Abstract
Nitrogen (N) loss as ammonia (NH3) from agricultural systems is one of the major sources of atmospheric pollutants and is responsible for more than 50% of global NH3 emissions. Ammonia volatilization from animal manures may be altered by amendment with chars derived from pyrolysis (pyrochars) or hydrothermal carbonization (hydrochars) by providing exchange sites for ammonium (NH4+) or changing the pH of manure. Pyrochar and hydrochar differ in chemical and structural composition, specific surface area, and pH and therefore may affect NH3 volatilization differently. In a laboratory incubation experiment, we investigated the effect of pyrochar (pH 9.0) and hydrochar (pH 3.8) from Miscanthus on NH3 emission after addition to poultry manure and cattle slurry. We analyzed manure treatments with and without char addition and acidification and determined the effect of char addition on immobilization of manure-derived NH4+. Ammonia emission from pure poultry manure amounted 84% of the applied NH4+-N, while 67% of the applied NH4+-N was lost as NH3 from cattle slurry. Addition of pyrochar or hydrochar had no or only marginal effects on NH3 emissions except for a reduction in NH3 emissions by 19% due to hydrochar application to CS (p < 0.05), which seems to be primarily related to the char pH. Sorption of NH4+ by admixture of chars to manure was generally small: between 0.1- and 0.5-mg NH4+-N g−1 chars were sorbed. This corresponds to between 0.1 and 3.5% of the NH4+ applied, which obviously was not strong enough to reduce emissions of NH3. Overall, our results do not provide evidence that addition of pyrochar or hydrochar to cattle slurry and poultry manure is an effective measure to reduce NH3 volatilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural systems are responsible for more than 90% of total ammonia (NH3) emissions in Europe (Erisman et al. 2008) and more than 50% of the NH3 emissions worldwide (Bouwman et al. 1997). Emissions of NH3 result in atmospheric deposition of nitrogen (N), which can destabilize natural and semi-natural terrestrial ecosystems, where biomass growth is often N limited and native species are adapted to low N availability. Application of animal manures such as cattle slurry (CS) or poultry manure (PM) to agricultural soils is one of the major sources of NH3 emission that results in various threats to the environment (Beusen et al. 2008). Atmospheric deposition of NH3 contributes to water eutrophication and soil acidification and promotes indirect emission of the greenhouse gas nitrous oxide (N2O) (Karaca et al. 2004). In the “Thematic Strategy on Air Pollution” (CEC 2005), the European Commission aims at reducing around 30% of agricultural NH3 emissions in the EU25 by 2020 as compared to 2005. Common ways for the reduction of NH3 emissions in barns are filter systems and covering of storage tanks. For spreading on fields, low-emission field application techniques (e.g., direct injection, immediate incorporation, or surface-banded application with a trailing shoe (Horlacher and Marschner 1990; Webb et al. 2010), amendments of manure or soil with tannins (Jordan et al. 2015) or zeolithe (Kithome et al. 1999), as well as manure acidification are recommended mitigation measures (Kai et al. 2008). Still, NH3 remains a problem and new technologies are required to decrease NH3 volatilization from organic manure applied to soils and enhance the nutrient use efficiency of agricultural plant production.
Amendment of manure with char is discussed as a promising option to decrease NH3 volatilization, retain nutrients, and prevent leaching (Lehmann and Joseph 2009). Chars are the solid charcoal product of thermal conversion of a variety of organic feedstock materials, such as wood and other forestry as well as agricultural residues or sewage sludge (Hale et al. 2013; Yao et al. 2012). At present, the two main processes for the production of chars intended for use in agriculture are slow pyrolysis and hydrothermal carbonization (HTC). Pyrolysis is the combustion and conversion of biomass at high process temperatures under oxygen-free conditions. HTC implements a low-temperature transformation process (temperatures between 180 and 300 °C) combined with high pressure (2 to 2.5 MPa) and water for several hours (Libra et al. 2011). In the following, we will refer to the solid product derived from pyrolysis and HTC as pyrochar and hydrochar, respectively. Large specific surface areas (SSA) as well as a high degree of aromaticity (Keiluweit et al. 2010; Lehmann et al. 2006) and recalcitrance against degradation or mineralization characterize pyrochars (Glaser et al. 2002; Schimmelpfennig and Glaser 2012). Hydrochars have a lower degree of carbonization and thus more aliphatic carbon (C) and smaller amounts of aromatic C as well as lower SSA compared to pyrochars (Eibisch et al. 2013; Libra et al. 2011; Titirici et al. 2007). Further, the pH of hydrochars is generally acidic, while pyrochars tend to have an alkaline pH. In addition to these general differences between pyrochar and hydrochar, their properties differ strongly depending on the specific feedstock, carbonization process parameters, and subsequent thermochemical reactions (Cantrell et al. 2012; Cao et al. 2011; Eibisch et al. 2015; Yao et al. 2012).
High NH3 emissions occur in particular when urea is converted to ammonium (NH4+) and CO3− via hydrolysis by microbial urease and when materials have high NH4+ contents and pH. Addition of char to manure can influence NH3 emissions either by affecting the pH, which in turn shifts the NH3(aq)/NH4+-equilibrium, or by sorbing NH4+ as a precursor of NH3. The amount of sorption depends on the SSA and the amount of acidic functional groups on the chars’ surface, which in turn is also pH-dependent (Kastner et al. 2009). Several studies focusing on soil-char mixtures have shown that leaching of nitrate (NO3−) and NH4+ from soils amended with pyrochar or hydrochar was reduced by sorption on the respective char (Bargmann et al. 2014; Ding et al. 2010; Laird et al. 2010; Sarkhot et al. 2012). Sorption of mineral N (i.e., NO3− and NH4+) was often found to be higher for pyrochars than hydrochars (Bandosz and Petit 2009; Ding et al. 2010; Gronwald et al. 2015). The addition of char to organic materials prone to NH3 volatilization reduced NH3 emissions due to surface interactions with NH4+ (e.g., Doydora et al. 2011; Malińska et al. 2014; Spokas et al. 2012; Steiner et al. 2010). However, these studies mainly focused on pyrochar, while only very few studies investigated hydrochar effects. For example, Schimmelpfennig et al. (2014) assessed the initial N losses via NH3 emissions after slurry application with and without hydrochar and pyrochar from Miscanthus × gigantheus and found that hydrochar reduced NH3-N losses to a significantly greater extent than pyrochar. They ascribed this to the acidic pH of hydrochar (pH 5.1 compared to a pH of 10 of pyrochar) and to the capacity of hydrochar for N microbial immobilization. Overall, studies concerning the effect of pyrochar and hydrochar on soils and manure-derived NH3 emissions in the temperate zone are rare, and the mechanisms underlying the interaction of different char-manure mixtures are not yet fully understood. Furthermore, most studies focused on pyrochar, and knowledge on the effect of pyrochar vs. hydrochar on the potential reduction of NH3 emissions is scarce.
The objectives of this study are (i) to investigate the effects of an admixture of pyrochar and hydrochar produced from the same substrate (Miscanthus × gigantheus) to different manure types (i.e., CS and PM) on NH3 emissions, (ii) to determine the effect of char type on pH and NH4+ sorption, and (iii) to analyze the effects of char admixture on dynamics of NH3 emission from CS and PM applied to the surface of a sandy loam. For this purpose, we conducted a laboratory incubation experiment under controlled conditions. We hypothesized that sorption of NH4+ will reduce NH3 emission more strongly than the char-induced pH effect. Second, we hypothesized that when applied to the soil surface, NH3 emissions from manure and char-amended manure are generally lower than for pure manure due to physicochemical interactions with the soil matrix. We expected the reduction of NH3 volatilization to be higher for pyrochar compared to hydrochar treatments.
