Abstract
Climate changes are altering precipitation to more frequent extreme precipitation events that have strong impacts on the structure and functions of grassland ecosystems. We conducted a rain simulation experiment combined with in situ 15 N labeling of three nitrogen (N) forms (NO3−, NH4+, glycine) to investigate how the frequency of extreme precipitation influences plant productivity and N acquisition (N uptake, 15 N recovery, and preference for N form) by the dominant species Stipa grandis and soil microorganisms in the temperate steppe. Extreme precipitation had three frequencies (1, 3, and 6 events for low, medium, and high frequency) with the same total rain amount in 1-month cycle. The low frequency reduced the S. grandis biomass by 39%, whereas the high ones raised the S. grandis biomass by 43% and increased plant and microbial N uptake up to 6.3-fold and 5.1-fold of those under ambient precipitation, respectively. Plants preferred NO3− and microorganisms preferred NH4+ under low frequency, but they showed similar preference for three N forms, leading to chemical niche overlap for NO3−, NH4+, and glycine under high frequency. This indicated that high precipitation frequency effectively reduced the proportion of each N form, which plants and microorganisms competed for as the available N pool increased. Overall, the increase of precipitation frequency (decreasing intensity) shifted the extreme (low frequency but high intensity) to optimal conditions for plant productivity and N acquisition by plants and microorganisms in the temperate steppe. These findings provide new insights for understanding the diverse responses of ecosystem functions to extreme climate events.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the major concerns regarding future climate change is the frequency and magnitude of extreme climate events (Easterling and Mearns 2000; Donat et al. 2016; Grant 2017; Raghavendra et al. 2018), including extreme drought and rainfall. The models used by the Intergovernmental Panel on Climate Change (IPCC) consistently predict the changes in the magnitude and frequency of extreme precipitation events as the climate warms (IPCC 2013), and these scenarios have occurred at local, regional, and global scales (Knapp et al. 2008; IPCC 2013; Donat et al. 2016; Wang et al. 2017). In Inner Mongolia, a region dominated by continental temperate steppe, the extreme precipitation magnitude has varied little or even slightly decreased (0–6 mm per decade) (Zhai et al. 2005), but it increased the frequency and intensity of short-term extreme precipitation over the last 50 years, indicating unstable condition of precipitation under ongoing climate change (Chen et al. 2012; Fu et al. 2013). Extreme precipitation accounts for a large proportion of the total annual precipitation (Chen et al. 2012) and leads to high precipitation variability; this further aggravates the soil water deficit in Inner Mongolia steppe.
In arid and semiarid areas, precipitation is a key driver of ecosystem structure and function. Altered precipitation patterns have strong effects on the plant species composition and net primary production (NPP) by changing soil water availability (Knapp et al. 2008; Li et al. 2019). Therefore, soil water availability is a crucial factor linking precipitation with ecological response (Wu et al. 2011; Knapp et al. 2016). In arid ecosystem, extreme precipitation via greater infiltration increases the effective precipitation and available soil water for plants (Knapp et al. 2008). An increase in effective precipitation stimulates plant photosynthesis by maximizing light capture through opened stomata and faster CO2 exchange and leads to NPP increase (Joslin and Wolfe 1998). Changes in soil water due to dynamic precipitation differentially affect N turnover (Cregger et al. 2014) and plant N acquisition in arid ecosystem and further influence plant productivity (McCulley et al. 2009; Kong et al. 2013). After extreme precipitation, soil nutrients are solubilized, and a large pulse of N is released owing to the soil aggregate disruption (Borken and Matzner 2009; Navarro-Garcia et al. 2012) or even the microbial cell burst (Blagodatsky et al. 1987; Blagodatsky and Yevdokimov 1998). In temperate grasslands, increased soil water availability promoted N mineralization and improved soil N availability for plants (Wang et al. 2006; Lü and Han 2010). Microbial N uptake also has various responses to the soil moisture fluctuations. In arid land, microbial metabolism and N uptake are supposed to increase with moisture due to water limitation and slow substrate diffusion (Borken and Matzner 2009). However, the moisture range required for soil microbial metabolism is very wide, and thus, microbial N uptake could be constant by the moisture changed from suboptimal to optimal condition (Collins et al. 2008; Zhang et al. 2019). And when soil is waterlogged by the extreme precipitation events, oxygen deficit and sudden changes in water potential in soil limit or terminate microbial metabolic activities and decline their N uptake (Kieft et al. 1987; Zhang et al. 2019). Many studies indicate that extreme precipitation causes N leaching (Yahdjian and Sala 2010; Hess et al. 2020; Zheng et al. 2020); however, soil N pulse varies with the intensity, duration time, and intervals between events, or even with plant species. In the semiarid steppe and tallgrass community, soil N availability increases about 40–60% with a shift from 10- to 30-day interval between extreme precipitation but changes little in mixed grasslands (Heisler-White et al. 2009). Numerous studies investigate the effect of soil water and N fluctuation induced by extreme precipitation on plant productivity (Heisler-White et al. 2009; Kang et al. 2013; Kong et al. 2013; Wilcox et al. 2017), while few studies focus on the further changes in plant-microbial N acquisition strategy on plant growth in water and N-limited arid land.
Plant and microbial N acquisition is critical for understanding NPP because N is the often limiting nutrient in many ecosystems (LeBeaur and Treseder 2008). In N-limited ecosystem, plant species often perform chemical niche differentiation to minimize competition and facilitate coexistence (McKane et al. 2002; Ashton et al. 2010; Xu et al. 2011a, b). In Arctic tundra, the most abundant N form was first used by the most productive species, and the less abundant N form was largely used by sub-productive species (McKane et al. 2002). Plants often maximize resource utilization to increase their competitive abilities. Many studies on plant-microbial competition for N demonstrated that microorganisms acquire N more efficiently than plants in temperate and alpine grasslands (Schimel et al. 1989; Ouyang et al. 2016; Xu et al. 2011b; Liu et al. 2016). Consequently, the intensity of plant-microbial competition for N and niche differentiation (i.e., spatial, temporal and chemical) could be changed by the N limitation arising from the altered precipitation. Plants and microorganisms increase N uptake and acquire more NH4+ than NO3− under intense precipitation and drying-rewetting conditions (Månsson et al. 2014). This can be connected to (1) slower nitrification under very high soil moisture due to the limited oxygen diffusion (Grundmann et al. 1995; Chen et al. 2013) and (2) the preferences for N form in plants and microorganisms under higher water availability (Houlton et al. 2007; Wang and Macko 2011; Kuzyakov and Xu 2013; Hong et al. 2019). However, it remains unclear how the chemical niche differentiation of plants and microorganisms varies with intense precipitation. Besides chemical niche differentiation, plants and microorganisms demonstrate spatial niche differentiation to weaken the competition (Xu et al. 2011b; Liu et al. 2016). The root biomass and microbial biomass (Xu et al. 2011b; Pabst et al. 2013), as well as soil nutrient content (Farley and Fitter 1999), decrease with soil depth. In alpine grasslands, plants competed effectively with microorganisms for NO3− in 5 cm depth, while microorganisms strongly outcompeted plants for NH4+ below 5 cm soil depth (Xu et al. 2011b). The frequency of extreme precipitation could affect N localization in soil depth and further influence N acquisition by plants and microorganisms.
