Abstract
Nitrification is believed to be one of the major sources of N2O production emitted from soil. Previous studies showed that both ammonia-oxidising bacteria (AOB) and archaea (AOA) can produce N2O via nitrification but their relative contributions are still poorly defined. Here, we used acetylene, an inhibitor of AOB and AOA ammonia monooxygenase (AMO), and 1-octyne, a selective inhibitor that specifically inhibits AOB AMO, to investigate how AOB versus AOA contribute to N2O emissions in two distinct arable soils. Soil amended with ammonium (NH4 +) increased N2O emissions to a greater extent than nitrate (NO3 −), and acetylene had a greater impact on N2O emissions in NH4 +-treated soils than that in NO3 −-amended soils, which indicated that nitrification was the dominant N2O emitting process in these two arable soils. In the alluvial and red soil, the percentage of evolved N2O after application of NH4 + by AOB were 70.5 ~ 78.1 % and 18.7 ~ 19.7 % by AOA, respectively. Quantitative PCR revealed that NH4 + addition stimulated AOB growth, and the growth could be significantly inhibited by acetylene or 1-octyne in the two soils. The stimulation of N2O emissions by NH4 + and the relative suppression by inhibitors paralleled fluctuations in the AOB growth. In addition, cumulative N2O emissions were not correlated with AOA abundance in the two soils. Our results revealed that AOB could contribute more to soil N2O production than AOA in the NH4 +-amended arable soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O) is a powerful greenhouse gas that results in global warming and stratospheric ozone depletion. Increasing application of mineral N fertiliser and the expansion of arable soils contributes to over half of anthropogenic N2O production over the past few decades (Davidson 2009; Smith et al. 2012; Shcherbak et al. 2014). Therefore, it is necessary to understand the microbial mechanism of N2O emission from soil in order to develop future mitigation strategies and achieve sustainable agriculture practices.
Nitrification is an important N cycling process in agricultural ecosystems, which oxidises ammonia (NH3) to nitrate (NO3 −) via intermediate products, i.e. hydroxylamine (NH2OH) and nitrite (NO2 −) in a two-step process, and can consequently emit N2O as a by-product under certain soil conditions (Wrage et al. 2001). Ammonia oxidation, the oxidation of NH3 to NO2 − via NH2OH, is the rate-limiting step in nitrification and can be carried out by both ammonia-oxidising bacteria (AOB) and ammonia-oxidising archaea (AOA). Nitrification-related processes (the oxidation of NH2OH and NO2 − reduction) mediated by AOB are recognised to be a main pathway of N2O emission from arable soils (Gӧdde and Conrad 1999; Zhu et al. 2013; Huang et al. 2014a). The traditional viewpoint that soil ammonia oxidation and associated N2O emission is exclusively carried out by AOB has been challenged by the discovery of amoA and nirK genes in AOA strains (Venter et al. 2004; Lund et al. 2012) and by the demonstration of the N2O production capacity of AOA enriched or isolated from arable soils and marine ecosystem (Jung et al. 2011, 2014; Santoro et al. 2011; Loscher et al. 2012). In most soils, AOA outnumbers AOB abundance, a common feature in multiple ecosystems (Leininger et al. 2006; He et al. 2007; Hu et al. 2013), and a high AOA ammonia oxidation activity has been assessed in certain soils (Yao et al. 2011; Zhang et al. 2012; Hu et al. 2015). These results suggested that AOA could play a greater role than AOB in ammonia oxidation and N2O production in some soils (Nicol et al. 2008; Stieglmeier et al. 2014; Hink et al. 2016). However, by characterising the conserved amoA gene, which encodes a subunit of the ammonia monooxygenase (AMO), it has been observed that the populations of ammonia oxidisers are largely controlled by soil type, NH4 + concentration, pH and water content (Nicol et al. 2008; Di et al. 2009; Chen et al. 2010; Norton and Stark 2011). In ammonium-rich soils, AOB abundance and activity increased whereas AOA abudance is unaffected or inhibited in response to a high concentration of ammonium fertilisers (Jia and Conrad 2009; Di et al. 2009; Di and Cameron 2011; Sterngren et al. 2015; Ouyang et al. 2016). In unfertilised or acidic soils, AOA abundance and metabolic activity are much higher than those of AOB (Offre et al. 2009; Gubry-Rangin et al. 2010; Zhang et al. 2012). Thus, the role of AOB and AOA in overall ammonia oxidation and N2O emission is not very well understood across different soils.
