Abstract
Niche partitioning by time, space and chemical forms has been suggested as an important mechanism to maintain species coexistence. Climate warming is assumed to increase soil nutrient availability through enhancing mineralization of soil organic matter in a variety of terrestrial ecosystems. However, few studies have yet examined how dominant plant species contribute to species coexistence when nutrient enrichment occurs in native ecosystems. We studied a single fairy ring (5 m diameter) in a Kobresia meadow in the Tibetan Plateau. This kind of rings is caused by a basidiomycete fungus Agaricus campestris, and is evidenced by dark-green vegetation boundaries. Nutrient enrichment occurs due to enhanced decomposition of soil organic matter (SOM) in the fungus growth zone of these rings. We conducted a short-term 15N labelling experiment and found that dominant plant species shifted their N uptake patterns and preferred N form (NO −3 , NH +4 , and amino acid N) in response to nutrient enrichment in an N-limited alpine meadow. The legume Gueldenstaedtia diversifolia had the lowest aboveground biomass among the five plant species studied at low available N level, although it mainly utilized ammonium (the most abundant N form). The two graminoids (Elymus nutans and Stipa aliena) demonstrated similar aboveground biomass at low and high available N levels, showing a similar pattern switching from NH +4 /NO −3 uptake outside the ring to glycine uptake in the annulus zone of the ring. The biomass of the forb Gentiana straminea differed significantly at low and high available N levels, but its N uptake pattern almost remained unchanged. Species therefore differed in their response to nutrient enrichment, most species showing chemical niche shifts instead of niche conservatism. This finding has important implications with regard to understanding the mechanisms responsible for species coexistence when natural nutrient enrichment is induced by climate warming in terrestrial ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The control of how plant diversity is maintained is a central topic in ecology, mainly focusing on species coexistence in ecosystems (Chesson 2000; Silvertown 2004). Several mechanisms have been invoked to explain plant species coexistence in N-limited ecosystems, including variation in resource requirements of individual species and partitioning of N acquisition in time, space and chemical forms (Schoener 1974; Tilman 1982; Chesson 2000; McKane et al. 2002; Miller and Bowman 2002; Silvertown 2004). Recent fertilization experiments showed a tendency for species loss with increasing soil nutrient availability (Gough et al. 2000; Stevens et al. 2004; Harpole and Tilman 2007; Clark and Tilman 2008; Manning et al. 2008). Experiments showed that N enrichment can modify plant community composition through altering plant-soil feedbacks (Manning et al. 2008). Moreover, numerous field and laboratory experiments showed that soil nutrient availability can be enhanced by soil warming through stimulating decomposition of SOM (Lükewille and Wright 1997; Rustad et al. 2001; Sardans et al. 2006), and thereby may result in species losses (Klein et al. 2004). A possible mechanistic explanation of grassland species loss caused by nutrient enrichment was put forward by Harpole and Tilman (2007), who suggested that species loss resulted from decreasing their niche dimension. However, few studies have been conducted to examine how dominant plant species coexist when nutrient enrichment occurs in native ecosystems.
The Tibetan Plateau has been regarded as “the third pole of the Earth”, covering over 2.5 million km2 with an average altitude of more than 4000 m above sea level. Approximately 35% of its area is alpine meadows (Zheng 2000). The high OM status of these alpine meadow soils is due to harsh climatic conditions. This leads to the trapping of N in forms unavailable to plants in alpine Kobresia humilis meadows (Song et al. 2007). As a result, plant growth is strongly limited by soil-available N in this type of meadow (Zhou 2001). Several lines of evidence suggest that the Tibetan Plateau is experiencing climatic warming (Thompson et al. 1993; French and Wang 1994) and the Plateau has been predicted to undergo greater than average increases in temperatures in the future (Giorgi et al. 2001). This indicates that climate warming might enhance mineralization of SOM and give rise to nutrient enrichment in alpine meadows. Therefore, an important question arises: how do dominant plant species acclimate to nutrient enrichment and how does this contribute to species coexistence in alpine meadows? It was shown that plant species occupy distinct niches with regard to their relative N uptake (Kahmen et al. 2006). We here advance a hypothesis that dominant plant species shift their N uptake patterns (here referred as chemical niche) in response to nutrient enrichment and contribute to species coexistence in these alpine meadows.
