodeling as an “upstream therapy” for the AF substrate, so that aliskiren was started 2 weeks prior to the start of rapid pacing (Fig. 1).
Hemodynamic parameters
At the end of the each protocol, the following hemodynamic parameters were evaluated by catheterization: systemic blood pressure (BP), pulmonary arterial pressure (PAP), pulmonary arterial wedge pressure (PAWP), central venous pressure (CVP), and cardiac output in all 28 dogs.
Histology
After hemodynamic evaluation, all 28 dogs were put down via pentobarbital overdose and small portions of LA-free walls were excised from the area apart from the electrodes and fixed for histological analysis. Tissue fibrosis was evaluated by Azan staining, and the degree of fibrosis was quantified by measuring the mean %area in digitized images.
Quantitative real-time reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was prepared from the small excised portion of the LA-free wall using a total RNA isolation kit (SV Total RNA Isolation System, Promega, Madison, WI, USA). Complementary deoxyribonucleic acid (cDNA) was synthesized from 3 μg of the total RNA with reverse transcriptase (Invitrogen, San Diego, CA, USA) in a final volume of 20 μl. mRNA levels of the matricellular protein-related and signaling molecules, i.e., fibronectin 1 (FN1), Periostin, type 3 collagen (COL3), monocyte chemotactic protein (MCP1), and transforming growth factor β (TGF-β), were evaluated by quantitative real-time RT-PCR. These factors were selected as upregulated factors in global DNA profiling during our pilot study. The level of beta-actin was also evaluated as an internal control. Real-time RT-PCR was performed with a QuantiTect™ SYBR Green PCR Master Mix (Qiagen, Valencia, CA, USA) and CFD 3240 Chromo4™ Detection System (Bio-Rad Lab, Inc., Richmond, CA, USA) using each primer pair. For the standard curve, the standard plasmid for each molecule was constructed as previously described [23]. Serially diluted standard plasmids were analyzed at the same time, and the absolute copy numbers were calculated.
Immuno-fluorescence staining evaluations of fibronectin 1 and type 3 collagen
Immuno-fluorescence staining of FN1 and COL3 was performed as follows. Small sections of LA tissue in all dogs were fixed in 2 % paraformaldehyde for 1 h and then replaced by 30 % saccharose and embedded in OCT compound. Frozen 4-µm sections were rinsed using phosphate-buffered saline (PBS) and blocked using 0.2 % bovine serum albumin for 30 min. Then, each section was activated by anti-fibronectin antibody [IST-9] (ab6328; Abcam plc, Cambridge, UK) or by anti-collagen type 3 antibody (Quartett GmbH, Berlin, Germany) for 1 h. Finally, all sections for both FN1 and COL3 were activated by Alexa Fluor 594 goat anti-mouse IgG antibody for 45 min, and observed by Olympus DP70 (Olympus, Japan) fluorescence microscope.
All objective analyses were performed by two blinded investigators.
Statistical analysis
All data are expressed as mean ± SE. ANOVA test was applied to comparison among the three groups. The data between the unpaired two groups were compared using Student’s t test. Paired t test was applied for comparing data at each measurement time points with day 0 in each group. A p value <0.05 was considered significant.
Results
Changes in the electrophysiological parameters
Figure 2 exhibits the changes in AERP and 1/CT, i.e., ΔAERP and %1/CT, along the time course of rapid atrial pacing. In the pacing control group, AERP was rapidly shortened in the first week of rapid pacing and continued till the end of the protocol as previously reported [24, 25]. In the pacing + aliskiren group, the AERP was shortened similarly (Fig. 2a–c). The degree of AERP shortening in the pacing + aliskiren group was consistently smaller than that in the pacing control group, but differences reached significance only in the first 3 weeks of BCL 200 ms protocol (Fig. 2b). The %1/CT decreased gradually from baseline to the end of the study in both pacing control and pacing + aliskiren groups (Fig. 2d–f). The degree of %1/CT decrease was generally smaller in the pacing + aliskiren group than in the pacing control group, and the differences were significant in the later phases of the 6-week protocol in BCLs of 150 and 200 ms.
