Abstract
Chronic kidney disease (CKD) status might modify the predictive effect of peripheral endothelial dysfunction on cardiovascular events after percutaneous coronary intervention (PCI). The aim of this study was to examine the differential effect of peripheral endothelial dysfunction on clinical outcome after PCI between CKD and non-CKD patients. We conducted a cohort study of 435 patients following PCI. CKD was defined as estimated glomerular filtration rate <60 mL/min/1.73 m2. Peripheral endothelial dysfunction was examined using reactive hyperemia-peripheral arterial tonometry index (RHI), and we divided patients into low- and high-natural logarithmic RHI (Ln-RHI) group. The endpoint was a composite of cardiovascular death, nonfatal myocardial infarction, ischemic stroke, hospitalization due to unstable angina pectoris, and coronary revascularization. A total of 56 patients had a cardiovascular event. Patients who suffered a cardiovascular event had significantly lower Ln-RHI than other patients in the non-CKD group (0.46 ± 0.18 versus 0.60 ± 0.25; P = 0.002). Kaplan–Meier analysis demonstrated a significantly higher probability of cardiovascular events in low Ln-RHI patients in the non-CKD group (log-rank test: P = 0.003). Multivariate Cox proportional hazards analysis identified Ln-RHI as an independent and significant predictor of future cardiovascular events in the non-CKD group (HR: 0.096; 95 % CI 0.02–0.47; P = 0.004) but not in the CKD group. There was a differential effect of peripheral endothelial dysfunction on clinical outcome after PCI between CKD and non-CKD patients, and peripheral endothelial dysfunction significantly correlates with subsequent cardiovascular events after PCI in non-CKD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Percutaneous coronary intervention (PCI) is performed in patients with coronary heart disease to improve symptoms and clinical prognosis, and dual antiplatelet therapy (DAPT) is currently recommended for the prevention of adverse cardiovascular events in patients following coronary stents [1–3]. Patients following coronary stent implantation have a high risk of subsequent cardiovascular events, including myocardial infarction, stroke, and cardiovascular death. All patients after coronary intervention should receive secondary preventive interventions, but determination of their risk factor is still insufficient.
Reactive hyperemia-peripheral arterial tonometry (RH-PAT), which is used to measure the digital hyperemic response, is a noninvasive, automatic, and less operator-dependent test that is clinically used to evaluate endothelial function [4, 5]. It is reported that the RH-PAT index (RHI) predicted adverse cardiovascular events in patients without known coronary artery disease [6], and we have reported that the RHI was useful for identifying patients who were at high risk for ischemic heart disease [7, 8].
Chronic kidney disease (CKD) is thought to be associated with atherosclerosis, cardiovascular events, increased platelet activation, and reduced platelet inhibition by DAPT [9, 10], and coronary heart disease is a major cause of death for CKD patients [11]. Recently, we have demonstrated that endothelial function is significantly correlated with the presence of coronary heart disease and is an independent predictor of future cardiovascular events in the CKD patients [12]. The predictive effect of peripheral endothelial function might vary between CKD and non-CKD patients, and the association of peripheral endothelial dysfunction with clinical outcome following PCI according to the presence of CKD is poorly understood.
The aim of the present study was thus to investigate the differential predictive effect of peripheral endothelial dysfunction on future subsequent cardiovascular events in patients undergoing PCI between CKD and non-CKD.
Materials and methods
The study complied with the Declaration of Helsinki regarding investigation in humans was approved by our institutional review committee and was conducted in accordance with the guidelines of an ethics committee. Written informed consent was obtained from all patients.
Study population
This was a single-center study and a total of 600 consecutive patients who underwent PCI from January 2009 to November 2012 in our hospital were eligible. We excluded patients treated with thrombolytic agents, ticlopidine, sarpogrelate, or cilostazol and patients with deep vein thrombosis, atrial fibrillation, collagen disease, liver dysfunction, and malignant diseases. All patients underwent cardiac catheterization and PCI during hospitalization, and they received DAPT with maintenance doses of 100 mg/day of aspirin and 75 mg/day of clopidogrel after a loading dose of 300 mg of clopidogrel. The estimated glomerular filtration rate (eGFR) was calculated according to the new Japanese equation: eGFR (mL/min/1.73 m2) = 194 × serum creatinine (−1.094) × age (−0.287) × 0.739 (if female) [13]. The time point of eGFR measurement was before the PCI. We defined CKD as eGFR <60 mL/min/1.73 m2 in this study.