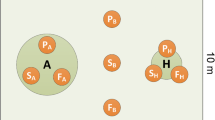
Fairy rings in grasslands are manifestations of basidiomycete activities, and are often observed due to close-cropped and homogeneous vegetation. They are often classified as three types according to whether vegetation is killed at the ring margin, grows more vigorously or is unaffected (Griffith and Roderick 2008). In typical alpine Kobresia meadows, the basidiomycete fungus Agaricus campestris develops fairy rings. The vegetation in the fungus growth zone grows vigorously, which is evidenced by dark-green vegetation boundaries (Fig. 1) and circular fruit body distributions. Basidiomycete fungi grow on dead organic matter (Cooke and Rayner 1984) and return nutrients to the soil, leading to higher N availability in annular areas of rings than outside the rings (Kaiser 1998; Edwards 1988; Gramss et al. 2005; Griffith and Roderick 2008). This provides a unique environment for testing how dominant plant species acclimate to nutrient enrichment in alpine meadows, circumventing manipulations by fertilizer addition. To test the hypothesis above, a short-term 15N experiment was conducted in the fungus growth zone and outside the fairy ring, focusing on chemical niches (i.e. N uptake preferences) of five common dominant grassland species.
The basidiomycete fungus develops a fairy ring as evidenced by a dark-green vegetation boundary a, showing three zones: (a) outside the fairy ring not yet colonized by the fungus, (b) in the annulus zone with fungus growth, and (x) the inner zone where the Agaricus has already grown through. Sampling points within the fairy ring were positioned in the centre of the fungal growth zone b: white rectangles were injected with water as the control, diagonal rectangles with 15N-glycine, dotted rectangles with 15N-NO −3 , and hatched rectangles with 15N-NH +4
Materials and methods
Study site
The experiment was conducted in an alpine meadow at the Haibei Alpine Meadow Ecosystem Station of the Chinese Academy of Sciences, Qinghai Province (37° 36′ 60″ N, 101° 19′ 14″ E, 3215 m asl). 25-year means for temperature and rainfall were −1.7 °C and 600 mm, respectively. Dominant species are Kobresia humilis Serg., Elymus nutans Griseb., Stipa aliena Keng., Poa sp., Festuca ovina Linn., Gentiana aristata Maxim., Gentiana straminea Maxim., Saussurea superba Anth., and Gueldenstaedtia diversifolia Maxim. (Zhou 2001). The soil is classified as Mat Cryo-gelic Cambisol (Chinese Soil Taxonomy Research Group 1995) corresponding to Gelic Cambisol (WRB 1998). There were on average 28 plant species within 25 cm × 25 cm quadrats outside the fairy ring and 23 species in the fungus growth zone. The five selected dominant species accounted for 69% of total aboveground biomass in the outer zone not yet colonized by the fungus and 56% in the annulus zone of the ring. The height of herbaceous plants averaged about 20 cm in the outer zone and 26 cm in the fungus growth zone of the fairy ring.
Experimental layout
Only a single ring (5 m diameter) was investigated in this study. Twenty-four 10 × 20 cm plots were set up in a Kobresia humilis meadow in July, 2007. Twelve plots were positioned in the centre of the 50 cm wide annulus area (Fig. 1b) developed by Agaricus campestris, the other twelve plots were located more than 25 m distant from the fairy ring within the alpine meadow. Five dominant species in the annulus area and outside the fairy ring were selected as target species: one sedge (Kobresia humilis), two graminoids (Elymus nutans and Stipa aliena), and two forbs (Gueldenstaedtia diversifolia and Gentiana straminea).
