Abstract
Hypertrophic cardiomyopathy (HCM) with systolic dysfunction carries a poor prognosis. Although late gadolinium enhancement (LGE) on cardiac magnetic resonance is associated with adverse cardiac events in HCM and is inversely related to left ventricular ejection fraction (LVEF), it is unknown whether LGE or LVEF more accurately predicts adverse cardiac events in HCM with systolic dysfunction. We retrospectively assessed the extent of LGE with a threshold of 6 standard deviations in 46 consecutive HCM patients with systolic dysfunction defined as LVEF <50 % (average 35 ± 12 %) who underwent cardiac magnetic resonance (35 males, mean age 59 ± 14 years). They were followed up over 1755 ± 594 days. The composite adverse cardiac events end point included cardiovascular death, lethal arrhythmia, cardioembolic stroke, and unplanned heart failure hospitalization. LGE was detected in all patients, and the mean extent was 30 ± 15 %. Twenty-nine patients developed adverse cardiac events. Multivariate Cox proportional hazard analysis revealed the extent of LGE as a good independent predictor of adverse cardiac events. Risk increased with the extent of LGE (hazard ratio = 1.62/10 % increase in LGE, 95 % confidence interval = 1.23–2.15, p < 0.001). LVEF was inversely related to the extent of LGE (r = −0.44; p = 0.002) and was also an independent predictor of adverse cardiac events. Risk decreased with LVEF (hazard ratio = 0.68/10 % increase in LVEF, 95 % confidence interval = 0.51–0.91, p = 0.010). The Akaike information criterion evaluating the fit of a model demonstrated that the extent of LGE was a better independent predictor of MACE than LVEF (Akaike information criterion = 172.20 and 178.09, respectively).The extent of LGE was a good independent predictor of adverse cardiac events and reflected mortality and morbidity more precisely than LVEF in HCM with systolic dysfunction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiovascular disease. HCM is widely regarded as being associated predominantly with hyperdynamic left ventricular (LV) systolic function and has a generally benign clinical course, specifically a 1 % annual mortality rate, little or no disability, and normal life expectancy in almost all patients [1–5]. LV myocardial fibrosis, a hallmark of HCM, has been implicated in promoting heart failure (HF) as well as risk of sudden arrhythmic death [6, 7]. Risk stratification in HCM is still largely limited by relatively low positive predictive values of classical clinical risk factors, due in part to low mortality rates, because the clinical markers of myocardial fibrosis have not been defined [8–11]. Recently, late gadolinium enhancement (LGE) by cardiac magnetic resonance (CMR) has emerged as the gold standard for imaging of myocardial fibrosis in vivo [12, 13]. Several studies and meta-analyses revealed that LGE has value in predicting adverse cardiac events and is useful in risk stratification in clinical practice, and thus might be a much better predictor than established classical clinical risk factors [10, 11, 14–20]. Although the presence and extent of LGE (% LGE) are clinically relevant, the optimal strategy for quantitating LGE in HCM is not still well defined (i.e., threshold of 2 or 6 standard deviations (SD) and full width at half maximum method) [16, 21]. On the other hand, HCM with overt systolic dysfunction (so-called end-stage phase, defined as LV ejection fraction (LVEF) <50 %) occurs in about 5 % of HCM patients, is a risk factor for adverse cardiac events, and results in an annual mortality rate of over 10 %. One of the cellular mechanisms for HCM with overt systolic dysfunction is marked LV fibrosis, which is detected by LGE [15, 22–26]. % LGE has shown an inverse relationship to LVEF in the general HCM population; however, in HCM patients with LVEF <50 %, the relationship between % LGE and LVEF is unclear because of small sample sizes in previous studies [15, 26]. % LGE in HCM with an LVEF <50 % was found to be distributed over a broad range, while that defined by a threshold of 6SD was reported to constitute a median value of 29 % of the LV volume (interquartile range 16–40 %) [26]. A recent study of a large cohort of 1293 HCM patients demonstrated that % LGE with a threshold of 6SD was a strong predictor of sudden cardiac death even in patients considered to be at lower risk for sudden cardiac death using conventional clinical risk factors. Although this study included 58 (4.5 %) HCM patients with an LVEF <50 %, the prognostic significance of % LGE with a threshold of 6SD in HCM with systolic dysfunction was not investigated [18]. Both systolic dysfunction and % LGE may be novel clinical risk factors useful for risk stratification in HCM; however, it remains unclear whether % LGE or LVEF more precisely predicts mortality and morbidity in HCM with systolic dysfunction [10, 11, 14–18, 23, 27]. Furthermore, the optimal threshold of % LGE for this purpose also remains unresolved.
Therefore, this study aimed to assess the relationship between % LGE and LVEF, as well as the prognostic value of LVEF and % LGE at several thresholds in predicting cardiac events in patients with HCM and systolic dysfunction.
