Abstract
Bepridil is an effective antiarrhythmic drug on supraventricular and ventricular arrhythmias, and inhibitor of calmodulin. Recent investigations have been elucidating that bepridil exerts antiarrhythmic effects through its acute and chronic application for patients. The aim of this study was to identify the efficacy and the potential mechanism of bepridil on the inward-rectifier potassium channel in neonatal rat cardiomyocytes in acute- and long-term conditions. Bepridil inhibited inward-rectifier potassium current (I K1) as a short-term effect with IC50 of 17 μM. Bepridil also reduced I K1 of neonatal cardiomyocytes when applied for 24 h in the culture medium with IC50 of 2.7 μM. Both a calmodulin inhibitor (W-7) and an inhibitor of calmodulin-kinase II (KN93) reduced I K1 when applied for 24 h as a long-term effect in the same fashion, suggesting that the long-term application of bepridil inhibits I K1 more potently than that of the short-term application through the inhibition of calmodulin kinase II pathway in cardiomyocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bepridil is an effective drug for a wide range of supraventricular and ventricular tachyarrhythmias. Although bepridil has originally been recognized as a class IV antiarrhythmic agent, an improved understanding of the pharmacological effects of this drug has reinforced the characteristics; bepridil is referred to as a multichannel blocker nowadays. In view point of pharmacodynamics, bepridil is a highly lipophilic drug (log P = 5.49, pKa = 9.16 at 37 °C) of which protein binding is approximately 99 % [1], and therapeutic plasma concentration may range from 0.5 to 5.0 µM [2]. In electropharmacological investigations, reported short-term effects of bepridil have included the blocking of various types of ion channels and transporters, such as inward-rectifier potassium current (I K1) [3], transient outward potassium current (I to) [3], rapid component of delayed rectifier potassium current (I Kr), slow component of delayed rectifier potassium current (I Ks), ultra-rapid component of delayed rectifier potassium current (I Kur) [4, 5], muscarinic acetylcholine-activated K+ current (I K,ACh), Na+-activated K+ current (I K,Na), sarcolemmal ATP-sensitive K+ current (I K,ATP) [6–8], voltage-gated sodium channel current (I Na), L-type calcium channel current (I Ca.L), T-type calcium channel current (I Ca.T) [2] and Na+–Ca2+ exchanger current [9–11]. More recently, long-term effects of bepridil on ionic currents have been recognized [12, 13]. Also several clinical researches have demonstrated that bepridil could be effective for treatment of persistent atrial fibrillation (AFib) and for the maintenance of normal sinus rhythm [14]. Remarkably, bepridil terminated AFib in 2 weeks after starting the administration in this clinical study, which suggests that bepridil has a long-term effect to reverse atrial electrical remodeling. Also several in vivo animal studies demonstrated that a long-term administration of bepridil prevented the shortening of the effective refractory period in the atrium when high-frequent electrical pacing was applied [15, 16]. These results also suggest that bepridil has a long-term antiarrhythmic effect besides its inhibitory action on various ionic channels, although the underlying mechanisms remain unclear. We hypothesized that bepridil might exert long-term electrophysiological effects on cardiomyocytes preferable as an antiarrhythmic drug. Because the inward-rectifier K+ channel is a major K+ channel responsible for the remodeling in the atrium with persistent AFib, we investigated a long-term effect of bepridil focusing on I K1 in comparison with its short-term effect on the same ionic channel by use of rat neonatal cardiomyocytes.
Materials and methods
The experimental protocol was approved in advance by the Ethics Review Committee for Animal Experimentation of Oita University School of Medicine.
Cell preparation
Neonatal rat cardiomyocytes (NRCMs) were prepared from 1–3-day-old Wistar rats as described previously [17, 18]. Isolated cardiomyocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10 % fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin at 37 °C in a 95 % O2-5 % CO2 incubator. Bepridil hydrochloride was a kind gift from Daiichi-Sankyo Company (Tokyo, Japan). Bepridil was prepared as 1 mM stock solution in distilled water. W-7, a calmodulin inhibitor, and KN93, an inhibitor of Ca2+/calmodulin-dependent kinase type II, purchased from Wako Pure Chemical Industries (Osaka, Japan), were dissolved in distilled water as 10 and 1 mM stock, respectively.