A mixture of glycine, NH +4 , and NO −3 (1:1:1 glycine-N/NH +4 -N/NO −3 -N) was injected into 5 cm soil depth. Before N was injected, each 10 × 20 cm plot was divided into eight 5 × 5 cm subplots. One milliliter solution was injected at the center of each subplot, yielding 19 μg N g−1 d.w. soil for each 10 × 20 cm plot. The amount and forms of N added in the three treatments were identical, but only one of the three N forms was labeled with 15N in each case (98.2 at% 15N enrichment for NO −3 , 98.4 at% 15N enrichment for NH +4 , and 95.0 at% 15N enrichment for glycine). Three replicate plots received each 15N form treatment. Six untreated plots (three in the fungus growth zone and three in the outer zone of the ring) which were not injected with 15N tracer were supplied with equivalent amounts of water and were taken as controls. When 15N tracers were injected into soils, great attention was given to an even distribution of the 15N labeled solution in the soil.
Because of rapid turnover of amino acids in soil (Jones and Kielland 2002), plants and soil were collected three hours after 15N tracer injection. The plots were completely sampled to 10 cm depth because over 80% of the roots are concentrated within this horizon (Zhou 2001) and immediately transferred to the laboratory. The whole 10 × 20 cm plot was excavated, from which roots were carefully separated so that ‘intact’ plant individuals were collected. The mycelium was invisible to the naked eye when these plots were excavated and broken up. Plants were sorted to species level. They were rinsed shortly after with water, then for 30 min with 0.5 mmol L−1 CaCl2 solution, and again with distilled water to remove 15N absorbed on the surface of plants. Plant material was dried at 60 °C for 48 h, weighed for total dry mass, N content, and 15N/14N ratio measurements. After roots were carefully removed from soil cores, the remaining soils were sieved to through 2 mm and stored at 4 °C until measurements of available N.
Sample analysis
Soil NO −3 -N and NH +4 -N were determined by autoanalyser in 0.5 M K2SO4 extracts. Soil glycine concentrations were measured by high-performance liquid chromatography (Waters 515) on the same extracts (Nasholm et al. 1987).
Dried intact plants including roots and shoots were ground to a fine powder using a ball mill (MM200, Retsch). Aliquots (2 mg) of ground plant material were weighed into tin capsules for analyzing total N, C and atom% 15N by a isotope ratio mass spectrometry (Mat253, Finnigan MAT). Atom% excess 15N (APE) was calculated as the atom% 15N difference between plants from 15N treated and from control plots.
N uptake calculation
Uptake of 15N (mg 15N m−2) of individual plant species was calculated by multiplying N content (mg N g−1 d.w.), APE, and biomass (g m−2). Uptake of available N species corresponding to the 15N treatment was calculated following McKane et al. (2002):
where mlabelled is the total mass (g m−2) of 15N-labelled N injected per plot, munlabelled is the mass of available N species measured in soils. Ulabelled is uptake (g m−2) of 15N from the source mlabelled, and Uunlabelled is uptake of available N from the source munlabelled.
Quantification of mycorrhizal colonization
To test whether arbuscular mycorrhizal fungi (AMF) contribute to shifts in N uptake pattern, mycorrhizal colonization was determined for the five dominant plant species. We collected fresh roots of the observed five plant species close to our 15N treatment plots. The roots after gentle washing were stored in 50% ethanol until measurements of AMF colonization. When measuring mycorrhizal colonization, roots were rinsed with tap water and then cleared in 10% KOH solutions for 45 min at 90 °C. After clearing, roots were boiled (95 °C) for at least 3 min in the staining solution consisting of 5% ink diluted in 5% acetic acid. A gridline-intersection method was used to quantify extent of mycorrhizal colonization (Vierheilig et al. 2005).
Results
N availability was higher in the annular area than outside the fairy ring (Table 1). Soil total N and available N such as ammonium, nitrate, and glycine in the annulus area were significantly higher than outside the fairy ring. Compared with the outer zone of the ring, C/N increased about 11% in the fungus growth zone while soil pH showed no significant difference.
K. humilis and G. straminea showed significantly higher total biomass in the annulus area than outside the ring, whereas no significant difference in total biomass was observed for E. nutans, S. aliena and G. diversifolia (Fig. 2).