Changes in electrophysiological parameters. a–c The changes in ΔAERP, and d–f exhibit changes in %1/CT with 3 BCLs. Over the course of the protocol, AERP shortening (negative ΔAERP) and decrease in %1/CT were observed in all BCLs. When comparing the pacing control (n = 12) and pacing + aliskiren groups (n = 12), the degree of ΔAERP changes and %1/CT decrease were generally smaller in the pacing + aliskiren group than in the pacing control group, although the significant differences were observed only at limited points
AF inducibility and the duration of AF
Figure 3 shows changes in AF inducibility over the course of the pacing protocol. AF inducibility tended to increase gradually over time in both groups, reaching significantly higher levels in the pacing control group in the later phase, i.e., 3–6 weeks, of the 6-week protocol (Fig. 3). In later phases of the 6-week protocol during the analysis of AF duration, incidence of long AF was significantly higher in the pacing control group than in the pacing + aliskiren group (Fig. 3). All episodes of induced AF were spontaneously terminated, and chronic AF was not induced in this study.
Changes in AF inducibility along the time course. The left and right bars in each pair of bars indicate the data of the pacing control (n = 12 in 0–3 weeks, n = 8 in 4–6 weeks) and pacing + aliskiren groups (n = 12 in 0–3 weeks, n = 8 in 4–6 weeks), respectively. The solid, gray and white colors indicate induced AF durations. The AF inducibility tended to increase gradually over time in both groups, and was significantly higher in the pacing control group in the later phase of the 6-week protocol. The incidence of long AF was significantly higher in the pacing control group compared with that in the pacing + aliskiren group during the later phase
Parameters in echocardiography
Figure 4 shows echocardiographic outcomes. In the pacing control group, both 3- (n = 4) and 6- (n = 8) week-paced dogs exhibited slight enlargements of LAV, LAD, and LVDd and slight decreases in LVEF after pacing. Therefore, ΔLAV, ΔLAD, and ΔLVDd were positive and ΔLVEF was negative. The pacing + aliskiren group outcomes were similar to those of the pacing control group, except that the degree of LA enlargement tended to be smaller. The ΔLAV was significantly smaller in the pacing + aliskiren group than in the pacing control group in both 3- (n = 4) and 6- (n = 8) week-paced dogs.
Changes in echocardiographic parameters. In the pacing control group, both 3- (n = 4) and 6- (n = 8) week-paced dogs exhibited slight enlargement of LAV, LAD, and LVDd and slight decrease in LVEF after pacing. The pacing + aliskiren group showed similar tendency, but the degree of LA enlargement tended to be smaller. The ΔLAV was significantly smaller in the pacing + aliskiren group than that in the pacing control group in both 3- and 6-week-paced dogs
Hemodynamic parameters
Hemodynamic evaluation at the end of the 6-week protocol resulted in no significant differences among pacing control and pacing + aliskiren groups or between the 3- (n = 4) and 6- (n = 4) week-paced dogs for BP, PAP, CO, PAWP, and CVP.
Histological changes
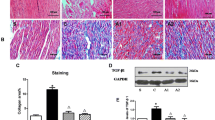
Figure 5 shows atrial histological changes in HE and Azan staining (a, b). In comparison with the sham group (n = 4), the pacing control group (n = 4) exhibited slight misalignment of myocytes, and interstitial fibrosis at the 3-week time point, which worsened at the 6-week time point (n = 8) in the Azan staining. In contrast, in the pacing + aliskiren group, histological findings were negated at both 3-week (n = 4) and 6-week time points (n = 8). When comparing the fibrotic area as %area in digitized images (c), the degree of fibrosis was increased in the pacing control group compared with that in the sham group, and it was significantly suppressed in the pacing + aliskiren group at both 3- and 6-week time points in comparison with the pacing control group.