A key functional enzyme in both bacterial and archaeal ammonia oxidisers is AMO. Acetylene (C2H2) is a suicide substrate for AMO (Hynes and Knowles 1978), and thus inhibits ammonia oxidation by autotrophic nitrifiers (Jia and Conrad 2009) at low concentration (10–100 Pa) and inhibits N2O reductase during denitrification at high concentration (10 KPa) (Huang et al. 2014b; Cui et al. 2016). Inhibition of soil ammonia oxidation by C2H2 is also used in routine methods to distinguish N2O production by nitrifiers and denitrifiers in soils (Bateman and Baggs 2005; Zhu et al. 2013). Recently, it has been shown that AOB is more sensitive to 1-octyne, a C8 alkine inhibitor, compared with AOA (Taylor et al. 2013, 2015). Moreover, Lu et al. (2015) and Ouyang et al. (2016) distinguished AOB and AOA contribution to nitrification by using 1-octyne in soil microcosm incubation and found that 1-octyne selectively inhibited AOB growth rather than AOA in forest and arable soils. Therefore, we could use 1-octyne to assess the relative contribution of AOB and AOA to N2O emission from two different arable soils. We hypothesised that nitrification was the main process of N2O emission in arable soils and AOB rather than AOA was the major contributor to soil N2O production in ammonium-rich arable soils.
Material and methods
Soil samples
Two arable soils from Luancheng county (40°7′34″N, 119°11′27″E), Hebei Province in northern China and Qiyang county (26°24′26″N, 112°00′45″E), Hunan Province in southern China were collected from the 0 to 20 cm layer: an upland alluvial soil and an upland red soil, respectively. The alluvial soil from Luancheng is classified as an aquic inceptisol according to US soil taxonomy (USDA 1994); its main properties are as follows: total N, 0.42 g kg−1; dissolved organic C (DOC) 26 mg kg−1; pH 8.0 (1:2.5 H2O); exchangeable NH4 +-N 2.4 mg kg−1; exchangeable NO3 −-N 117.8 mg kg−1. The red soil from Qiyang is classified as paleudults in the USDA Soil Taxonomy; its main properties are as follows: total N, 1.51 g kg−1; dissolved organic C (DOC) 33 mg kg−1; pH 6.0 (1:2.5 H2O); exchangeable NH4 +-N and NO3 −-N concentrations were 3.0 and 27.8 mg kg−1, respectively.
Microcosm incubation
To evaluate the contribution of different microbial groups to N2O emission, soils were subjected to microcosms incubation with acetylene and 1-octyne as selective inhibitors. The soil samples were sieved (<2 mm) and stored at 4 °C. The incubation experiments were conducted in 120-ml serum bottles containing 20 g of dry weight soil. The soil samples were pre-incubated for 7 days at 28 °C to stabilize the microbial population at 40 % water filled pore space (WFPS). After pre-incubation, soil was adjusted to 55 % WFPS following amendment with sterilised water (control, no N fertiliser or inhibitors) or a solution of 150 mg N as (NH4)2SO4 or KNO3 kg−1 soil in the presence or absence of 1-octyne (Oct) (5 μM aqueous) as described by Taylor et al. (2013) or acetylene (Ace) (0.1 % v/v). In total, nine treatments were described as follows: control, Oct, Ace, NH4 +, NH4 + + oct, NH4 + + ace, NO3 −, NO3 − + oct and NO3 − + ace. Each treatment was replicated three times. As acetylene was able to completely inhibit ammonia oxidation by autotrophic nitrifiers (AOA and AOB), N2O emisison from AOA and AOB (AOA + AOB) was calculated by substracting N2O emisison in the NH4 + + ace treatment from values measured in the NH4 + only treatment. N2O production by AOA was determined by substracting N2O production in the NH4 + + ace treatment from that detected in the NH4 + + oct treatment, since 1-Octyne specifically inhibits AOB ativity but not AOA. N2O production from AOA plus AOB (AOA + AOB) minus N2O emission by AOA was attributed to AOB. The relative contribution of N2O emission by AOA and AOB was therefore deduced.