Materials and methods
Production and general properties of pyrochars and hydrochars
Both hydrochar and pyrochar were produced from Miscanthus × giganteus. Miscanthus is an energy crop for use in the sustainable production of renewable fuels and chemicals. It has recently attracted considerable attention because of its great potential for biomass yield and because it is non-invasive (Brosse et al. 2012). In terms of biochar production, it may thus represent a low-cost byproduct available for use. Pyrochar was derived from 0.75-h pyrolysis at 750 °C in a Pyreg reactor. Hydrochar was carbonized in a tabular reactor (3 m−3) with water (1:10, w/w) for 11 h at 2 MPa and 200 °C by AddLogicLabs/SmartCarbon (Jettingen, Germany). To catalyze the dehydration process and to increase the C content in the solid product, citric acid powder was added to Miscanthus (Wang et al. 2010). All chars were dried at 40 °C and sieved (< 2 mm). The C and N contents were determined via dry combustion (TruSpec, LECO Corp., St. Joseph (MI), USA). Water-extractable organic C (WEOC) contents of the chars were quantified after extraction with deionized water (1:10, w/v) and 0.45-μm filtration by thermal oxidation with a TOC analyzer (DIMATOC2000, DIMATEC Essen, Germany). Hydrogen and oxygen contents of pyrochar and hydrochar as well as uncarbonized Miscanthus were determined with an elementary analyzer (Vario EL3, Elementag, Hanau, Germany). Element contents of chars (e.g., P, Ca, Mg, Na, K, S) were analyzed using inductively coupled plasma-optical emission spectroscopy (Varian Liberty 150). The pH value of the chars was determined in 0.01-M CaCl2 with a ratio of 1:5 (volume char/volume solution). Surface acidity was determined by base adsorption as described by Cheng and Lehmann (2009). Basic characteristics of feedstock, pyrochar, and hydrochar are listed in Table 1.
Soil properties
Soil was sampled from the Ap horizon (0–30 cm) of a Cambic Planosol at a cropland site in the North German lowland in December 2014 (mean annual temperature 8.8 °C, around 600-mm precipitation, 52° 36′ N, 10° 89′ E, 105 m a.s.l.). We took soil from four randomly distributed places on the cropland site with shovels. The material was transported in boxes. The site is managed according to common regional practice with conventional tillage and fertilization. Crop rotation was barley (Hordeum vulgare L.) (2012), mustard (Sinapis arvensis L.) (cover crop), sugar beet (Beta vulgaris L. ssp. vulgaris) (2013), and followed by winter wheat (Triticum aestivum L.) (2014). In preparation to the experiment, all four soil samples were mixed to one composite sample. The soil was sieved (< 4 mm) and stored for 4 weeks at 4 °C until the start of the experiment. The soil pH was measured in 0.01-M CaCl2 with a ratio of 1:5 (volume soil:volume solution). The water content was determined gravimetrically. Carbon and N contents were determined using dry combustion with an elemental analyzer (TruMac CN, LECO Corp., St. Joseph (MI), USA). Soil texture was determined by the combined sieve and pipette method. The soil had a pH of 6.5 and a water content of 28%. Organic C and total N concentrations were 11.6 and 1.2 g kg−1, respectively (C/N 9.9). The texture was sandy loam (67.1% sand, 21.7% silt, 11.2% clay).
Manure properties and preparation
As manure amendments, CS (dairy) and PM (layer) were used. Four subsamples of 6-L CS were collected from a covered slurry storage tank next to a research cowshed of the Friedrich-Loeffler-Institute of Animal Nutrition in Braunschweig (Germany) and mixed to obtain a representative slurry sample. The tanks had been filled continuously within a period of 2 weeks before sampling resulting in a storage time of CS of between 1 day to 2 weeks. The pH in CS was measured undiluted. After sampling, CS was stored closed for 3 days in a cold storage room at 4 °C until the start of the incubation experiment. Four 1-kg subsamples of PM were taken from one manure pit under the poultry coop of the Friedrich-Loeffler-Institute of Animal Welfare and Animal Husbandry in Celle (Germany) and were mixed afterwards to receive a representative PM sample. Storage time of PM was ≤ 24 h, because the manure pit is emptied daily. Before the pH measurement of PM, we diluted 5 g with 25 g deionized (DI) water (1:5). After sampling, PM was stored close for 4 days at 4 °C until the start of the incubation experiment.
Both manures were stirred and pH was measured after 10 min waiting time with a pH electrode. Dry matter content was determined gravimetrically after drying the manures at 105 °C for 24 h. Total N was measured on fresh manures using the Kjeldahl method. The NH4+ content in the manures was determined via steam distillation and titration using 10-g manure and 50-mL DI water. The manures’ properties are provided in Table 2.
NH3 volatilization experiment
Experimental design
An incubation experiment under controlled conditions in a climate chamber was conducted during a 144-h period, starting on day 1 at 6 a.m. and ending on day 7 at 6 a.m. The experimental duration was used in accordance to recent studies, which showed that most of NH3 volatilization from manures occurs during the first 7 to 10 days (Chen et al. 2013; Chowdhury et al. 2014; Jordan et al. 2015). Temperature was set to 10-°C air according to typical soil and air temperature in temperate climate at the time of manure field application in spring. In total, 26 soil/char/manure/acid treatments were established; each run in quadruplicate (Table 3).
Treatments without soil were carried out in order to evaluate the influence of the chars on NH3 volatilization from the pure manures. Therefore, 250-mL glass jars were used (manure layer thickness was ~ 3 to 4 mm; resulting headspace volume was around 240 mL). The applied amount of manure equaled 60 kg Nt ha−1 (2.41 g (dw) CS with 75.6-mg Nt; 2.59-g (dw) PM with 66.2-mg Nt). Char-CS mixtures were produced by either mixing 0.24 g of pyrochar or hydrochar with 2.41-g (dw) CS. Char-PM mixtures were produced by either mixing 0.26 g of pyrochar or hydrochar with 2.59-g (dw) PM. The added char amounts correspond to a 10% char addition to manures’ dry weight. We chose a relatively low char application rate, because high amounts of char (a) would not be implementable for farmers due to too high cost and (b) were found to lead to negative environmental impacts such as heavy metal accumulation, decrease in plant growth, or raising soil pH (e.g., Van Zwieten et al. 2010; Zimmerman et al. 2011). Since we wanted to keep the rate high enough to still be effective, we chose an application ratio in between the two tested by Steiner et al. (2010): they found a significant reduction of NH3 after application to poultry litter at a ratio of 1:4 but not at a ratio of 1:19. Therefore, we decided to keep our ratio in between these two application rates, but more close to the “effective” ratio of 1:4. To improve the mixing process of chars and PM, we added DI water 1:1 (fw/g water) to the mixture. Subtreatments with acid addition to the char/manure mixtures were conducted in order to shift the NH3(aq)/NH4+-equilibrium toward NH4+ to distinguish an additional pH effect induced by chars from NH4+ sorption due to their corresponding high SSA and to obtain more detailed information on pH as control of NH3 emission from char-manure mixtures. In the acidified treatments, sulfuric acid (H2SO4) was applied to one part of the manures 24 h before the experiment started. The target pH of the acidified manures was 5.5 to 5.8. With regard to the dry matter content of CS, we added a 25% H2SO4 to CS (0.02-mL acid:1-g fresh weight (fw) CS). Regarding to the dry matter contents of PM, we added 12.5% H2SO4 to PM (0.25-mL acid:1-g fw PM:0.75-mL H2O). Just before manures were applied to the jars, the pH was re-measured and adjusted back to the target pH of 5.5–5.8 (pH of acidified manures had increased up to 1–1.5 pH units for PM and 0.5–1 pH units for CS during the 24 h between first acidification and start of the incubation). To avoid a destruction of organic compounds in the manures during mixing, 98% sulfuric acid was diluted with DI water.