Smoking status was determined via an interview. Subjects were classified as having hypertension if they were receiving drug treatment for hypertension or if they had a systolic pressure of at least 140 mmHg or a diastolic pressure of at least 90 mmHg. Dyslipidemia was defined as low-density lipoprotein ≥140 mg/dL, high-density lipoprotein <40 mg/dL, or triglyceride ≥150 mg/dL and diabetes as a 2-h glucose tolerance test finding of at least 200 mg/dL or a fasting glucose level of ≥126 mg/dL, HbA1c ≥6.5 %, physician-diagnosed diabetes, and/or the use of diabetic medication. Patients who had an ankle-brachial index value of <0.90 in either leg were categorized as having peripheral arterial disease (PAD). Acute coronary syndrome (ACS) was defined as either an acute myocardial infarction (ST-elevation myocardial infarction or non-ST-elevation myocardial infarction) or unstable angina pectoris according to the American College of Cardiology/American Heart Association guidelines [2, 3].
Assessment of endothelial function by reactive hyperemia-peripheral arterial tonometry
Peripheral endothelial function was assessed by reactive hyperemia-peripheral arterial tonometry (RH-PAT) using the EndoPAT2000 system (Itamar Medical, Caesarea, Israel). RH-PAT measurement is largely operator-independent, and a computerized algorithm with an online system automatically calculated the RH-PAT index (RHI); thus, there was minimal interoperator and intraoperator variability. The RH-PAT studies were performed as described previously [7]. Since RHI values are not normally distributed, we calculated the natural logarithmic (Ln)-RHI values for statistical analyses. [7, 14]. Previous studies demonstrated that RH-PAT technology has excellent reproducibility [15, 16].
Follow-up
After coronary stent implantation, patients were followed prospectively at outpatient clinics until October 2013 or until an endpoint occurred. We performed follow-up angiography 6 to 9 months after the procedure. Cardiovascular events were ascertained from a review of medical records and confirmed by direct contact with the patients, their families, and physicians. The endpoint was a composite of cardiovascular death, nonfatal myocardial infarction, ischemic stroke, hospitalization due to unstable angina pectoris, and coronary revascularization. Cardiovascular death was defined as death due to myocardial infarction, congestive heart failure, or documented sudden cardiac death. We used the universal definition of myocardial infarction in this study [17]. The diagnosis of ischemic stroke was based on clinical and radiological evidence of stroke. Coronary revascularization was defined as emergent revascularization for unexpected hospitalization. Revascularization therapy based only on angiographic data, including PCI-mediated restenosis, was not counted as a cardiovascular event. For subjects who had more than 2 cardiovascular events, only the first event was considered in the analysis.
Statistical analysis
The Shapiro–Wilk test was used to assess the normal distribution of continuous data. Continuous variables with a normal distribution are expressed as the mean ± SD. Categorical data are presented as numbers or percentages. Differences between 2 groups were tested using Fisher’s exact test for categorical variables. Differences in continuous variables were analyzed by the unpaired t test or the Mann–Whitney U test, as appropriate. We used the Kaplan–Meier method to estimate the cardiovascular event probabilities at 1730 days, and we also used the log-rank test to compare distributions of survival times among groups. Cox proportional hazard models were used to calculate hazard ratios (HRs) and to test for the interaction between CKD and RHI. The results of this analysis are expressed as HRs for comparison of risk with 95 % confidence intervals (CIs). Univariate analysis was performed using clinical variables that are considered to be associated with cardiovascular events (RHI, male, age, body mass index, ACS, hypertension, dyslipidemia, diabetes, current smoking, previous myocardial infarction, previous stroke, and PAD). Clinical variables with a P value <0.05 were subsequently entered into multivariate analysis. A P value <0.05 was considered to denote the presence of a statistically significant difference, and the P value <0.1 was considered significant for the interaction analysis. Statistical analyses were performed using SPSS version 22 software (IBM Institute Inc., USA).