N2O and soil sampling
Gas samples (10 ml) were collected from the headspace with syringe at 1, 2, 3, 4, 7, 10, 14 and 21 days after the start of incubation. N2O concentration was analysed by a gas chromatograph (HP7890A, Agilent Technologies, CA, USA). Cumulative N2O emission during the incubation period was estimated according to Ma et al. (2009). Aerobic conditions were maintained by opening bottles for 20 min at intervals of 2–3 days when water content and 1-octyne or acetylene were supplemented (Offre et al. 2009; Taylor et al. 2013; Hink et al. 2016). Three bottles per treatment were destructively sampled after incubation for 0, 7, 14 and 21 days. Exchangeable NH4 +-N and NO3 −-N were extracted with 0.01 M CaCl2, under shaking for 1 h, and then, soil suspensions were filtered through Whatman no. 42 filter papers. Extracts were analysed colorimetrically by flow injection analyser (Skalar SAN++). The soil samples for molecular analysis were immediately stored at −80 °C.
DNA extraction and quantitive PCR (qPCR) analyses
Soil DNA was extracted from 0.4 g dry soil with MoBio Powersoil™ DNA isolation kit (MoBio Laboratories Inc, Carlsbad, CA) according to the manufacturer’s protocol. Concentration and quality of the extracted DNA were measured using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Soil DNA extracts were stored at −80 °C.
Quantification of amoA for AOB and AOA were determined using amoA1f/amoA2r (Rotthauwe et al. 1997) and crenamoA23f/crenamoA616r (Hu et al. 2015), respectively. PCR reactions were conducted on a CFX96 (Bio-rad, USA). Each 25-μl reaction mixture included 12.5 μl 2 × Supermix (Bio-rad, USA), 0.5 μl BSA, 0.5 μl of each primer (10 mM) and 2 μl 10-fold diluted DNA and 9 μl sterilised deionised water. The thermocycling conditions for AOB and AOA were as follows: denaturation at 95 °C for 3 min, 40 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 40 s, extension at 72 °C for 50 s and data collection at 83 °C for 15 s. Products’ specificity was checked by agarose gel electrophoresis and melting curve analysis. Plasmids as prepared as previously (He et al. 2007) were used as standards for AOB and AOA qPCR, respectively. The efficiencies for AOB and AOA amoA amplification ranged from 90 to 98 %, and the coorelation coefficient (R 2) was between 0.993 and 0.999 for both genes.
Statistical analysis
The statistical analysis was performed using SPSS 17.0 software (IBM Co., Armonk, NY, USA). ANOVA was performed to assess the significance of different treatments on N2O production, amoA gene abundance and exchangeable NH4 +-N and NO3 −-N concentrations. Correlations of cumulative N2O emissions with AOB or AOA amoA gene abundance were assessed by Pearson’s correlation procedure.
Results
N2O emission
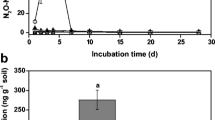
In the two soils, there was no clear difference in N2O production among control, Oct and Ace treatments after 21 days of incubation (Fig. 1). In the alluvial soil, N2O emission significantly increased with NH4 + addition and reached 287.9 ng N g−1 soil (P < 0.01). When NH4 + was applied with 1-octyne or acetylene, cumulative N2O production was dramatically reduced by 78.1 and 97 %, respectively. Nitrate-associated treatments did not significantly affect N2O emission (relative to the control) (P > 0.05). In the red soil, N2O emission was stimulated with the application of NH4 +, but to a lesser extent, i.e. reaching 84.9 ng N g−1 soil (P < 0.01). N2O emissions in the NH4 + + oct or NH4 + + ace treatments were reduced by 70.5 and 90 %, respectively. No significant difference in N2O emissions in acetylene-treated soil samples with or without NH4 + addition (P > 0.05) were observed. Amendment with NO3 − slightly stimulated N2O emission compared to the control. In the NO3 −-amended soil, acetylene addition inhibited N2O emission, but 1-octyne had no signficant inhibitory effect on it. In addition, the cumulative N2O emission from the NO3 − treatment was 36.4 and 4.7 times lower than that from the NH4 + treatment in the alluvial and red soils, respectively. These results indicated that nitrification rather than heterotrophic denitrification might be the main sources of N2O emssion in the two soils.