Plant in semiarid temperate steppe is extremely vulnerable and sensitive to the variation of soil water availability (Christensen et al. 2004). A lack of water in the temperate steppe usually limited soil available N; therefore, plants and microorganisms diverge their N sources (N forms) of acquisition to reduce competition and achieve resource partition. Plants mainly use NO3−, and microorganisms mainly take up NH4+ and NO3− in the temperate grassland with low soil moisture (Liu et al. 2016). After extreme precipitation, high rainfall use efficiency in arid land and increased soil water and N mineralization (Knapp et al. 2008) could alter the initial N uptake strategy of plants and microorganisms. We hypothesize that (1) increase in soil N availability induced by extreme precipitation with high frequency will alleviate N limitation for plant and microorganisms and thus increase their N uptake and plant productivity and (2) increased N availability will reduce the proportion of each N form which plants and microorganisms compete for, so plants and microorganisms will acquire N based on their preference, thus leading to strong chemical niches overlap. Overall, extreme precipitation will alter the ratio of N incorporated into microorganisms and plants and change their preference for N form. To test these hypotheses, we conducted an extreme precipitation simulation with different frequencies, combined with pulse 15 N labeling in a temperate steppe of North China. The frequency regulates the influence of extreme precipitation, a low to a high frequency describes a change from extreme to optimal condition for plant growth. The low precipitation frequency represents an extreme condition due to the largest wetting after long drying, and it is a striking situation for the ecosystem caused by ongoing climate change; the medium precipitation frequency represents occasional extreme precipitation; and the high precipitation frequency represents a relatively stable and optimal condition which can alleviate limitations of water and nutrient by frequent dry-rewetting.
Materials and methods
Study site
The study was conducted at the Xilingol Grassland Ecosystem Research Station (116° 20.718′ E, 44° 16.385′ N) (XGERS), Institute of Grassland Research, Chinese Academy of Agricultural Sciences. The area is characterized by a semiarid continental climate and is a temperate steppe dominated by Stipa grandis, and other plant species (Table S1) include Leymus chinensis, Cleistogenes squarrosa, Melissilus ruthenicus, Carpesium abrotanoides, Saussurea japonica, and Allium fistulosum. The dominant species of S. grandis was selected as the target species. Mean annual temperature (MAT) at the study site is 0 °C, with the highest monthly air temperature in July (18.3 °C) and the lowest in January (− 22 °C). Mean annual precipitation ranges from 350 to 450 mm year−1, and more than 70% of precipitation falls during June and August. The soil is chestnut soil, corresponding to a Haplic or Calcic Kastanozem (World Reference Base for Soil Resources 2014). Soil organic C is 1.11%, total N is 0.12%, C/N ratio is 9.35, and soil pH is 7.5.
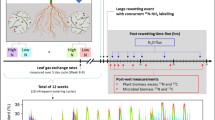
Simulating extreme precipitation events
Sixteen 2.5 m × 2.5 m plots were randomly set, with at least a 10 m buffer distance between every two plots (Fig. S1). The simulation of extreme precipitation was conducted from July 14 to August 13 of 2015, and natural precipitation was absent during the 3 days preceding the initial of the precipitation simulation experiment (Fig. S2). The ambient precipitation during the simulation period was 32 mm, and all the plots were under natural rain-fed condition and received ambient rainfall in addition to the water treatments.
The daily extreme precipitation defined as that larger than its 95th percentile for the year, from the period of past 50 years, and it usually ranged from 10 to 20 mm per day in Inner Mongolia (Zhai et al. 2005). Extreme precipitation events (≥ 10 mm per day) account for more than 50% of total annual precipitation, and 70–80% of extreme precipitation occurs in July and August in Inner Mongolia, corresponding to 60–90 mm per month at our study site. Besides, the maximum of an extreme precipitation event was approximately 100 mm during the past two decades (1993–2013) at Xilinhot based on precipitation data obtained from Xilinhot meteorological station close to our study site (Liu et al. 2018). Therefore, a total of 105 mm water was supplied in this study as the amount of extreme precipitation.
Different frequency (interval) of extreme precipitation events alters soil moisture through differing dry–wet cycling of soil, in return affects plant growth, microbial activity, and nutrient cycling. Here, the total amount of water applied to the experimental plots was held constant (105 mm) but delivered with different frequency. Four precipitation treatments were applied, no additional water (ambient rainfall), low precipitation frequency (low: 105 mm water supplied over one day per month), medium precipitation frequency (medium: 35 mm water supplied every 10 days, or 3 times per month), and high precipitation frequency (high: 17.5 mm water supplied every 5 days, or 6 times per month), and each precipitation treatment had four replicated plots. The hourly extreme precipitation in the semiarid ecosystem of Inner Mongolia was about 12.5 mm h−1 based on 111 stations in summer (June to August) during 1991 to 2015. In this study, water was applied to each plot in 6 h (6:00–12:00 a.m.) to low, 2 h (6:00–8:00 a.m.) to medium, and 1 h (6:00–7:00 a.m.) to high (corresponding to 17.5 mm h−1 in all treatments). Even water distribution was ensured each time by using four sprinklers placed about 20 cm above the plot surface. The water spray intensity was controlled by adjusting the nozzle size and water pressure. The intensity of simulated precipitation (17.5 mm h−1) has reached the extreme precipitation level in this area.