Results
A total of 435 patients were enrolled in this study, and patients were divided into the CKD group (n = 150) and the non-CKD group (n = 285). The mean eGFRs in the CKD and non-CKD groups were 46 ± 11 and 76 ± 13, respectively (P < 0.001). The mean Ln-RHI was 0.58 ± 0.24 and the median Ln-RHI was 0.54 in the total subjects. Patients were subsequently divided by the median Ln-RHI into low- and high-RHI patients. Table 1 lists the clinical characteristics of the CKD group and the non-CKD group according to the RHI state. Clinical characteristics were similar between low- and high-RHI subjects in both CKD and non-CKD groups, except for medication of ACE-I/ARB in the non-CKD group. The mean Ln-RHI in the CKD and non-CKD groups were 0.59 ± 0.24 and 0.58 ± 0.25, respectively (P = 0.91). There were no patients who experienced contrast-induced nephropathy.
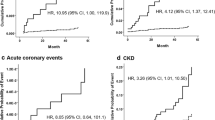
The data of 435 patients were available for analyzing cardiovascular events. The mean follow-up period was 927 days and the median follow-up period was 923 days. We followed patients up to 1730 days in this study. A total of 56 patients suffered a cardiovascular event. Details of the cardiovascular events are as follows: cardiovascular death (n = 4), nonfatal myocardial infarction (n = 4), stroke (n = 5), unstable angina (n = 12), and coronary revascularization (n = 31). The number of events for each group was 22 in the CKD group and 34 in the non-CKD group, respectively. Patients who suffered a cardiovascular event had significantly lower Ln-RHI than other patients in the non-CKD group (0.46 ± 0.18 versus 0.60 ± 0.25; P = 0.002). There was no significant difference in Ln-RHI between patients with and without cardiovascular events in the CKD group (0.56 ± 0.27 versus 0.59 ± 0.24; P = 0.58). In the Kaplan–Meier analysis, a significantly higher incidence of cardiovascular events in the low-RHI group was observed in the non-CKD group (log-rank test: P = 0.003) (Fig. 1a) but not observed in the CKD group (log-rank test: P = 0.753) (Fig. 1b).
Kaplan–Meier curves for primary composite endpoints during the follow-up period between low- and high-RHI groups in the CKD group (a) and the non-CKD group (b). Kaplan–Meier curves did not show a significant difference between low- and high-RHI patients in the CKD group (log-rank test: P = 0.753). On the other hand, patients with low RHI had a worse prognosis than patients with high RHI in the non-CKD group (log-rank test: P = 0.003). CKD chronic kidney disease, RHI reactive hyperemia-peripheral arterial tonometry (RH-PAT) index
The results of the univariate and multivariate Cox proportional hazards analysis for cardiovascular events are summarized in Tables 2 and 3. Multivariate Cox proportional hazard analysis identified Ln-RHI as an independent and significant predictor of future cardiovascular events only in the non-CKD group (HR: 0.096; 95 % CI 0.02–0.47; P = 0.004) but not in the CKD group. The P value of the interaction between CKD and RHI was 0.059.
Discussion
This is the first study to demonstrate the differential predictive effect of endothelial dysfunction on subsequent cardiovascular events after PCI between CKD and non-CKD. The findings of the present study are as follows: (1) A significantly higher incidence of cardiovascular events was observed in low-RHI patients in the non-CKD group. (2) Endothelial dysfunction is a significant and independent predictor of major cardiovascular events after PCI in the non-CKD group but not in the CKD group. Although there are several evidences of the relationship between endothelial dysfunction and atherosclerosis [18, 19], the effect of endothelial dysfunction in the subsequent cardiovascular events after PCI was still unclear. Previous studies have shown the correlation between endothelial function assessed by RH-PAT and coronary heart disease in high-risk patients, or it was reported that impaired endothelial function assessed by flow-mediated dilation (FMD) was associated with the risk of coronary restenosis after PCI [20], but there was insufficient data on the association between endothelial dysfunction and subsequent cardiovascular events in patients undergoing PCI.