Histological findings of HE staining (a) and Azan staining (b). In comparison with the sham group, the pacing control group exhibited slight misalignment of myocytes, fatty degeneration, and interstitial fibrosis even at 3-week time point (n = 4), which became more prominent at the 6-week time point (n = 8). In contrast, in the pacing + aliskiren group, histological findings were almost totally negated. In the comparison of fibrotic area (c), the degree of fibrosis increased in the pacing control group in comparison with the sham group (n = 4), and it was significantly suppressed in the pacing + aliskiren group at both 3 (n = 4) and 6 (n = 8) weeks
Expressions of fibrosis- and inflammation-related genes
Figure 6 shows mRNA expression levels of inflammation- and fibrosis-related genes. At the 3-week time point, mRNA expressions of MCP1 and fibronectin 1 were upregulated in the pacing control group (n = 4) in comparison with the sham group (n = 4), but these upregulations were suppressed in the pacing + aliskiren group (n = 4). At the 6-week time point, aliskiren tended to suppress these upregulations, but the difference between the pacing control (n = 8) and pacing + aliskiren groups (n = 8) became insignificant. In contrast, upregulation of COL3 was insignificant at week 3, but became significant at week 6. This upregulation of COL3 was also suppressed by aliskiren. TGF-β did not exhibit any significant changes at both 3- and 6-week time points.
Comparison of mRNA expressions of fibrosis- and inflammation-related genes. At the 3-week time point, mRNA expressions of MCP1 and fibronectin 1 were upregulated in the pacing control group (n = 4) in comparison with the sham group (n = 4), but these upregulations were suppressed in the pacing + aliskiren group (n = 4). At the 6-week time point, aliskiren tended to suppress upregulations but the difference between the pacing control (n = 8) and pacing + aliskiren groups (n = 8) became insignificant. The upregulation of COL3 was insignificant at 3 weeks, but became significant at 6 weeks. This upregulation of COL3 was also suppressed by aliskiren. TGF-β did not exhibit any significant changes at both 3- and 6-week time points
Expression of FN1 and COL3 were also evaluated by immuno-fluorescent staining (Fig. 7). Expression of FN1 seemed to be enhanced in the pacing control group in comparison with the sham group. However, this enhancement was not observed in the pacing + aliskiren group. At the 6-week time point, expression of FN1 decreased in total in comparison with the 3-week time point in the pacing control group, but the complexity of FN1 was enhanced and polymerized, and was localized in the specific area of interstitial connective tissue. In contrast, expression of COL3 was not observed at the 3-week time point, but clearly appeared at the 6-week time point in the pacing control group. This expression of COL3 was almost totally negated in the pacing + aliskiren group even at 6-week time point. This result was almost similar to the results obtained in the examination of mRNA expressions.
Immuno-fluorescent staining of FN1 (a) and COL3 (b). The expression of FN1 was enhanced in the pacing control group (n = 4) in comparison with the sham group (n = 4), but this enhancement was not observed in the pacing + aliskiren group (n = 4). At the 6-week time point, expression of FN1 appeared to be decreased in total in comparison with the 3-week time point in the pacing control group (n = 8), but the complexity of FN1 was enhanced and polymerized, and was localized in a specific area of the interstitial connective tissue. The expression of COL3 was not observed at the 3-week time point, but clearly appeared at the 6-week time point in the pacing control group (n = 8). The expression of COL3 was negligible in the pacing + aliskiren group (n = 8)
Discussion
This study evaluating the effects of aliskiren, a direct renin inhibitor, on the canine AF model has exhibited several interesting findings. First, aliskiren suppressed electrophysiological changes, such as AERP shortening, 1/CT decrease, and increase in AF inducibility, in the LA. Second, aliskiren also suppressed the increase in LAV, i.e., structural remodeling of the LA. Third, aliskiren suppressed tissue fibrosis at both 3- and 6-week time points. Fourth, the time course of the upregulations of tissue fibrosis mediators, such as FN-1, MCP-1, and periostin, was initially documented in the earlier phase, but these changes were rather converged in the later phase. In contrast, the upregulations of COL3 were observed more prominently at the later phase of the protocol, which suggests that upregulation of COL3 may be preceded by temporal upregulations of tissue fibrosis mediators. Finally, aliskiren appeared to suppress upregulations of various mediators such as COL3.