Materials and methods
Patient population
This study was a retrospective analysis of data acquired in patients with HCM who underwent gadolinium-enhanced CMR for further evaluation at the National Cerebral and Cardiovascular Center between February 2006 and August 2011. Of 114 HCM patients who underwent gadolinium-enhanced CMR, 56 consecutive HCM patients with systolic dysfunction (defined as LVEF <50 %) were identified. Of these, 10 were excluded, for the following reasons: 4 had documented prior myocardial infarction, 2 had a history of post-septal reduction therapy, 1 had a history of LV assist device removal, 1 had a history of mitral annuloplasty, and 2 had incomplete follow-up without sufficient data. The final study group consisted of 46 HCM patients with systolic dysfunction (35 males, mean age 59 ± 14 years).Of these, 40 underwent coronary angiography and were found to have no significant coronary artery disease (defined as >50 % stenosis in 1 major artery). In the 6 patients who did not undergo coronary angiography, we ruled out ischemic heart disease because the distribution of their LGE was not consistent with coronary vascular territories and did not exhibit a subendocardial pattern; 4 of these 6 who underwent stress myocardial scintigraphy showed no stress-induced ischemia. The diagnosis of HCM with systolic dysfunction was based on global LVEF <50 % at CMR acquisition with (1) the presence (or previous documentation) of an unexplained asymmetrical hypertrophied left ventricle on 2-dimensional echocardiography or CMR (maximal wall thickness ≥13 mm) in the absence of another disease that could account for the hypertrophy and/or cellular disarray in endomyocardial biopsy, or with (2) proven familial HCM with at least 1 relative who had an unequivocal diagnosis [1, 8, 25, 28]. Patients with coexisting HCM and hypertension were included in the study given the high prevalence of hypertension in a previous HCM cohort [29]. Patients’ events were reported by their cardiologists, who reviewed their medical records after hospital stays or attendance at outpatient clinics. Variables included general characteristics and clinical risk factors for sudden cardiac death, such as family history of sudden cardiac death, non-sustained ventricular tachycardia (VT) and syncope, as well as follow-up results. Maximal wall thickness >30 mm was not adopted because of their low prevalence (n = 1), and abnormal blood pressure response was not used because 42 of the 46 patients were over 40 years old and the exercise test was performed in very few of them [2, 9]. LV outflow tract obstruction was defined as >30 mmHg, measured by continuous-wave Doppler under resting conditions. The start of follow-up was defined as the date of the initial CMR. Only new events from the time of CMR acquisition were considered as primary or secondary end points. This retrospective study was approved by the Institutional Ethics Committee of the National Cerebral and Cardiovascular Center (M26-07) and conformed to the principles of the Declaration of Helsinki. The institutional review board waived the need of written informed consent from the patients in this study.
CMR protocol
CMR imaging was performed using a 1.5-T MR scanner (Magnetom Sonata; Siemens Medical solutions, Erlangen, Germany) with a standardized clinical protocol. All CMR images were electrocardiographically gated and obtained during repeated breath-holds. Cine images were acquired with the true-fast imaging with steady-state free precession (FISP) sequence with the following parameters: repetition time, 3.2 ms; echo time, 1.5 ms; flip angle, 90°; matrix, 128 × 128; field of view, 200 × 200 mm; section thickness, 6 mm; and section interval, 10 mm. After localization of the heart, cine images of 9–12 contiguous short-axis sections encompassing the entire LV were collected, along with 2-, 3-, and 4-chamber long-axis projections. Gadopentetate meglumine (0.15 mmol/kg; Magnevist; Schering AG, Berlin, Germany) was then administered at a rate of 3–4 mL/s using a power injector. LGE images were acquired 10 min after the injection with an inversion-recovery prepared true-FISP sequence with inversion time of 300 ms. Among the 9–12 short-axis slices, we excluded both ends of the apex and the base because the scans of these sections did not include the LV muscle or the beveled myocardium, which caused incorrect signal intensities. Then, seven adjacent slices in the middle of the remaining slices were obtained by using localizer of LV long-axis, as previously reported [30, 31]. Other imaging variables consisted of 65 segments, echo time 1.74 ms, flip angle 60°, field of view 340 × 255 mm, matrix 256 × 129, and voxel size 1.3 × 2.0 × 8.0 mm3.