15 N pulse labeling experiment
Plant N uptake has diurnal dynamics, and N uptake is high at 9:00–12:00 during a day combined with the high leaf photosynthetic rates (Rusjan and Mikoš 2010; Peng et al. 2014; Liu et al. 2021). 15 N pulse labeling was conducted at 9:00 a.m. on August 16 of 2015. After the precipitation simulation finished, eight 10 cm × 10 cm subplots were randomly set in each plot with at least 50 cm apart, and totally 128 subplots were set in all the treatments (Fig. S1). The medium-sized S. grandis was selected for 15 N labeling in this study, and most roots were within the subplot. Considering the short labeling time (3 h) and the short diffusion distance (several cm per hour) of labeling solution, no interactive N was up taken by the S. grandis from neighboring subplot. Four 15 N-labeled treatments (i.e., the addition of 15NO3−, 15NH4+, glycine-15 N, and none) were applied separately at two 15 N-injected soil depths (i.e., 0–5 cm and 5–15 cm in separate subplot) in each subplot. The total N amount added in each plot was 0.60 g·N m−2, with the same amounts (0.20 g·N m−2) of NO3−, NH4+, and glycine, but only one N form was labeled with 15 N (15NO3− (99.14 atom% 15 N), 15NH4+ (99.19 atom% 15 N), or glycine-15 N (99.04 atom% 15 N). A total of 60 mg of all three N forms was dissolved in 500 ml of distilled water, with identical N amounts (corresponding to 0.80 µg N·g−1 d.w. soil) of NH4+, NO3−, and glycine. Forty-five milliliters of solutions was injected into each subplot. To ensure that the 15 N solution was distributed evenly, each subplot (10 cm × 10 cm) was equally divided into 9 quadrats, and 5 ml solutions was injected at the center of each quadrat. 15 N solution was injected at 2.5 cm for 0–5 cm and at 10 cm for 5–15 cm soil depth, and both roots and soils were sampled to the depth of 15 cm.
Sampling and analysis
Plant and soil samples were collected 3 h after the 15 N solution injection. Rapid soil sampling was performed to ensure that 15 N for plant and microbial uptake is in intact injected form (Xu et al. 2011b; Moran-Zuloaga et al. 2015). Samples were excavated as soil blocks of 10 cm × 10 cm × 15 cm from each plot, all of the soil and the whole plant including shoots and roots within the block were collected. After washing, roots and shoots were carefully separated, and root samples were soaked in a 0.5 mM CaCl2 solution for 30 min and rinsed with distilled water to clear the 15 N adsorbed onto the root surface (Wang et al. 2016). Shoots and roots were dried at 65 °C for 48 h and weighed to determine the dry mass of aboveground and belowground plant biomass and ground to a fine powder using a ball mill (MM2, Fa. Retsch, Haan, Germany). Approximately 2 mg of plant material was weighed into tin capsules to analyze the N content and 15 N atom% using a PDZ Europa ANCA-GSL elemental analyzer interfaced with a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK).
Soil samples were quickly transferred to the laboratory, sieved through a 2 mm mesh, and stored at 4 °C (Wang et al. 2016) until analysis. Chloroform fumigation-extraction procedure was used to measure N content and 15 N atom% of the microbial biomass (Brooks and Schmidt 1998; Liu et al. 2016). Approximately 10 g of soil was fumigated with alcohol-free chloroform for 24 h and extracted with 40 ml 0.05 M K2SO4 solution (Ouyang et al. 2016). Another 10 g of soil was extracted without fumigation, and the extracts were quickly freeze-dried for subsequent analysis of the N content and 15 N atom% using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK). The concentrations of NH4+ and NO3− in native soils were measured by an autoanalyzer (AA3, Bran-Luebbe, Germany), and glycine-N was measured by high-performance liquid chromatography (Waters 515, Waters Inc., USA).
Calculation and statistics
15 N atom% excess (APE) was calculated as the excess compared with the atom% of the control treatment (Fry 2006):
where atom%plant−labeled indicates the atom percent in the 15 N-labeled plant and atom%plant−control indicates the atom percent in the same control plant.
15 N uptake by plants (μg·N m−2) was calculated by multiplying the N content (μmol N g−1 dry mass), APE, relative molecular mass of 15 N (g mol−1), and total plant biomass (g m−2), according to the following equation (McKane et al. 2002):
where UP-labeled indicates the total amount of 15 N uptake by plant (μg·N m−2), Ncontent indicates the plant N content (μmol g−1 dry mass), APE indicates the atom percent excess (%), 15 indicates the atomic mass of 15 N, and B indicates the total plant biomass (aboveground + belowground biomass) (g m−2) collected from each subplot (10 cm × 10 cm) at 0–15 cm soil depth.
15 N uptake by microorganisms was calculated as the difference between the 15 N recovered from fumigated and non-fumigated soil samples according to the following equation (Zogg et al. 2000):
where UM-labeled indicates the total amount of 15 N uptake by microorganisms and Rfum and Rnon-fum indicate the total amounts of 15 N recovered from fumigated and non-fumigated soil extracts, respectively.
Nitrogen uptake by plants or microorganisms was calculated by 15 N and corrected with the soil-native N content according to the following equation (McKane et al. 2002):
where Uunlabeled indicates the total amount of N uptake by plants or microorganisms, Ulabeled indicates the total amount of 15 N uptake by plants or microorganisms, mlabeled indicates the total amount of 15 N added to soil in each subplot, and munlabeled indicates the soil-native N concentration from the controlled plot.
15 N recovery by plants (Rplant, %) or microorganisms (RMB, %) was calculated by the following equation (Zogg et al. 2000; Clemmensen et al. 2008):
where Ulabeled indicates the amount of 15 N uptake by plants or microorganisms and 15Nadded indicates the amount of 15 N added to the soil per square meter. 15 N recovery for the total three forms of 15 N is described as the total 15 N recovery. The competition for 15 N between plants and microorganisms (RMB/Rplant) is presented as the ratio of 15 N recovery in microorganisms (RMB) to 15 N recovery in plants (Rplant).
The total content of three N forms (NH4+, NO3−, and glycine) in soil was calculated to indicate the soil N availability under different precipitation frequencies.