Total biomass of dominant plant species both in the annulus zone and outside the fairy ring in an alpine meadow in the Tibetan Plateau. Values are means (±1 SE) of 12 replicates. Asterisks indicate significant differences between in the annulus area and outside the fairy ring at 0.05 error probability level
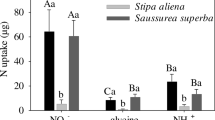
The sedge K. humilis used mainly nitrate (the least abundant N-form in the soil) outside the fairy ring, but in the annulus zone there was a switch to uptake of glycine and ammonium (Table 2, Fig. 3). The legume G. diversifolia utilized the most abundant N form (ammonium) outside the ring but the least abundant N form (nitrate) under high N availability. The graminoids E. nutans and S. aliena both showed a similar pattern switching from NH +4 /NO −3 uptake outside to glycine uptake in the annulus zone (Table 2, Fig. 3). They mainly acquired N from both the most and the least abundant N forms outside the ring while utilizing preferentially the second most abundant N form at higher N availability. Uptake patterns of the forb G. straminea were similar in both areas (Table 2, Fig. 3).
Chemical niche shifts in terms of N acquisition of dominant plant species between in the annulus zone and outside the fairy ring in an alpine meadow in the Tibetan Plateau. The axes of x, y and z represent the contribution of ammonium, nitrate and glycine to total N uptake (as %). Gray areas refer to chemical niches of different dominant plant species outside the fairy ring (low N availability), whereas areas enclosed by dark lines indicate the same species in the fungus growth zone of the fairy ring (enhanced N availability). Values are means (±1SE) of 3 replicates. Asterisks indicate significant difference between in the fungus growth zone and outside the fairy ring at 0.01 (*) and 0.001 (**) error probability level
Outside the fairy ring, inorganic N made a major contribution to total N uptake for all five plant species (Fig. 4a). Compared to outside the ring, the five dominant plant species acquired more amino acid N in the annulus zone of the ring (Fig. 4b). The sedge (K. humilis) and graminoids (E. nutans and S. aliena) showed a significant difference in N uptake between in the annulus zone and outside the ring, whereas the forbs (G. diversifolia and G. straminea) didn’t show significant differences (Table 2).
Contribution of N from glycine or inorganic N (NO −3 +NH +4 ) to total N uptake by dominant plant species in an alpine meadow in the Tibetan Plateau a outside the fairy ring and b in the fungus growth zone of the fairy ring. The two extra plots to the right present concentrations of available soil inorganic and organic N c outside the fairy ring and d in the fungus growth zone of the fairy ring. Asterisks indicate significant differences between inorganic N and glycine uptake at the same site at 0.05 error probability level
The roots of K. humilis and G. diversifolia were not colonized by AMF, while the other three species were colonized by AMF. G. straminea had a very low mycorrhizal colonization, ranging between 5% (in the annulus zone of the fairy ring) and 8% (outside the fairy ring). S. aliena (in vs. outside, 65% vs. 70%) and E. nutans (in vs. outside, 70% vs. 56%) showed higher rates of mycorrhizal colonization.
Discussion
We observed different patterns than those reported by McKane et al. (2002) for an arctic tundra ecosystem, i.e. that the most productive species preferentially utilized the most abundant N form while less productive species used less abundant N forms in an arctic tundra ecosystem. In our study G. diversifolia had the lowest total biomass among the five plant species studied at low available N level (Fig. 2), although it utilized the most abundant N form (ammonium) (Fig. 3). The two graminoids demonstrated similar total biomass at low and high available N levels (Fig. 2), and showed a similar pattern switching from NH +4 /NO −3 uptake outside the ring to glycine uptake in the fungus growth zone of the ring (Fig. 3). In contrast, total biomass of G. straminea differed significantly at low and high available N levels, but its N uptake pattern almost remained unchanged (Fig. 3). Only one out of five dominant plant species showed niche conservatism (G. straminea), indicating that chemical niche shifts or niche conservatism vary in a species-specific manner. Nonetheless, different patterns of N uptake by dominant plant species under different available N levels mean that dominant plant species can switch their chemical niches for different N forms to acclimate to nutrient enrichment in alpine meadows (Fig. 3).
