Abstract
A 4-month field experiment was conducted in an organic vegetable production system; two crops, Brassica chinensis and Cichorium endivia L., were cultivated successively and treated with 0 (CKC), 30 (PC1), 60 (PC2), or 120 (PC3) t ha−1 of pig manure-based compost. The replicated and heavy application of pig manure-based compost increased the total amounts of some heavy metals (Zn, Cu, Pb, and Cd) in the short term in soil. Despite the Shannon (bacterial diversity), Chao1 (richness), and evenness indices of different samples calculated after pyrosequencing analysis were similar, changes occurred in the bacterial community composition in soils amended with different rates of compost. The joint cluster and principal component analysis (PCA) indicated that heavy rates of compost may not change bacterial diversity in the short term and, in some cases, even produce a lower genetic diversity. The optimum rate, as well as the period, for compost application should be evaluated to promote the sustainable development of organic agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional agriculture, which is dependent on intensive inputs of synthetic fertilizers, herbicides, and pesticides, has created many environmental problems including soil erosion, salinization, organic matter depletion, biodiversity reduction, groundwater contamination, and water eutrophication, with problems in the quality and safety of the agricultural products (Horrigan et al. 2002; Zhou et al. 2005). However, applications of chemical products are completely prohibited in organic farming, which is characterized by organic inputs, recycling for nutrients, biological approaches, and careful cropping system design for pest management (Tu et al. 2006). Thus, organic agriculture has gained worldwide acceptance and undergone vigorous development in the last decades for providing lower environmental pressure and safer agricultural products than conventional agriculture.
Composts are obtained by the biological decomposition of organic materials, and during the composting process, chemical stabilization of organic substrates is obtained without the presence of weed seeds and human and plant pathogens (Trewavas 2004). Animal manures from the livestock industry are the most popular materials being used for composting in China. However, the presence of high concentrations of heavy metals, that are derived from salt and additives provided for improving animal health and productivity, as well as the composting process itself, may cause heavy metal contamination of the compost (Baldantoni et al. 2010). Therefore, the long-term application of manure-based compost may cause accumulation of heavy metals in soils especially at high rates (Wong et al. 1999; Zhou et al. 2005; Achiba et al. 2009, 2010). Unfortunately, overuse of manure-based compost in organic agriculture systems in China is common, especially during the transition period from conventional to organic systems (Ju et al. 2007). However, the changes of heavy metal concentration caused by excessive application of manure-based compost are generally ignored. Moreover, the accumulation of potentially toxic heavy metals might also have a detrimental influence on activity, biomass, and composition of microbial communities (Singh and Kalamdhad 2013).
Soil microorganisms, which are involved in nutrient cycling, transformation processes, and soil aggregate formation, as well as in plant pathology or plant growth promotion, play a vital role in defining soil quality and productivity (Widmer et al. 2006). It is necessary to describe the dynamics of microbial communities and identify the possible functions of these populations when amendments are applied to cultivated fields. A 12-year crop rotation field experiment conducted by Ros et al. (2006) showed that application of composts produced from urban organic wastes, green wastes, manure, and sewage sludge increased the diversity of ammonia oxidizers and bacteria of soil, as shown by the denaturing gradient gel electrophoresis (DGGE) analysis of 16S rDNA. However, in a short-term (2-year) study of soil amended with municipal solid waste (MSW) compost, no distinct influence occurred in bacterial community compositions even though the addition had a positive influence on the crop production (Convertini et al. 1999). On the other hand, Sun et al. (2014) showed that the 10 % manure treatment produced a more diverse bacterial community composition, as determined by DNA-based pyrosequencing, in apple rhizosphere soil than other manure rates in a 3-year pot experiment. The studies mentioned above demonstrated that both the application rate and period of organic amendments can affect the composition of soil microflora.
However, little information is available concerning the short-term effects of applying high rates of manure-based compost on the total heavy metal accumulation and bacterial community composition of soil. We hypothesized that heavy and replicated application of manure-based compost in the short term may change heavy metal content and composition of soil. For this reason, a 4-month field experiment was conducted in an organic vegetable production system (i) to characterize the total heavy metal accumulation in amended soils and (ii) to determine the bacterial diversity by 454 pyrosequencing in soil treated with different rates of pig manure-based compost. It has been shown that only a small portion of soil microbial diversity has been identified or adequately characterized (Myrold and Nannipieri 2014) and it depends in the used method (Crecchio et al. 2004; Bakken and Frostegård 2006; Van Elsas et al. 2014).
Materials and methods
Field experiment
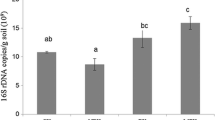
The field experiment was conducted from March 13 to July 12 of 2013 at the organic farm of Jiangyang at Yangzhou in Jiangsu Province, China, located at longitude 119° 7′ 8.52″ W and latitude 32° 23′ 1.55″ N. Brassica chinensis and Cichorium endivia L. were cultivated for 4 months. The compost produced from pig manure mainly was applied prior to the first and second crop, at the rates of 0 (CKC), 30 t ha−1 crop−1 (PC1), 60 t ha−1 crop−1 (PC2), and 120 t ha−1 crop−1 (PC3). The used compost rates were those employed by Wong et al. (1999), who conducted a field experiment to evaluate the growth of B. chinensis and Zea mays L. on loamy soil. Each treatment was replicated three times. The main properties of the loamy soil (sand 43.9 %, silt 36.4 %, clay 19.7 %) and compost are summarized in Table 1. The bacterial community composition of the applied pig manure-based compost was analyzed using 454 pyrosequencing (Fig. 1 and Table s1). The experimental plan included 12 plots and each plot consisted of a 4 × 5-m square, which was separated from each neighboring plot by a neutral zone of 0.5 m. All plots were arranged in a complete randomized design.