The study was designed as a three-factorial experiment: (1) 4 levels of precipitation, (2) three 15 N forms + unlabeled reference, and (3) two soil depths. The effects of the precipitation pattern, N form, soil depth, and their interactions on N uptake by plants and microorganisms, RMB, Rplant, and RMB/Rplant were tested using mixed-effect model analysis in the SPSS 20.0 software package (SPSS Inc., Chicago, IL, USA). The mixed-effect model analyses were performed with fixed effect of precipitation frequency (P), N form (N), soil depth (D), and their interactions (P × N, P × D, N × D, P × N × D), and random effect of plot. All the differences were tested at p < 0.05. The standard errors of means are shown in the figures and tables.
Results
Biomass and root-to-shoot ratio of S. grandis
A total of 105 mm of extra rainfall was added in the three experimental treatments compared to the ambient precipitation (32 mm). After rainfall addition, soil water content increased under medium and high precipitation frequencies at 0–5 cm soil depth and increased only in the high frequency at 5–15 cm (p < 0.05, Table 1). The water content under high frequency was 8.63% and 9.60% at 0–5 cm and 5–15 cm soil depths, which were 2.7- and 1.9-fold of those under the ambient precipitation, respectively. Compared to the ambient precipitation, the aboveground biomass of S. grandis decreased under low precipitation frequency and increased under high precipitation frequency (p < 0.05, Fig. 1). The root-to-shoot ratio of S. grandis under high precipitation frequency (0.32 ± 0.02) was lower than that in the other treatments.
Aboveground and belowground biomass (0–15 cm soil depth) of S. grandis in the temperate steppe of North China. The values on the zero line are root-to-shoot ratios. Precipitation treatments include ambient (no additional water), low (low precipitation frequency, single rainfall event per month), medium (medium precipitation frequency, 3 rainfall events per month), and high (high precipitation frequency, 6 rainfall events per month). Uppercase letters indicate significant differences (p < 0.05) between precipitation treatments. Bars show means ± SE (n = 32)
Nitrogen uptake by plants and microorganisms
The total N uptake of all three N forms (glycine, NH4+, and NO3−) by S. grandis was the lowest under low frequency, intermediate under medium frequency, and highest under high frequency of extreme precipitation at 0–5 cm soil depth (p < 0.05) but was independent of extreme precipitation at 5–15 cm soil depth (Fig. 2a). Plant N uptake increased under high frequency of extreme precipitation for all three N forms at 0–5 cm soil depth and for NH4+ at 5–15 cm soil depth (p < 0.05, Fig. S3a, b). NH4+ uptake increased the most among three N forms.
N uptake by S. grandis and microorganisms at two soil depths under different precipitation treatments in the temperate steppe of North China. Precipitation treatments include ambient (no additional water), low (low precipitation frequency, single rainfall event per month), medium (medium precipitation frequency, 3 rainfall events per month), and high (high precipitation frequency, 6 rainfall events per month). Soil depths included 0–5 cm and 5–15 cm. Uppercase letters indicate significant differences (p < 0.05) between precipitation treatments at the same soil depth, and lowercase letters indicate significant differences (p < 0.05) between soil depths under the same precipitation frequency. Bars show means ± SE (n = 4)
An increase in N uptake by microorganisms occurred under high precipitation frequency compared with other precipitation treatments at 0–5 cm soil depth (p < 0.05, Fig. 2b). Microbial N uptake of NO3− and NH4+ increased with high precipitation frequency at 0–5 cm soil depth and was increased to 4.9-fold and 5.7-fold of those with ambient precipitation, respectively (Fig. S3c). At 5–15 cm soil depth, microbial uptake of NH4+ and NO3− was constant between precipitations, whereas glycine uptake decreased under low and high precipitation frequency compared with that under ambient precipitation (Fig. S3d). Among all three N forms, glycine uptake was consistently the lowest both by plants and microorganisms (Fig. S3).
Nitrogen uptake in the temperate steppe suggested niche differentiation for N forms between plants and microorganisms. Plants and microorganisms most take up mineral N (NH4+ and NO3−) rather than organic N (glycine) (Fig. 3 and Fig. S3). Under ambient precipitation, plants and microbial preference for N form were similar at 0–5 cm soil depth, but plant favored more NO3− and microorganisms favored more NH4+ at 5–15 cm soil depth (Fig. 3). Under the extreme precipitation treatments, plants favored NO3−, but with increasing precipitation frequency, plants increased NH4+ uptake and showed a similar preference for NO3− and NH4+ under high precipitation frequency (Fig. 3). Microorganisms favored NH4+ versus NO3−, although this preference weakened under high precipitation frequency (Fig. 3). The preferences of plants and microorganisms for three N forms intensely differentiated under low and medium frequency but converged under high precipitation frequency. Plants and microorganisms overlapped the niches for N forms under high precipitation frequency independent of soil depth (Fig. 3).
Shifts in plants and microbial niche differentiation for the uptake of three N forms (glycine, NO3−, and NH4+) at two soil depths in the temperate steppe. The triangles present contribution of glycine, ammonium, and nitrate to total N uptake (%) by plants and microorganisms (total sum of 3 N forms in microorganisms and plants = 100%). The area enclosed by a blue solid line represents N chemical niche of microorganisms, and the green shadowed area represents that of plants. The area overlapping between the black solid triangle and the shadowed area represents the relative intensity of niche overlapping between microorganisms and plants for N—presented as the red value inside. Bold red values (%) show the percentage of niche overlapping. The larger the value, the lower portion of N competed for, because of the increased pool size of available N for plants and microorganisms at the high precipitation frequency. Note a clear movement of the green triangle in the direction of NH4+ uptake with the high frequency at 0–5 cm soil depth, and with the medium and high frequency of extreme precipitation at 5–15 cm soil depth, as the increasing water availability. Note that the microorganisms took 11–174 times more 15 N compared to plants (see text and Fig. 4)
15 N recovery in plants and microorganisms
15 N recovery in plants was greatly affected by extreme precipitation frequencies (p = 0.002, Table S2). As precipitation frequency increased, 15 N recovery in plants for three N forms changed, especially for mineral N forms (Fig. S4a, b). Compared with ambient precipitation, 15 N recovery under high precipitation frequency increased in all three 15 N forms except for 15 N-glycine recovery at 5–15 cm soil depth. In microorganisms, 15 N recovery was independent of precipitation (p = 0.303, Table S2, Fig. S4b). Microbial 15 N recovery in 15 N-glycine was higher than other two 15 N forms under ambient precipitation (p < 0.05, Fig. S4c, d), while microbial 15 N recovery in three N forms was constant under extreme precipitation treatments, except for it under medium precipitation frequency at 5–15 cm soil depth.