Cumulative N2O emissions after 21 days of laboratory incubation in an alluvial soil (a) and a red soil (b) which were amended with distilled sterilised water, NH4 + or NO3 − (control, NH4 + or NO3 −), with 1-octyne (Oct, NH4 + + oct or NO3 − + oct) or with acetylene (Ace, NH4 + + ace or NO3 − + ace). Data are means with standard deviations (n = 3). Different letters above the bars denote significant difference and the same letters denote no significant difference
Exchangeable NH4 +-N and NO3 −-N concentration
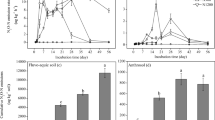
In the two soils, exchangeable NH4 +-N concentration was consistently low in the control, and 1-octyne alone had no significant effect on it (P > 0.05) (Fig. 2). In all acetylene-treated soils, nitrification was completely blocked and exchangeable NH4 +-N concentration strongly increased due to accumulation of mineralisation-derived NH3 after 21 days of incubation. In the alluvial soil, exchangeable NH4 +-N concentration in the NH4 + treatment decreased from 143.7 to 2.7 mg N kg−1 soil and was almost completely consumed due to continued oxidation by the ammonia oxidisers in the first 7 days. After that, the exchangeable NH4 +-N concentration showed no significant difference from the control (P > 0.05). In the red soil, the exchangeable NH4 +-N concentration gradually decreased to approach that of the control at day 21. After application of 1-octyne with NH4 +, exchangeable NH4 +-N concentrations declined more slowly in the two soils. Amendment with NO3 − alone did not significantly affect the change in exchangeable NH4 +-N concentration. No obvious difference in exchangeable NH4 +-N concentration was observed between NO3 − and NO3 − + oct treatments (P > 0.05).
The dynamics of NH4 + and NO3 − during incubation for 21 days in an alluvial soil (a, b) and a red soil (c, d) which were amended with distilled sterilised water, NH4 + or NO3 − (control, NH4 + or NO3 −), with 1-octyne (Oct, NH4 + + oct or NO3 − + oct) or with acetylene (Ace, NH4 + + ace or NO3 − + ace). Data are means with standard deviations (n = 3)
In the two soils, the exchangeable NO3 −-N concentration in the control slightly increased due to nitrification of NH3 derived from organic N mineralisation during the incubation (Fig. 2). 1-Octyne addition alone had no significant effect on soil exchangeable NO3 −-N concentration, but acetylene completely suppressed exchangeable NO3 −-N production. Ammonium amendment substantially increased exchangeable NO3 −-N concentrations (P < 0.01), whereas 1-octyne partly slowed exchangeable NO3 − formation in the NH4 + + oct treatment (Fig. 2). Exchangeable NO3 −-N concentrations in the NO3 − and NO3 − + oct treatments were similar after 21 days of incubation.
Abundance of AOB and AOA amoA genes
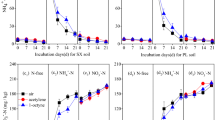
After 21 days, the AOB amoA gene abundance in the control was about 1.1 × 107 copies g−1 soil in the alluvial soil and 2.8 × 106 copies g−1 soil in the red soil, respectively (Fig. 3). No significant difference of AOB amoA gene abundance was observed among the control, Oct, Ace, NO3 −, NO3 − + oct and NO3 − + ace treatments in the two soils (P > 0.05). The application of NH4 + significantly increased AOB amoA gene abundance, reaching 3.1 × 107 copies g−1 soil in the alluvial soil and 7.2 × 106 copies g−1 soil in the red soil, respectively (P < 0.01). However, AOB amoA gene abundance in the NH4 + + oct and NH4 + + ace treatments was obviously lower than those in the NH4 + treatment. No significant variation of AOB amoA gene abundance was observed between NH4 + + oct and NH4 + + ace treatments (P > 0.05).
AOB and AOA amoA genes abundance after 0 and 21 days of laboratory incubation in an alluvial soil (a, b) and a red soil (c, d) which were amended with distilled sterilised water, NH4 + or NO3 − (control, NH4 + or NO3 −), with 1-octyne (Oct, NH4 + + oct or NO3 − + oct) or with acetylene (Ace, NH4 + + ace or NO3 − + ace). Data are means with standard deviations (n = 3). Different letters above the bars denote significant difference and the same letters denote no significant difference
In the alluvial soil, AOA amoA gene abundance ranged from 1.7 × 108 to 1.9 × 108 copies g−1 soil, showing no significant difference among all the treatment groups (P > 0.05). In the red soil, AOA amoA gene abundance between the control and Oct treatments was similar, at around 3.1 × 107 copies g−1 dry soil. However, NH4 + or NO3 − addition significantly reduced AOA amoA gene abundance, showing a possible inhibitory effect of inorganic N on the AOA populations in the acidic red soil.
The regression analysis between the AOB and AOA amoA gene abundance and cumulative N2O emission from different treatments is illustrated in Fig. 4. Cumulative N2O emissions were positively correlated with AOB abundance in the alluvial soil (y = 1.46e0.17x, R 2 = 0.98, P < 0.001) and the red soil (y = 3.06e0.048x, R 2 = 0.85, P < 0.001). However, AOA abundance was not correlated with cumulative N2O emissions in either of the two soils.