Suppressive effect of aliskiren on the electrical remodeling in the LA
To the best of our knowledge, this report is the first documentation of suppressive effects of aliskiren on AF in a canine atrial rapid pacing model. The degree of suppression of AERP shortening was less prominent and insignificant in part, but it was similar to the efficacy of other anti-RAS medicines [9, 24, 25]. The suppressive effect of aliskiren on AF inducibility was considered to mainly depend on the suppression of 1/CT decrease and/or interstitial fibrosis. In our canine model of AF, we have previously reported several possible medicines, including olmesartan, which could suppress atrial remodeling, but significant suppression was observed mainly in the right atrium and sometimes not in the LA in part [9, 10, 24, 26]. The mechanism of the difference between the RA and LA is still unclear [9, 10, 24, 26], but at least in the present study, aliskiren suppressed the atrial remodeling in the LA. Aliskiren was the first medicine that suppressed the atrial remodeling of LA at least in our canine model of AF. Considering LA is the main stage of AF substrate, aliskiren may be an effective therapy for early-stage AF. Recently, Zhao et al. [27] have reported that aliskiren would exhibit suppressive effect on the changes in ion channel expressions in the atrial tachycardia-induced atrial fibrillation model. In our present study, we cannot discuss about the ion channel expressions which were not evaluated in this study, but the ERP was not significantly modified by aliskiren. The reason of this discordance is unclear, but it may depend on the slight difference in the model itself. The mechanism of suppressive effect of aliskiren should be considered to appear through its effect on the conduction property and the expressions of matricellular proteins and collagen at least in our present study.
Suppressive effect of aliskiren on the atrial fibrosis and structural remodeling
Our results indicate that aliskiren suppressed atrial structural remodeling being independent of improvements in left ventricular function [24, 26]. Moreover, histological evaluation revealed the suppressive effect of aliskiren on interstitial fibrosis and degeneration of myocytes. This is consistent with the findings of De Mello et al. who reported that there is a correlation between 1/CT decrease and interstitial fibrosis, indicating that the suppressive effects of aliskiren on tissue fibrosis may be involved in the mechanism of suppression of 1/CT decrease [28]. This mechanism appears to be similar to the effects of ARBs on such structural remodeling in AF model.
Mechanism of suppression of atrial tissue fibrosis
Quantification of the expression of mRNA revealed the upregulation of some matricellular proteins and extracellular matrix-related genes, such as FN-1, MCP-1, or periostin. Interestingly, such upregulations appeared in the relatively earlier phase in this study protocol, i.e., at 3 weeks, preceding the appearance of COL3 proliferation. This clearly indicates that the upregulation of these mediators are the mechanism of later tissue fibrosis at least in this model of AF. Immuno-fluorescent staining also revealed that FN-1, one of the multifunctional matricellular proteins, showed marked increase in the pacing control group at 3 weeks. In addition, consistent with mRNA analysis, expression of FN-1 decreased at 6 weeks, but it appeared to be polymerized and localized. Because FN-1 is also an important mediator of inflammation, subsequent appearance of myocyte degeneration and tissue fibrosis may be explained by FN-1 upregulation during the early phase [29, 30]. Although aliskiren may potentially suppress COL3 upregulation during the later phase, suppression of the upregulation of matricellular proteins during the early phase is probably a main mechanistic influence of aliskiren on the suppression of atrial remodeling. The recent reports also suggest the suppressive effect of aliskiren on tissue fibrosis through the suppression of intracellular RAS, even in other organs [31]. Other reports also evaluated the effect of aliskiren on pulmonary-atrial conduction triggering AF, and they documented suppression of CV decrease and an increase in AF inducibility [28, 32]. These findings are compatible with the results of our present study.
Limitations
The present study has several limitations. First, we did not evaluate the ion-channels or transporters, which may influence ERP. Second, we did not evaluate the localization and expression of connexin families, which can strongly influence 1/CT. Third, 1/CT was not the actual reflection of the conduction velocity because the distance between two electrodes was not measured and fixed by enlargement of atrial geometry. Forth, we did not evaluate the systemic effect of aliskiren particularly on the levels of RAS components. Finally, the sampling time point was totally limited, but it was difficult to design a study using a large number of medium-sized animals. In future, these limitations should be studied with different designs.