Ratios of 15 N recovery in microorganisms-to-plants (RMB/Rplant) among three N forms ranged from 11.27 to 174.25 at 0–5 cm and ranged from 21.39 to 123.02 at 5–15 cm (Fig. 4), indicating that microorganisms strongly outcompeted plants for N over very short time. RMB/Rplant greatly affected by precipitation frequency (p = 0.007), gradually declined with increasing precipitation frequency, did not differ between soil depths (p = 0.378), and differed for each 15 N form (p < 0.001, Table S2). RMB/Rplant were higher in 15NH4+ and 15 N-glycine than in 15NO3− (Fig. 4).
Ratio of 15 N recovery in microorganisms (RMB) to plants (Rplant) at two soil depths under different precipitation treatments. Precipitation treatments include ambient (no additional water), low (low precipitation frequency, single rainfall event per month), medium (medium precipitation frequency, 3 rainfall events per month), and high (high precipitation frequency, 6 rainfall events per month). Soil depths included 0–5 cm and 5–15 cm. Uppercase letters indicate significant differences (p < 0.05) between precipitation treatments in the same N form, and lowercase letters indicate significant differences (p < 0.05) between N forms under the same precipitation frequency. Bars show means ± SE (n = 4)
Discussion
Biomass of S. grandis and plant-microorganism N uptake
N uptake by plants and microorganisms increased under high precipitation frequency (Fig. 2), relating to the improved water and N availability in soil which control the N acquisition strategies of plants and microorganisms (Heisler-White et al. 2009; Lü and Han 2010). In the temperate steppe, low water availability prevented N from being available for both plants and microorganisms (Harpole et al. 2007; Ma et al. 2018). A high frequency of precipitation offers constant water recharge for soil, effectively alleviating water stress in the temperate steppe (Table 1). Increased soil water availability stimulates microbial activity, results in more rapid microbial biomass turnover and N mineralization, and enhances N availability in soil (Fierer and Schimel 2002; Månsson et al. 2014). Additionally, soil water stimulates rhizosphere processes, such as fine root and mycorrhizal growth, increases the uptake surface of roots, and promotes nutrient acquisition (Green et al. 2005). N and C cycle interacts strongly, and increased N uptake improves plant growth in most N-limited ecosystems (Lebauer and Treseder 2008). The precipitation frequency substantially changes dry–wet cycles of soil, influences nutrient pools and turnover, and leads to biomass decrease or increase (Knapp et al. 2008). In our study, aboveground biomass decreased under low frequency, remained stable under medium frequency, and increased under high frequency, compared with that under ambient precipitation (p < 0.05, Fig. 1). We argue that the (indirect) N effect on plant growth was driven initially by an increase in soil water availability that accelerated N cycling under high precipitation frequency. In contrast, low precipitation frequency is the extreme condition with the largest amplitude of dry-rewetting that often results in water and nutrient losses (Knapp et al. 2008). Moreover, such striking water pulse may have inhibited plant and microbial activity or damage plant roots for their normal metabolism (Vicente-Serrano et al. 2013). Overall, frequency altered the effects of extreme precipitation on the ecosystem because a low to high frequency converted the feeble or negative impacts of extreme precipitation to a relatively unstressed and stable state to activate plant and microbial N uptake and plant growth. This fully supports our first hypothesis.
Chemical niches and ratios of 15 N recovery in microorganisms to plants
Diverse N forms in soil can be taken up by plants and microorganisms (Harrison et al. 2008; Xu et al. 2011b) and differ with altered precipitation. Many studies have indicated that both plants and microorganisms prefer to absorb NH4+ over NO3− as for assimilation of NH4+ is more energetically efficient, so NO3− has been reduced in roots or shoots (Gavrichkova and Kuzyakov 2008; Xu et al. 2011b). Nevertheless, owing to the higher mobility of NO3− and slower NH4+ delivery to the root surface, especially at low soil moisture content (Wang and Macko 2011; Liu et al. 2016), there may be no sufficient NH4+ for plants and microorganisms to take up in arid soil. Under such condition, microorganisms preferentially utilize absorbable N form like NH4+ owing to their higher efficiency in N acquisition than plants (Schimel et al. 1989; Xu et al. 2011b), and thus, plants have to absorb more NO3− for growth. Such differentiation of N sources between plants and microorganisms was confirmed under arid conditions at 5–15 cm soil depth because plants acquired more NO3− and microorganisms acquired more NH4+ under ambient precipitation (Fig. 3). When the extreme precipitation occurred, plants and microorganisms differentiated intensely their acquisition of N forms under low precipitation frequency, while under high precipitation frequency, plants absorbed more NH4+ and showed a similar preference for NO3− or NH4+ to that of microorganisms and resulted in chemical niches overlap for plants and microorganisms (Fig. 3). Nitrogen loss under low frequency and relatively low water and N fluctuations under medium frequency caused plant and microorganisms still severely compete for N, and thus, they remained original N acquisition strategy (distinct preference for N form) and reduced competition by chemical niche differentiation. High precipitation frequency supplied constant water and (1) increased available N pool in soil decreasing the proportion of each N form which plants and microorganisms competed for but (2) promoted plant N uptake capacity to compete for their preferential N form. Our results were consistent with the previous studies demonstrating that plants switched N sources from NO3− to NH4+ along an environmental gradient from drier to wetter (Houlton et al. 2007; Wang and Macko 2011; Månsson et al. 2014). Supporting our second hypothesis, high precipitation frequency moderated the striking influence of extreme precipitation, increased N availability that could satisfy both plants and microorganisms for taking up N according to their preference, and lead to the same chemical niche.
Besides inorganic N, it has been well demonstrated over past years that organic N in terms of amino acid can be absorbed by plants and is an important N source for plants and microorganisms in N-limited grassland (Ouyang et al. 2016; Wang et al. 2016; Tian et al. 2020). In our study, plant and microbial glycine uptake was extremely lower than inorganic N (Fig. 3 and Fig. S3), though relatively high 15 N-glycine was recovered by plant and microorganisms (Fig. S4). This indicates that plants and microorganisms in temperate steppe have high potential but low actual organic N uptake due to the low concentration of glycine in the soil (Kahmen et al. 2009; Liu et al. 2016) and rapid organic N decomposition by microorganisms (Ma et al. 2021). And the glycine content was unresponsive to the extreme precipitation (Table 1). Therefore, our study suggests that organic N contributes little to N uptake of S. grandis and microorganisms in Inner Mongolia temperate steppe.