The contribution of AOB and AOA to N2O production in two arable soils
Octyne-sensitive (AOB) N2O emissions varied significantly between soil types and NH4 + amendment (Fig. 5). In the NH4 + treatment, the fraction of octyne-sensitive (AOB) N2O emission was much higher than that of octyne-resistent (AOA) N2O emission in the two soils, and this effect depended on soil type. For example, octyne-sensitive (AOB) N2O emission in the alluvial soil was 78.1 %, higher than in the red soil (70.5 %). The percentage of octyne-resistent (AOA) N2O emission was 19.7 % in the alluvial soil and 18.7 % in the red soil, respectively. In unamended soils, no significant difference between the percentage of octyne-resisitent (AOA) N2O emission and the fraction of octyne-sensitive (AOB) N2O emission was observed (data not shown).
Discussion
Nitrification and denitrification are considered to be the two primary processes of N2O production in arable soils (Wrage et al. 2001). It is commonly suggested that nitrification was the major process of N2O emission in soils below 60–70 % WFPS, whereas denitrification would be the main source N2O production above 70 % WFPS (Bateman and Baggs 2005; Braker and Conrad 2011; Ma et al. 2015). Here, we postulated that nitrification was the major source of N2O emission because: (1) soil treated NH4 + resulted in much higher N2O production compared to that in NO3 −-treated soils; (2) applications of acetylene, which inhibits autotrophic ammonia oxidisers (AOB or AOA), reduced N2O emission by 90–97 % in our soils, highlighting the importance of nitrifiers to N2O emission; (3) all experiments were performed under aerobic conditions, and soil water content was kept at 55 % WFPS, which was more conductive to nitrification than denitrification (Bateman and Baggs 2005). Hence, these results indicated that N2O was mainly produced from nitrification in these two agricultural soils.
Clearly, soil treated with NH4 + strongly stimulated AOB growth rather than AOA in the two soils. AOA did not respond to the application of NH4 + in the alluvial soil, while it was inhibited with respect to the control in the red soil, thus confirming what it was obtained in agricultural soils (Wu et al. 2011; Ai et al. 2013; Habteselassie et al. 2013; Di et al. 2014, 2014; Chen et al. 2015). Generally, AOB growth is favored by high NH4 +-N soil conditions (Jia and Conrad 2009; Verhamme et al. 2011; Taylor et al. 2012), whereas AOA growth is not affected or suppressed (Di et al. 2009, 2010; Di and Cameron 2011; Di et al. 2014). Actually, the AOA population only grew in the control in the red soil, where NH4 + was generated from mineralised organic N, suggesting that AOA growth is favored in low fertility status soils (Nicol et al. 2008; Di et al. 2009, 2010; Zhang et al. 2012). Here, the stimulation and inhibition of AOB growth by NH4 + and inhibitors, respectively, occurred. Consequently, AOB amoA gene abundance was significantly correlated with cumulative N2O emissions in the two soils. Similar studies also showed that AOB must play a critical role in N2O production in high urea-N soils (Cui et al. 2013; Dai et al. 2013).
1-Octyne is a potentially selective inhibitor of AOB activity in agricultural soils (Taylor et al. 2013), and by using this selective inhibition, we showed that AOB had a great potential of contributing to soil N2O production in high NH4 +-N agricultural soils despite the presence of abundant AOA in these soils. Two mainly N2O-yielding can explain the reason for the higher N2O production by AOB than AOA, NH2OH oxidation and nitrifier denitrification (Shaw et al. 2006; Stein 2011; Schreiber et al. 2012). It was reported that N2O emission from the oxidation of NH2OH contributed very little to total N2O production and nitrifier denitrification was believed to be the predominant process for N2O production in soils with 50 % < WFPS < 70 % (Kool et al. 2010, 2011). Recently, Vajrala et al. (2013) found that NH2OH was an intermediate of ammonia oxidation in AOA, but they would not be able to produce N2O like AOB probably due to lack of genes for a homologue of hydroxylamine oxidoreductase (HAO) known to be responsible for N2O formation in AOB (Hooper and Terry 1979). In addition, AOA also lack genes encoding a potential NO-reductase (NOR), which is involved in nitrifier-denitrification and thus N2O production in AOB (Stein 2011; Tourna et al. 2011). Therefore, we suggested that N2O emission from the two tested soil probably depended on denitrification by AOB.