Electrophysiological recording
I K1 in NRCMs were recorded by whole-cell patch clamp using an EPC-9 amplifier controlled by Pulse ver. 8 software (HEKA Eletronik, Lambrecht, Germany). Patch pipettes were pulled from 75-mm plain capillary tubes (Drummond Scientific Co., Broomall, PA, USA) by Model P-97 (Sutter Instrument Co., Novato, CA, USA), and were heat-polished subsequently to achieve the pipette resistance at 2–4 MΩ when filled with the pipette solution. Series resistance was compensated by at least 80 % and was continually monitored throughout the experiment. The I K1 current was elicited by 1000 ms depolarizing steps from a holding potential of −40 mV to potentials ranging from −120 to +30 mV in 10-mV increments. All the current measurements were done at room temperature (20–23 °C). For the current recording, the chamber was filled with bath solution contained (mM) NaCl 140, KCl 5.4, MgCl2 1, CaCl2 1.8, HEPES 10, glucose 10 (pH 7.4 by NaOH). To suppress potential interference of I to, I Ks and I Ca.L to the measurement of IK1, 4-aminopyridine (2 mM), chromanol 293B (10 μM) and CdCl2 (0.3 mM) were added to the bath solution. The patch electrodes were filled with pipette solution consists of (mM) KCl 140, MgCl2 1, EGTA 10, HEPES 10, and Mg-ATP 5 (pH 7.2 by KOH).
Statistical analysis
All data are presented mean ± SEM. Statistical analysis was performed by one-way ANOVA with Student–Newman–Keuls or Fisher LSD. IC50 values were estimated using non-linear least square curve-fitting programs in Sigma plot software ver. 10 (SPSS, Chicago, IL, USA). Differences were considered significant when p values were less than 0.05 if nothing else is mentioned.
Results
Acute effect of bepridil on I K1
We first examined the acute effect of bepridil on I K1 in NRCMs. The cells were superfused with normal Tyrode’s solution and the membrane potential was held at −80 mV followed by a short prepulse (−40 mV, 300 ms) to establish a block of the voltage-gated sodium (Na+) channel and the transient outward potassium (K+) channel. To begin with, we applied Ba2+ into the bath solution to confirm that the evoked currents were mainly composed of I K1 (Fig. 1b). Figure 1a illustrates typical acute effects of bepridil (1, 5, 10 µM) on I K1 as sequentially applied on the same cardiomyocyte, demonstrating a dose-dependent inhibition of I K1 by acute application of bepridil. After application of bepridil in 5 min, the terminal current of I K1 was decreased by 15 % (1 µM), by 24 % (5 µM) and by 32 % (10 µM) as assessed at −120 mV in this cardiomyocyte. The acute effects of bepridil on I K1 were plotted against the membrane potentials imposed (I–V relationship), and summarized in Fig. 1b: bepridil of 1, 5, 10 and 30 µM inhibited I K1 by 16.4, 31, 39.6 and 53.6 % at −100 mV, respectively. Slope conductance of I K1 at −100 mV was decreased by bepridil in a dose-dependent manner; bepridil at concentrations of 1, 5, 10 and 30 µM decreased the conductance to 0.56 ± 0.03, 0.52 ± 0.06, 0.46 ± 0.05, 0.36 ± 0.02 nS/pF, respectively, from the control value of 0.68 ± 0.06 nS/pF (Fig. 1c). Slope conductance of I K1 at +20 mV was also decreased by bepridil in a dose-dependent manner; bepridil at 1, 5, 10 and 30 µM decreased the conductance to 0.031 ± 0.01, 0.028 ± 0.004, 0.004 ± 0.003, 0.006 ± 0.001 nS/pF, respectively, from the control value of 0.062 ± 0.004 nS/pF (Fig. 1d).