15 N recovery was calculated to reflect partitioning of added 15 N between plants and microbial biomass, without considering the role of native N pool. In general, 15 N recovery in plants increased as the precipitation frequency increase, whereas microbial 15 N recovery differed little (Fig. S4), and RMB/Rplant reduced under high precipitation frequency (Fig. 5). In arid ecosystem, low soil moisture limits plant N uptake. But when the soil moisture increased with the precipitation frequency (Table 1), plant roots and mycorrhizas could actively absorb more N (Green et al. 2005). Unlike the plant, microbial N uptake might be less limited under ambient precipitation due to the large surface-volume ratio and fast growth rates of microorganisms, which is conducive to N acquisition in short-time labeling (Schwinning and Sala 2004; Wilkinson et al. 2014). Lower soil moisture is needed for microorganisms to acquire enough N to grow than plants (Schwinning and Sala 2004; Collins et al. 2008). It is likely that the initial soil moisture of this area have already fully activated microbial 15 N uptake; hence, the additional precipitation did not increase total 15 N recovery in microorganisms. The increased N availability under high precipitation frequency reduced RMB/Rplant, confirms high precipitation frequency influencing the relationship between plants and microorganisms, and greatly increased the plant competitive capacity (Fig. 5). Overall, extreme precipitation with a high frequency alters N absorption by plants and microorganisms and shifts their preferences to the same N form in the temperate steppe (Fig. 6).
Ratios of total 15 N recovery in microorganism (RMB) to plant (Rplant), N availability (NO3− + NH4+ + glycine-N), and water content (bottom blue arrow) at 0–15 cm soil depth under extreme precipitation treatments. The inset plot shows the linear relationship between N availability and RMB/Rplant. Precipitation treatments include ambient (no additional water), low (low precipitation frequency, single rainfall event per month), medium (medium precipitation frequency, 3 rainfall events per month), and high (high precipitation frequency, 6 rainfall events per month). Uppercase letters indicate significant differences (p < 0.05) in RMB/Rplant between precipitation treatments, and lowercase letters indicate significant differences (p < 0.05) in N availability of soil between precipitation treatments. Bars show means ± SE (n = 4)
Conclusions
Aboveground plant biomass and N acquisition by plants and microorganisms were affected by the precipitation frequency. A low to a high frequency of extreme precipitation changed the conditions from extreme to optimal for plant growth and N acquisition, linking to the increase of soil N availability resulting in more stable soil moisture. The extreme precipitation with low frequency reduced S. grandis growth, whereas the extreme precipitation with high frequency raised the growth and N acquisition of S. grandis and promoted microbial N uptake. In the temperate steppe, plants mainly absorb NO3−, and microorganisms mainly take up NH4+. However, due to the increase in water and N availability under high precipitation frequency, the proportion of each N form which plants and microorganisms compete for is reduced, and plants took up more NH4+ compared with the ambient conditions. Plants and microorganisms absorbed N forms according to their preferences and converge their chemical niche under high precipitation frequency. Mineral N is the primary source of plant and microbial N uptake, and glycine contributes little due to the small concentration in the soil. A high precipitation frequency increased the ratio between 15 N incorporated in plants and microorganisms. On the whole, extreme precipitation shifted the extreme scenario from a very long dry–wet cycling to a relatively mildest stable conditions for plant growth in the temperate steppe and increased plant biomass by altering N acquisition of plants and microorganisms. This study clarifies the effects of extreme precipitation on the N acquisition by plants and microorganisms through a single sampling. To better understand the effects of extreme precipitation on temperate grasslands, long-term spatio-temporal N acquisition by plants and microorganisms combined with changes in productivity and species diversity should be investigated under a variety of extreme precipitation patterns.
References
Ashton IW, Miller AE, Bowman WD, Suding KN (2010) Niche complementarity due to plasticity in resource use: plant partitioning of chemical N forms. Ecology 91:3252–3260
Blagodatsky SA, Yevdokimov IV (1998) Extractability of microbial N as influenced by C: N ratio in the flush after drying or fumigation. Biol Fertil Soils 28:5–11
Blagodatsky SA, YeV B, Gorbenko AYu, Panikov NS (1987) A rehydration method of determining the biomass of microorganisms in soil. Sov Soil Sci 19:119–126
Borken W, Matzner E (2009) Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Global Change Biol 15:808–824
Brooks PD, Schmidt SK (1998) Inorganic nitrogen and microbial biomass dynamics before and during spring snowmelt. Biogeochemistry 43:1–15
Chen H, Sun J, Chen X, Zhou W (2012) CGCM projections of heavy rainfall events in China. Int J Climatol 32:441–450
Chen Y, Xu Z, Hu H, Hu Y, Hao Z, Jiang Y, Chen B (2013) Response of ammonia-oxidizing bacteria and archaea to nitrogen fertilization and precipitation increment in a typical temperate steppe in Inner Mongolia. Appl Soil Ecol 68:36–45
Christensen L, Coughenour MB, Ellis JE, Chen ZZ (2004) Vulnerability of the Asian typical steppe to grazing and climate change. Clim Change 63:351–368
Clemmensen KE, Sorensen PL, Michelsen A, Jonasson S, Ström L (2008) Site-dependent N uptake from N-form mixtures by arctic plants, soil microbes and ectomycorrhizal fungi. Oecologia 155:771
Collins SL, Sinsabaugh RL, Grenshaw C, Green L, Porras-Alfaro A, Stursova M, Zeglin LH (2008) Pulse dynamics and microbial processes in aridland ecosystems. J Ecol 96:413–420
