Abstract
Soil amendment with biochar is shown to be a low-cost carbon sequestration option while its properties contribute to nutrient dynamics. The properties of biochar can be purposefully modified using different techniques including chemical oxidation. A change in biochar functionality, to an equivalent state of natural aging, could alter plant nutrient use efficiency, particularly nitrogen (N) and phosphorus (P), which however has not been examined yet. Here, we examined 15N recovery and P uptake in mixtures of clover (Trifolium repens) and ryegrass (Lolium perenne) after biochar amendment at different oxidation states (i.e., fresh, 5%OBC and 15%OBC, oxidized with 5 and 15% H2O2, respectively) and addition of 15NH4+-N and 15NO3−-N. Overall, after 30 days of growth, the total 15N recovery in plant and soil was 41% greater with NH4+-N than with NO3−-N addition. However, the 15N recovery from NH4+-N addition was greater in the oxidized biochar treatments than the control and fresh biochar treatments (a significant biochar × N interaction). Chemical oxidation also increased leaching of NO3−-N and phosphate-P while greater plant P uptake occurred in the 15%OBC treatment with NH4+-N addition. An increase in cation exchange sites with oxidized biochar may have increased NH4+-N retention and biomass production (N uptake) and, thus, 15N recovery. The increase in P availability may further have increased plant biomass production in the 15%OBC treatment with NH4+-N. Our results suggest that oxidized biochar could increase primary productivity by increasing NH4+-N use efficiency and P availability while increasing the risk of NO3−-N and phosphate-P leaching.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biochar, a form of pyrolyzed biomass, is considered as one of the carbon (C)-negative technologies (IPCC 2018). When applied to soil, it interacts with soil particles, organic matter, and nutrients affecting nutrient dynamics in the soil (Atkinson et al. 2010; Kookana et al. 2011). The role of biochar in manipulating nutrient dynamics depends on its surface properties including surface charge and surface area. However, the surface functionality in the freshly prepared biochar is often low (Mia et al. 2017a). Thus, it can be regarded as inactive/inert having a little influence on soil fertility and crop productivity (Jeffery et al. 2017, 2015). Therefore, enhancing functionality of biochar has been considered a strategy to increase its role to nutrient cycling (Mia et al. 2017a; Wang et al. 2015).
The surface functionality of biochar can potentially be enhanced with physical, chemical, and biological treatments while chemical treatments had large effects (Cheng and Lehmann 2009; Mia et al. 2017a). For example, chemical treatment of biochar with hydrogen peroxide (H2O2) increased cation exchange capacity including NH4+ sorption (Wang et al. 2015). Therefore, application of oxidized biochar to soil can have a significant impact on nutrient dynamics. However, there is no study so far about how chemically oxidized biochar may affect nutrient dynamics in soil-plant systems. Moreover, pyrogenic carbon (similar in properties to biochar) after retaining in the environment for a long time (i.e., aged chars) also showed similar properties to that of oxidized biochar (Cheng et al. 2008). Therefore, an understanding of nutrient dynamics after oxidized biochar amendment may provide some insight into long-term biochar effects in the environment.
Nitrogen (N) is a vital nutrient comprising up to 5% of the plant biomass, and therefore, it is required in relatively large quantities for crop production (Hawkesford et al. 2011). Plant productivity is often limited by soil N supply since the reactive N is lost from soils through leaching, volatilization, and denitrification (Cassman et al. 2002; Lassaletta et al. 2014). Management practices including organic amendments and, more recently, biochar amendments have been used to increase reactive surfaces for retaining reactive N in the soil rooting zone.
Biochar having diverse surface properties showed variable effects on N dynamics, i.e., N retention, volatilization, leaching, plant growth, and N uptake and microbial N cycling in the soil (Esfandbod et al. 2017; Gronwald et al. 2018; Güereña et al. 2013; Lee et al. 2017; Prendergast-Miller et al. 2014). For example, enhanced soil N retention was attributed to a greater NH4+-N and NO3−-N sorption, respectively, to the positive and negative surface functional groups of biochar (Clough et al. 2013; Singh et al. 2010). For similar reasons, biochar with different surface functionality may alter N retention when N is added as NH4+-N or NO3−-N. To our knowledge, effects of oxidized biochar on N retention and leaching from the two different N sources have not been examined yet in plant-soil systems.
Phosphorus (P) is another important essential nutrient that regulates enzyme activities and constitutes plant and microbial cellular structures and, therefore, is equally important for maintaining primary productivity (Elser et al. 2007; Hawkesford et al. 2011). Fresh biochar amendment can increase P availability in soil through several ways, including (a) increasing mycorrhizal association and P mining, (b) P sorption onto biochar’s surface, and (c) altering soil pH (Gao et al. 2019; Madiba et al. 2016; Warnock et al. 2007; Yao et al. 2012; Zhang et al. 2017). Biochar with positive surface functional groups (often the freshly prepared) may adsorb some P on its surface while P sorption on oxidized biochar may be minimal (Lawrinenko et al. 2016; Mia et al. 2017b). Additionally, an increased plant available P in anthropogenic soils containing oxidized char suggest that oxidized biochar may increase the availability of P in the soil (Liang et al. 2006) but may also increase P leaching. Moreover, P speciation and availability can also be affected by NH4+ and NO3− mediated changes in rhizosphere pH (Neumann and Römheld 2011; Shen et al. 2011). However, studies investigating oxidized biochar effects on plant P availability, uptake, and leaching have not been reported yet.