AMF are mainly associated with P uptake, but numerous studies suggest that they can participate in N acquisition by plants from OM (e.g., Hodge et al. 2001; He et al. 2003; Govindarajulu et al. 2005; Lambers et al. 2008; Hodge and Fitter 2010). This can not fully explain the chemical niche shifts observed here. The roots of K. humilis and G. diversifolia were not colonized by AMF either in the annulus zone or outside the fairy ring, though both species showed strong and opposite N uptake patterns. At high available N level K. humilis shifted to more abundant N forms while G. diversifolia shifted to the least abundant N form. G. diversifolia had the lowest biomass among the five dominant plant species. A possible explanation for these differences in niche trajectory therefore is that G. diversifolia preferentially acquired NO −3 , the least preferred N form at low available N levels, to reduce competition with the other dominant plant species. Several lines of evidence indicate that some grassland species preferentially utilize NO −3 (Miller et al. 2007; von Felten et al. 2009). K. humilis has been suggested as a keystone species in this type of meadow, but this is mainly caused by grazing stress because of its stronger tolerance of over-grazing by yaks and sheep and nutrient-poor environments (Kaiser et al. 2008). Field observations have shown that graminoids are gradually becoming dominant when grazing is stopped. Under such conditions K. humilis preferentially acquired N from the least available form (nitrate) although it had the highest total biomass (Fig. 2). When N enrichment occurred, K. humilis shifted towards using the more abundant N forms. Compared with K. humilis and G. diversifolia, the roots of the other three species were colonized by AMF. G. straminea had a very low mycorrhizal colonization, whereas both S. aliena and E. nutans exhibited higher rates of mycorrhizal colonization. However, the lack of large differences in mycorrhizal colonization between in the annulus zone and outside the fairy ring, suggests that the shifts in N uptake patterns of these three species were not caused by altered AMF colonization. Overall, colonization of AMF clearly did not object our major finding that the dominant plant species in these alpine meadows showed chemical niche shifts and differed in their strategy to acquire available soil N under different nutrient levels.
To our knowledge, this is the first report showing shifts in N uptake patterns of dominant plant species in response to nutrient enrichment induced by basidiomycete fungi in native ecosystems, completely different from previous studies. First, most studies about the effects of N enrichment on plant diversity were manipulated by adding extraneous N (Gough et al. 2000; Stevens et al. 2004; Harpole and Tilman 2007; Clark and Tilman 2008; Manning et al. 2008; Duprè et al. 2010; Bobbink et al. 2010). Additions of extraneous N are a good approach to unravel the effects of N deposition on plant diversity. A number of mechanisms have been identified, i.e. long-term negative effect of ammonia and ammonium, soil-mediated effects of acidification and increased susceptibility to secondary stress, disturbance factors and direct toxicity of N gases (Bobbink et al. 2010). Apparently, however, this is not appropriate for investigating the effects of soil N availability on plant diversity through adding extraneous N. Besides, microbial community and activities are also affected by N deposition (Johnson et al. 1998; Waldrop et al. 2004; Bradley et al. 2006; Allison et al. 2007). Consequently, the conclusions obtained from N fertilization observation can not represent the effects of N enrichment derived from enhanced SOM decomposition by soil warming. Second, studies in this regard mainly focused on loss of plant diversity caused by N enrichment. Harpole and Tilman (2007) ascribed grassland plant species loss to reduced niche dimension caused by nutrient enrichment. The expanding of high stature (or N-demanding) species at the expense of low stature (or less competitive) species in grassland ecosystems was regarded as a second mechanism (Berendse and Elberse 1990; Klanderud 2008). In contrast, in this study we focused on species coexistence among dominant plant species