CMR data analysis
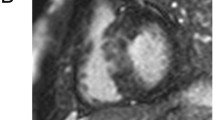
Two experienced radiologists used dedicated software to perform cine image analysis (Argus Function; Siemens Medical Solution, Germany) with calculations of LV volume, mass, and function. LGE images were analyzed by one experienced radiologist and one cardiologist on a workstation (Ziostation 2, Ziosoft, Tokyo, Japan). Regions of LGE in 7 short-axis LGE imaging slices were automatically defined as those exhibiting signal intensity above a pre-determined threshold. We defined thresholds of 2SD and 6SD above the mean signal intensity of remote non-damaged myocardium. We used 2SD because it was previously validated in detecting scars in ischemic heart disease and was utilized in several major reports regarding HCM and LGE [6, 11–13]. We used 6SD because semi-automated LGE gray-scale thresholding using 6SD greater than the mean of visually normal remote myocardium was previously shown to provide the best agreement with visual assessment and, therefore, was the most reliable method for assessing % LGE in LV myocardium in HCM [32, 33]. The mass of LGE was calculated using the area of LGE obtained from the 7 LGE imaging slices. % LGE was expressed as a percentage of LV mass according to the following equation: % LGE = [LGE mass (g)]/[LV mass (g)] × 100. The method used for assessing % LGE and representative image is shown in Fig. 1.
Quantification of the extent of LGE in CMR. a Gray-scale LGE, b A region of interest was defined within the remote normal myocardium. The endocardial and epicardial borders were planimetered on the short-axis view. Two gray-scale thresholds of 2SD and 6SD exceeding the mean signal intensity for normal remote myocardium were used to define areas of LGE. LGE volume was derived by summation of discs. Green area indicates LGE with a threshold of ≥2SD and <6SD, yellow area indicates LGE with a threshold of ≥6SD, and the sum of them indicates LGE with a threshold of ≥2SD. Abbreviations as in Table 1
Histopathology
We examined the pathological correlation of LGE with CMR to determine the etiology of LGE with a threshold of 2SD or 6SD in 3 patients with HCM with systolic dysfunction who underwent CMR and heart transplantation.
Statistical analysis
Results were summarized as mean ± SD or frequency with percentage for continuous and categorical variables, respectively. Continuous variables were compared by the unpaired t test or Mann–Whitney U test was used, as appropriate. Categorical values were compared by Fisher exact test. The primary composite end point was major adverse cardiac events (MACE), including cardiovascular death, lethal arrhythmia, stroke due to cardiac emboli, and unplanned HF hospitalization. Separate secondary end point was unplanned HF hospitalization alone. Patients were censored at the time of their first event or the time of their last clinical follow-up regarding each end point. Statistical analysis was performed using the number of patients who experienced an index composite outcome and time to the index event. When patients experienced several cardiac events during follow-up, the first cardiac event for each end point was regarded as an index outcome. Demographic, clinical, and CMR scan characteristics were all first tested with univariate Cox proportional hazard analysis for each end point. The presence of LV outflow tract obstruction was excluded from univariate analysis because all 4 patients with this condition underwent de novo septal reduction therapy during the follow-up period without preceding cardiovascular events. For these 4 patients, the end of follow-up was regarded as the time of septal reduction therapy [20]. Multivariate Cox proportional hazards analysis was applied to separately evaluate the influence of LVEF and % LGE with a threshold of 2SD or 6SD on MACE or unplanned HF hospitalization. The covariates included in the multivariate model were selected on the basis of univariate analysis of each end point using a forward stepwise method with entrance and stay criteria of p < 0.10; these covariates were limited in number due to the small number of observed events. Because there was strong colinearity (correlation coefficient > 0.6) between LV end-diastolic volume index, end-systolic volume index, and LVEF, only LVEF was included in the multivariate models. History of previous stroke was also excluded from the multivariate analysis for MACE due to low prevalence (n = 3). The fits of multivariate models were evaluated by the Akaike information criterion. A p value <0.05 was considered statistically significant. All analyses were performed with JMP for Windows version 11 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical and CMR characteristics
Forty-six patients were retrospectively followed for 1755 ± 594 days (4.8 ± 1.6 years). Their baseline characteristics are summarized in Table 1. LVEF was 35.3 ± 12.3 %, ranging from 8 to 49 %. LGE was present in all patients; the mean % LGE with thresholds of 2SD and 6SD were 53.1 ± 14.6 and 30.0 ± 15.1 %, respectively. % LGE with a threshold of 6SD was inversely related to LVEF (r = −0.44; p = 0.002), but that with a threshold of 2SD was not (r = −0.28, p = 0.060) (Fig. 2).
Relationship between LVEF and the extent of LGE. a LVEF and % LGE with a threshold of 2SD, b LVEF and % LGE with a threshold of 6SD. % LGE with a threshold of 6SD was inversely related to LVEF (r = −0.44; p = 0.002), but that with a threshold of 2SD was not (r = −0.28, p = 0.060). Abbreviations as in Table 1
Histopathological correlation with LGE
We performed histopathological examinations of transverse sections of explanted hearts from 3 patients who underwent heart transplantation. There was excellent agreement between the location and quantity of fibrosis in the explanted hearts and LGE on pre-explant CMR (Fig. 3). Intriguingly, LGE defined using a threshold of 6SD aligned closely with the distribution of replacement fibrosis, LGE with a threshold of 2SD reflected a mixture of both interstitial and replacement fibrosis, and areas with no LGE also demonstrated diffuse interstitial fibrosis (Fig. 4).