Treatments with soil were carried out in order to quantify the effect of either pyrochar or hydrochar on NH3 volatilization from manure applied to the soil surface. For this purpose, 797-g soil (dw, sieved to < 4 mm) was filled in a glass jar and compacted to a bulk density of 1.2 g cm−3 (soil layer thickness was ~ 20 cm; surface area was 88.25 cm2, headspace volume was around 250 mL). In the soil treatments, manure, manure-char, manure-acid, and manure-char-acid mixtures were applied to the soil surface in order to simulate surface-banded application of CS and spreading of PM on the soil surface as done in common field praxis. The viscosity of CS was comparable to a liquid yogurt. Therefore, the manure infiltrated about 3 to 5 mm into the soil. PM was more solid and therefore infiltrated only marginally into the soil. Viscosity of manures remained similar upon acidification.
All mixtures were filled into the glass jars that were closed with airtight lids, and a controlled fresh air flow (humidified air with a relative air humidity of 90% in order to prevent drying) was installed. During incubation, the water content of the soil was checked by weighing and corrected at days 3 and 5. Soil-manure treatments were irrigated with 2- to 4-g DI water to compensate the water loss of 0.25 to 0.5% caused by the air exchange. In the pure manure treatments, we added 1-g DI water to PM (drying of 19%) and 4 g to CS (drying of 16%).
Measurements and calculations
To determine NH3 emissions, we used the volatilization-diffusion experimental setup with forced-draft system similar to Rachhpalsingh and Nye (1986) and Roelcke et al. (1996). Humidified air (90% relative air humidity) was passed over the soil surface in the jars with an air flow of 16.3 exchange volumes min−1 (corresponding to a flow rate of 3.9 L min−1 per 250-mL jar and 4.1 L min−1 per 1-L jar) in accordance with Roelcke et al. (1996). The air-tight screw cap lids for every jar were designed similar to those of Pacholski et al. (2006). Ammonia was trapped as NH4+ in a subsequent gas wash flask containing 200 mL of 0.0125 NH3PO4. In a pre-study, a second trap was connected to the exhaust air of the first wash flask in order to establish that emitted NH3 would be completely trapped in the first wash flask. Samples from the flasks were analyzed for NH4+-N using a photometric continuous flow analyzer (SKALAR San++ Continuous-Flow Analyzer, Breda, The Netherlands). The amount of NH4+-N in the gas wash flask (NGWF) was calculated as follows:
where NCGWF and NBlind are the measured NH4+-N concentrations in the gas wash flask and blind vessel [mg L−1] and V is the volume of H3PO4 in the gas wash flask [L].
Manure pH during the 7-day incubation period
An additional set of samples was incubated under conditions similar to the volatilization experiment during the 7-day incubation period in order to determine the change of manures’ pH over time. Therefore, char/manure/acid treatments without soil were established in triplicate by using 250- and 1000-mL jars for PM and CS mixture, respectively. The jars were filled up with 300-g (fw) CS (corresponds to ca. 300 mL) and 40-g (fw) PM. Char and acid were applied in the same ratio as used in the NH3 volatilization experiment. The same forced-draft system was used to flush the headspace in the jars, but no subsequent gas wash flask was coupled. The pH values for manures were determined once a day during the 7-day period. For CS, pH was measured directly in the jar. To determine the pH of PM, a subsample of 5 g (fw) was diluted with 25-g deionized (DI) water (1:5) and shaken for 10 min. Afterwards, the pH was measured in the dilution with a pH electrode.
Sorption of NH4 +
Mineral nitrogen (Nmin = NO3−-N + NH4+-N) was determined for pure manure and manure-char treatments with and without acidification after 24 h. Therefore, an additional sample set of manure/char/acid mixtures without soil, similar to that used in the incubation experiment, was set up closed and stored airtight/unventilated for a 24 h at 10 °C. All samples were extracted by shaking for 1 h to a ratio 1:20 (sample:extracting agent) with 0.01-M CaCl2 as well as with 0.01-M KCl. Nitrogen concentrations of the extracts were determined by using photometric continuous flow analyzer (SKALAR San++ Continuous-Flow Analyzer, Breda, The Netherlands). The CaCl2 extracts were carried out in order to determine the NH4+ in solution, while the KCl extracts will extract sorbed Nmin as well as Nmin in solution. The difference in NH4+-concentration between extracts will give information on the amount of NH4+ sorbed to the chars.
Statistical analyses
Statistical analyses were conducted using R 3.4.2 (RCoreTeam 2017). A generalized additive model (Wood 2004, 2011; R package mgcv 1.8-20) was fit to the time response values of pH. Parametric effects as well as separate smoothers were used for different manure × acidification treatments and for different char treatments. Repeated measures were considered as random effects. When multiple pairwise comparisons were done, p values were adjusted according to Holm (1979).
Since the time course of cumulative NH3 emissions of non-acidified treatments followed a saturation curve, the asymptotic model
where F are the cumulative NH3 emissions (mg g−1 manure), A (% applied NH4+-N) denotes the asymptote of the saturation curve, t is time (hours), and κ (1/h) is an exponential decay constant fit to this data. We are aware that the chosen exponential saturation model for the description of time courses of NH3 emissions is not capable to account for sigmoidal time courses as observed for acidified treatments. However, fitting the curves to a sigmoidal-based model was tested but not possible. Therefore, model parameters cannot be compared for all treatments. The fit was done simultaneously to data from all non-acidified treatments using the gnls function from R package nlme (Pinheiro and Bates 2000; Pinheiro et al. 2015). This allowed the parameters to be modeled depending on the different treatment factors, i.e., manure type, soil, char type, and acidification. Repeated measures were neglected here, since including random effects precluded successful convergence. However, diagnostic plots indicated that their variance would be very small and they could thus safely be neglected. The resulting analysis of variance (ANOVA) table can be found in Table S1. Table S2 shows the parameters of the fitted asymptotic model including the half-life of the applied NH4+-N in the soil-manure system. Temporal dynamics of NH3 emissions from acidified treatments did not follow an obvious and common model and were therefore not analyzed in this way.
Significance of differences between treatments’ cumulated NH3 emissions were analyzed using ANOVA followed by Tukey’s post hoc test (p < 0.05).
Results
Properties of the used chars and manures
The produced hydrochar had a pH of 3.8 and a SSA of 5.2 m2 g−1, while the pyrochar had a pH of 9.0 and a SSA of 279 m2 g−1 (Table 1). Surface acidity was lower for pyrochar (0.36 mmol H+ g−1) than hydrochar (5.84 mmol H+ g−1) (p < 0.001). Hydrochar had a lower ash content and lower C concentration compared to pyrochar, but a higher amount of water-extractable organic C (210 mg L−1 for hydrochar compared to 7 mg L−1 for pyrochar) (p < 0.001). The O/C and H/C ratios of pyrochar were smaller than for hydrochar, which reflects their higher degree of carbonization (p < 0.05). Concentrations of P, Ca, Mg, and K were higher in pyrochar than hydrochar. Only Na was abundant in lower amounts in pyrochar than hydrochar.
The used manures had dry matter contents of 10.3 (CS) and 50.4% (PM; Table 2). The pH value of CS was 7.2 and it contained 3.1-mg total N g−1 manure (fresh weight), 40% of which were NH4+-N, and PM had a pH value of 8.2 and contained 12.8-mg total N g−1 manure (fresh weight), 30% of which were NH4+-N.