when natural nutrient enrichment occurred. Actually, this indicates that nutrient enrichment may have greater impacts on non-dominant plant species than dominant plant species. Recent warming experiments also showed rapid loss of non-dominant species in alpine meadows (Klein et al. 2004; Li et al. 2004). Therefore, further studies should focus on responses of non-dominant species to nutrient enrichment for a better understanding of effects of nutrient enrichment on plant species diversity, as species-specific responses to warming and nutrient addition were observed in alpine ecosystems (Klanderud 2008). Third, so far the observation regarding niche shift of dominant plant species is obtained from empirical studies in invasive species ecology (Dietz and Edwards 2006; Broennimann et al. 2007; Harrington et al. 2009). Invasive plants often produce certain biochemicals that depress the growth and development of other neighboring plants (Callaway and Aschehoug 2000; Bais et al. 2003; Thorpe et al. 2009). In this case, it is difficult to obtain real processes of niche shift in native ecosystems. Nonetheless, our results also suffered from some deficiencies. First, in this study we only studied a single fairy ring produced by Agaricus because it was difficult to find several fairy rings at the same site. Second, biotic and abiotic changes occurred with decomposer fungal growth and activity which influence interactions between plant species. A previous study in the Tibetan alpine meadow showed that Agaricus significantly increased inorganic N content and available phosphorus in soil, but didn’t alter pH in this type of meadow. The aboveground biomass of graminoids, sedges, legumes and forbs was significantly higher in the annulus zone than outside the fairy ring (Liu 1997). In this study we also observed an increase of total biomass between in the annulus zone compared with the outer zone of the fairy ring. This implies that the basidiomycete fungus Agaricus campestris doesn’t depress the growth of the five dominant plant species (Fig. 2). Therefore, our results at least reflect how dominant plant species acclimate to high available N level. As the development of fairy rings of this size normally takes decades, we can assume that the observed chemical niche shifts occur within annual to decadal time scales. This indicates that chemical niche shifts can occur rapidly in alpine meadows, and challenges the core of niche-based geographical models which have employed niche conservatism as an important assumption (Peterson et al. 1999; Pearman et al. 2008).
In summary, this study demonstrates that dominant species can acclimate to N enrichment via chemical niche shifts which may indirectly contribute to the maintenance of species coexistence. This finding has important implications with regard to understanding the mechanisms responsible for species coexistence when nutrient enrichment is induced by climate warming. Additionally, because of rapid chemical niche shifts in terrestrial ecosystems as shown here greater caution should be taken when drawing conclusions using niche-based geographical models to predict how species respond to global warming. Considering there are still some deficiencies, further research needs to investigate more fairy rings and effects of interactions between AMF and basidiomycete fungi on plant N acquisition.
References
Allison SD, Hanson CA, Treseder KK (2007) Nitrogen fertilization reduces diversity and alters community structure of active fungi in boreal ecosystems. Soil Biol Biochem 39:1878–1887
Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM (2003) Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 301:1377–1380
Berendse F, Elberse WT (1990) Competition and nutrient availability in heathland and grassland ecosystems. In: Grace JB, Tilman D (Eds) Perspectives on Plant Competition, Academic Press, pp 93–116
Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, Erisman J-W, Fenn M, Gilliam F, Nordin A, Pardo L, De Vries W (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20:30–59
Bradley K, Drijber RA, Knops J (2006) Increased N availability in grassland soils modifies their microbial communities and decreases the abundance of arbuscular mycorrhizal fungi. Soil Biol Biochem 38:1583–1595