Acute effect of bepridil on inward rectifier potassium current (I K1). a Current traces of I K1 in the control and during the acute (5 min) application of bepridil (1, 5, 10 µM) are shown. Outward I K1 traces at the potentials of +20 mV at the terminal phase indicated by box are shown in an inset. b I–V relationships constructed by using group data in control and during the application of 1, 5, 10 and 30 µM bepridil in 5 min. The current density of I K1 at −100 mV was reduced to −9.9 ± 0.6 mV at 1 µM (p = 0.027), −8.2 ± 1 mV at 5 µM (p = 0.003), −7.1 ± 0.7 mV at 10 µM (p = 0.001) and −5.5 ± 0.4 mV at 30 µM (p = 0.001 vs. control). c, d The slope conductance in the control and during the acute effect of 1, 5, 10 and 30 µM bepridil at −100 mV (c) and +20 mV (d). *p < 0.05, **p < 0.01 compared with control
Acute effect of W-7 and KN93 on I K1
Because bepridil is a potent inhibitor of calmodulin, we next studied the impact of acute calmodulin inhibition on I K1. Representative traces in the absence or presence of a calmodulin inhibitor (W-7) or an inhibitor of calmodulin kinase type II (KN93) are illustrated in Fig. 2a. The acute application of W-7 (10, 20 µM) was without effect on I K1 (Fig. 2a, b), and so was KN93 (10 µM). There was no significant change in the slope conductance of I K1 at −100 and +20 mV by blocking calmodulin with W-7 or by blocking calmodulin kinase type II with KN93 (Fig. 2c, d).
Acute effect of calmodulin inhibition on I K1. a Current traces of I K1 in the control and during the acute (5 min) application of W-7, a calmodulin inhibitor, and KN93, an inhibitor of calmodulin kinase type II, are shown. Outward I K1 traces at the potentials of +20 mV at the terminal phase indicated by box are shown in an inset. b I–V relationships constructed by using group data in control and during the acute application of W-7 (10, 20 µM) and KN93 (10 µM). c, d The slope conductance in the control and during the acute effect of W-7 (10, 20 µM) and KN93 (10 µM) at −100 mV (c) and +20 mV (d)
Long-term effect of bepridil on I K1
We then assessed the action of bepridil as applied in the culture medium for 24 h on I K1 in NRCMs. Figure 3a indicates representative samples of I K1 recorded with or without actions of bepridil (1, 5, 10 µM) for 24 h, demonstrating a long-term inhibitory effect of bepridil on I K1. Note that cardiomyocytes were incubated with bepridil-free culture medium for 1 h prior to the electrophysiological study, and that the bath solution was without bepridil in this patch-clamp study. The I–V relationships revealed a dose-dependent long-term reduction of I K1 by bepridil for 24 h; I K1 was reduced by 27 % (0.3 µM), 34 % (1 µM), 61 % (5 µM) and 84 % (10 µM) as assessed at −120 mV (Fig. 3b). Slope conductances of I K1 at −100 and +20 mV were also dose-dependently decreased by incubation with bepridil for 24 h (Fig. 3c, d). Importantly, reduction ratios of outward components and inward components of I K1 were nearly comparable (Fig. 3c, d).