Grasslands in Australia cover more than 90% of agricultural lands (ABS 2016) with a relatively lower productivity compared to many European grasslands. One of the possible reasons for the lower productivity could be the low-input pasture management. Relatively higher N and P supply in combination with generation of reactive surfaces through functionalized biochar application may enhance its pasture productivity while storing C in the soils for longer terms. For large-scale adoption of such practices, an in-depth examination is needed to consolidate our confidence. However, there are no studies investigating the effects of biochar with variable surface functionality on N and P dynamics in grasslands, particularly where high-input agriculture is practiced. Here, we examined N and P uptake and leaching in a Dermosol (Cambisol) grown with a clover grass mixture after fresh and chemically oxidized biochar application with and without 25 g P m−2, and with 50 g N m−2 applied either as NH4+ or as NO3−. The applied N was labeled with 15N; therefore, we were able to measure 15N recovery in plants, soil, and leachates. We hypothesized that soil amendment with an oxidized biochar will recover more 15N in plants and soil with NH4+-N than NO3−-N application (H1), while oxidized biochar will increase soil P availability and plant uptake, and the risk of P leaching (H2).
Materials and methods
Experimental setup—biochar oxidation treatment
The biochar oxidation procedure and oxidation-induced changes in biochar properties have been reported in Mia et al. (2017b). Briefly, a wood-derived biochar (produced at 550 °C) was oxidized with 5% and 15% H2O2 in a water bath for 6 h. The oxidized biochar was washed thoroughly with deionized water after removal of excess H2O2 through evaporation at 120 °C to dryness and pH adjustment to that of fresh biochar (pH = 6.2). The fresh biochar was also washed thoroughly to remove ash and nutrients (EC < 5 μS cm−1). The cation exchange capacity (CEC) of fresh biochar (FBC), 5% oxidized biochar (5%OBC), and 15% oxidized biochar (15%OBC) were 24, 76, and 99 cmolc kg−1, respectively (Mia et al. 2017b). For our experiment, we used four biochar treatments (BC): FBC, 5%OBC, and 15%OBC, all applied at a rate of 20 t ha−1, and a control (C, i.e., no biochar addition). The soil used in this experiment was a Dermosol, according to the Australian soil classification (Isbell 2002), which is equivalent to a Cambisol in the FAO soil classification. The Dermosol contained 51.5% sand, 19.7% silt, and 28.8% clay and had a pH of 6.81 (Mia et al. 2017c). The exchangeable Ca, Mg, K, and Na concentrations in the soil were 6.3, 1.2, 1.1, and 0.1 cmol kg−1 respectively (Keith et al. 2016). The CEC of the soil was 8.57 cmolc kg−1 (measured with the ammonium/sodium acetate compulsive replacement method at pH = 7.0) (Mia et al. 2017c). For each biochar treatment, we filled 16 pots (diameter 6.5 cm, height 20 cm). All pots received an initial supply of macronutrients (N, P, K Ca, Mg, and S at 2, 2, 4, 2.15, 2.9, and 0.9 g m−2 respectively) and micronutrients (Cu, Mn, Zn, B, and Fe at 0.01, 0.08, 0.05, 0.01, and 0.02 g m−2 respectively) (Mia et al. 2018). A mixture of the grass (Lolium perenne) and the legume (Trifolium repens) was sown at a 1:1 ratio and pots were kept in a growth chamber. In the growth chamber, the temperature was maintained at 25 °C/15 °C (day/night) while the light intensity kept at 1000 W using metal halide lamps that were on for 12 h each day. The relative humidity in the growth chamber was 60%. The pots were regularly watered for replenishing moisture loss. After 42 days of growth, the aboveground plant biomass was harvested for a biological N fixation study (Mia et al. 2018). We continued using the same pots for the current study.
Nitrogen and P treatments
For the N treatment (N), we added 50 g N m−2 of NH4+-N (AN) as (NH4)2SO4 to half of the pots (8 pots for each biochar treatment) and NO3−-N (NN) as KNO3 to the other half. The tracers were added to surface of the pots as solutions. The added N from both N sources was enriched in 15N (4 atom%). For the P treatment (P), we added 25 g P m−2 to half of the pots (as a mixture of KH2PO4 and K2HPO4 in a solution resembling to the soil pH) and no P to the other half of the pots. The biochar (FBC, 5%OBC, and 15%OBC with a control), N (AN and NN), and P (with or without) treatments were applied in a full factorial design with 4 replicates for each treatment combination. Although the N and P application rates were relatively high compared to the commonly used rates in Australian grasslands, here we wanted to identify how the N and P addition interacted with the biochar treatments in a relatively short-term pot experiment, rather than mimicking field conditions where these interactions occur over a much longer timeframe and within a greater soil volume. The N and P additions occurred 1 week after the first harvest. Soil moisture content was brought to field capacity before treatment intervention to maximize homogenous mixing of tracers. Soil moisture content in the pots was then maintained at 60% water holding capacity by frequent watering to account for weight loss due to evapotranspiration. No drainage occurred during these watering events.
Leaching events and leachate analysis
One hour after N and P addition (T1), we added 100 mL of water (equivalent to a 300-mm rain event) and leachate was collected under gravitational force into collection tubes (for details see SI, Fig. S1). The second (T2) and third (T3) leaching events were conducted with the same volume of water for each pot after 24 h and 20 days of N and P addition. The leachates were analyzed for NH4+-N and NO3−-N concentration on a flow injection analyzer (QuickChem FIA+, Lachat Instruments, Loveland, CO, USA) while P concentration was measured following a colorimetric (ammonium paramolybdate/stannous chloride) method (Bray and Kurtz 1945; Mia et al. 2017b). We also measured leachate pH. To determine 15N recovery in the leachates, N was trapped into acidified filter paper discs (Stark and Hart 1996) only for the first and second leaching events since the N concentration in the leachate collected at the third leaching event was below the detection limit (0.02 mg N L−1) in most cases. Briefly, two small filter paper discs were soaked with 10 μL 2.5 M KHSO4 and enveloped inside polytetrafluoroethylene (PTFE) and sealed. An aliquot of leachate was taken (containing between 20 and 100 μg inorganic N) into a 100-mL plastic container and water was added to reach a final volume of 40 mL. Then, 0.2 g MgO and 0.4 g of Devarda’s alloy were added to convert all inorganic N into NH3. Next, the PTFE traps were added, and the containers were quickly closed. The containers were kept at room temperature for 5 days while they were swirled every day. The paper filter discs were then dried under 98% H2SO4 in a desiccator and packed into tin foil for 15N analysis on an isotope ratio mass spectrophotometer (IRMS, Thermo Fisher Scientific, Bremen, Germany).