Broennimann O, Treier UA, Müller-Schärer H, Thuiller W, Peterson AT, Guisan A (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–709
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523
Chesson P (2000) Mechanisms of maintenance of species diversity. Ann Rev Ecolog Syst 31:343–366
Chinese Soil Taxonomy Research Group (1995) Chinese soil taxonomy. Science Press, Beijing, pp 58–147
Clark CM, Tilman D (2008) Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 451:712–715
Cooke RC, Rayner ADM (1984) The ecology of saprotrophic fungi. Longman, London
Dietz H, Edwards PJ (2006) Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology 87:1359–1367
Duprè C, Stevens CJ, Ranke T, Bleeker A, Peppler-Lisbach C, Gowing DJG, Dise NB, Dorland E, Bobbink R, Diekmann M (2010) Changes in species richness and composition in European acidic grasslands over the past 70 years: the contribution of cumulative atmospheric nitrogen deposition. Glob Chang Biol 16:344–357
Edwards PJ (1988) Effects of the fairy ring fungus Agaricus arvensis on nutrient availability in grassland. New Phytol 110:377–381
French HM, Wang B (1994) Climate controls on high altitude permafrost, Qinghai-Xizang (Tibet) Plateau, China. Permafr Periglac Process 5:87–100
Giorgi F, Hewitson B, Christensen J, Hulme M, Von Storch H, Whetton P, Jones R, Fu C et al (2001) Climate change 2001: regional climate information—evaluation and projections. In: Houghton JT, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA (eds) Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 585–636
Gough L, Osenberg CW, Gross KL, Collins SL (2000) Fertilization effects on species density and primary productivity in herbaceous plant communities. Oikos 89:428–439
Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Gramss G, Voigt K-D, Bergmann H (2005) Factors influencing water solubility and plant availability of mineral compounds in the tripartite fairy rings of Marasmius oreades (Bolt.:Fr.) Fr. J Basic Microbiol 45:41–54
Griffith GW, Roderick K (2008) Saprotrophic basidiomycetes in grasslands: distribution and function. In: Boddy L, Frankland JC, van West P (eds), Ecology of saprotrophic basidiomycetes. British Mycological Society Symposia Series. Elsevier Ltd., pp 275–297
Harpole WS, Tilman D (2007) Grassland species loss due to reduced niche dimension. Nature 446:791–793
Harrington LA, Harrington AL, Yamaguchi N, Thom MD, Ferreras P, Windham TR, Macdonald DW (2009) The impact of native competitor on an alien invasive: temporal niche shifts to avoid interspecific aggression. Ecology 90:1207–1216
He X, Critchley C, Bledsoe C (2003) Nitrogen transfer within and between plants through common mycorrhizal networks (CMNs). Crit Rev Plant Sci 22(6):531–567
Hodge A, Fitter A (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. PNAS 107:13754–13759
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic matter. Nature 413:297–299
Johnson D, Leake JR, Lee JA, Campbell CD (1998) Changes in soil microbial biomass and microbial activities in response to 7 years simulated pollutant nitrogen deposition on heathland and two grasslands. Environ Pollut 103:239–250
Jones DL, Kielland K (2002) Soil amino acid turnover dominates the nitrogen flux in permafrost-dominated taiga forest soils. Soil Biol Biochem 34:209–219
Kahmen A, Renker C, Unsicker SB, Buchmann N (2006) Niche complementarity for nitrogen: an explanation for the biodiversity and ecosystem functioning relationship? Ecology 87(5):1244–1255
Kaiser P (1998) Relations of Leucopaxillus giganteus, basidiomycete of fairy rings, with soil microflora and grassland plants. Cryptogam Mycol 19:45–61
Kaiser K, Miehe G, Barthelmes A, Ehrmann O, Scharf A, Schult M, Schlütz F, Adamczyk S, Frenzel B (2008) Turf-bearing topsoils on the central Tibetan Plateau, China: pedology, botany, geochronology. Catena 73:300–311
Klanderud K (2008) Species-specific responses of an alpine plant community under simulated environmental change. J Veg Sci 19(3):363–372
Klein JA, Harte J, Zhao X (2004) Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau. Ecol Lett 7:1170–1179
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23(2):95–103
Li Y, Zhao L, Zhao X, Zhou H (2004) Effects of a 5-year mimic temperature increase to the structure and productivity of Kobresia humilis meadow. Acta Agrestia Sin 12(3):236–239