Conclusion
Aliskiren suppressed AF inducibility in canines. This effect was accompanied by and possibly resulted from the suppression of tissue fibrosis. Moreover, aliskiren suppressed the excess MCP1 and FN1 in relatively early phase of the atrial remodeling, and it was considered to result in the later suppression of COL3 upregulation and subsequent suppression of atrial tissue fibrosis.
References
Wolf PA, Mitchell JB, Baker CS, Kannel WB, D’Agostino RB (1998) Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med 158:229–234
Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D (1998) Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98:946–952
Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ (2003) Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 107:2920–2925
Hamatani Y, Iguchi M, Nakamura M, Ohtani R, Yamashita Y, Takagi D, Unoki T, Ishii M, Masunaga N, Ogawa H, Hamatani M, Abe M, Akao M (2015) Incidence and predictors of ischemic stroke during hospitalization for congestive heart failure. Heart Vessels. doi:10.1007/s00380-015-0719-4
Yang YJ, Yuan JQ, Fan CM, Pu JL, Fang PH, Ma J, Guo XY, Li YS (2015) Incidence of ischemic stroke and systemic embolism in patients with hypertrophic cardiomyopathy, nonvalvular atrial fibrillation, CHA2DS2-VASc score of ≤1 and without anticoagulant therapy. Heart Vessels. doi:10.1007/s00380-015-0718-5
Members of the Sicilian Gambit (2001) New approaches to antiarrhythmic therapy. Part I: emerging therapeutic applications of the cell biology of cardiac arrhythmias. Circulation 104:2865–2873
Patlolla V, Alsheikh-Ali AA, Al-Ahmad AM (2006) The renin–angiotensin system: a therapeutic target in atrial fibrillation. Pacing Clin Electrophysiol 29:1006–1012
Schneider MP, Hua TA, Böhm M, Wachtell K, Kjeldsen SE, Schmieder RE (2010) Prevention of atrial fibrillation by renin–angiotensin system inhibition a meta-analysis. J Am Coll Cardiol 55:2299–2307
Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K (2003) Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol 41:2197–2204
Kataoka N, Nishida K, Kinoshita K, Sakamoto T, Nakatani Y, Tsujino Y, Mizumaki K, Inoue H, Kinugawa K (2016) Effect of irbesartan on development of atrial fibrosis and atrial fibrillation in a canine atrial tachycardia model with left ventricular dysfunction, association with p53. Heart Vessels. doi:10.1007/s00380-016-0853-7
GISSI-AF Investigators, Disertori M, Latini R, Barlera S, Franzosi MG, Staszewsky L, Maggioni AP, Lucci D, Di Pasquale G, Tognoni G (2009) Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med 360:1606–1617
The AFFIRM Investigators, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD (2002) A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 347:1825–1833
J-RHYTHM II Investigators, Yamashita T, Inoue H, Okumura K, Kodama I, Aizawa Y, Atarashi H, Ohe T, Ohtsu H, Kato T, Kamakura S, Kumagai K, Kurachi Y, Koretsune Y, Saikawa T, Sakurai M, Sato T, Sugi K, Nakaya H, Hirai M, Hirayama A, Fukatani M, Mitamura H, Yamazaki T, Watanabe E, Ogawa S (2011) Randomized trial of angiotensin II-receptor blocker vs. dihydropyridine calcium channel blocker in the treatment of paroxysmal atrial fibrillation with hypertension (J-RHYTHM II Study). Europace 13:473–479
McKelvie RS, Yusuf S, Pericak D, Avezum A, Burns RJ, Probstfield J, Tsuyuki RT, White M, Rouleau J, Latini R, Maggioni A, Young J, Pogue J (1999) Comparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD Pilot Study Investigators. Circulation 100:1056–1064
Schjoedt KJ, Andersen S, Rossing P, Tarnow L, Parving HH (2004) Aldosterone escape during blockade of the renin–angiotensin–aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia 47:1936–1939
Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Cabrini G, Bonizzato A, Graziani M, Anker SD, Coats AJ, Zardini P (2001) Failure of aldosterone suppression despite angiotensin-converting enzyme (ACE) inhibitor administration in chronic heart failure is associated with ACE DD genotype. J Am Coll Cardiol 37:1808–1812