Long-term effect of bepridil on I K1. a Current traces of I K1 in the control (vehicle for 24 h) and after the long-term (24 h) treatment with bepridil (1, 5, 10 µM) are shown. Outward I K1 traces at the potentials of +20 mV at the terminal phase indicated by box are shown in an inset. During the records of I K1, bepridil was exclude from the bath solution. b I–V relationships constructed by using group data in control and after the treatment with 0.3, 1, 5, and 10 µM bepridil. The current density of I K1 at −100 mV was reduced to −9.8 ± 0.4 mV at 0.3 µM (p = 0.173), −8.1 ± 0.8 mV at 1 µM (p = 0.036), −5.6 ± 1.3 mV at 5 µM (p < 0.001) and −2.1 ± 0.4 mV at 10 µM (p < 0.001 vs. control). c, d The slope conductance in the control and after the long-term treatment with 0.3, 1, 5, and 10 µM bepridil at −100 mV (c) and +20 mV (d). # p < 0.1, *p < 0.05, **p < 0.01 compared with control
Long-term effect of W-7 and KN93 on I K1
Impact of long-term inhibition of calmodulin and calmodulin kinase type II on I K1 was accordingly assessed under the same experiment condition. As shown in Fig. 4a, representative traces of I K1 demonstrated long-term inhibitory effects of W-7 and KN93 which did not exert any acute inhibitory effect on I K1 (Fig. 2). In the presence of 10 and 20 µM W-7 in the culture medium, I K1 was reduce by 36 and 42 %, respectively, as assessed at −120 mV. Slope conductance of I K1 was also reduced by 10 µM W-7 (by 35 %) and by 20 µM W-7 (by 42 %) at −100 mV (Fig. 4c, d). A inhibitor of calmodulin kinase type II (KN93, 10 µM) which does not have any inhibitory effect on calmodulin per se, also significantly reduced I K1 by 71 % at −100 mV by a long-term application for 24 h in the culture medium (Fig. 4a, b). Note that reduction ratios of I K1 by 10 µM W-7 (35 %), 1 µM bepridil (33 %), and 10 µM W-7 with 1 μM bepridil (33 %) were all nearly comparable (Fig. 4c).
Long-term effect of bepridil on I K1. a Current traces of I K1 in the control (vehicle for 24 h) and after the long-term (24 h) treatment with bepridil (1, 5, 10 µM) are shown. Outward I K1 traces at the potentials of +20 mV at the terminal phase indicated by box are shown in an inset. During the records of I K1, bepridil was exclude from the bath solution b I–V relationships constructed by using group data in control and after the treatment with 0.3, 1, 5, and 10 µM bepridil. The current density of I K1 at −100 mV was reduced to −9.8 ± 0.4 mV at 0.3 µM (p = 0.173), −8.1 ± 0.8 mV at 1 µM (p = 0.036), −5.6 ± 1.3 mV at 5 µM (p < 0.001) and −2.1 ± 0.4 mV at 10 µM (p < 0.001 vs. control). c, d The slope conductance in the control and after the long-term treatment with 0.3, 1, 5, and 10 µM bepridil at −100 mV (c) and +20 mV (d). # p < 0.1, *p < 0.05, **p < 0.01 compared with control
Comparison of short-term and long-term effects of bepridil on I K1
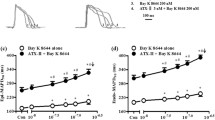
Concentration-dependent short-term (5 min) and long-term (24 h) inhibitory effects of bepridil on I K1 were assessed and compared in Fig. 5. Bepridil reduced I K1 by a short-term effect with IC50 of 17 µM, whereas by a long-term effect with IC50 of 2.7 µM.
Comparison of the short-term (5 min) and long-term (24 h) inhibitory effect of bepridil on I K1. Fractional changes in I K1 at −100 mV were plotted against the concentration for bepridil (open circles for the short-term effect and closed circles for the long-term effect). Each plot indicates mean ± SE. Solid lines were drawn by fitting the Hill plot equation to the experimental data, demonstrating the half maximal inhibitory concentration (IC50) of 17 µM for the short-term effect and 2.7 µM for the long-term effect of bepridil on I K1
Discussion
In the present study, we found that an antiarrhythmic drug bepridil not only inhibited I K1 as a short-term effect (5 min) but also reduced I K1 density as a long-term effect (24 h) in NRCMs. The drug’s potency to inhibit I K1 was approximately 6 times greater in a long-term effect than that in a short-term effect. A calmodulin inhibitor (W-7) and an inhibitor of calmodulin kinase type II (KN93), both of which have no direct effect on I K1, reduced I K1 density as a long-term effect. Reductions of I K1 by bepridil and by bepridil with W-7 were nearly comparable when applied for 24 h, which suggests that a long-term inhibitory effect of bepridil on I K1 depends upon the modulation of calmodulin activity in cardiomyocytes. A multichannel blocker bepridil may exert its antiarrhythmic effects not only through blocking ionic currents acutely but also through regulating calmodulin signals so as to possibly modulate cellular potassium channel expression as a long-term action.