LGE with histopathological correlation in three patients who underwent CMR and heart transplantation. a Histopathological transverse sections of explanted heart from 26-year-old male who underwent CMR 2.6 years before heart transplantation, b 28-year-old male, 4.5 years before, c 48-year-old male, 6.4 years before. Right panels Masson’s trichrome staining, center panels gray-scale LGE, left panels LGE with thresholds of 2SD and 6SD. Abbreviations as in Table 1
A representative case of histopathological extent of fibrosis and extent of LGE of CMR. a Masson’s trichrome stain ×2, b LGE with thresholds of 2SD and 6SD, and c gray-scale LGE. d Histopathological finding of area of no LGE, e LGE with a threshold of 2SD, and f LGE with a threshold of 6SD. LGE defined using a threshold of 6SD aligned closely with the distribution of replacement fibrosis, LGE with a threshold of 2SD reflected a mixture of both interstitial and replacement fibrosis, and areas with no LGE also demonstrated diffuse interstitial fibrosis. Abbreviations as in Tables 1 and 2
Clinical outcomes
During follow-up, percutaneous transluminal septal myocardial ablation was performed in all 4 patients with LV outflow tract obstruction. Implanted cardioverter-defibrillators (ICD) with or without cardiac resynchronization therapy (CRT) were implanted in 11 (24 %) and 10 (22 %) of patients, respectively. In 2 patients receiving ICDs without CRT, the ICDs were upgraded to ICDs with CRT. The reason for ICD implantation in the 21 patients was primary prevention in 15 (71 %) and secondary prevention in 6 (29 %). There were several lethal arrhythmic events and strokes due to cardiac emboli, as listed in Table 2. Overall, 23 of 46 (50 %) patients experienced several instances of unplanned HF hospitalization. Of these, 7 died of HF and 6 received implantable LV assist devices; of those receiving assist devices, 3 underwent heart transplantation and 2 died of HF. On the other hand, in patients without unplanned HF hospitalization, there was 1 sudden cardiac death and 2 HF deaths. Overall, 11 of 46 patients died of HF (Table 2). The number of adverse cardiac events included as index events in primary composite MACE outcome analysis are listed in center column of Table 2. Among the 29 patients with index primary composite MACE events, additional subsequent events were common. The cumulative number of each MACE event over the follow-up period is listed in the right column of Table 2.
MACE
During follow-up, 29 of 46 (63 %) patients met the primary end point of MACE. The breakdown of MACE is shown in Table 2. The univariate Cox proportional hazard analysis comparing different clinical and CMR characteristics is displayed in Table 3. The parameters of hypertension, New York Heart Association class III or IV and plasma B-type natriuretic peptide (BNP) levels reached statistically significant levels. In addition to CMR parameters reflecting LV remodeling, % LGE with threshold of 6SD reached statistically significant levels. Investigation of the medications being used at the time of CMR revealed that diuretics, spironolactone, and warfarin were administered significantly more frequently among patients with MACE than those without. Significant correlations were observed between LV end-diastolic volume index and end-systolic volume index and between end-systolic volume index and LVEF (r = 0.99, p < 0.0001 and r = −0.78, p < 0.0001, respectively). As a result, LV end-diastolic and end-systolic volume indices were omitted while LVEF was selected for multivariate analysis. Multivariable Cox proportional hazard analysis revealed that % LGE with a threshold of 2SD, % LGE with a threshold of 6SD, and LVEF were independent predictors of MACE (hazard ratio = 1.36/10 %, 95 % confidence interval = 1.00–1.84, p = 0.049; hazard ratio = 1.62/10 %, 95 % confidence interval = 1.23–2.15, p < 0.001; hazard ratio = 0.68/10 %, 95 % confidence interval = 0.51–0.91, p = 0.010, respectively). The Akaike information criterion evaluating the fit of a model demonstrated that % LGE with a threshold of 6SD was a better independent predictor of MACE than % LGE with a threshold of 2SD or LVEF (Akaike information criterion = 172.20, 178.97 and 178.09, respectively) (Table 4).