At present study, we have revealed that the effect of peripheral endothelial dysfunction on clinical outcome is more important in the non-CKD patients than in the CKD patients. CKD is thought to be associated with atherosclerosis, increased platelet activation, reduced platelet inhibition by DAPT, and cardiovascular events [9], and in other studies, it was suggested that clinical factors like diabetes and CKD might modify the predictive effect of surrogate markers [21, 22]. Similarly, we hypothesized that the effect of peripheral endothelial dysfunction on clinical outcome might be different between CKD and non-CKD patients, and evidence for the association between endothelial dysfunction and clinical outcome according to the presence or absence of CKD was insufficient. Thus, in the present study, we have demonstrated that CKD status modifies the prognostic effect of peripheral endothelial dysfunction in patients undergoing PCI, and the role of endothelial function as a surrogate marker of clinical outcome is more important in non-CKD patients.
It is reported that peripheral endothelial dysfunction correlates with the presence of coronary heart disease and is an independent predictor of adverse cardiovascular event in CKD patients with at least one coronary risk factor [12]. Because there are not enough reports on this issue, it is unclear why peripheral endothelial dysfunction is associated with increased risk of cardiovascular events only in the non-CKD group but not in the CKD group in the present study. The presence of CKD is reported to be one of the predictive factors of clinical outcome in patients with stent implantation [23], and clinical studies have suggested that CKD itself contributes to high residual platelet reactivity [9]. Thus, it is possible that the contribution of CKD status may be considerable and might have outweighed the influence and impact of peripheral endothelial dysfunction in CKD patients at the present study.
Endothelial function is associated with the maintenance of vascular tone, thrombosis, platelet adhesion, vasculature-blood cell homeostasis, and impaired endothelial function is an early and fundamental event in the development of atherosclerosis [19]. Assessment of endothelial function by FMD or RH-PAT might be more useful for the patients without co-morbidities such as coronary heart disease. However, at the present study, we have shown that impairment of endothelial function is independently associated with clinical outcome in high-risk patients requiring PCI. This observation suggests that endothelial dysfunction is still a useful surrogate marker in such high-risk patients and more careful management of cardiovascular risk factors is indicated in patients with endothelial dysfunction undergoing PCI. However, the present study suggested that the predictive effect of endothelial function varied according to the presence or absence of CKD. Thus, more careful observation is needed to follow up patients without co-morbidities such as CKD and with endothelial dysfunction. Endothelial function could be improved by appropriate medications and lifestyle interventions [24, 25], and treatment of coronary risk factors by optimal medications could lead to improvement of endothelial dysfunction, attenuate enhanced platelet aggregation, and reduce cardiovascular events. Further study is needed to clarify our findings.
Study limitations
One limitation of this study is that it was performed in a single center. Compared with previous Western studies, the number of patients was small, and the study may have been underpowered to detect a difference in the clinical event rate. Therefore, a large multiracial and multicenter study is required to confirm our results. In addition, patients characteristics of medications of ACE-I/ARB were different between low- and high-RHI patients in the non-CKD group. At the study design, we did not plan to use drug prescriptions as variables of cox proportional hazard model, and we cannot deny that this difference might influence clinical outcome. In this study, we did not take the severity of coronary artery disease into account nor did compare that between high- and low-RHI groups. The severity of coronary artery disease might influence the incidence of composite endpoint. Moreover, the implantation of drug-eluting stent might worsen endothelial function. Thus, we cannot deny the possibility that the use of drug-eluting stent might influence the study results. Further continuous clinical studies are necessary.
Conclusions
There was a differential effect of peripheral endothelial dysfunction on clinical outcome after PCI between CKD and non-CKD patients, and peripheral endothelial dysfunction significantly correlates with subsequent cardiovascular events after PCI in non-CKD patients.