Manure pH and changes during the 7-day incubation period
The pH in pure manures (i.e., without soil and amendments) increased from 8.2 to 9.7 for PM and from 7.2 to 7.5 for CS during the 144 h of incubation (Fig. 1a, b). The application of hydrochar (pH 3.8) and pyrochar (pH 9.0) to either CS or PM did not lead to a change in the initial pH for either manure type. However, it changed the development of pH during the experimental time (p < 0.05): hydrochar application to both manure types slowed down the rate and extent of pH increase, while pyrochar application accelerated the rate and extent of pH increase ending up with lower (manure + hydrochar) and higher (manure + pyrochar) pH values, respectively, compared to manures without char amendment.
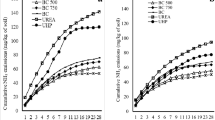
Temporal dynamics of pH values for manure and manure-char mixtures for a poultry manure and b cattle slurry and corresponding cumulative NH3-N lost in mg g−1 manure (dry weight) during 144 h of incubation without soil (c, d) and after application to soil (e, f). Means and standard errors (n = 4). For pH: the lines are the results from the fit to the time response values of pH modeled using the generalized additive model. For NH3-N emission: Solid lines indicate modeled NH3-N emission based on the fitted asymptotic model; dashed lines connect measured values for better visualization after cumulating
For the acidified treatments, the pH likewise increased over time from the adjusted pH of 5.5 to 5.8 to values of 6.2 to 6.6 (CS treatments) and 7.5 to 7.6 (PM treatments) after 144 h, respectively. For PM, the pH increase in the acidified treatments, i.e., with and without char, was most pronounced during the first 25 h of the experiment (increase from pH 5.5 to 5.8 to pH ~ 7) and only slightly increased further thereafter (Fig. 1a). In the CS treatments, the pH increased more or less linearly during the experimental time and remained < pH 7.0.
An F test comparison between the full generalized additive model and a model without the char effects indicated a highly significant char effect (p < 1e−13). Pairwise comparisons between char treatments were conducted by fitting models to data subsets with only two treatments. They confirmed that pH-time curves were different between all char treatments (p < 0.05, adjusted for multiple comparisons). Overall, pH values were highest for the pyrochar treatment, slightly lower for the treatment without char addition and considerably lower for the hydrochar treatment (Fig. 1a, b). The pyrochar and non-char treatments differed only by their mean pH values, whereas the shapes of the curves were not significantly different. The pH values of the hydrochar treatments followed a curve that was not only offset but also significantly (p < 1e−5) different in its shape compared to the other two treatments.
NH3 volatilization
Ammonia emissions from the treatments without soil (Fig. 1c, d) ranged from 0.3 to 8.4-mg NH3-N g−1 manure during the experimental time of 144 h. Highest emissions evolved from the pure manures. In pure PM, 84% of the applied NH4+-N was emitted as NH3-N during the incubation period. This corresponds to 25% of the applied Nt (Table 4). From pure CS, 67% of the applied NH4+-N was lost as NH3 within 144 h, corresponding to 27% of applied Nt. The modeled half-life of NH3 emissions from pure PM and CS was 9 and 16 h, respectively (Table S2). In the non-acidified treatments, the major part of the NH3 was volatized during the first 3 days of incubation. The application of char to the manures had no significant effect on the NH3 volatilization except for application of hydrochar to CS, which led to a reduction in NH3 volatilization from 8.4-mg g−1 manure to 6.8-mg g−1 manure (p < 0.05), corresponding to a reduction of 19% compared to pure CS (Fig. 2a). Still, the NH3 lost in this treatment corresponded to 54% of the NH4-N applied or 22% of the Nt applied (Table 4).
Percentual change in NH3 emissions compared to pure manure for manure-char and manure-acid-char composites a without and b with soil for poultry manure (PM) and cattle slurry (CS). Means and standard errors (n = 4). Different letters indicate significant differences within same manure treatment (p < 0.05)
The acidification of pure manures induced a lag-time of about 25 h with no or just very low NH3 volatilization for both treatments with and without char application (Fig. 1c, d). After the lag-time, small but continuous NH3 emission rates were observed, resulting in a linear increase of cumulative NH3-N losses. Emissions were higher in the acidified PM treatments than the acidified CS treatments. After 144 h, the NH3 emissions in the acidified treatments had not yet reached a plateau similar to that of the non-acidified treatments at the end of the experimental time. Overall, cumulative NH3 loss in acidified pure manures amounted 34 and 3% of the applied NH4-N for PM + acid and CS + acid (Table 4). This corresponds to an overall reduction in NH3 volatilization by 59% (PM + acid) and 95% (CS + acid) upon acidification compared to the respective pure manures during the observation period of 144 h (Fig. 2a). Also in manure-char mixtures, acidification generally led to a marked decrease of NH3 volatilization of between 50 and 75% compared to pure PM and 93 to 97% compared to pure CS (Fig. 2). Char addition to acidified manures did not lead to significant changes in NH3 emission compared to pure acidified manure (Figs. 1c, d and 2a).
For the manure + soil treatments, the NH3 volatilization was generally lower than from pure manure with and without char (Fig. 1c–f). From PM + soil, 3.5-mg NH3-N g−1 manure was lost compared to 6.4-mg NH3-N g−1 manure in pure PM, which corresponds to a reduction of 46% due to application to soil (Fig. 1c, e). Application of CS to soil decreased the NH3 volatilization from 8.4- to 3.3-mg NH3-N g−1 manure (reduction of 61%; Fig. 1d, f). Overall, this corresponds to a loss of 26% of the NH4+-N applied or 11% of Nt, Table 4). The modeled half-life of NH3-emissions was 13 and 20 h for PM + soil and CS + soil, respectively (Table S2). When hydrochar was applied to the manures, 45 to 47% and 25 to 26% of the applied NH4+-N were lost from PM and CS treatments, respectively (Table 4). For pyrochar, no effect on NH3 emission was observed for PM (loss of 48% cp. to 45% of the applied NH4+-N), but a significantly higher NH3 emission was observed from CS + pyro + soil (32.5% of the applied NH4+-N) than CS + soil (26.4% of the applied NH4+-N).
Acidification of the manure + char + soil mixtures resulted in significantly lower NH3-N emissions compared to mixtures without acid (Fig. 1e, f). Overall, the reductions upon acidification were up to 85% for PM mixtures and 82% for CS mixtures when compared to non-acidified manure + char + soil (Fig. 2b). Overall, only 4 to 7% and 7 to 10% of the applied NH4+-N was lost in acidified CS and PM treatments, respectively (Table 4). However, a significant effect of hydrochar or pyrochar application to acidified manures was not found. Cumulative NH3-N emissions continued to increase slightly after manure application until the end of the experiment in the treatments with the addition of acid. During this period, we observed constant low emission rates at every sampling time.
Sorption of NH4 + on hydrochar and pyrochar
Concentrations of NH4+-N in KCl extracts ranged from 11.4- to 14.4-mg g−1 manure (dw) for CS and from 5.2- to 7.1-mg g−1 manure for PM. Amounts of NO3−-N in the manure/char mixtures were negligible (≤ 0.02-mg g−1 manure for all investigated samples, data not shown). Overall, neither the applied pyrochar nor hydrochar sorbed significant amounts of NH4+-N from CS and PM with or without acid (0.4 to 3.5% of the applied NH4+-N were sorbed corresponding to amounts of between 0.1- and 0.5-mg sorbed NH4+-N g−1 char) (Fig. 3).