Unplanned HF hospitalization
Overall, 23 of 46 (50 %) patients had unplanned HF hospitalizations (Table 2). Results of the univariate Cox proportional hazard analysis comparing different clinical and CMR characteristics are displayed in Table 5. Age at diagnosis, moderate to severe mitral regurgitation, New York Heart Association class III or IV, and plasma BNP reached statistical significance. In addition to CMR parameters reflecting LV remodeling, % LGE with thresholds of both 2SD and 6SD reached statistical significance. Investigation of the medications being used at the time of CMR revealed that diuretics and spironolactone were administered significantly more frequently among patients with unplanned HF hospitalization than those without. Multivariable Cox proportional hazard analysis revealed that % LGE with a threshold of 2SD and % LGE with a threshold of 6SD were independent predictors of unplanned HF hospitalization (hazard ratio = 1.62/10 %, 95 % confidence interval = 1.19–2.25, p = 0.002; hazard ratio = 1.68/10 %, 95 % confidence interval = 1.30–2.16, p < 0.0001, respectively); however, LVEF was not an independent predictor(hazard ratio = 0.71/10 %, 95 % confidence interval = 0.47–1.07, p = 0.098). The Akaike information criterion evaluating the fit of a model demonstrated that % LGE with a threshold of 6SD was a better independent predictor of unplanned HF hospitalization than % LGE with a threshold of 2SD (Akaike information criterion = 136.62 and 142.61, respectively) (Table 6). Several patients experienced recurrent unplanned HF hospitalization; the number of such hospitalizations per patient was related not to LVEF (r = −0.06; p = 0.798) nor % LGE with a threshold of 2SD (r = 0.22; p = 0.303), but to % LGE with a threshold of 6SD (r = 0.44; p = 0.037) (Fig. 5).
Relationship between LVEF, the extent of LGE, and number of recurrent unplanned heart failure hospitalizations. a LVEF and number of recurrent unplanned HF hospitalizations, b % LGE with a threshold of 6SD and number of recurrent unplanned HF hospitalizations. The number of recurrent hospitalizations per patient was related not to LVEF (r = −0.06, p = 0.798) but to % LGE with a threshold of 6SD (r = 0.44; p = 0.037). Abbreviations as in Table 1
Discussion
The major findings of this study were that % LGE with a threshold of 6SD was a good independent predictor of primary composite MACE and secondary unplanned HF hospitalization end points and reflected both mortality and morbidity more precisely than either % LGE with a threshold of 2SD or LVEF in patients with HCM and systolic dysfunction.
Although an inverse relationship was observed between LVEF and % LGE with a threshold of 6SD even in HCM with systolic dysfunction (Fig. 2b), the relationship in HCM with systolic dysfunction (r = −0.44) was slightly stronger than that in the general HCM population (r = −0.3) as shown in a previous report [15]. In the present study, the prognostic value of LVEF was lower than that of % LGE with a threshold of 6SD. This seems to be contradictory, but it is not. In this study population, % LGE with a threshold of 6SD was 30.0 ± 15.1 %, comparable to data from published reports [15, 26]. It was also reported that there was substantial overlap of % LGE with a threshold of 6SD among LVEF subgroups of <50, 50–65, and >65 % [26], and this overlap tendency of % LGE with a threshold of 6SD among LVEF subgroups (i.e., <20, 20–40, and >40 %) was also observed even in HCM with systolic dysfunction as shown in Fig. 2b. Therefore, % LGE with a threshold of 6SD cannot be estimated accurately by the value of LVEF; in other words, the extent of fibrosis implicated in adverse cardiac events cannot be estimated accurately by the value of LVEF. Furthermore, % LGE with a threshold of 6SD is an absolute value that is reflective of myocardial fibrosis, while global LVEF values may vary because of fluctuating heart rate, afterload and/or preload, concomitant conduction disturbance, or mitral regurgitation. These factors suggest that % LGE with a threshold of 6SD is a more accurate predictor than LVEF despite the inverse relationship between % LGE and LVEF. In addition, we investigated only HCM with systolic dysfunction carrying poor prognosis, which may have reduced the prognostic value of LVEF.
Although the risk factors for sudden cardiac death in general HCM patients have been identified, there are limited data for identifying the risk factors for MACE and/or progression of HF in patients with HCM and systolic dysfunction [8, 9]. In fact, we found no significant correlation between any single clinical risk factor and the occurrence of MACE or unplanned HF hospitalization. It is important to identify predictive risk factors for MACE, however, and given that the fibrosis detected by LGE may have prognostic significance in ischemic heart disease and dilated cardiomyopathy [12, 34], it may play a similarly important role in HCM, especially that accompanied by systolic dysfunction. HCM is characterized by diffuse histopathological abnormalities involving the entire LV, including replacement and interstitial fibrosis [21].