Bepridil decreased I K1 as a short-term effect
Bepridil inhibited I K1 in NRCMs as a short-term effect in a dose-dependent manner with IC50 of 17 µM (Fig. 5). A previous study reported that bepridil blocked I K1 in sheep Purkinje fibers by the two-microelectrode technique with IC50 of <1.8 µM [3]. Electropharmacology of bepridil revisited by our study indicates that the potency of the drug on I K1 channel might depend upon species differences. On the other hand, the two-electrode voltage-clamp technique reportedly faces limits in two important application areas: the penetration by a second electrode in small cells often caused damage that resulted in leakage of cellular contents and a large electrical conductance across the membrane, and in some preparations the cells were commonly out of sight and it was difficult to drive the second electrode into the same cell [19]. Particularly the first limitation could be interfering the accurate measurement of non-voltage gated ionic currents such as I K1. Consequently, patch-clamp study may offer some advantages over microelectrode techniques for this type of electropharmacological evaluation of the drug on I K1.
Bepridil is therefore relatively less potent in inhibition of I K1 as a short-term effect (IC50 of 17 µM) in comparison with its effect on other ionic channels. While bepridil inhibits other cardiac ionic channels or transporters relatively potently (IC50): L-type Ca2+ channel (I Ca.L) (1.6 µM) [7], T-type Ca2+ channel (I Ca.T) (0.4–10.6 µM) [2], voltage-gated Na+ channel (I Na) (4–96 µM) [7, 12], delayed rectifier K+ channels (6.6 µM for ultrarapid component (I Kur) [5], 13.2 µM for rapid component (I Kr) [4], 6.2 µM for slow component (I Ks) [4]), Na+-activated K+ channel (I K,Na) (2.2 µM) [6], transient outward current (I to) (~3 µM) [3], ATP-sensitive K+ channel (I K,ATP) (6.6–10.0 µM) [6], and Na+–Ca2+ exchanger (8.1 µM) [11].
Bepridil decreased I K1 as a long-term effect
Recent investigations have shown that chronic administration of some antiarrhythmic drugs results in various long-term effects besides blocking channels/receptors as a short-term effect. Class I antiarrhythmic drugs, which have definite inhibitory action on the voltage-gated Na+ channel, resulted in up-regulation of cardiac Na+ channel expression [20]. Also recent studies indicate that bepridil upregulated I Na density and Kv1.5 channel expression in a dose-dependent manner apart from its acute blocking effects [12, 13]. However, to our knowledge, there are no published studies regarding the long-term effect of bepridil on the I K1 channel. In this context, our present study first demonstrates that bepridil significantly decreases I K1 by a long-term effect in a dose-dependent manner in cardiomyocytes. Importantly, the IC50 value of long-term I K1 inhibition by bepridil was 2.7 µM, suggesting that bepridil inhibits I K1 by a long-term effect with greater potency than that by a short-term effect (IC50 value of 17 µM).
To investigate the cellular mechanism, we observed I K1 after inhibition of calmodulin or calmodulin kinase type II in the same fashion, because bepridil is a potent calmodulin inhibitor [21–23]. This study clearly demonstrated that a calmodulin inhibitor (W-7) and an inhibitor of calmodulin kinase type II (KN93) greatly reduced I K1 as a long-term effect. Because the long-term I K1 inhibition by 10 µM W-7 was comparable to the long-term inhibition by 1 µM bepridil, and because long-term application of 10 µM W-7 with 1 µM bepridil reduced I K1 as much as either of 10 μM W-7 alone or 1 µM bepridil alone, it is conceivable that bepridil down-regulated I K1 via the calmodulin-dependent pathway. We have previously reported that a long-term application of bepridil upregulated I Na through the inhibition of calmodulin action [12]. Taken together, it is suggested that an antiarrhythmic drug bepridil is a modulator of ion channel expression in cardiomyocytes depending upon the Ca2+—calmodulin/calmodulin kinase II pathway.