In addition, NI addition has limitations and the effectiveness of inhibitors in N2O emission, and asssociated microbe studies may depend on soil conditions (Hatch et al. 2005). Stable isotope enrichment approaches should be applied to identify N2O production during different processes following application of 15N-labelled NH4 + or NO3 − and 18O-labelled H2O and NO3 − in short-term experiments (Cheng et al. 2014; Li and Lang 2014; Zhu et al. 2013). Zhu et al. (2013) carried out a dual labelling approach that employed an 18O-, 15N-enrichment method for distinguishing among nitrous oxide (N2O) production from nitrifier nitrification, nitrifier denitrification and heterotrophic denitrification. A dual labelling method may improve our ability to discrimate between the contributions of nitrification and denitrification to total N2O production. A combination of the stable isotopic enrichment approach coupled with molecular method may be required to estimate the contributions of all known N2O-genic processes in the future.
In conclusion, we compared relative bacterial and archaeal contribution to soil N2O production from two contrasting arable soils. By using inhibitors for specific micobial groups and metablic pathways, we demonstrated that nitrification was the predominant driving process of N2O emission and AOB dominated in N2O production when NH4 + was applied in the alluvial and red soils. The relative contribution of N2O emission from AOA is lower than that from AOB under this condition. Moreover, we also observed that the changes of N2O emissions among all treatments were correlated with variation of AOB abundance but not with AOA abundance in the two tested arable soils. Therefore, AOB may play a more important role than AOA in N2O emission in a high NH4 +-N concentration arable soils.
References
Ai C, Liang GQ, Sun JW, Wang XB, He P, Zhou W (2013) Different roles of rhizosphere effect and long-term fertilization in the activity and community structure of ammonia oxidizers in a calcareous fluvo-aquic soil. Soil Biol Biochem 57:30–42
Bateman EJ, Baggs EM (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fertil Soils 41:379–388
Braker G, Conrad R (2011) Diversity, structure, and size of N2O-producing microbial communities in soils-what matters for their functioning? Adv Appl Microbiol 75(75):33–70
Chen X, Zhang LM, Shen JP, Xu ZH, He JZ (2010) Soil type determines the abundance and community structure of ammonia-oxidizing bacteria and archaea in flooded paddy soils. J Soils Sediments 10:1510–1516
Chen QH, Qi LY, Bi QF, Dai PB, Sun DS, Sun CL, Liu WJ, Lu LL, Ni WZ, Lin XY (2015) Comparative effects of 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD) on ammonia-oxidizing bacteria and archaea in a vegetable soil. Appl Microbiol Biotechnol 99:477–487
Cheng Y, Wang J, Wang SQ, Zhang JB, Cai ZC (2014) Effects of soil moisture on gross N transformations and N2O emission in aci subtropical forest soils. Bio Fertil Soils 50:1099–1108
Cui PY, Fan FL, Yin C, Li Z, Song AL, Wan Y, Liang YC (2013) Urea- and nitrapyrin-affected N2O emission is coupled mainly with ammonia oxidizing bacteria growth in microcosms of three typical Chinese arable soils. Soil Biol Biochem 66:214–221
Cui PY, Fan FL, Yin C, Song AL, Huang PR, Tang YJ, Zhu P, Peng C, Li T, Wakelin SA, Liang YC (2016) Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biol Biochem 93:131–141
Dai Y, Di HJ, Cameron KC, He JZ (2013) Effects of nitrogen application rate and a nitrification inhibitor dicyandiamide on ammonia oxidizers and N2O emissions in a grazed pasture soil. Sci Total Environ 465:125–135
Davidson EA (2009) The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat Geosci 2:659–662
Di HJ, Cameron KC (2011) Inhibition of ammonium oxidation by a liquid formulation of 3,4-Dimethylpyrazole phosphate (DMPP) compared with a dicyandiamide (DCD) solution in six new Zealand grazed grassland soils. J Soils Sediments 11:1032–1039
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci 2:621–624
Di HJ, Cameron KC, Sherlock RR, Shen JP, He JZ, Winefield CS (2010) Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea. J Soils Sediments 10:943–954
Di HJ, Cameron KC, Podolyan A, Robinson A (2014) Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol Biochem 73:59–68