Thus, we examined the pathological correlation with CMR and found that LGE defined using a threshold of 6SD aligned closely with the distribution of replacement fibrosis, but LGE with a threshold of 2SD reflected a mixture of both interstitial and replacement fibrosis, as previously reported [21] (Figs. 3, 4). These qualitative differences in fibrosis patterns were considered to result in a gradient of gadolinium deposition and could be distinguished by the LGE threshold used (i.e., 2SD or 6SD). On the other hand, LGE performed using the threshold method cannot visualize diffuse interstitial fibrosis because this type of fibrosis is “nulled” to highlight focal scarring [35]. Indeed, areas with no LGE also had diffuse interstitial fibrosis (Fig. 4). We considered that % LGE with a threshold of 6SD reflects the extent of severe replacement fibrosis in LV masses, providing greater prognostic value than the 2SD threshold. % LGE with a threshold of 6SD was a better independent predictor of MACE and unplanned HF hospitalization and was more significantly associated with recurrent unplanned HF hospitalization than % LGE with a threshold of 2SD. Our data underscore the potential value of assessing the extent and quality of LGE rather than only the presence of LGE for predicting outcomes in HCM with systolic dysfunction.
In this study population, 21 of 46 (46 %) patients underwent implantation of ICDs with or without CRT. These device therapies might have modified clinical outcomes because they can easily detect arrhythmic events, prevent sudden cardiac death due to lethal arrhythmia, and reduce unplanned HF hospitalization. The differences in baseline CMR parameters and cumulative number of cardiac events between patients receiving and not receiving device therapies are shown in Table 7. Patients receiving device therapies not only had significantly younger age, lower LVEF, and larger LV volume index, but also showed a tendency toward larger extent of LGE. Indeed, arrhythmic events were more frequently detected in patients receiving device therapies, while there was 1 sudden cardiac death in device-free patients. The frequencies of HF death, LV assist device implantation, and heart transplantation did not vary according device therapy status; however, the frequencies of both the primary and secondary end points (MACE and unplanned HF hospitalization, respectively) were significantly higher in patients receiving device therapies. Therefore, patients who were receiving such therapies had a poorer prognosis than patients who were not. This might be influenced by adverse LV remodeling in patients with implantable devices. ICD therapy was confirmed to effectively prevent sudden cardiac death in HCM patients [36, 37]. On the other hand, a previous observational study reported that end-stage HF was the main cause of cardiac death in HCM patients receiving ICD therapy. Furthermore, it was reported that severe HF symptoms and indications for CRT were independent predictors of mortality in HCM patients with ICDs [38]. Both the present study and those mentioned above might demonstrate that the efficacy of ICDs with or without CRT therapy for preventing HF death was limited in HCM patients with systolic dysfunction. Although one study reported that CRT improved HF symptoms and was associated with reverse remodeling of LV in HCM with systolic dysfunction [39], the advantage of CRT in HCM with systolic dysfunction is still unclear [36, 37].
Limitations
The present study had several limitations. First, it used a retrospective design and relatively small sample size due to the low prevalence of HCM with concomitant systolic dysfunction. Second, this single-center study in a tertiary hospital-based population was probably influenced by referral bias because of this population’s high-risk status, presence of serious conditions such as heart transplantation requiring specialized care, and known increased risk of adverse outcomes including mortality and re-hospitalization. Further multicenter collaborative clinical trials are required to identify whether our findings are applicable to a community-based population. Third, in our center, gadolinium-enhanced CMR is performed in patients with HCM based on the judgment of the attending physicians, and not all patients with HCM undergo this procedure. This may result in selection bias. Patients with ICD, pacemakers, or renal insufficiency (estimated glomerular filtration rate <30 ml/min/1.73 m2) were not pre-included in this study population because of contraindications to gadolinium-enhanced CMR, thus reducing the number of subjects prone to cardiovascular events, especially arrhythmic events. Arrhythmic events were more frequently detected in patients who were receiving device therapies than in those who were not, and there was a possibility that arrhythmic events in patients not receiving device therapies were overlooked. This in turn might have modified clinical outcomes. Fourth, we performed endomyocardial biopsy in 35 of 46 patients and excluded metabolic or infiltrative storage disorders characterized by LV hypertrophy, such as amyloidosis and Fabry disease. Regarding the residual 11 patients, we diagnosed them with HCM based on unexplained asymmetrical LV hypertrophy and a family history of HCM. Although this population had no symptoms that suggested a multisystem disorder, it is possible that one or more had a rare storage disease with only cardiac involvement. Fifth, we compared the histopathological findings and LGE results in only 3 patients. Sixth, the diffuse interstitial fibrosis in HCM currently cannot be determined using the threshold method mentioned above. T1 mapping techniques might provide better and more accurate evaluation of diffuse myocardial fibrosis [40, 41]. Finally, we evaluated LGE of only “single shot” CMR; assessing LGE of repeat CMR examinations over time could be useful because HCM-associated fibrosis is usually progressive [42, 43].