Cregger MA, McDowell NG, Pangle RE, Pockman WT, Classen AT (2014) The impact of precipitation change on nitrogen cycling in a semi-arid ecosystem. Funct Ecol 28:1534–1544
Donat MG, Lowry AL, Alexander LV, O’gorman PA, Maher N (2016) More extreme precipitation in the world’s dry and wet regions. Nat Clim Change 6:508–513
Easterling DR, Mearns LO (2000) Climate extremes: observations, modeling, and impacts. Science 289:2068–2074
Farley RA, Fitter AH (1999) Temporal and spatial variation in soil resources in a deciduous woodland. J Ecol 87:688–696
Fierer N, Schimel JP (2002) Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34:777–787
Fry B (2006) Stable Isotope Ecology. Springer, New York
Fu G, Yu J, Yu X, Ouyang R, Zhang Y, Wang P, Liu W, Min L (2013) Temporal variation of extreme rainfall events in China, 1961–2009. J Hydrol 487:48–59
Gavrichkova O, Kuzyakov Y (2008) Ammonium versus nitrate nutrition of Zea mays and Lupinus albus: effect on root-derived CO2 efflux. Soil Biol Biochem 40:2835–2842
Grant PR (2017) Evolution, climate change, and extreme events. Science 357:451–452
Green JJ, Dawson LA, Proctor J, Duff EI, Elston DA (2005) Fine root dynamics in a tropical rain forest is influenced by rainfall. Plant Soil 276:23–32
Grundmann GL, Renault P, Rosso L, Bardin R (1995) Differential effects of soil water content and temperature on nitrification and aeration. Soil Biol Biochem 59:1342–1349
Harpole WS, Potts DL, Suding KN (2007) Ecosystem responses to water and nitrogen amendment in a California grassland. Global Change Biol 13:2341–2348
Harrison KA, Bol R, Bardgett RD (2008) Do plant species with different growth strategies vary in their ability to compete with soil microbes for chemical forms of nitrogen. Soil Biol Biochem 40:228–237
Heisler-White JL, Blair JM, Kelly EF, Harmoney K, Knapp AK (2009) Contingent productivity responses to more extreme rainfall regimes across a grassland biome. Global Change Biol 15:2894–2904
Hess LJT, Hinckley ES, Robertson GP, Matson PA (2020) Rainfall intensification increases nitrate leaching from tilled but not no-till cropping systems in the U.S. Midwest. Agr Ecosyst Environ 290:106747
Hong J, Qin X, Ma X, Xu X, Wang X (2019) Seasonal shifting in the absorption pattern of alpine species for NO3– and NH4+ on the Tibetan Plateau. Biol Fertil Soils 55:801–811
Houlton BZ, Sigman DM, Schuur EA, Hedin LO (2007) A climate-driven switch in plant nitrogen acquisition within tropical forest communities. P Natl Acad Sci USA 104:8902–8906
IPCC (2013) Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Contribution of Working Group 1 to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York
Joslin JD, Wolfe MH (1998) Impacts of water input manipulations on fine root production and mortality in a mature hardwood forest. Plant Soil 204:165–174
Kahmen A, Livesley SJ, Arndt SK (2009) High potential, but low actual, glycine uptake of dominant plant species in three Australian land-use types with intermediate N availability. Plant Soil 325:109–121
Kang M, Dai C, Ji W, Jiang Y, Yuan Z, Chen HYH (2013) Biomass and its allocation in relation to temperature, precipitation, and soil nutrients in Inner Mongolia grasslands. China. Plos One 8:e69561
Kieft TL, Soroker E, Firestone MK (1987) Microbial biomass response to a rapid increase in water potential when dry soil is wetted. Soil Biol Biochem 19:119–126
Knapp AK, Beier C, Briske DD, Classen AT, Luo Y, Reichstein M, Smith MD, Smith SD, Bell JE, Fay PA, Heisler JL, Leavitt SW, Sherry R, Smith B, Weng E (2008) Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 58:811–821
Knapp AK, Avolio ML, Beier C, Carroll CJW, Collins SL, Dukes JS, Fraser LH, Griffin-Nolan RJ, Hoover DL, Jentsch A, Loik ME, Phillips RP, Post AK, Sala OE, Slette IJ, Yahdjian L, Smith MD (2016) Pushing precipitation to the extremes in distributed experiments: recommendations for simulating wet and dry years. Global Change Biol 23:1774–1782
Kong D, Lü X, Jiang L, Wu HF, Miao Y, Kardol P (2013) Extreme rainfall events can alter inter-annual biomass responses to water and N enrichment. Biogeosciences 10:8129–8138
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656
Lebauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Li L, Zheng Z, Biederman JA, Xu C, Xu Z, Che R, Wang Y, Cui X, Hao Y (2019) Ecological responses to heavy rainfall depend on seasonal timing and multi-year recurrence. New Phytol 223:647–660
Liu Q, Qiao N, Xu X, Xin X, Han JY, Tian Y, Ouyang H, Kuzyakov Y (2016) Nitrogen acquisition by plants and microorganisms in a temperate grassland. Sci Rep 6:22642
Liu T, Wang L, Feng XJ, Zhang JB, Ma T, Wang X, Liu ZG (2018) Comparing soil carbon loss through respiration and leaching under extreme precipitation events in arid and semiarid grasslands. Biogeosciences 15:1627–1641
Liu M, Xu X, Nannipieri P, Kuzyakov Y, Gunina A (2021) Diurnal dynamics can modify plant-microbial competition for N uptake via C allocation. Biol Fertil Soils 57:949–958
Lü X, Han X (2010) Nutrient resorption responses to water and nitrogen amendment in semi-arid grassland of Inner Mongolia, China. Plant Soil 327:481–491
Ma L, Liu G, Xu X, Xin X, Bai W, Zhang L, Chen S, Wang R (2018) Nitrogen acquisition strategies during the winter-spring transitional period are divergent at the species level yet convergent at the ecosystem level in temperate grassland. Soil Biol Biochem 122:150–159
Ma Q, Hill PW, Chadwick DR, Wu L, Jones DL (2021) Competition for S-containing amino acids between rhizosphere microorganisms and plant roots: the role of cysteine in plant S acquisition. Biol Fertil Soils 57:825–836
Månsson KF, Olsson MO, Falkengrengrerup U, Bengtsson G (2014) Soil moisture variations affect short-term plant-microbial competition for ammonium, glycine, and glutamate. Ecol Evol 4:1061–1072
McCulley RL, Burke IC, Lauenroth WK (2009) Conservation of nitrogen increases with precipitation across a major grassland gradient in the Central Great Plains of North America. Oecologia 159:571–581
Mckane RB, Johnson LC, Shaver GR et al (2002) Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415:68–71