Impact of I K1 block by bepridil
I K1 plays a critical role in shaping action potentials in ventricular cardiomyocytes, especially the final phase of repolarization, and stabilizing repolarized resting membrane potential [24]. Reduction in the outward potassium currents prolongs action potential duration (APD) and effective refractory period (ERP), which may result in the destabilization and early termination of reentrant-based arrhythmias [25]. Standing on these viewpoints, we speculate that bepridil decreases I K1 both in short- and long-term application to prolong APD and promote early termination of ventricular arrhythmias. Bepridil is also known to suppress supraventricular arrhythmias potently. Indeed, a nominative effect of bepridil in a canine model of rapid electrical pacing for 2 weeks has been reported; bepridil suppressed the shortening of atrial effective refractory period (AERF) in the first week and further restored AERP to the pre-pacing level in the second week [16]. In this article, a beneficial effect of bepridil was considered in the context of bepridil-induced AERP shortening, although the underlying mechanism was not identified. The long-term inhibitory effect of I K1 by bepridil may account for the mechanisms of AERP prolongation in the pathological condition of the heart such as in rapid electrical pacing animal models.
Kir2.1, the major isoform of I K1 present in the heart, is mainly expressed in the ventricular tissue [26]. However, recent studies have recognized that Kir2.1/I K1 increases in the atrial tissue obtained from patients with chronic atrial fibrillation (AFib) and in animal models of AFib [27–30]. Although AFib is a complex arrhythmias with multiple mechanisms [31, 32], I K1 is a particularly important mechanistic determinant of AFib-supporting reentry. Enhanced I K1 may shorten APD and AERP by accelerating the membrane repolarization, and may also increase Na+ current availability which results in acceleration and stabilization of reentrant rotor [33, 34] in the atrium. Hence, the long-term application of bepridil would be counteracting the upregulation of I K1 in the atrium caused by AFib, and accordingly beneficial for the termination of chronic AFib. Currently, bepridil is one of the pharmacological options for paroxysmal AFib and persistent AFib as well. J-BAF Study has demonstrated that bepridil effectively converted to sinus rhythm in patients with persistent AFib [14]. A long-term inhibitory effect of I K1 in cardiomyocytes by bepridil, presented in this study, may account for the active mechanism of this drug for AFib treatment.
Limitations
There are several limitations in the present study. Although we have obtained IC50 values of bepridil to inhibit I K1, a simple comparison of drug concentrations between short-term and long-term effects may not be appropriate. Because bepridil is a highly lipophilic drug, assessment of drug efficacy on I K1 by an in vitro experiment without serum proteins and/or serum lipids may not represent the effect of bepridil in in vivo application. Furthermore, a caution would be needed to extrapolate these neonatal rat heart experiments to human therapeutics. Although our study has identified plausible mechanism for the down-regulation of I K1 as a long-term effect of bepridil, there is a need for additional evidence on the levels of channel transcription and translation to reveal the molecular pharmacological actions of bepridil.
Conclusion
In summary, our study revealed that the long-term application of bepridil inhibits I K1 more potently than that of the short-term effect through the inhibition of calmodulin kinase II pathway in cardiomyocytes, which may explain the hitherto unknown pharmacological mechanism of bepridil when applied for a long-term period to exert its relevant antiarrhythmic actions for the treatment of chronic AFib patients.