References
King SB 3rd, Smith SC Jr, Hirshfeld JW Jr, Jacobs AK, Morrison DA, Williams DO, Feldman TE, Kern MJ, O’Neill WW, Schaff HV, Whitlow PL, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW, ACC/AHA/SCAI (2008) 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol 51:172–209
Kushner FG, Hand M, Smith SC Jr, King SB 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO (2009) 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 54:2205–2241
Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS (2011) 2011 ACCF/AHA focused update of the Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 57:1920–1959
Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE (2003) Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 146:168–174
Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P (2006) Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol 101:545–548
Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A (2010) Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J 31:1142–1148
Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, Matsubara J, Sumida H, Kaikita K, Kojima S, Nagayoshi Y, Yamamuro M, Izumiya Y, Iwashita S, Matsui K, Jinnouchi H, Kimura K, Umemura S, Ogawa H (2010) Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol 55:1688–1696
Matsuzawa Y, Sugiyama S, Sumida H, Sugamura K, Nozaki T, Ohba K, Matsubara J, Kurokawa H, Fujisue K, Konishi M, Akiyama E, Suzuki H, Nagayoshi Y, Yamamuro M, Sakamoto K, Iwashita S, Jinnouchi H, Taguri M, Morita S, Matsui K, Kimura K, Umemura S, Ogawa H (2013) Peripheral endothelial function and cardiovascular events in high-risk patients. J Am Heart Assoc 2:e000426
Gremmel T, Müller M, Steiner S, Seidinger D, Koppensteiner R, Kopp CW, Panzer S (2013) Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol Dial Transplant 28:2116–2122
Matsue Y, Matsumura A, Abe M, Ono M, Seya M, Nakamura T, Iwatsuka R, Mizukami A, Setoguchi M, Nagahori W, Ohno M, Suzuki M, Hashimoto Y (2013) Prognostic implications of chronic kidney disease and anemia after percutaneous coronary intervention in acute myocardial infarction patients. Heart Vessels 28:19–26
Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, Jamus MT, Hemmelgarn BR, Network Alberta Kidney Disease (2012) Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 380:807–814
Hirata Y, Sugiyama S, Yamamoto E, Matsuzawa Y, Akiyama E, Kusaka H, Fujisue K, Kurokawa H, Matsubara J, Sugamura K, Maeda H, Iwashita S, Jinnouchi H, Matsui K, Ogawa H (2014) Endothelial function and cardiovascular events in chronic kidney disease. Int J Cardiol 173:481–486
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ (2008) Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 117:2467–2474
Selamet Tierney ES, Newburger JW, Gauvreau K, Geva J, Coogan E, Colan SD, de Ferranti SD (2009) Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr 154:901–905
Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, Schnall RP, Holmes DR, Higano ST, Lerman A (2003) Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol 41:1761–1768
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction (2012) Third universal definition of myocardial infarction. Circulation 126:2020–2035
Cox DA, Vita JA, Treasure CB, Fish RD, Alexander RW, Ganz P, Selwyn AP (1989) Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation 80:458–465
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809
Patti G, Pasceri V, Melfi R, Goffredo C, Chello M, D’Ambrosio A, Montesanti R, Di Sciascio G (2005) Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation 111:70–75
Mizobe M, Hokimoto S, Akasaka T, Arima Y, Kaikita K, Morita K, Miyazaki H, Oniki K, Nakagawa K, Ogawa H (2014) Impact of CYP2C19 polymorphism on clinical outcome following coronary stenting is more important in non-diabetic than diabetic patients. Thromb Res 134:72–77
Tabata N, Hokimoto S, Akasaka T, Arima Y, Kaikita K, Kumagae N, Morita K, Miyazaki H, Oniki K, Nakagawa K, Matsui K, Ogawa H (2014) Chronic kidney disease status modifies the association of CYP2C19 polymorphism in predicting clinical outcomes following coronary stent implantation. Thromb Res 134:939–944
Kaneko H, Yajima J, Oikawa Y, Tanaka S, Fukamachi D, Suzuki S, Sagara K, Otsuka T, Matsuno S, Funada R, Kano H, Uejima T, Koike A, Nagashima K, Kirigaya H, Sawada H, Aizawa T, Yamashita T (2014) Impact of aging on the clinical outcomes of Japanese patients with coronary artery disease after percutaneous coronary intervention. Heart Vessels 29:156–164
Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R (2002) Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 40:505–510
Bonetti PO, Lerman LO, Lerman A (2003) Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23:168–175
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Tabata, N., Hokimoto, S., Akasaka, T. et al. Differential impact of peripheral endothelial dysfunction on subsequent cardiovascular events following percutaneous coronary intervention between chronic kidney disease (CKD) and non-CKD patients. Heart Vessels 31, 1038–1044 (2016). https://doi.org/10.1007/s00380-015-0713-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-015-0713-x