Conclusions
% LGE with a threshold of 6SD was a better independent predictor of MACE and unplanned HF hospitalization and reflected mortality and morbidity more precisely than both LVEF and % LGE with a threshold of 2SD in HCM with systolic dysfunction. We suggest that quantification of LGE with a threshold of 6SD is useful for predicting MACE and recurrent unplanned HF hospitalization and can contribute to further risk stratification in patients with HCM and systolic dysfunction.
References
Maron BJ (2002) Hypertrophic cardiomyopathy: a systematic review. JAMA 287:1308–1320
Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ (2000) Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 36:2212–2218
Cannan CR, Reeder GS, Bailey KR, Melton LJ 3rd, Gersh BJ (1995) Natural history of hypertrophic cardiomyopathy. A population-based study, 1976 through 1990. Circulation 92:2488–2495
Kawashiri MA, Hayashi K, Konno T, Fujino N, Ino H, Yamagishi M (2014) Current perspectives in genetic cardiovascular disorders: from basic to clinical aspects. Heart Vessels 29:129–141
Fujita E, Nakanishi T, Nishizawa T, Hagiwara N, Matsuoka R (2013) Mutations in the cardiac troponin T gene show various prognoses in Japanese patients with hypertrophic cardiomyopathy. Heart Vessels 28:785–794
Kwon DH, Smedira NG, Rodriguez ER, Tan C, Setser R, Thamilarasan M, Lytle BW, Lever HM, Desai MY (2009) Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol 54:242–249
Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Colan SD, Seidman JG, Díez J, Seidman CE (2010) Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med 363:552–563
Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH 3rd, Spirito P, Ten Cate FJ, Wigle ED, Task Force on Clinical Expert Consensus Documents, American College of Cardiology, Committee for Practice Guidelines. European Society of Cardiology (2003) American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 42:1687–1713
McKenna WJ, Behr ER (2002) Hypertrophic cardiomyopathy: management, risk stratification, and prevention of sudden death. Heart 87:169–176
O’Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli-Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK (2010) Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol 56:867–874
Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, Nassenstein K, Schlosser T, Sabin GV, Sechtem U, Mahrholdt H (2010) Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 56:875–887
Kwong RY, Farzaneh-Far A (2011) Measuring myocardial scar by CMR. JACC Cardiovasc Imaging 4:157–160
Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ (2004) The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 43:2260–2264
Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA, Gersh BJ (2010) Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail 3:51–58
Maron MS, Appelbaum E, Harrigan CJ, Buros J, Gibson CM, Hanna C, Lesser JR, Udelson JE, Manning WJ, Maron BJ (2008) Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail 1:184–191
Green JJ, Berger JS, Kramer CM, Salerno M (2012) Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy JACC Cardiovasc. Imaging 5:370–377
Alla VM, Koneru S, Hunter C, Mooss A (2012) LGE and the risk of sudden death in HCM. JACC Cardiovasc Imaging 5:761–762
Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, Udelson JE, Rowin E, Lombardi M, Cecchi F, Tomberli B, Spirito P, Formisano F, Biagini E, Rapezzi C, De Cecco CN, Autore C, Cook EF, Hong SN, Gibson CM, Manning WJ, Appelbaum E, Maron MS (2014) Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 130(6):484–495
Hen Y, Iguchi N, Machida H, Takada K, Utanohara Y, Sumiyoshi T (2013) High signal intensity on T2-weighted cardiac magnetic resonance imaging correlates with the ventricular tachyarrhythmia in hypertrophic cardiomyopathy. Heart Vessels 28:742–749
Hen Y, Iguchi N, Utanohara Y, Takada K, Machida H, Takayama M, Sumiyoshi T (2014) Prognostic value of late gadolinium enhancement on cardiac magnetic resonance imaging in Japanese hypertrophic cardiomyopathy patients. Circ J 78:929–937
Moravsky G, Ofek E, Rakowski H, Butany J, Williams L, Ralph-Edwards A, Wintersperger BJ, Crean A (2013) Myocardial fibrosis in hypertrophic cardiomyopathy: accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging 6:587–596
Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ (2006) Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 114:216–225
Fernández A, Vigliano CA, Casabé JH, Diez M, Favaloro LE, Guevara E, Favaloro RR, Laguens RP (2011) Comparison of prevalence, clinical course, and pathological findings of left ventricular systolic impairment versus normal systolic function in patients with hypertrophic cardiomyopathy. Am J Cardiol 108:548–555