Gӧdde M, Conrad R (1999) Immediate and adaptational temperature effects on nitric oxide production and nitrous oxide release from nitrification and denitrification in two soils. Biol Fertil Soils 30:33–40
Gubry-Rangin C, Nicol GW, Prosser JI (2010) Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol Ecol 74:566–574
Habteselassie MY, Xu L, Norton JM (2013) Ammonia-oxidizer communities in an agricultural soil treated with contrasting nitrogen sources. Front Microbiol 4:326
Hatch D, Trindade H, Cardenas L, Carneiro J, Hawkins J, Scholefield D, Chadwick D (2005) Laboratory study of the effects of two nitrification inhibitors on greenhouse gas emissions from a slurry-treated arable soil: impact of diurnal temperature cycle. Biol Fertil Soils 41:225–232
He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG, Di H (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Hink L, Nicol GW, Prosser JI (2016) Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environ Microbiol. doi:10.1111/1462-2920.13282
Hooper AB, Terry KR (1979) Hydroxylamine oxidoreductase of Nitrosomonas production of nitric-oxide from hydroxylamine. Bioch Et Bioph Act 571:12–20
Hu HW, Zhang LM, Yuan CL, He JZ (2013) Contrasting Euryarchaeota communities between upland and paddy soils exhibited similar pH-impacted biogeographic patterns. Soil Biol Biochem 64:18–27
Hu HW, Macdonald CA, Trivedi P, Holmes B, Bodrossy L, He JZ, Singh BK (2015) Water addition regulates the metabolic activity of ammonia oxidizers responding to environmental perturbations in dry subhumid ecosystems. Environ Microbiol 17:444–461
Huang T, Gao B, Hu XK, Lu X, Well R, Christie P, Bakken LR, Ju XT (2014a) Ammonia-oxidation as an engine to generate nitrous oxide in an intensively managed calcareous fluvo-aquic soil. Sci Rep 4:3950
Huang Y, Li YY, Yao HY (2014b) Nitrate enhances N2O emission more than ammonium in a highly acidic soil. J Soils Sediments 14:146–154
Hynes RK, Knowles R (1978) Inhibition by acetylene of ammonia oxidation in Nitrosomonas-Europaea. FEMS Microbiol Lett 4:319–321
Jia ZJ, Conrad R (2009) Bacteria rather than archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11:1658–1671
Jung MY, Park SJ, Min D, Kim JS, Rijpstra WIC, Damste JSS, Kim GJ, Madsen EL, Rhee SK (2011) Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural Soil. Appl Environ Microbiol 77:8635–8647
Jung MY, Well R, Min D, Giesemann A, Park SJ, Kim JG, Kim SJ, Rhee SK (2014) Isotopic signatures of N2O produced by ammonia-oxidizing archaea from soils. ISME J 8:1115–1125
Kool DM, Wrage N, Zechmeister-Boltenstern S, Pfeffer M, Brus D, Oenema O, Van Groenigen JW (2010) Nitrifier denitrification can be a source of N2O from soil: a revised approach to the dual-isotope labelling method. Eur J Soil Sci 61:759–772
Kool DM, Dolfing J, Wrage N, Van Groenigen JW (2011) Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol Biochem 43:174–178
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Li P, Lang M (2014) Gross nitrogen transformations and related N2O emissions in uncultivated and cultivated black soil. Biol Fertil Soils 50:197–20
Loscher CR, Kock A, Konneke M, LaRoche J, Bange HW, Schmitz RA (2012) Production of oceanic nitrous oxide by ammonia-oxidizing archaea. Biogeosciences 9:2419–2429
Lu XD, Bottomley PJ, Myrold DD (2015) Contributions of ammonia-oxidizing archaea and bacteria to nitrification in Oregon forest soils. Soil Biol Biochem 85:54–62
Lund MB, Smith JM, Francis CA (2012) Diversity, abundance and expression of nitrite reductase (nirK)-like genes in marine thaumarchaea. ISME J 6:1966–1977
Ma J, Ma E, Xu H, Yagi K, Cai ZC (2009) Wheat straw management affects CH4 and N2O emissions from rice fields. Soil Biol Biochem 41:1022–1028
Ma L, Shan J, Yan XY (2015) Nitrite behavior accounts for the nitrous oxide peaks following fertilization in a fluvo-aquic soil. Biol Fert soils 51:563–572
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978
Norton JM, Stark JM (2011) Regulation and measurement of nitrification in terrestrial systems. Methods Enzymol 486:343–368
Offre P, Prosser JI, Nicol GW (2009) Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol Ecol 70:99–108
Ouyang Y, Norton JM, Stark JM, Reeve JR, Habteselassie MY (2016) Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil. Soil Biol Biochem 96:4–15