MacFadyen RJ, Lee AF, Morton JJ, Pringle SD, Struthers AD (1999) How often are angiotensin II and aldosterone concentrations raised during chronic ACE inhibitor treatment in cardiac failure? Heart 82:57–61
Baker KM, Kumar R (2006) Intracellular angiotensin II induces cell proliferation independent of AT1 receptor. Am J Physiol Cell Physiol 291:C995–C1001
Re RN (2003) Intracellular renin and the nature of intracrine enzymes. Hypertension 42:117–122
Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC (2012) Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol 302:R518–R530
Nussberger J, Wuerzner G, Jensen C, Brunner HR (2002) Angiotensin II Suppression in humans by the orally active renin inhibitor aliskiren (SPP100) comparison with enalapril. Hypertension 39(1):E1–E8
Kojima Jisho, Niwano Shinichi, Moriguchi Masahiko, Ikeda Kazuko, Inuo Kimiatsu, Saito Junko, Izumi Toru (2003) Effect of pilsicainide on atrial electrophysiologic properties in the canine rapid atrial stimulation model. Circ J 67:340–346
Sato Daisuke, Niwano Shinichi, Imaki Ryuta, Masaki Yoshihiko, Sasaki Sae, Yuge Masaru, Hirasawa Shoji, Sasaki Takeshi, Moriguchi Masahiko, Niwano Hiroe, Yoshimura Hirokuni, Izumi Tohru (2006) Bepridil inhibits sub-acute phase of atrial electrical remodeling in canine rapid atrial stimulation model. Circ J 70:206–213
Fukaya H, Niwano S, Niwano H, Masaki Y, Kiryu M, Hirasawa S, Sato D, Moriguchi M, Izumi T (2012) Combined effects of up- and downstream therapies on atrial fibrillation in a canine rapid stimulation model. Int J Cardiol 157:197–206
Fukaya H, Niwano S, Satoh D, Masaki Y, Niwano H, Kojima J, Moriguchi M, Izumi T (2008) Inhomogenic effect of bepridil on atrial electrical remodeling in a canine rapid atrial stimulation model. Circ J 72:318–326
Nakashima H, Kumagai K (2007) Reverse-remodeling effects of angiotensin II type 1 receptor blocker in a canine atrial fibrillation model. Circ J 71:1977–1982
Zhao Z, Wang X, Li J, Yang W, Cheng L, Chen Y, Liu T, Liu E, Chen K, Li G (2014) Protective effects of aliskiren on atrial ionic remodeling in a canine model of rapid atrial pacing. Cardiovasc Drugs Ther 28(2):137–143
De Mello W, Rivera M, Rabell A, Gerena Y (2013) Aliskiren, at low doses, reduces the electrical remodeling in the heart of the TGR(mRen2)27 rat independently of blood pressure. J Renin Angiotensin Aldosterone Syst 14:23–33
Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP (2011) Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res 108:582–592
Choi MG, Hynes RO (1979) Biosynthesis and processing of fibronectin in NIL.8 hamster cells. J Biol Chem 254:12050–12055
Singh VP, Le B, Khode R, Baker KM, Kumar R (2008) Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 57:3297–3306
Tsai CF, Chen YC, Lin YK, Chen SA, Chen YJ (2011) Electromechanical effects of the direct renin inhibitor (aliskiren) on the pulmonary vein and atrium. Basic Res Cardiol 106:979–993
Acknowledgments
This study was supported by a Grant for scientific research from the Ministry of Education Science and Culture of Japan (No. 23591061).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There was no financial support from any specific company for this study or any conflict of interest. No specific unapproved use of any compound or product occurred.
Disclosure
Aliskiren (RaslilezR tablets, for oral use) was supplied by Novartis Pharma, Co. Ltd.
Rights and permissions
About this article
Cite this article
Satoh, A., Niwano, S., Niwano, H. et al. Aliskiren suppresses atrial electrical and structural remodeling in a canine model of atrial fibrillation. Heart Vessels 32, 90–100 (2017). https://doi.org/10.1007/s00380-016-0874-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-016-0874-2