Sorption of NH4+-N in the manure-char (a, c) and manure-char-acid (b, d) composites for poultry manure (PM) and cattle slurry (CS) mixed with pyrochar or hydrochar after 24 h as mg g−1 manure (dry weight). Means and standard errors (n = 4). Different letters indicate significant differences within same manure treatment (p < 0.05)
Discussion
Influence of pyrochar and hydrochar amendment of manure on NH3 emissions
Generally, NH3 volatilization from animal manure is influenced by the NH4+ content, the manure pH, the NH3(aq)/NH4+-equilibrium, the N mineralization intensity, and physical factors affecting volatilization rates of NH3 (Martins and Dewes 1992). Poultry litter differs considerably in composition from other manures: it provides greater P and N concentrations as well as other micro- and macronutrients (Mitchell and Tu 2006; Stephenson et al. 1990). Despite greater total N concentration in the PM used in the present study compared to CS, the proportion of NH4+-N was lower in PM (27% of total N) than CS (43%), which was also reflected in lower NH3 emissions from pure PM than pure CS.
Char application may influence NH3 volatilization from manure by shifting the NH3(aq)/NH4+ equilibrium through pH changes or by sorption of NH4+ onto the chars’ surfaces. The sorption capacity of char results from cation and anion exchange, as well as SSA, internal porosity, and surface functional groups (Hale et al. 2013; Laird et al. 2010; Lehmann and Joseph 2009). Especially pyrochars, which have an up to 50-fold higher SSA compared to hydrochars, are thought to promote sorption of NH4+ (Eibisch et al. 2015; Lehmann and Joseph 2009; Schimmelpfennig and Glaser 2012). In contrast to pyrochars, only marginal capacity of hydrochar for NH4+-N sorption was found (Gronwald et al. 2015; Yao et al. 2012). A reduction in NH3 emissions upon application of hydrochar therefore likely results from their low pH.
While several authors reported high sorption potential of pyrochar for NH3 (e.g., Asada et al. 2002; Doydora et al. 2011; Kastner et al. 2009; Steiner et al. 2010; Taghizadeh-Toosi et al. 2011), application of pyrochar in our study had no effect on NH3 emissions and only sorbed marginal amounts of NH4+. In a previous study, Gronwald et al. (2015) investigated the sorption behavior of the chars and soil used in this study as well as other sandy and loamy soils and chars from different feedstock materials. In a batch experiment, they found that sorption of NH4+ on pyrochar from Miscanthus correlated with pore volume and ash content (p ≤ 0.01) and was strongly non-linear with increasing nutrient solution. The latter indicates a limited number of cation exchange sites of the char (Hale et al. 2013). Further, during field incubation, Gronwald et al. 2015 found that the sorptive capacity of pyrochar diminished within months. These findings may partly explain the ineffectiveness of pyrochar to reduce NH3 emissions observed here. Due to the limited number of cation exchange sites of the char, the high amounts of manure-derived NH4+ may have exceeded the sorptive capacity of the char used. In accordance with this, previous studies that reported significant reduction of NH3 emissions used higher char application rates. While we applied chars to the manures at a ratio of 1:10 (dw/dw), Steiner et al. (2010) used a mixture of 1:4 (dw/dw) and Doydora et al. (2011) even added acidified pyrochars from pine chips and peanut hulls to poultry litter at a ratio of 1:1 (dw/dw). Consequently, a markedly lower number of functional groups and SSA was available for NH4+ sorption in our char treatments. In their second treatment with an application of pyrochar/poultry litter of 1:19 (dw/dw), Steiner et al. (2010) also found no significant reduction of NH3 emissions. Another reason may be differences in the pyrolysis temperature: while the pyrochar used in the present study was produced at 750 °C, the chars used in the above mentioned studies which reported a reduction in NH3 emissions were produced at temperatures < 500 °C. A high pyrolysis temperature was chosen in this study, because we hypothesized that pyrochar would reduce NH3 emissions due to sorption of NH4+ and that sorption would increase with SSA as suggested in a range of studies (e.g., Hale et al. 2013; Keiluweit et al. 2010; Lehmann et al. 2006). While SSA and cation exchange capacity (CEC) increase with increasing pyrolysis temperature, the amount of acidic surface functional groups decreases. This is also reflected in the pyrochars’ pH (Chun et al. 2004; Lehmann 2007). Taghizadeh-Toosi et al. (2012) found an increasing pH and a decrease in acidic functional groups with increasing production temperature of pyrochar from pine chips with increasing temperature from 300 to 500 °C and reported a close relationship between pyrochar pH/surface acidity and the amount of NH4+-N taken up. The pyrochar used in this study had a surface acidity of 0.36 mmol H+ g−1, which is considerably lower than that observed for low-temperature pyrochars (1.3 to 2.8 mmol H+ g−1, e.g., Chun et al. 2004; Taghizadeh-Toosi et al. 2012). Similar to our study, Subedi et al. (2015) found no reduction of NH3 emissions after pyrochar addition to pig slurry. Their char was produced under conditions comparable to our pyrochar and had similar physicochemical properties (surface acidity and SSA of 249.9 m2 g−1 compared to 279 m2 g−1 and a pH of 10.1 compared to pH 9.0 of our pyrochar). Therefore, we assume that surface acidity is more important for mitigation of NH3 emissions than a high SSA. Consequently, production temperature is a major factor determining the efficacy of pyrochar to reduce NH3 emissions.
Only few studies so far investigated the NH3 mitigation potential of hydrochar application to manure. While pyrochar treatments showed no reduction of NH3 emissions regardless of the manure type used, the application of hydrochar had no effect on NH3 emissions for PM but reduced NH3 emissions from CS by 19%. Since sorption of NH4+ was found to be marginal for both, CS and PM, the reduction likely resulted from the low pH of hydrochar. Similar to our results, Schimmelpfennig et al. (2014) reported significantly lower NH3 emissions from pig slurry amended with hydrochar from Miscanthus compared to pyrochar-amended pig slurry and assumed hydrochar acidity (pH 5.1) to be responsible for the lower NH3 emissions. Reasons for the different effectiveness of hydrochar application between PM and CS may be differences in manure pH (CS 7.2, PM 8.2) or different buffering capacities of the two types of manure, which will be discussed in the following chapter.
Development of NH3 emissions in acidified hydrochar and pyrochar treatments
Generally, animal slurries have a strong buffering capacity. The major components influencing the pH system are total ammoniacal N (TAN), total inorganic C (TIC), volatile fatty acids (VFA), and the solid phases of calcite (CaCO3) and struvite (MgNH4PO4·6H2O) (Sommer and Husted 1995a). The pH is mainly governed by the species NH4+/NH3, CO2/HCO3−/CO32−, and CH3COOH/CH3COO−. Calcite and struvite dissolve when the pH is < 7 and < 6, respectively, so that these solid phase components will only contribute to the buffering capacity at pH values below these levels. The high buffering capacity of the manures was reflected in our finding that application of hydrochar (with a pH of 3.8 compared to manure pHs of 7.2 and 8.2) had no direct effect on initial manure pH. The alkalinity of pyrochar as well as the amount of acids in the hydrochar were too low to directly change the manure pH. Further, considerable amounts of acid were needed to reach the target pH of 5.5 to 5.8 upon acidification (0.02-mL 25% H2SO4, 1-g fw CS; 0.25-mL 12.5% H2SO4:1-g fw PM:0.75-mL H2O), which again reflects a high buffering capacity. Overall, the amount of acid needed was higher for PM than CS, likely due to a higher buffering capacity and acid neutralizing capacity of PM. Consequently, very high amounts of hydrochar would be needed to cause a decrease of the initial pH.