Plant and soil analysis
Plants were harvested after 30 days of regrowth. The above ground biomass was separated into T. repens and L. perenne species, and the biomass was recorded after drying in an oven at 60 °C for 48 h. Roots were separated from the soil by sieving and the root biomass was recorded after washing and oven drying. Representative soil samples were taken after thorough mixing the soil in the pots. Inorganic N (exchangeable NH4+ and NO3−) was determined on a flow injection analyzer (QuickChem FIA+, Lachat Instruments, Loveland, CO, USA) after extraction with 0.05 M K2SO4, while available P was determined in 0.03 M NH4F–0.025 M HCl (1:10, w/v) extracts using a colorimetric method (Bray and Kurtz 1945). Microbial C and N were determined using the fumigation extraction method (Bruulsema and Duxbury 1996). Briefly, soil samples were extracted with 0.05 M K2SO4 following a protocol similar to inorganic N extraction after being exposed to chloroform vapor for 48 h under vacuum in a desiccator. Total C and N were analyzed using a TOC-VCPN analyzer (Shimadzu Scientific Instruments, Tokyo, Japan). The microbial biomass C and N were then determined from the difference in concentrations between fumigated and non-fumigated samples, while C and N extraction efficiencies of 0.45 and 0.54 were used, respectively (Beck et al. 1997; Brookes et al. 1985). Soil pH was measured in soil-water slurries (1:10, w/v) and CEC was determined using the ammonium acetate method (Chapman 1965). Briefly, 4 g of soil was shaken with 1 M CH3COONH4 (pH = 7.0) for 30 min and the solution was discarded after centrifugation. This step was repeated twice. The excess NH4+ not bound to the exchange sites was removed with three consecutive washings using 70% ethanol. Then, NH4+ was exchanged with Na+ using 1 M CH3COONa (pH = 7.0). This step was repeated twice and NH4+ concentration in the composited solution was measured as discussed above. Plant biomass and soil samples were ground with a ball mill. Ground aboveground plant biomass (0.5 g) was ashed at 500 °C for 4 h and then P was measured using a colorimetric method (ammonium paramolybdate/vanadate) (Jackson 1958). Shoots, roots, and soil samples were analyzed for total N and 15N using the IRMS. The 15N recovery was calculated using the following equation (Mia et al. 2017c):
where Nplant, 15Nlp, 15Nnlp, and 15Nl are the total N content in plant biomass, 15N atom% in the labeled plant biomass, 15N atom% in non-labeled plant biomass, and 15N enrichment in the N fertilizers (4 atom%), respectively. The non-labeled plant biomass 15N atom% values were used from the previous harvest. The 15N recovery in soil and leachate were calculated in a similar way. Since no drainage occurred during non-leaching events, gaseous loss of 15N was calculated as the difference in 15N applied and 15N recovered in the plant, soil, and leachates.
Statistical analysis
Plant and soil variables were analyzed following a full factorial design with B, N, and P treatment as the fixed factors. Data were transformed when the model assumptions were not met. We used Tukey’s HSD test to separate the means (p < 0.05). All analyses were performed using JMP (pro 11.0, SAS Institute, Cary, NC, USA).
Results
Soil properties
Soil pH was significantly lower by 1.5 units in the NH4+-N treatment (AN) than in the NO3−-N treatment (NN) while P addition reduced soil pH slightly (by 0.04 units, Table 1). Average across biochar treatments, the highest exchangeable soil NH4+-N concentration (0.70 mg kg−1) was in the AN and without P treatment while it was the lowest (0.30 mg kg−1) in pots that were amended with NN and P (N × P interaction). Overall, the FBC treatment contained the highest soil NO3−-N of all biochar treatments. In fact, all other interactions on soil NO3−-N, including BC × N × P, were significant. Available P was greater (41 mg kg−1) in soil with the AN treatment than with the NN treatment (36 mg kg−1). The P treatment significantly increased soil available P from 30 to 46 mg kg−1 at the harvest. Compared to the control treatment, the fresh and oxidized biochar treatments significantly increased soil CEC, but there was no difference between the fresh and oxidized biochar treatments (Fig. S2). The combined application of AN and P increased microbial biomass C by 22% and 12%, respectively (Fig. S3, p < 0.001). However, the overall microbial N was greater with NN than AN (24 vs.19 mg N kg−1 soil) with a larger difference with15%OBC, giving a significant BC × N interaction (Fig. S3, p = 0.012).
Biomass production
L. perenne aboveground biomass production was ~ 14% greater in the AN than in the NN treatment, with a larger effect in the oxidized biochar treatments causing a clear BC × N interaction (Fig. 1, Table S2). T. repens aboveground biomass production was 14% greater with AN (557 g m−2) than with NN addition. Overall, root biomass significantly increased by 8% with AN addition (581 g m−2) than with NN addition (Fig. S4). Combining aboveground (sum of L. perenne and T. repens) and root biomass, we observed a BC × N interactive effect, similar to that of L. perenne aboveground biomass production (Fig. S4).
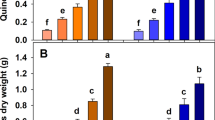
Aboveground biomass production of L. perenne (a) and T. repens (b) under different biochar and N treatments (average across P treatment). FBC, 5%OBC, and 15%OBC denote fresh biochar and biochar oxidized with 5% and 15% H2O2, respectively, while AN and NN denote NH4+-N and NO3−-N application, respectively. Error bars indicate standard error of means (n = 8). Different letters above the bars indicate significant difference in biomass production (p < 0.05)
Nitrogen and P uptake in aboveground biomass
The L. perenne and T. repens combined aboveground N uptake was significantly greater (by 23%) with AN than with NN amendment. Nitrogen uptake was greater in the oxidized biochar treatments receiving AN treatment than in the same biochar treatment but with NN (BC × N interaction, Fig. S5, Table S2). Similar to N uptake, the overall P uptake was significantly higher by 38% in the AN than in the NN treatment. However, this increase in P uptake with AN treatment was much higher in the oxidized biochar than in the FBC and control treatments, reaching the highest value in the 15%OBC treatment (9.8 g m−2) (significant BC × N interaction, Fig. 2). As expected, we observed an overall 14% increase in P uptake with P addition.