References
Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J (2006) DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 34:D668–D672
Uchino T, Lee TS, Kaku T, Yamashita N, Noguchi T, Ono K (2005) Voltage-dependent and frequency-independent inhibition of recombinant Cav3.2 T-type Ca2+ channel by bepridil. Pharmacology 74:174–181
Berger F, Borchard U, Hafner D (1989) Effects of the calcium entry blocker bepridil on repolarizing and pacemaker currents in sheep cardiac Purkinje fibers. Naunyn Schmiedebergs Arch Pharmacol 339:638–646
Wang JC, Kiyosue T, Kiriyama K, Arita M (1999) Bepridil differentially inhibits two delayed rectifier K+ currents, I Kr and I Ks, in guinea pig ventricular myocytes. Br J Pharmacol 128:1733–1738
Kobayashi S, Reien Y, Ogura T, Saito T, Masuda Y, Nakaya H (2001) Inhibitory effect of bepridil on hKv1.5 channel current: comparison with amiodarone and E-4031. Eur J Pharmacol 430:149–157
Li Y, Sato T, Arita M (1999) Bepridil blunts the shortening of action potential duration caused by metabolic inhibition via blockade of ATP-sensitive K+ channels and Na+-activated K+ channels. J Pharmacol Exp Ther 291:562–568
Hara Y, Nakaya H (1995) SD-3212, a new class I and IV antiarrhythmic drug: a potent inhibitor of the muscarinic acetylcholine-receptor operated potassium current in guinea-pig atrial cells. Br J Pharmacol 116:2750–2756
Sato T, Costa AD, Saito T, Ogura T, Ishida H, Garlid KD, Nakaya H (2006) Bepridil, an antiarrhythmic drug, opens mitochondrial KATP channels blocks sarcolemmal KATP channels, and confers cardioprotection. J Pharmacol Exp Ther 316:182–188
Sato N, Nishimura M, Kawamura Y, Ward CA, Kikuchi K (1996) Blocks of Na+ channel by bepridil in isolated guinea-pig ventricular myocytes. Eur J Pharmacol 314:373–379
Yatani A, Brown AM, Schwartz A (1986) Bepridil block of cardiac calcium and sodium channels. J Pharmcol Exp Ther 237:9–17
Watanabe Y, Kimura J (2001) Blocking effect of bepridil on Na+/Ca2+ exchange current in guinea pig cardiac ventricular myocytes. Jpn J Pharmacol 85:370–375
Kang L, Zheng MQ, Morishima M, Wang Y, Kaku T, Ono K (2009) Bepridil up-regulates cardiac Na+ channels as a long-term effect by blunting proteasome signals through inhibition of calmodulin activity. Br J Pharmacol 157:404–414
Suzuki S, Kurata Y, Li P, Notsu T, Hasegawa A, Ikeda N, Kato M, Miake J, Sakata S, Shiota G, Yoshida A, Ninomiya H, Higaki K, Yamamoto K, Shirayoshi Y, Hisatome I (2012) Stabilization of Kv1.5 channel protein by bepridil through its action as a chemical chaperone. Eur J Pharmacol 696:28–34
Yamashita T, Ogawa S, Sato T, Aizawa Y, Atarashi H, Fujiki A, Inoue H, Ito M, Katoh T, Kobayashi Y, Koretsune Y, Kumagai K, Niwano S, Okazaki O, Okumura K, Saku K, Tanabe T, Origasa H (2009) J-BAF Investigators. Dose-response effects of bepridil in patients with persistent atrial fibrillation monitored with transtelephonic electrocardiograms: a multicenter, randomized, placebo-controlled, double-blind study (J-BAF Study). Circ J 73:1020–1027
Kato R, Singh BN (1986) Effects of bepridil on the electrophysiologic properties of isolated canine and rabbit myocardial fibers. Am Heart J 111:271–279
Sato D, Niwano S, Imaki R, Masaki Y, Sasaki S, Yuge M, Hirasawa S, Sasaki T, Moriguchi M, Niwano H, Yoshimura H, Izumi T (2006) Bepridil inhibits sub-acute phase of atrial electrical remodeling in canine rapid atrial stimulation model. Circ J 70:206–213
Wang Y, Morishima M, Zheng M, Uchino T, Mannen K, Takahashi A, Nakaya Y, Komuro I, Ono K (2007) Transcription factors Csx/Nkx2.5 and GATA4 distinctly regulate expression of Ca2+ channels in neonatal rat heart. J Mol Cell Cardiol 42:1045–1053
Uchino T, Isomoto S, Noguchi T, Ono K (2013) Window current through the T-type Ca2+ channel triggers the mechanism for cellular apoptosis via mitochondrial pathways. Heart Vessels 28:658–666