Acidification strongly decreased total NH3 emissions during the first 6 days after application and changed emission dynamics. It reduced NH3 emissions from PM and CS by 59 to 96% compared to the respective non-acidified treatments, which is in the range reported by others (Kai et al. 2008; Petersen et al. 2012; Sommer et al. 2017). During the first 25 h of the experiment, a lag-time with pH values ≤ 6 and no NH3 volatilization were observed. With time of incubation, the pH values increased in all treatments. Potential processes leading to pH increases with the consequence of increasing potential of NH3 emissions are urea hydrolysis and degradation of slurry-borne VFA and slow pH buffer reactions (Ferguson et al. 1984; Sørensen 1998). The time span with low and constant NH3 emissions depends on the adjusted pH (Dai and Blanes-Vidal 2013) and on the buffering capacity of the manure (Cassity-Duffey et al. 2015; Sommer and Husted 1995b). Overall, in our study, the pH increase during the experimental time was much more pronounced in the acidified PM treatments than the CS treatments: in the PM treatments, a pH > 7 was already reached 24 h after the start of the experiment, while the pH remained < 7 throughout the experimental time in all acidified CS treatments. This quick pH increase in the PM + acid treatment also points to a higher buffering capacity of PM than CS. In CS + hydro + acid, the pH remained ≤ 6 during the first 120 h and only increased to 6.2 at the end of the experiment. The different developments of the pH values between PM and CS during the experimental time likely derive from a different composition of the buffer system and the resulting differences in the buffering capacity in these two manure types. With a wide range of both inorganic and organic components in poultry litter, there are probably many mechanisms participating in buffering, but the main mechanisms in poultry litter seem to be CaCO3, organic matter, and VFA (Cassity-Duffey et al. 2015).
Overall, the results from our study suggest that acidification results in much lower but more persistent NH3 emission rates. It remains unclear whether the total NH3 emissions in the acidified treatments will at some point equalize those from the non-acidified treatments, because a plateau was not yet reached during the experimental time of 144 h. Dai and Blanes-Vidal (2013) reported that NH3 emission rates in acidified treatments equalized to emission rates compared to treatments without acidification when summarized over a period of 264 h. However, Sommer et al. (2017) investigated NH3 emissions from acidified and non-acidified CS over a period of 47 days and found that overall NH3 emissions of acidified manure remained lower than from non-acidified manure during the whole time.
Soil application of pyrochar and hydrochar-amended manure
When applied to soil, NH3 emissions from our manure treatments were generally lower than without soil. The total NH3 loss in the treatments with soil was up to 46% lower for PM and up to 62% lower for CS compared to the manure treatments without soil. This applies for both pure manures and char-amended manures irrespective of the char type added. This reduction resulted most likely from infiltration of manure into the pore space, which was more intense for CS than PM due to its higher water content and therefore higher viscosity. Infiltration of manures into pore space lowers NH3 emissions due to decreasing contact between manure and the atmosphere and NH4+ sorption and pH buffering in the soil matrix (Webb et al. 2010). Overall, several processes are known that may reduce the availability of NH4+, a precursor of NH3 when the manure comes into contact with the soil: (i) N microbial immobilization of NH4+ from manure into soil organic matter (Burger and Jackson 2003), (ii) NH4+ sorption on mineral surfaces (Gronwald et al. 2015) or fixation in clay minerals (Juang et al. 2001), (iii) oxidation of NH4+ to NO2− and NO3− by nitrification (Huang et al. 2014), and (iv) gaseous N losses as N2O from nitrification or N2O and N2 from denitrification coupled to nitrification (Wrage et al. 2001). Another reason for reduced NH3 emissions may be interactions between reduced urea hydrolysis through the presence of calcium carbonate (CaCO3) in soil (Fenn et al. 1981). However, the latter does not apply for our soil, which had a pH of 6.5 and no carbonate.
In the treatments with soil application, NH3 emissions remained similar after addition of pyrochar to manure compared to pure PM (loss of 48% of the applied NH4+-N in PM + pyro + soil compared to 45% of the applied NH4+-N in PM + soil). For CS, 32.5% of the applied NH4+-N was lost in CS + pyro + soil compared to 26.4% of the applied NH4+-N in CS + soil, which corresponds to an increase of NH3 volatilization by 23%. Similar to our results, Subedi et al. (2015) observed slightly higher NH3 emission from pig slurry applied to pyrochar-amended soil (23.5% of NH4+-N applied was lost in the pyrochar treatment compared to 18.2% in the control). They ascribed this to the alkaline pH of the char (pH 10.1), which may also be the reason in our study (pyrochar pH 9.0), especially since this effect was only seen for CS—the manure with the lower buffer capacity. Application of hydrochar to the manures had no significant influence on NH3 emissions when compared to pure manure. However, NH3 emissions were significantly lower from CS + hydro + soil than CS + pyro + soil. This is in line with findings from Schimmelpfennig et al. (2014), who investigated application of pig slurry to soil with and without hydrochar and pyrochar admixture and reported significantly lower NH3 emissions after application of pig slurry to a hydrochar-soil mixture compared to soil + pyrochar + slurry. Schimmelpfennig et al. (2014) ascribed the higher NH3 emissions from pyrochar treatments to its alkaline pH (pH 10.1) and the lower ones from hydrochar treatments to its acidity (pH 5.1) as well as a possible N microbial immobilizing capacity from the high amounts of easily available C compounds from hydrochar. Contrary to our results, Subedi et al. (2015) found an increase in NH3 emissions from pig slurry applied to hydrochar-amended soil (38.7% of NH4+-N applied compared to 26.4% in the control during 50 h of investigation). They explained this with a different infiltration behavior due to a possible hydrophobicity of the hydrochar, which they had mixed with the soil prior to slurry we mixed with manure prior to soil application. We found no difference in infiltration behavior due to char application, which may explain the higher emissions found in the study from Subedi et al. (2015).
Conclusions
We found no evidence of a clear and consistent reduction of NH3 emission from CS and PM by admixture of hydrochar (pH 3.8) or pyrochar (pH 9.0). The minor and opposite effects of char addition on NH3 emissions seem to be driven almost exclusively by the char’s effects on pH, even if measured pH changes induced by char addition to manure were marginal. Sorption of NH4+ in slurry and manure by char addition was generally low and slightly larger for the treatment with pyrochar addition than hydrochar addition. However, these effects were obviously not strong enough to effectively reduce emission of NH3. Acidification of CS and PM strongly decreased total NH3 emissions during the first 144 h after application and changed emission dynamics. The results suggest that acidification results in much lower but more persistent NH3 emission rates. All in all, our results do not provide evidence that addition of pyrochar or hydrochar to CS and PM is an effective measure to reduce NH3 volatilization.