Phosphorus uptake in the aboveground biomass of L. perenne (bottom bars) and T. repens (upper bars) under different biochar and N addition treatments (average across the P treatment). Therefore, whole vertical bars represent total aboveground P uptake. For treatment abbreviations, see Fig. 1. Error bars represent standard error of the means (n = 8). Different letters above bars indicate significant differences in P uptake in grasses (p < 0.05) while different letters in the top bars represent significant differences in combined P uptake in grass and clover
Leachate pH and NH4 +-N, NO3 −-N, and PO4 3− concentrations
Overall, the leachate pH was significantly higher in the 15%OBC treatment compared to the control treatment at T1 and T2 (Table S1). AN treatment had lower leachate pH than NN treatment. Not surprisingly, the amount of NH4+-N leached at T1 was significantly higher with AN than NN treatment (3.3 vs. 2.4 mg m−2) while P addition increased leaching loss of NH4+-N by 37% compared to the control (2.4 mg m−2) (Fig. 3a, Table S3). At T2, the greatest amount of NH4+-N leaching (251 mg m−2) occurred in the control treatment combined with AN and without P, while all biochar treatments with NN regardless of P treatment had similar leaching of NH4+-N (BC × N × P interaction, Fig. 3b, Table S3). Overall, the total NH4+-N lost from combined leaching events was in the order of C > FBC > 5%OBC > 15%OBC, while AN and P addition increased NH4+-N leaching by 2613% and 104%, respectively. NH4+-N leaching was increased with P addition in all the fresh and oxidized biochar treatments receiving NH4+-N. However, NH4+-N leaching in all biochar treatments was similar when they received NO3−-N irrespective of P treatments (BC × N × P interaction, Fig. S6, Table S3).
Leaching of NH4+-N (a, b) and NO3−-N (c, d) after 1 h (T1) and 24 h (T2) of nutrient addition under different biochar and N addition treatments (average across the P treatment). For treatment abbreviations, see Fig. 1. Error bars in the boxes represent standard error of the means (n = 8). Different letters above bars indicate significant differences (p < 0.05)
The amount of NO3−-N leached at T1 with NN addition was the largest in the control treatment (1976 mg m−2) while the lowest leaching was observed in the FBC treatment but it increased with oxidation (Fig. 3c, Table S3). However, the biochar treatment had no effect on NO3−-N leaching when applied with NH4+-N. In the second leaching event (T2), NO3−-N leaching was significantly greater in the control and FBC treatment than in the oxidized biochar treatments when applied with NH4+-N (Fig. 3d, Table S3). In contrast to NH4+-N leaching, total NO3−-N leaching was in the order of C > 15%OBC > %OBC > FBC while it was 239 times greater (7514 vs. 31 mg m−2) with NN than with AN addition. Leachate concentrations of NH4+-N and NO3−-N during the third leaching event were all very low, often below the detection limit (0.02 mg N L−1), and are not reported.
Average across the N and P addition treatments, pots with 15%OBC caused the greatest amount of PO43−-P leaching at T1 (87 mg P m−2), while P addition increased PO43−-P leaching by 70% (42 mg P m−2) (Fig. 4a, Table S3). Moreover, we observed a significant linear relationship between leachate pH at T1 and P leaching (r2 = 0.33, p < 0.001, Fig. S7). All treatments and their interactive effects were significant at T2 (Table S3). At T3, PO43−-P leaching was significantly reduced with 5%OBC compared to 15%OBC, while P and AN addition increased PO43−-P leaching by 442% and 64%, respectively (Fig. 4c). When all three leaching events were combined, the 15%OBC treatment, averaged across the N and P addition treatments, caused the largest PO43−-P leaching (263 mg P m−2) while the least PO43−-P leaching was in the FBC treatment (147 mg P m−2). Surprisingly, the overall PO43−-P leaching increased with AN compared to NN addition, while it was also accelerated with P addition resulting in the largest P leaching in the combined NH4+-N and P application (N × P interaction, Fig. S8).
Leaching of PO43−-P after 1 h (T1), 24 h (T2), and 20 days (T3) of nutrient addition under different biochar and N addition treatments (average across the P treatment). For treatment abbreviations, see Fig. 1. Error bars in the bars represent standard error of the means (n = 8). Different letters above bars indicate significant differences (p < 0.05)
15N recovery in plant, soil, and leachates
The 15N recovery in the aboveground biomass of L. perenne with AN was largest in the 15%OBC treatment, while in the NN treatment, the biochar treatment had no effect (BC × N interaction, Fig. S9, Table S2). Total plant 15N recovery showed a similar BC × N interactive effect as observed in L. perenne aboveground biomass (Fig. 5a, Table S2). Across all other treatments, soil 15N recovery was the highest in 5%OBC treatment, while it was also greater in pots that received AN compared to pots receiving NN (Fig. 5a). Combining total plant and soil recovery, we observed a higher total 15N recovery with AN addition than with NN addition, with the greatest total 15N recovery (47 g m−2) obtained in the 15%OBC treatment with AN. However, total 15N recovery among biochar treatments did not differ when they received NN (significant BC × N interaction).