Liu ZK (1997) A comparison between mushroom sphere and plants outside the sphere and soil in alpine meadow. Pratacult Sci 14(3):68–70
Lükewille A, Wright RF (1997) Experimentally increased soil temperature causes release of nitrogen at a boreal forest catchment in southern Norway. Glob Chang Biol 3:13–21
Manning P, Morrison SA, Bonkowski M, Bardgett RD (2008) Nitrogen enrichment modifies plant community structure via changes to plant–soil feedback. Oecologia 157:661–673
McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B, Giblin AE, Kielland K, Kwiatkowski BL, Laundre JA, Murray G (2002) Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415:68–71
Miller AE, Bowman WD (2002) Variation in nitrogen-15 natural abundance and nitrogen uptake traits among co-occurring alpine species: do species partition by nitrogen form? Oecologia 130:609–616
Miller AE, Bowman WD, Suding KN (2007) Plant uptake of inorganic and organic nitrogen: neighbor identity matters. Ecology 88(7):1832–1840
Nasholm T, Sandberg G, Ericsson A (1987) Quantitative-analysis of amino-acids in conifer tissues by high-performance liquid-chromatography and fluorescence detection of their 9-fluorenylmethyl chloroformate derivatives. J Chromatogr 396:225–236
Pearman PB, Guisan A, Broennimann O, Randin CF (2008) Niche dynamics in space and time. Trends Ecol Evol 23(3):149–158
Peterson AT, Soberón J, Sánchez-Cordero V (1999) Conservatism of ecological niches in evolutionary time. Science 285:1265–1267
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JH, Gurevitch J, GCTE-NEWS (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Sardans J, Peñuelas J, Estiarte M (2006) Warming and drought alter soil phosphatase activity and soil availability in a Mediterranean shrubland. Plant Soil 289:227–238
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39
Silvertown J (2004) Plant coexistence and the niche. Trends Ecol Evol 19:605–611
Song MH, Xu XL, Hu QW, Tian YQ, Ouyang H, Zhou CP (2007) Interactions of plant species mediated plant competition for inorganic nitrogen with soil microorganisms in an alpine meadow. Plant Soil 297:127–137
Stevens CJ, Dise NB, Mountford JO, Gowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303:1876–1879
Thompson LG, Mosley-Thompson E, Davis M, Lin PN, Yao T, Dyurgerov M (1993) Recent warming: ice core evidence from tropical ice cores with emphasis on Central Asia. Glob Planet Change 7:145–156
Thorpe AS, Thelen GC, Diaconu A, Callaway RM (2009) Root exudate is allelopathic in invaded community but not in native community: field evidence for the novel weapons hypothesis. J Ecol 97:641–645
Tilman D (1982) Resource competition and community structure. Princeton Univ. Press, Princeton
Vierheilig H, Schweiger P, Brundrett M (2005) An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol Plant 125:393–404
von Felten S, Hector A, Buchmann N, Niklaus PA, Schmid B, Scherer-Lorenzen M (2009) Belowground nitrogen partitioning in experimental grassland plant communities of varying species richness. Ecology 90(5):1389–1399
Waldrop MP, Zak DR, Sinsabaugh RL (2004) Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36:1443–1451
WRB (1998) World reference base for soil resources. FAO/ISRIC/ISSS, Rome
Zheng D (2000) Mountain geoecology and sustainable development of the Tibetan Plateau. Kluwer, Dordrecht
Zhou XM (2001) Alpine Kobresia meadows in China. Science Press, Beijing, pp 51–62
Acknowledgements
We thank Dr. Douglas Schaefer for his English improvement. We also thank the two anonymous reviewers for their helpful comments that helped us to greatly improve the manuscript. This study was supported by the National Natural Science Foundation of China (Grant No. 30870424) and National Basic Research Program of China (Grant No. 2010CB833501).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Herbert J. Kronzucker.
Rights and permissions
About this article
Cite this article
Xu, X., Ouyang, H., Cao, G. et al. Dominant plant species shift their nitrogen uptake patterns in response to nutrient enrichment caused by a fungal fairy in an alpine meadow. Plant Soil 341, 495–504 (2011). https://doi.org/10.1007/s11104-010-0662-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-010-0662-1