References
Asada T, Ishihara S, Yamane T, Toba A, Yamada A, Oikawa K (2002) Science of bamboo charcoal: study on carbonizing temperature of bamboo charcoal and removal capability of harmful gases. J Health Sci 48:473–479. https://doi.org/10.1248/jhs.48.473
Bandosz TJ, Petit C (2009) On the reactive adsorption of ammonia on activated carbons modified by impregnation with inorganic compounds. J Colloid Interface Sci 338:329–345. https://doi.org/10.1016/j.jcis.2009.06.039
Bargmann I, Rillig MC, Kruse A, Greef JM, Kücke M (2014) Effects of hydrochar application on the dynamics of soluble nitrogen in soils and on plant availability. J Plant Nutr Soil Sci 177:48–58. https://doi.org/10.1002/jpln.201300069
Beusen AHW, Bouwman AF, Heuberger PSC, Van Drecht G, Van Der Hoek KW (2008) Bottom-up uncertainty estimates of global ammonia emissions from global agricultural production systems. Atmos Environ 42:6067–6077. https://doi.org/10.1016/j.atmosenv.2008.03.044
Bouwman AF, Lee DS, Asman WAH, Dentener FJ, Van Der Hoek KW, Olivier JGJ (1997) A global high-resolution emission inventory for ammonia. Glob Biogeochem Cycles 11:561–587. https://doi.org/10.1029/97GB02266
Brosse N, Dufour A, Meng X, Sun Q, Ragauskas A (2012) Miscanthus: a fast- growing crop for biofuels and chemicals production. Biofuels Bioprod Biorefin 6:580–598. https://doi.org/10.1002/bbb.1353
Burger M, Jackson LE (2003) Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol Biochem 35:29–36. https://doi.org/10.1016/S0038-0717(02)00233-X
Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428. https://doi.org/10.1016/j.biortech.2011.11.084
Cao XY, Ro KS, Chappell M, Li YA, Mao JD (2011) Chemical structures of swine-manure chars produced under different carbonization conditions investigated by advanced solid-state 13C nuclear magnetic resonance (NMR) spectroscopy. Energy Fuel 25:388–397. https://doi.org/10.1021/ef101342v
Cassity-Duffey K, Cabrera M, Mowrer J, Kissel D (2015) Titration and spectroscopic measurements of poultry litter pH buffering capacity. J Environ Qual 44:1283–1292. https://doi.org/10.2134/jeq2014.11.0463
Chen CR, Phillips IR, Condron LM, Goloran J, Xu ZH, Chan KY (2013) Impacts of greenwaste biochar on ammonia volatilisation from bauxite processing residue sand. Plant Soil 367:301–312. https://doi.org/10.1007/s11104-012-1468-0
Cheng CH, Lehmann J (2009) Ageing of black carbon along a temperature gradient. Chemosphere 75:1021–1027. https://doi.org/10.1016/j.chemosphere.2009.01.045
Chowdhury MA, de Neergaard A, Jensen LS (2014) Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere 97:16–25. https://doi.org/10.1016/j.chemosphere.2013.10.030
Chun Y, Sheng G, Chiou CT, Xing B (2004) Compositions and sorptive properties of crop residue-derived chars. Environ Sci Technol 38:4649–4655. https://doi.org/10.1021/es035034w
Commission of the European Communities (2005) Communication from the commission to the council and the European Parliament—thematic strategy on air pollution. URL: http://www.central2013.eu/fileadmin/user_upload/Downloads/Document_Centre/OP_Resources/03_Thematic_strategy_on_air_pollution_com_en.pdf
Dai XR, Blanes-Vidal V (2013) Emissions of ammonia, carbon dioxide, and hydrogen sulfide from swine wastewater during and after acidification treatment: effect of pH, mixing and aeration. J Environ Manag 115:147–154. https://doi.org/10.1016/j.jenvman.2012.11.019
Ding Y, Liu Y-X, Wu W-X, Shi D-Z, Yang M, Zhong Z-K (2010) Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Pollut 213:47–55. https://doi.org/10.1007/s11270-010-0366-4
Doydora SA, Cabrera ML, Das KC, Gaskin JW, Sonon LS, Miller WP (2011) Release of nitrogen and phosphorus from poultry litter amended with acidified biochar. Int J Environ Res Public Health 8:1491–1502. https://doi.org/10.3390/ijerph8051491
Eibisch N, Helfrich M, Don A, Mikutta R, Kruse A, Ellerbrock R, Flessa H (2013) Properties and degradability of hydrothermal carbonization products. J Environ Qual 42:1565–1573. https://doi.org/10.2134/jeq2013.02.0045
Eibisch N, Schroll R, Fuß R, Mikutta R, Helfrich M, Flessa H (2015) Pyrochars and hydrochars differently alter the sorption of the herbicide isoproturon in an agricultural soil. Chemosphere 119:155–162. https://doi.org/10.1016/j.chemosphere.2014.05.059
Erisman JW, Bleeker A, Hensen A, Vermeulen A (2008) Agricultural air quality in Europe and the future perspectives. Atmos Environ 42:3209–3217. https://doi.org/10.1016/j.atmosenv.2007.04.004
Fenn LB, Matocha JE, Wu E (1981) A comparison of calcium carbonate precipitation and pH depression on calcium-reduced ammonia loss from surface-applied urea. Soil Sci Soc Am J 45:1128–1131. https://doi.org/10.2136/sssaj1981.03615995004500060023x
Ferguson RB, Kissel DE, Koelliker JK, Basel W (1984) Ammonia volatilization from surface-applied urea: effect of hydrogen ion buffering capacity. Soil Sci Soc Am J 48:578–582. https://doi.org/10.2136/sssaj1984.03615995004800030022x
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fertil Soil 35:219–230. https://doi.org/10.1007/s00374-002-0466-4
Gronwald M, Don A, Tiemeyer B, Helfrich M (2015) Effects of fresh and aged chars from pyrolysis and hydrothermal carbonization on nutrient sorption in agricultural soils. Soil 1:475–489. https://doi.org/10.5194/soil-1-475-2015
Hale SE, Alling V, Martinsen V, Mulder J, Breedveld GD, Cornelissen G (2013) The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 91:1612–1619. https://doi.org/10.1016/j.chemosphere.2012.12.057
Holm S (1979) A Simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70. https://doi.org/10.2307/4615733
Horlacher D, Marschner H (1990) Schätzrahmen zur Beurteilung von Ammoniakverlusten nach Ausbringung von Rinderflüssigmist. J Plant Nutr Soil Sci 153:107–115. https://doi.org/10.1002/jpln.19901530209
Huang T, Gao B, Hu X-K, Lu X, Well R, Christie P, Bakken LR, Ju X-T (2014) Ammonia-oxidation as an engine to generate nitrous oxide in an intensively managed calcareous Fluvo-aquic soil. Sci Rep 4 doi:https://doi.org/10.1038/srep03950
Jordan G, Predotova M, Ingold M, Goenster S, Dietz H, Joergensen RG, Buerkert A (2015) Effects of activated charcoal and tannin added to compost and to soil on carbon dioxide, nitrous oxide and ammonia volatilization. J Plant Nutr Soil Sci 178:218–228. https://doi.org/10.1002/jpln.201400233
Juang TC, Wang MK, Chen HJ, Tan CC (2001) Ammonium fixation by surface soils and clays. Soil Sci 166:345–352. https://doi.org/10.1097/00010694-200105000-00005
Kai P, Pedersen P, Jensen JE, Hansen MN, Sommer SG (2008) A whole-farm assessment of the efficacy of slurry acidification in reducing ammonia emissions. Eur J Agron 28:148–154. https://doi.org/10.1016/j.eja.2007.06.004
Karaca S, Gurses A, Ejder M, Acikyildiz M (2004) Kinetic modeling of liquid-phase adsorption of phosphate on dolomite. J Colloid Interface Sci 277:257–263. https://doi.org/10.1016/j.jcis.2004.04.042
Kastner JR, Miller J, Das KC (2009) Pyrolysis conditions and ozone oxidation effects on ammonia adsorption in biomass generated chars. J Hazard Mater 164:1420–1427. https://doi.org/10.1016/j.jhazmat.2008.09.051
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253. https://doi.org/10.1021/es9031419
Kithome M, Paul JW, Bomke AA (1999) Reducing nitrogen losses during simulated composting of poultry manure using adsorbents or chemical amendments. J Environ Qual 28:194–201. https://doi.org/10.2134/jeq1999.00472425002800010023x
Laird D, Fleming P, Wang BQ, Horton R, Karlen D (2010) Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158:436–442. https://doi.org/10.1016/j.geoderma.2010.05.012