15N recovery in plant biomass and soil (a), in leachates (b), and 15N loss as gaseous emission (c). The upper bars in panel a represent total 15N recovery while the lower bars represent soil recovery under different biochar and N treatments (average across the P treatment). Therefore, the difference of these two bars indicates total 15N recovery in plants. The upper bars in panel b represent total 15N recovery in the leachates while the lower bars indicate 15N recovery during first leaching event (after 1 h of tracer addition). Therefore, the difference indicates 15N recovery during the second leaching event (after 24 h of tracer addition). The error bars represent standard error of means (n = 8). For explanation of treatment abbreviations, see Fig. 1. Different letters above bars indicate significant differences (p < 0.05)
The 15N recovery in leachates at T1 was small and similar in all biochar treatments when receiving AN, while when receiving NN, the greatest 15N recovery was in the control treatment (1.95 g m−2), followed by oxidized and fresh biochar treatments (BC × N interaction, Fig. 5b, Table S2). At T2, the leachate 15N recovery was similar in all biochar treatments when they received NN either with or without P while the recovery with AN addition was smallest with oxidized biochar and without P addition (BC × N × P interaction). When 15N recovery at T1 and T2 were combined, the 15N recovery from the NN treatment was smallest in the FBC treatment, but increased with oxidation reaching similar values to the control at 15%OBC, while the biochar treatments had no effect on 15N recovery with AN addition (BC × N interaction, Fig. 5b, Table S2).
The gaseous loss of 15N, calculated from the difference between total 15N added and 15N recovered in plants, soil, and leachates, was on average more than double (10.8 vs. 5.05 g m−2) with NN than with AN addition (Fig. 5c, Table S2). Moreover, the biochar treatment did not cause a significant effect on gaseous loss of 15N when NN was applied, while the loss was reduced in the oxidized biochar treatment when combined with AN addition (BC × N interaction, Fig. 5c, Table S2).
Discussion
15N retention and loss
Biochar with diverse functional surfaces may affect N use efficiency (i.e., total N retained in the plant-soil system), particularly when N is applied as oppositely charged ions (NH4+ vs. NO3−). We observed an increased total 15N recovery with the 15%OBC when co-applied with NH4+-N while FBC caused a slightly (although not significantly) larger total 15N recovery when added as NO3−-N. These results partially support our first hypothesis (H1). Chemically oxidized biochar may have contained more cation exchange sites than fresh biochar (Mia et al. 2017b), thereby providing more sites to retain 15NH4+-N, although the increase in soil CEC with oxidized biochar was not significant (Fig. S1). This increased retention may have led to a significant increase in plant biomass production, N uptake, and, thus, 15N recovery (Figs. 1, 5, and S5). Specifically, L. perenne biomass 15N recovery in the AN treatment was 24% greater in the 15%OBC than in the control (Fig. S9). A significant relationship between aboveground L. perenne N concentration and soil available NH4+-N concentration (p < 0.001, r2 = 0.21, data not shown) supports our argument. Although we did not find a significant biochar treatment effect on the leachate 15N recovery when co-applied with AN treatment, the NH4+-N leaching was significantly reduced in the oxidized biochar treatments (Fig. 3b) suggesting that the greater retention of AN at biochar’s surfaces might also have contributed to the reduced loss of NH4+-N through leaching. As a result, the greater retention and plant 15N recovery reduced gaseous loss of 15N in this treatment (Fig. 5c). While we found no reports on 15N recovery after oxidized biochar amendment, a greater NH4+-N retention was observed in soils that contained oxidized (bio)chars from historical inputs or from biochar amendments after several years of field aging (Esfandbod et al. 2017; Güereña et al. 2013; Lee et al. 2017; Steiner et al. 2008). However, fresh biochar with a high surface area and relatively high CEC could also increase retention of NH4+-N in soil, although this may be short-lived due to blocking of biochar’s pores (Hüppi et al. 2016; Singh et al. 2010; van Zwieten et al. 2010). Therefore, oxidized biochar (with large CEC) along with AN application may be one of the potential means of increasing N use efficiency (Güereña et al. 2013; Jones et al. 2012; Mia et al. 2017c; Singh et al. 2010).
While we did not find an increased total 15N recovery in the FBC treatment when co-applied with NN, the leachate 15N recovery and NO3−-N leaching were lowest in the FBC treatment at T1 (Figs. 5b and 3c), suggesting that NO3−-N leaching was lower in the freshly prepared biochar compared to its oxidized forms. The greater retention of NN in the fresh biochar treatment increased soil NO3−-N concentrations in this treatment compared to the control (although not significantly) (Table 1). As a consequence, N uptake was greater in the FBC treatment than the other treatments (Fig. S5). Similar to our results, FBC application with large surface area and positive charge retained more NO3−-N in the soil, often leading to an increased N uptake (Jin et al. 2016; Ventura et al. 2013; Yao et al. 2012). Chemical oxidation increased loss of NO3−-N from the soil (Fig. 3c, d). We believe that this was due to loss of positively charged functional groups such as pyridine and oxonium on biochar surfaces with chemical oxidation (Lawrinenko et al. 2016; Mia et al. 2017b). Moreover, microbial mechanisms (e.g., microbial immobilization) for increased NO3−-N retention or uptake with oxidized biochar were most likely not prominent in our relative short-term experiment. Biochar application could also change N transformations (Teutscherova et al. 2017) and therefore could potentially have affected nitrification in the AN treatment. However, we believe that nitrification in the AN treatment was relatively small and not affected by the biochar treatment, since NO3− leaching in the AN treatment was low at T1 and T2 (Fig. 3c, d) and below the detection limit at T3 (data not presented) for all biochar treatments, while the soil NO3− concentrations in the AN treatment were also similar for all biochar treatments (Table 1).
Although previous studies have suggested that P availability can affect N use efficiency (He and Dijkstra 2015; Mehnaz and Dijkstra 2016), we did not find a significant effect of P treatment on overall 15N recovery (Fig. 5a). However, total NH4+-N leaching increased with P addition with larger effects in the FBC and 5%OBC treatments (Fig. S6), suggesting that P application increased loss of NH4+-N possibly due to accelerated N mineralization with P supply (that was not studied here) (Chen et al. 2017; Mia et al. 2017c). It is also possible that some of the NH4+-N was attracted to negatively charged phosphate (counterion balance in the soil solutions) resulting in higher mobility and leaching. We also found an increased NO3−-N leaching with P addition after 24 h of nutrient addition (T2) possibly due to competition between anions on the soil anion exchange sites (Donn and Menzies 2005; Eick et al. 1999) while a reduced NO3−-N leaching was reported for longer interactions due to increased plant uptake (Stevens et al. 1993). These results suggest that P addition may affect N leaching and, thus, N use efficiency. However, further studies are needed to unravel the underlying mechanisms.