Moran-Zuloaga D, Dippold MA, Glaser B, Kuzyakov Y (2015) Organic nitrogen uptake by plants: reevaluation by position-specific labeling of amino acids. Biogeochemistry 125:359–374
Navarro-Garcia F, Casermeiro MA, Schimel JP (2012) When structure means conservation: effect of aggregate structure in controlling microbial responses to rewetting events. Soil Biol Biochem 44:1–8
Ouyang S, Tian Y, Liu Q, Zhang L, Wang R, Xu X (2016) Nitrogen competition between three dominant plant species and microbes in a temperate grassland. Plant Soil 408:121–132
Pabst H, Kühnel A, Kuzyakov Y (2013) Effect of land-use and elevation on microbial biomass and water extractable carbon in soils of Mt. Kilimanjaro Ecosystems. Appl Soil Ecol 67:10–19
Peng Y, Li C, Fritschi FB (2014) Diurnal dynamics of maize leaf photosynthesis and carbohydrate concentrations in response to differential N availability. Environ Exp Bot 99:18–27
Raghavendra A, Dai A, Milrad S, Cloutier-Bisbee SR (2018) Floridian Heatwaves and extreme precipitation: future climate projections. Clim Dynam 52:495–508
Rusjan S, Mikoš M (2010) Seasonal variability of diurnal in-stream nitrate concentration oscillations under hydrologically stable conditions. Biogeochemistry 97:123–140
Schimel JP, Jackson LE, Firestone MK (1989) Spatial and temporal effects on plant-microbial competition for inorganic nitrogen in a California annual grassland. Soil Biol Biochem 21:1059–1066
Schwinning S, Sala OE (2004) Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia 141:211–220
Tian Y, Yu M, Fan Z, Ouyang S, Xu X, Gao Q, Li X (2020) Uptake of amino acids and inorganic nitrogen by two dominant temperate grasses. Rhizosphere 14:100199
Vicente-Serrano SM, Gouveia C, Camarero JJ, Begueria S (2013) Response of vegetation to drought time-scales across global land biomes. P Natl Sci USA 110:52–57
Wang L, Macko SA (2011) Constrained preferences in nitrogen uptake across plant species and environments. Plant Cell Environ 34:525–534
Wang C, Wan S, Xing X, Zhang L, Han X (2006) Temperature and soil moisture interactively affected soil net N mineralization in temperate grassland in Northern China. Soil Biol Biochem 38:1101–1110
Wang R, Tian Y, Ouyang S, Xu X, Xu F, Zhang Y (2016) Nitrogen acquisition strategies used by Leymus chinensis and Stipa grandis in temperate steppes. Biol Fertil Soils 52:1–11
Wang G, Wang D, Trenberth KE, Erfanian A, Yu M, Bosilovich MG, Parr DT (2017) The peak structure and future changes of the relationships between extreme precipitation and temperature. Nat Clim Change 7:268–274
Wilcox KR, Shi Z, Gherardi LA, Lemoine NP, Koerner SE, Hoover DL, Bork E, Byrne KM Jr, Collins SL, Evans S, Gilgen AK, Holub P, Jiang L, Knapp AK, LeCain D, Liang J, Garcia-Palacios P, Peñuelas J, Pockman WT, Smith MD, Sun S, White SR, Yahdjian L, Zhu K, Luo Y (2017) Asymmetric responses of primary productivity to precipitation extremes: a synthesis of grassland precipitation manipulation experiments. Global Change Biol 23:4376–4385
Wilkinson A, Hill PW, Farrar JF, Jones DL, Bardgett RD (2014) Rapid microbial uptake and mineralization of amino acids and peptides along a grassland productivity gradient. Soil Biol Biochem 72:75–83
World Reference Base for Soil Resources (2014) International soil classification system for naming soils and creating legends for soil maps. Food and Agriculture Organization of The United Nations, Rome
Wu Z, Dijkstra P, Koch GW, PeÑuelas J, Hungate BA (2011) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Global Change Biol 17:927–942
Xu X, Ouyang H, Cao G, Richter A, Wanek W (2011a) Dominant plant species shift their nitrogen uptake patterns in response to nutrient enrichment caused by a fungal fairy in an alpine meadow. Plant Soil 341:495–504
Xu X, Ouyang H, Richter A, Wanek W, Cao G, Kuzyakov Y (2011b) Spatio-temporal variations determine plant and microbe competition for inorganic nitrogen in an alpine meadow. J Ecol 99:563–571
Yahdjian L, Sala OE (2010) Size of precipitation pulses controls nitrogen transformation and losses in an arid patagonian ecosystem. Ecosystems 13:575–585
Zhai P, Zhang X, Wan H, Pan X (2005) Trends in total precipitation and frequency of daily precipitation extremes over China. J Climate 18:1096–1108
Zhang S, Zheng Q, Noll L, Hu Y, Wanek W (2019) Environmental effects on soil microbial nitrogen use efficiency are controlled by allocation of organic nitrogen to microbial growth and regulate gross N mineralization. Global Change Biol 135:304–315
Zheng W, Wang S, Tan K, Lei Y (2020) Nitrate accumulation and leaching potential is controlled by land-use and extreme precipitation in a headwater catchment in the North China Plain. Sci Total Environ 707:136168
Zogg GP, Zak DR, Pregitzer KS, Burton AJ (2000) Microbial immobilization and the retention of anthropogenic nitrate in a northern hardwood forest. Ecology 81:1858–1866
Acknowledgements
The authors thank Ruixue Wang for her assistance in the fieldwork.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Nos. 41771325; 41877089), the Second Tibetan Plateau Scientific Expedition and Research Program (Grant No. 2019QZKK0405), the National Key Research and Development Program of China (Grant Nos. 2016YFC0500502; 2017YFA0604802), the Russian Government Program of Competitive Growth of Kazan Federal University and with the support of the RUDN University Strategic Academic Leadership Program.
Author information
Authors and Affiliations
Contributions
1. Yuqiang Tian and Xingliang Xu planned and designed the research.
2. Shuhai Wen and Shengnan Ouyang performed experiments and conducted fieldwork.
3. Shuhai Wen, Yuqiang Tian, Xingliang Xu, and Yakov Kuzyakov conducted data analysis and interpretation and wrote and revised the manuscript.
4. Shengnan Ouyang, Minghua Song, Xiaobing Li, Yong Zhang, and Si Gao revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wen, S., Tian, Y., Ouyang, S. et al. High frequency of extreme precipitation increases Stipa grandis biomass by altering plant and microbial nitrogen acquisition. Biol Fertil Soils 58, 63–75 (2022). https://doi.org/10.1007/s00374-021-01608-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-021-01608-7