Lehmann J (2007) Bio-energy in the black. Front Ecol Environ 5:381–387. https://doi.org/10.1890/1540-9295(2007)5[381:BITB]2.0.CO;2
Lehmann J, Joseph S (2009) Biochar for environmental management: science and technology. Earthscan, London
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems—a review. Mitig Adapt Strat Glob Chang 11:403–427. https://doi.org/10.1007/s11027-005-9006-5
Libra JA, Ro KS, Kammann C, Funke A, Berge ND, Neubauer Y, Titirici MM, Fühner C, Bens O, Kern J, Emmerich KH (2011) Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2:71–106. https://doi.org/10.4155/bfs.10.81
Malińska K, Zabochnicka-Świątek M, Dach J (2014) Effects of biochar amendment on ammonia emission during composting of sewage sludge. Ecol Eng 71:474–478. https://doi.org/10.1016/j.ecoleng.2014.07.012
Martins O, Dewes T (1992) Loss of nitrogenous compounds during composting of animal wastes. Bioresour Technol 42:103–111. https://doi.org/10.1016/0960-8524(92)90068-9
Mitchell CC, Tu S (2006) Nutrient accumulation and movement from poultry litter. Soil Sci Soc Am J 70:2146–2153. https://doi.org/10.2136/sssaj2004.0234
Pacholski A, Cai GX, Nieder R, Richter J, Fan XH, Zhu ZL, Roelcke M (2006) Calibration of a simple method for determining ammonia volatilization in the field—comparative measurements in Henan Province, China. Nutr Cycl Agroecosyst 74:259–273. https://doi.org/10.1007/s10705-006-9003-4
Petersen SO, Andersen AJ, Eriksen J (2012) Effects of cattle slurry acidification on ammonia and methane evolution during storage. J Environ Qual 41:88–94. https://doi.org/10.2134/jeq2011.0184
Pinheiro J, Bates D (2000) Mixed-effects models in S and S-PLUS vol 1. Springer, New York. https://doi.org/10.1007/b98882
Pinheiro J, Bates D, Saikat D, Sarkar D (2015) nlme: linear and nonlinear mixed effects models R package version 31-122
Rachhpalsingh, Nye PH (1986) A model of ammonia volatilization from applied urea. 2. Experimental testing. J Soil Sci 37:21–29. https://doi.org/10.1111/j.1365-2389.1986.tb00003.x
RCoreTeam (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Roelcke M, Han Y, Li SX, Richter J (1996) Laboratory measurements and simulations of ammonia volatilization from urea applied to calcareous Chinese loess soils. Plant Soil 181:123–129. https://doi.org/10.1007/bf00011298
Sarkhot DV, Berhe AA, Ghezzehei TA (2012) Impact of biochar enriched with dairy manure effluent on carbon and nitrogen dynamics. J Environ Qual 41:1107–1114. https://doi.org/10.2134/jeq2011.0123
Schimmelpfennig S, Glaser B (2012) One step forward toward characterization: some important material properties to distinguish biochars. J Environl Qual 41:1001–1013. https://doi.org/10.2134/jeq2011.0146
Schimmelpfennig S, Müller C, Grünhage L, Koch C, Kammann C (2014) Biochar, hydrochar and uncarbonized feedstock application to permanent grassland—effects on greenhouse gas emissions and plant growth. Agric Ecosyst Environ 191:39–52. https://doi.org/10.1016/j.agee.2014.03.027
Sommer SG, Husted S (1995a) The chemical buffer system in raw and digested animal slurry. J Agric Sci 124:45–53. https://doi.org/10.1017/S0021859600071239
Sommer SG, Husted S (1995b) A simple model of pH in slurry. J Agric Sci 124:447–453. https://doi.org/10.1017/S0021859600073408
Sommer SG, Clough TJ, Balaine N, Hafner SD, Cameron KC (2017) Transformation of organic matter and the emissions of methane and ammonia during storage of liquid manure as affected by acidification. J Environ Qual 46:514–521. https://doi.org/10.2134/jeq2016.10.0409
Sørensen P (1998) Carbon mineralization, nitrogen immobilization and pH change in soil after adding volatile fatty acids. Eur J Soil Sci 49:457–462. https://doi.org/10.1046/j.1365-2389.1998.4930457.x
Spokas KA, Novak JM, Venterea RT (2012) Biochar’s role as an alternative N-fertilizer: ammonia capture. Plant Soil 350:35–42. https://doi.org/10.1007/s11104-011-0930-8
Steiner C, Das KC, Melear N, Lakly D (2010) Reducing nitrogen loss during poultry litter composting using biochar. J Environ Qual 39:1236–1242. https://doi.org/10.2134/jeq2009.0337
Stephenson AH, McCaskey TA, Ruffin BG (1990) A survey of broiler litter composition and potential value as a nutrient resource. Biol Wastes 34:1–9. https://doi.org/10.1016/0269-7483(90)90139-J
Subedi R, Kammann C, Pelissetti S, Taupe N, Bertora C, Monaco S, Grignani C (2015) Does soil amended with biochar and hydrochar reduce ammonia emissions following the application of pig slurry? Eur J Soil Sci 66:1044–1053. https://doi.org/10.1111/ejss.12302
Taghizadeh-Toosi A, Clough TJ, Condron LM, Sherlock RR, Anderson CR, Craigie RA (2011) Biochar incorporation into pasture soil suppresses in situ nitrous oxide emissions from ruminant urine patches. J Environ Qual 40:468–476. https://doi.org/10.2134/jeq2010.0419
Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM (2012) Biochar adsorbed ammonia is bioavailable. Plant Soil 350:57–69. https://doi.org/10.1007/s11104-011-0870-3
Titirici M-M, Thomas A, Antonietti M (2007) Back in the black: hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J Chem 31:787–789. https://doi.org/10.1039/b616045j
Van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246. https://doi.org/10.1007/s11104-009-0050-x
Wang LL, Guo Y, Zhu Y, Li Y, Qu Y, Rong C, Ma X, Wang Z (2010) A new route for preparation of hydrochars from rice husk. Bioresour Technol 101:9807–9810. https://doi.org/10.1016/j.biortech.2010.07.031
Webb J, Pain B, Bittman S, Morgan J (2010) The impacts of manure application methods on emissions of ammonia, nitrous oxide and on crop response—a review. Agric Ecosyst Environ 137:39–46. https://doi.org/10.1016/j.agee.2010.01.001
Wood SN (2004) Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Statist Assoc 99:673–686. https://doi.org/10.1198/016214504000000980
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B (Stat Methodol) 73:3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x
Wrage N, Velthof GL, van Beusichem ML, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33:1723–1732. https://doi.org/10.1016/S0038-0717(01)00096-7
Yao Y, Gao B, Zhang M, Inyang M, Zimmerman AR (2012) Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 89:1467–1471. https://doi.org/10.1016/j.chemosphere.2012.06.002
Zimmermann AR, Gao B, Ahn M-Y (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179. https://doi.org/10.1016/j.soilbio.2011.02.005
Acknowledgements
We thank Frank Hegewald and Dagmar Wenderoth for taking NH3 samples and for support on construction of the incubation system; Regina Lausch and Silke Weiss for sample preparation; Ute Tambor, Sabine Wathsack, and Monika Zerbian for laboratory analyses and Stefan Burkart for technical instructions and support. We thank the Friedrich-Loeffler-Institute of Animal Nutrition (Braunschweig, Germany) and the Friedrich-Loeffler-Institute of Animal Welfare and Animal Husbandry (Celle, Germany) for providing the manures.
Funding
This project was financed by the German Research Foundation (DFG-Research Training Group 1397 “Regulation of soil organic matter and nutrient turnover in organic agriculture,” University of Kassel).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Gronwald, M., Helfrich, M., Don, A. et al. Application of hydrochar and pyrochar to manure is not effective for mitigation of ammonia emissions from cattle slurry and poultry manure. Biol Fertil Soils 54, 451–465 (2018). https://doi.org/10.1007/s00374-018-1273-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-018-1273-x