Phosphorus availability and uptake
Soil amendment with fresh biochar can increase P availability and uptake in plants (Madiba et al. 2016) while anthropogenic soils containing oxidized char often show a high plant available P contents (Araújo et al. 2015; Liang et al. 2006). In our experiment, we did not find a significant increase in available soil P after fresh or oxidized biochar amendment at the end of the experiment. However, we observed a significant increase in plant P uptake in the 15%OBC treatment when co-applied with AN (Fig. 2). The greater P uptake was mainly observed due to increased biomass production in this treatment (Fig. S4) since the biochar treatment did not affect P concentration in the aboveground biomass of both species (data not shown). While FBC reduced overall total P leaching compared to the control, it increased with oxidation causing a significantly higher P loss in the 15%OBC treatment compared to its initial state (FBC) (Fig. 4). These results suggest that biochar oxidation can increase mobility and uptake of P. Therefore, our hypothesis (H2) is substantiated.
Changes in the functionality of biochar with chemical oxidation could affect P retention (Laird et al. 2010; Lawrinenko et al. 2016). While positive functional groups (oxonium and pyridine) in fresh biochar might have contributed to increased P retention and reduced leaching (Laird et al. 2010; Mia et al. 2017b), the creation of negative surface functional groups (carboxylic and phenolic groups) and loss of positive surface functional groups with oxidation of biochar may have caused the reduced retention and greater leaching of P (Lawrinenko et al. 2016; Mia et al. 2017b). Therefore, the higher P uptake in the 15%OBC with NH4+-N was most likely not caused by changes in P retention.
Biochar-mediated changes in soil pH often play a vital role in P availability (Schneider and Haderlein 2016). It is possible that buffering of soil solution pH (leachate pH ~ 7.0 at T3, Table S1) with the carboxylic and phenolic functional groups in the oxidized biochars (Magdoff and Bartlett 1985; Mia et al. 2017b; Singh et al. 2017) may have contributed to the higher P leaching and availability in the 15%OBC with AN, even though NH4+-N uptake and oxidation of NH4+ to NO3− usually cause soil acidification (Neumann and Römheld 2011), as observed in our study. The significantly higher leachate pH in the 15%OBC treatment (Table S1) and the positive relationship (r2 = 0.33, p < 0.001) between leachate pH and P leaching at the first leaching event (T1) (Fig. S7B) support this view. Moreover, the significantly larger P leaching in the 15%OBC with AN treatment compared to NN treatment at T3 (20 days after nutrient addition) suggests that P availability was higher in this treatment for most of the time of the experiment. Since the N recovery is ~ 93% in the 15%OBC, the oxidation of NH4+ to NO3− and then leaching of NO3− likely were minimal in this treatment. Therefore, changes in soil pH attributed to oxidation of NH4+ were most likely also small.
Competitive interactions between soil organic matter and fixed P is one of the dominant mechanisms controlling P availability in P-fixing soils (Chassé and Ohno 2016). Since the Dermosol contained substantial Fe/Al oxide minerals (Viscarra Rossel et al. 2009), it is likely that competitive interactions between fixed phosphate and oxidized biochar (containing a large negative surface area) caused some of the fixed P to go into solution (Hiemstra et al. 2013; Schneider and Haderlein 2016). Moreover, it is also possible that enhanced microbial P cycling might have contributed to the increase in plant P uptake in 15%OBC treatment, which is supported by the significant positive relationship between plant P uptake and microbial biomass C (r2 = 0.28, p < 0.001, Fig. S7A). Additionally, it is possible that oxidation of NH4+ to NO3− in the AN treatment could have intensified competitive interactions between two negatively charged ions (NO3− and PO43−) resulting in a greater availability and leaching of P in the 15%OBC treatment. However, we believe that biochar effects on the competitive interactions between these two anions would be small, since we did not find large increases of NO3−-N concentration in the oxidized biochar treatment receiving AN (Table 1).
Implications and limitations of the study
Although chemical oxidation of biochar with H2O2 may not be exactly similar to the natural aging process, chemically oxidized biochar carries somewhat similar properties such as large surface carboxylic and phenolic groups to that of naturally aged chars (Cheng et al. 2008; Mao et al. 2012; Mia et al. 2017a). During natural aging, a significant part of biochar surface might be blocked with soil organic matter and minerals (de la Rosa et al. 2018; Sorrenti et al. 2016). However, part of the fresh and oxidized biochar pores may have been blocked during first phase of growth (50 days) before treatments started, particularly in the clay-rich soils intensively grown with grass and clover mixture. Moreover, with our previous studies, we observed that surface charge is more important than surface area in retaining NH4+ (Mia et al. 2017b). Therefore, we think that results from our study using chemically oxidized biochar provide valuable information about the future role of naturally aged biochar on nutrient dynamics.
The fertilizer application rates in this study were purposefully high, although not uncommon for grasslands that are intensively grazed. The pots we used were also small and three large leaching events were conducted equivalent to 300 mm rain events each. We therefore caution with extrapolating our results to field situations. Rather, our results provide a mechanistic understanding of how N and P dynamics are affected by fresh and oxidized biochar in a plant-soil system.
Conclusions
We observed a coupled increase of 15N recovery and plant P uptake in the oxidized biochar treatments (5%OBC and particularly 15%OBC) receiving AN-based fertilizer due to increased retention of NH4+-N at cation exchange sites and greater P availability, possibly because the oxidized biochar buffered soil solution pH or intensified the competition between oxidized biochar and phosphate for positively charged mineral surfaces. Therefore, our results suggest that combined application of oxidized biochar and AN-based fertilizers could potentially increase agricultural productivity. We underscore the fact that oxidation of biochar could increase the risk of phosphate and nitrate leaching from top soil layers with potential consequences for water pollution.