Axon Instruments, Inc. (2012) The axon guide: electrophysiology and biophysics laboratory techniques, 3rd edn. Chapter 1: Bioelectricity, pp 17–38
Duff HJ, Offord J, West J, Catterall WA (1992) Class I and IV antiarrhythmic drugs and cytosolic calcium regulate mRNA encoding the sodium channel alpha subunit in rat cardiac muscle. Mol Pharmacol 42:570–574
Itoh H, Ishikawa T, Hidaka H (1984) Effects on calmodulin of bepridil, an antianginal agent. J Pharmacol Exp Ther 230:737–741
Zimmer M, Hofmann F (1987) Differentiation of the drug-binding sites of calmodulin. Eur J Biochem 164:411–420
Schaeffer P, Lugnier C, Stoclet JC (1991) Interactions of calmodulin antagonists with calcium antagonists binding sites. Eur J Pharmacol 206:325–332
Kawashiri M, Hayashi K, Konno T, Fujino N, Ino H, Yamagishi M (2014) Current perspectives in genetic cardiovascular disorders: from basic to clinical aspects. Heart Vessels 29:129–141
Yamazaki M, Honjo H, Nakagawa H, Ishiguro YS, Okuno Y, Amino M, Sakuma I, Kamiya K, Kodama I (2007) Mechanisms of destabilization and early termination of spiral wave reentry in the ventricle by a class III antiarrhythmic agent, nifekalant. Am J Physiol Heart Circ Physiol 292:H539–H548
Dhamoon AS, Jalife J (2005) The inward rectifier current (I K1) controls cardiac excitability and is involved in arrhythmogenesis. Heart Rhythm 2:316–324
Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U (2005) The G protein-gated potassium current I KACh is constitutively active in patients with chronic atrial fibrillation. Circulation 112:3697–3706
Gaborit N, Steenman M, Lamirault G, Le Meur N, Le Bouter S, Lande G, Léger J, Charpentier F, Christ T, Dobrev D, Escande D, Nattel S, Demolombe S (2005) Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation 112:471–481
Girmatsion Z, Biliczki P, Bonauer A, Wimmer-Greinecker G, Scherer M, Moritz A, Bukowska A, Goette A, Nattel S, Hohnloser SH, Ehrlich JR (2009) Changes in microRNA-1 expression and I K1 up-regulation in human atrial fibrillation. Heart Rhythm 6:1802–1809
Luo X, Pan Z, Shan H, Xiao J, Sun X, Wang N, Lin H, Xiao L, Maguy A, Qi XY, Li Y, Gao X, Dong D, Zhang Y, Bai Y, Ai J, Sun L, Lu H, Luo XY, Wang Z, Lu Y, Yang B, Nattel S (2013) MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest 123:1939–1951
Soeki T, Bando S, Uematsu E, Matsuura T, Niki T, Ise T, Kusunose K, Hotchi J, Ueda Y, Tomita N, Yamaguchi K, Yagi S, Fukuda D, Taketani Y, Iwase T, Yamada H, Wakatsuki T, Shimabukuro M, Sata M (2014) Pentraxin 3 is a local inflammatory marker in atrial fibrillation. Heart Vessels 229:653–658
Okada A, Kashima Y, Tomita T, Takeuchi T, Aizawa K, Takahashi M, Ikeda U (2014) Characterization of cardiac oxidative stress levels in patients with atrial fibrillation. Heart Vessels. doi:10.1007/S00380-014-0582-8
Pandit SV, Berenfeld O, Anumonwo JM, Zaritski RM, Kneller J, Nattel S, Jalife J (2005) Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J 88:3806–3821
Katsouras G, Sakabe M, Comtois P, Maguy A, Burstein B, Guerra PG, Talajic M, Nattel S (2009) Differences in atrial fibrillation properties under vagal nerve stimulation versus atrial tachycardia remodeling. Heart Rhythm 6:1465–1472
Acknowledgments
This study was supported in part by Japanese Ministry of Education, Science, and Culture Grant-in-aid (KAKEN) #25460292 (to K. Ono).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Ma, F., Takanari, H., Masuda, K. et al. Short- and long-term inhibition of cardiac inward-rectifier potassium channel current by an antiarrhythmic drug bepridil. Heart Vessels 31, 1176–1184 (2016). https://doi.org/10.1007/s00380-015-0762-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-015-0762-1