Biagini E, Coccolo F, Ferlito M, Perugini E, Rocchi G, Bacchi-Reggiani L, Lofiego C, Boriani G, Prandstraller D, Picchio FM, Branzi A, Rapezzi C (2005) Dilated-hypokinetic evolution of hypertrophic cardiomyopathy: prevalence, incidence, risk factors, and prognostic implications in pediatric and adult patients. J Am Coll Cardiol 46:1543–1550
Olivotto I, Cecchi F, Poggesi C, Yacoub MH (2012) Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ Heart Fail 5:535–546
Olivotto I, Maron BJ, Appelbaum E, Harrigan CJ, Salton C, Gibson CM, Udelson JE, O’Donnell C, Lesser JR, Manning WJ, Maron MS (2010) Spectrum and clinical significance of systolic function and myocardial fibrosis assessed by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol 106:261–267
Yacoub MH, Olivotto I, Cecchi F (2007) ‘End-stage’ hypertrophic cardiomyopathy: from mystery to model. Nat Clin Pract Cardiovasc Med 4:232–233
Hamada T, Kubo T, Kitaoka H, Hirota T, Hoshikawa E, Hayato K, Shimizu Y, Okawa M, Yamasaki N, Matsumura Y, Yabe T, Takata J, Doi YL (2010) Clinical features of the dilated phase of hypertrophic cardiomyopathy in comparison with those of dilated cardiomyopathy. Clin Cardiol 33:E24–E28
Argulian E, Messerli FH, Aziz EF, Winson G, Agarwal V, Kaddaha F, Kim B, Sherrid MV (2013) Antihypertensive therapy in hypertrophic cardiomyopathy. Am J Cardiol 111:1040–1045
Kono AK, Yamada N, Higashi M, Kanzaki S, Hashimura H, Morita Y, Sakuma T, Noguchi T, Naito H, Sugimura K (2011) Dynamic late gadolinium enhancement simply quantified using myocardium to lumen signal ratio: normal range of ratio and diffuse abnormal enhancement of cardiac amyloidosis. J Magn Reson Imaging 34:50–55
Ise T, Hasegawa T, Morita Y, Yamada N, Funada A, Takahama H, Amaki M, Kanzaki H, Okamura H, Kamakura S, Shimizu W, Anzai T, Kitakaze M (2014) Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart 100:1165–1172
Harrigan CJ, Peters DC, Gibson CM, Maron BJ, Manning WJ, Maron MS, Appelbaum E (2011) Hypertrophic cardiomyopathy: quantification of late gadolinium enhancement with contrast-enhanced cardiovascular MR imaging. Radiology 258:128–133
Spiewak M, Malek LA, Misko J, Chojnowska L, Milosz B, Klopotowski M, Petryka J, Dabrowski M, Kepka C, Ruzyllo W (2010) Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur J Radiol 74:e149–e153
Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ (2006) Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 48:1977–1985
Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC (2010) Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 122:138–144
Authors/Task Force members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H (2014) 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 35:2733–2779
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines (2011) 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 58:e212–e260
Vriesendorp PA, Schinkel AF, Van Cleemput J, Willems R, Jordaens LJ, Theuns DA, van Slegtenhorst MA, de Ravel TJ, ten Cate FJ, Michels M (2013) Implantable cardioverter-defibrillators in hypertrophic cardiomyopathy: patient outcomes, rate of appropriate and inappropriate interventions, and complications. Am Heart J 166:496–502
Rogers DP, Marazia S, Chow AW, Lambiase PD, Lowe MD, Frenneaux M, McKenna WJ, Elliott PM (2008) Effect of biventricular pacing on symptoms and cardiac remodelling in patients with end-stage hypertrophic cardiomyopathy. Eur J Heart Fail 10:507–513
Brouwer WP, Baars EN, Germans T, de Boer K, Beek AM, van der Velden J, van Rossum AC, Hofman MB (2014) In-vivo T1 cardiovascular magnetic resonance study of diffuse myocardial fibrosis in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 16:28
Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, Nagel E (2013) Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging 6:475–484
Quarta G, Grasso A, Pasquale F, Flett AS, Sado DM, Bonini E, Ariti C, Prasad SK, Elliott PM, Moon JC (2011) Evolution and clinical importance of fibrosis in HCM. JACC Cardiovasc Imaging 4:1221–1223
Todiere G, Aquaro GD, Piaggi P, Formisano F, Barison A, Masci PG, Strata E, Bacigalupo L, Marzilli M, Pingitore A, Lombardi M (2012) Progression of myocardial fibrosis assessed with cardiac magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 60:922–929
Acknowledgments
The present study was supported by an Intramural Research Fund (24-6-29) for Cardiovascular Diseases from the National Cerebral and Cardiovascular Center.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Funada, A., Kanzaki, H., Noguchi, T. et al. Prognostic significance of late gadolinium enhancement quantification in cardiac magnetic resonance imaging of hypertrophic cardiomyopathy with systolic dysfunction. Heart Vessels 31, 758–770 (2016). https://doi.org/10.1007/s00380-015-0670-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-015-0670-4