References
Araújo SR, Söderström M, Eriksson J, Isendahl C, Stenborg P, Demattê JAM (2015) Determining soil properties in Amazonian Dark Earths by reflectance spectroscopy. Geoderma 237–238:308–317
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Australian Bureau of Statistics (ABS) (2016) Land management and farming in Australia, 2015–16. Available at http://www.abs.gov.au/ausstats/abs@.nsf/mf/4627.0. Accessed 5 Sep 2017
Beck T, Joergensen RG, Kandeler E, Makeshin E, Nuss E, Oberholzer HR, Scheu S (1997) An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol Biochem 29:1023–1032
Bray RH, Kurtz LT (1945) Determination of total, organic and available forms of phosphorus. Soil Sci 59:39–45
Brookes PC, Landman A, Prudent G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Bruulsema TW, Duxbury JM (1996) Simultaneous measurement of soil microbial nitrogen, carbon, and carbon isotope ratio. Soil Sci Soc Am J 60:1787
Cassman KG, Dobermann A, Walters DT (2002) Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31:132–140
Chapman HD (1965) Cation-exchange capacity. In: Black CA (ed) Methods of soil analysis—chemical and microbiological properties. Agronomy 9:891–901
Chassé AW, Ohno T (2016) Higher molecular mass organic matter molecules compete with orthophosphate for adsorption to iron (oxy)hydroxide. Environ Sci Technol 50:7461–7469
Chen H, Zhang W, Gurmesa GA, Zhu X, Li D, Mo J (2017) Phosphorus addition affects soil nitrogen dynamics in a nitrogen-saturated and two nitrogen-limited forests. Eur J Soil Sci 68:472–479
Cheng C-H, Lehmann J (2009) Ageing of black carbon along a temperature gradient. Chemosphere 75:1021–1027
Cheng C-H, Lehmann J, Engelhard MH (2008) Natural oxidation of black carbon in soils: changes in molecular form and surface charge along a climosequence. Geochim Cosmochim Acta 72:1598–1610
Clough T, Condron L, Kammann C, Müller C (2013) A review of biochar and soil nitrogen dynamics. Agronomy 3:275–293
de la Rosa JM, Rosado M, Paneque M, Miller AZ, Knicker H (2018) Effects of aging under field conditions on biochar structure and composition: implications for biochar stability in soils. Sci Total Environ 613–614:969–976
Donn MJA, Menzies NWA (2005) The effect of ionic strength variation and anion competition on the development of nitrate accumulations in variable charge subsoils. Australian J Soil Res 43:43–50
Eick MJ, Brady WD, Lynch CK (1999) Charge properties and nitrate adsorption of some acid southeastern soils. J Environ Qual 28:138–144
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seablom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Esfandbod M, Phillips IR, Miller B, Rashti MR, Lan ZM, Srivastava P, Singh B, Chen CR (2017) Oxidized acidic biochar increases nitrogen retention and decreases ammonia volatilization in alkaline bauxite residue sand. Ecol Eng 98:157–165
Gao S, DeLuca T, Cleveland C (2019) Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: a meta-analysis. Sci Total Environ 654:463–472
Gronwald M, Helfrich M, Don A, Fuß R, Well R, Flessa H (2018) Application of hydrochar and pyrochar to manure is not effective for mitigation of ammonia emissions from cattle slurry and poultry manure. Biol Fertil Soils 54:451–465
Güereña D, Lehmann J, Hanley K, Enders A, Hyland C, Riha S (2013) Nitrogen dynamics following field application of biochar in a temperate North American maize-based production system. Plant Soil 365:239–254
Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Møller IS, White P (2011) Functions of macronutrients. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, London, pp 191–248
He M, Dijkstra FA (2015) Phosphorus addition enhances loss of nitrogen in a phosphorus-poor soil. Soil Biol Biochem 82:99–106
Hiemstra T, Mia S, Duhaut PB, Molleman B (2013) Natural and pyrogenic humic acids at goethite and natural oxide surfaces interacting with phosphate. Environ Sci Technol 47:9182–9189
Hüppi R, Neftel A, Lehmann MF, Krauss M, Six S, Leifeld J (2016) N use efficiencies and N2O emissions in two contrasting, biochar amended soils under winter wheat—cover crop—sorghum rotation. Environ Res Lett 11:84013
IPCC (Intergovernmental Panel on Climate Change) (2018) Mitigation pathways compatible with 1.5°C in the context of sustainable development in global warming of 1.5°C. pp 93–174
Isbell R (2002) The Australian soil classification, 4th edn. CSIRO Publishing, Collingwood, Vicotira, Australia, pp 1–144
Jackson ML (1958) Soil chemical analysis. Prentice-Hall, Inc, Englewood Cliffs, NJ
Jeffery S, Abalos D, Spokas K, Verheijen FGA (2015) Biochar effects on crop yields. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science, technology and implementation. Earthscan, London, pp 301–326
Jeffery S, Abalos D, Prodana M, Bastos A, van Groenigen JW, Hungate B, Verheijen F (2017) Biochar boosts tropical but not temperate crop yields. Environ Res Lett 12:053001
Jin Z, Chen X, Chen C, Tao P, Han Z, Zhang X (2016) Biochar impact on nitrate leaching in upland red soil, China. Environ Earth Sci 75:1–10
Jones DL, Rousk J, Edwards-Jones G, DeLuca TH, Murphy DV (2012) Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol Biochem 45:113–124
Keith A, Singh B, Dijkstra FA, Van Ogtrop F (2016) Biochar field study: greenhouse gas emissions, productivity, and nutrients in two soils. Agron J 108:1–11
Kookana RS, Sarmah AK, Van Zwieten L, Krull E, Singh B (2011) Biochacar application to soil: agronomic and environmental benefits and unintended consequences. Adv Agron 112:103–143
Laird D, Fleming P, Wang B, Horton R, Karlen D (2010) Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158:436–442