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Santoro AE, Buchwald C, McIlvin MR, Casciotti KL (2011) Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science 333:1282–1285
Schreiber F, Wunderlin P, Udert KM, Wells GF (2012) Nitric oxide and nitrous oxide turnover in natural and engineered microbial communities: biological pathways, chemical reactions, and novel technologies. Front Microbiol 3:372
Shaw LJ, Nicol GW, Smith Z, Fear J, Prosser JI, Baggs EM (2006) Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ Microbiol 8:214–222
Shcherbak I, Millar N, Robertson GP (2014) Global meta-analysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc Natl Acad Sci U S A 111:9199–9204
Smith KA, Mosier AR, Crutzen PJ, Winiwarter W (2012) The role of N2O derived from crop-based biofuels, and from agriculture in general, in Earth’s climate. Phil Trans R Soc B 367:1169–1174
Stein LY (2011) Surveying N2O-producing pathways in bacteria. In: Klotz MG (ed) Methods in enzymology, vol 486. Academic Press, CA, pp 131–152
Sterngren AE, Hallin S, Bengtson P (2015) Archaeal ammonia oxidizers dominate in numbers, but bacteria drive gross nitrification in N-amended grassland soil. Front Microbiol 6:1350
Stieglmeier M, Mooshammer M, Kitzler B, Wanek W, Zechmeister-Boltenstern S, Richter A, Schleper C (2014) Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J 8:1135–1146
Taylor AE, Zeglin LH, Wanzek TA, Myrold DD, Bottomley PJ (2012) Dynamics of ammonia-oxidizing archaea and bacteria populations and contributions to soil nitrification potentials. ISME J 6:2024–2032
Taylor AE, Vajrala N, Giguere AT, Gitelman AI, Arp DJ, Myrold DD, Sayavedra-Soto L, Bottomley PJ (2013) Use of aliphatic n-Alkynes to discriminate soil nitrification activities of ammonia-oxidizing thaumarchaea and bacteria. Appl Environ Microbiol 79:6544–6551
Taylor AE, Taylor K, Tennigkeit B, Palatinszky M, Stieglmeier M, Myrold DD, Schleper C, Wagner M, Bottomley PJ (2015) Inhibitory effects of C-2 to C-10 1-Alkynes on ammonia oxidation in two Nitrososphaera Species. Appl Environ Microbiol 81:1942–1948
Tourna M, Stieglmeier M, Spang A, Konneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A, Schleper C (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci U S A 108:8420–8425
USDA (1994) Keys to soil taxonomy. United States Department of Agriculture, Soil Conservation Service, 6th edn., p 306
Vajrala N, Martens-Habbena W, Sayavedra-Soto LA, Schauer A, Bottomley PJ, Stahl DA, Arp DJ (2013) Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc Natl Acad Sci U S A 110:1006–1011
Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, Wu DY, Paulsen I, Nelson KE, Nelson W, Fouts DE, Levy S, Knap AH, Lomas MW, Nealson K, White O, Peterson J, Hoffman J, Parsons R, Baden-Tillson H, Pfannkoch C, Rogers YH, Smith HO (2004) Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66–74
Verhamme DT, Prosser JI, Nicol GW (2011) Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J 5:1067–1071
Wrage N, Velthof GL, van Beusichem ML, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33:1723–1732
Wu YC, Lu L, Wang BZ, Lin XG, Zhu JG, Cai ZC, Yan XY, Jia ZJ (2011) Long-term field fertilization significantly alters community structure of ammonia-oxidizing bacteria rather than archaea in a paddy soil. Soil Sci Soc Am J 75:1431–1439
Yao H, Gao Y, Nicol GW, Campbell CD, Prosser JI, Zhang L, Han W, Singh BK (2011) Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl Environ Microbiol 77:4618–4625
Zhang LM, Hu HW, Shen JP, He JZ (2012) Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J 6:1032–1045
Zhu X, Burger M, Doane TA, Horwath WR (2013) Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc Natl Acad Sci U S A 110:6328–6333
Acknowledgments
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15020200) and by the Natural Science Foundation of China (41322007 and 41371265).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Q., Zhang, LM., Shen, JP. et al. Nitrogen fertiliser-induced changes in N2O emissions are attributed more to ammonia-oxidising bacteria rather than archaea as revealed using 1-octyne and acetylene inhibitors in two arable soils. Biol Fertil Soils 52, 1163–1171 (2016). https://doi.org/10.1007/s00374-016-1151-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-016-1151-3