Lassaletta L, Billen G, Grizzetti B, Angade J, Garnier J (2014) 50 year trends in nitrogen use efficiency of world cropping systems: the relationship between yield and nitrogen input to cropland. Environ Res Lett 9:105011
Lawrinenko M, Laird DA, Johnson RL, Johnson RL, Jing D (2016) Accelerated aging of biochars: impact on anion exchange capacity. Carbon 103:217–227
Lee SCK, Rosenani AB, Ishak CF, Rahim KA, Meyer-Aurich A (2017) 15N-labelled fertiliser recovery by maize (Zea mays L.) and leaching of nutrients as influenced by oil palm empty fruit bunch biochar in a mini-lysimeter under controlled tropical environment. Arch Agron Soil Sci 63:1711–1724
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O'Neill B, Skjemstad JO, Thies J, Luizão FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719
Madiba OF, Solaiman ZM, Carson JK, Murphy DV (2016) Biochar increases availability and uptake of phosphorus to wheat under leaching conditions. Biol Fertil Soils 52:439–446
Magdoff FR, Bartlett RJ (1985) Soil pH buffering revisited. Soil Sci Soc Am J 49:145–148
Mao J-D, Johnson RL, Lehmann J, Olk DC, Veves EG, Thompson ML, Schmidt-Rohr (2012) Abundant and stable char residues in soils: implications for soil fertility and carbon sequestration. Environ Sci Technol 46:9571–9576
Mehnaz KR, Dijkstra FA (2016) Denitrification and associated N2O emissions are limited by phosphorus availability in a grassland soil. Geoderma 284:34–41
Mia S, Dijkstra FA, Singh B (2017a) Long-term aging of biochar: a molecular understanding with agricultural and environmental implications. Adv Agron 141:1–51
Mia S, Dijkstra FA, Singh B (2017b) Aging induced changes in biochar’s functionality and adsorption behavior for phosphate and ammonium. Environ Sci Technol 51:8359–8367
Mia S, Singh B, Dijkstra FA (2017c) Oxidized biochar affects gross nitrogen mineralization and recovery: a 15N study in two contrasting soils. GCB Bioenergy 9:1196–1206
Mia S, Dijkstra FA, Singh B (2018) Enhanced biological nitrogen fixation and competitive advantage of legumes in mixed pastures diminish with biochar aging. Plant Soil 424:639–651
Neumann G, Römheld V (2011) Rhizosphere chemistry in relation to plant nutrition. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Presss, Elsevier Ltd., Tokyo, pp 347–368
Prendergast-Miller MT, Duvalla M, Sohia SP (2014) Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur J Soil Sci 65:173–185
Schneider F, Haderlein SB (2016) Potential effects of biochar on the availability of phosphorus—mechanistic insights. Geoderma 277:83–90
Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen Z, Zhang W, Zhang F (2011) Phosphorus dynamics: from soil to plant. Plant Physiol 156:997–1005
Singh BP, Hatton BJ, Singh B, Cowie AL, Kathuria A (2010) Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J Environ Qual 39:1224–1235
Singh B, Dolk MM, Shen Q, Camps-Arbestain M (2017) Biochar pH, electrical conductivity and liming potential. In: Singh B, Camps-Arbestain M, Lehmann J (eds) Biochar: a guide to analytical methods, 1st edn. CRSIRO Publishing, Melbourne, Australia, pp 23–38
Sorrenti G, Masiello CA, Dugan B, Toselli M (2016) Biochar physico-chemical properties as affected by environmental exposure. Sci Total Environ 563–564:237–246
Stark JM, Hart SC (1996) Diffusion technique for preparing salt solutions, Kjeldahl digests, and persulfate digests for nitrogen-15 analysis. Soil Sci Soc Am J 60:1846
Steiner C, Glaser B, Teixeira WG, Lehmann J, Blum WEH, Zech W (2008) Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J Plant Nutr Soil Sci 171:893–899
Stevens PA, Harrison AF, Jones HE, Williams TG, Hughes S (1993) Nitrate leaching from a Sitka spruce plantation and the effect of fertilization with phosphorus and potassium. For Ecol Manag 58:233–247
Teutscherova N, Vazquez E, Masaguer A, Navas M, Scow KM, Schmidt R, Benito M (2017) Comparison of lime- and biochar-mediated pH changes in nitrification and ammonia oxidizers in degraded acid soil. Biol Fertil Soils 53(7):811–821
van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
Ventura M, Sorrenti G, Panzacchi P, Goerge E, Tonon G (2013) Biochar reduces short-term nitrate leaching from a horizon in an apple orchard. J Environ Qual 42:76
Viscarra Rossel RA, Cattle SR, Ortega A, Fouad A (2009) In situ measurements of soil colour, mineral composition and clay content by vis-NIR spectroscopy. Geoderma 150:253–266
Wang B, Lehmann J, Hanley K, Hestrin R, Enders A (2015) Adsorption and desorption of ammonium by maple wood biochar as a function of oxidation and pH. Chemosphere 138:120–126
Warnock DD, Lehmann J, Kuyper TW, Rilling MC (2007) Mycorrhizal responses to biochar in soil—concepts and mechanisms. Plant Soil 300:9–20
Yao Y, Gao B, Zhang M, Inyang M, Zimmerman AR (2012) Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 89:1467–1471
Zhang KL, Chen L, Li Y, Brookes PC, Xu JM, Luo Y (2017) The effects of combinations of biochar, lime, and organic fertilizer on nitrification and nitrifiers. Biol Fertil Soils 53(1):77–87
Acknowledgments
We are grateful to Dr. Claudia Keitel for her help in analyzing our samples.
Funding
Shamim recognizes the funding of the Ph.D. project by The University of Sydney.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1156 kb)
Rights and permissions
About this article
Cite this article
Mia, S., Singh, B. & Dijkstra, F.A. Chemically oxidized biochar increases ammonium-15N recovery and phosphorus uptake in a grassland. Biol Fertil Soils 55, 577–588 (2019). https://doi.org/10.1007/s00374-019-01369-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-019-01369-4