Different rates of compost were evenly spread to the soil surface by hand and incorporated to a depth of 15–20 cm by manual hoeing prior to each planting. The seedlings of B. chinensis were grown for 20 days in a greenhouse and then transplanted with 20 × 20 cm row plant spacings and irrigated once every 24 h until they were established. The plots were weeded manually every month. After B. chinensis harvesting, the seedlings of C. endivia L., grown for 30 days in a greenhouse, were also transplanted with 20 × 20 cm row and plant spacings.
Soil sampling and total heavy metal analysis
Soil samples were collected at a depth of 0–20 cm in each plot after each crop harvest at five different spots using a hand auger (5 cm diameter); the mixed samples were immediately divided into two parts: one part was stored at −80 °C and the other was air-dried immediately. The air-dried compost and soil samples were pulverized, sieved (<2 mm), and digested by an acid mixture (HNO3 65 % and HF 50 %, 2:1 = v/v) for determining the total amounts of Zn, Cu, Pb, and Cd. Heavy metal concentrations were determined with an ICP-OES (Spectroflame Modula E Spectro Analytical Instruments, Kleve, Germany). Details of the methods are described by Baldantoni et al. (2010).
DNA extraction and quantitation, 16S ribosomal RNA gene amplification, and pyrosequencing
The total DNA was extracted from 0.5 g thawed soil using an E.Z.N.A. Soil DNA Kit (Omega Bio-Tek Inc., Norcross, GA, USA) following the manufacturer’s instructions. The extracted DNA was checked on a 1 % agarose gel and the A260/A280 ratio of DNA was determined using a NanoDrop ND-2000 spectrophotometer (NanoDrop, Wilmington, DE, USA). The DNA concentration was adjusted to 5 ng μL−1 for each sample and was amplified by the bacterial 16S ribosomal RNA (rRNA) primers 27F (5′-GCCTTGCCAGCCCGCTCAG-TC-AGAGTTTGATCCTGGCTCAG-3′) and 533R (5′-GCCTCCCTCGCGCCATCAG-AC-NNNNNNNNNN-TTACCGCGGCTGCTGGCAC-3′). This fusion primer included Roche-454 A/B adapters (shown in italics) and a 2-bp linker sequence (shown in bold) followed by a unique, error-correcting barcode sequence (Ns) (Zhao et al. 2014).
The PCRs were replicated three times; the 20-μL mixture contained 2 μM of each primer, 0.25 μM dNTPs (Takara), 4 μL 5× FastPfu Buffer (TransGen, TransGen Biotech Co., Ltd., Beijing, China), 1 U of FastPfu DNA polymerase (2.5 U μL−1, TransGen), and 10 ng soil DNA template; a negative control was also included. The amplification program consisted in an initial denaturation at 95 °C for 2 min followed by 25 cycles at 95 °C for 30 s (denaturation), at 55 °C for 30 s (annealing), and at 72 °C for 30 s (extension); the final extension was at 72 °C for 5 min. The PCR products were visualized on 2 % agarose gels, and all PCR products of each sample were pooled and purified by a PCR Clean-up Kit (Axygen Bio, USA).
Pyrosequencing data and statistical analysis
The pyrosequencing was performed on a 454 GS-FLX Titanium System (Roche, Switzerland) by Majorbio Bio-pharm Technology Co., Ltd (Shanghai, China). Raw sequence data were processed using Mothur (version 1.29.2) according to the Schloss standard operating procedure (Schloss et al. 2009). The details were as follows: sequences having a minimum flow length of 450 flows were denoised using the Mothur-based reimplementation of the PyroNoise algorithm with the default parameters (Quince et al. 2011). Denoised sequences were sorted based on sample-specific tags and eliminated if they could not be sorted by a tag, they were shorter than 50 bases, or they contained more than 1 undefined base (Bibby et al. 2010). The unique sequences with the tag and primer sequences removed were aligned against the Silva 106 database (Pruesse et al. 2007). A distance matrix was built using the eliminated chimer sequences and by considering a distance threshold of 0.2. The remaining sequences of each sample were clustered to operational taxonomic units (OTUs) based on 97 % sequence similarity criterion; the representative sequences of each OTU were compiled and compared to the Ribosomal Database Project II (RDP II) (Cole et al. 2007) using the “Naive Bayesian rRNA Classifier” to identify the nearest phylogenetic homologue (confidence level of 80 %). The relative abundance was defined as the number of sequences of the same phylogenetic group divided by the total number of the target phyla or genera per sample. Additionally, the evenness, Simpson, Chao1, abundance-based coverage estimator (ACE), and Good’s nonparametric coverage indices were also calculated to describe the α-diversity of each sample. Canoco 4.5 was used to run principal component analysis (PCA).
Means, standard deviation, and analysis of variance (ANOVA) were determined by the SPSS computer package (version 11.0). The means were compared using the Duncan test with a significance level of p < 0.05. The mean values of three triplicates and standard errors were shown in figures.
Results and discussion
Total heavy metal concentration
At the end of the first crop, no significant effect on the Pb and Cd contents of soil (0–20 cm) was observed for all rates of applied pig manure compost, but the concentrations of both Zn and Cu were increased after incorporation of 120 t ha−1 crop−1 (Fig. 2). However, after the second application of pig manure-based compost at the rate of 120 t ha−1 crop−1, the content of Pb was also significantly increased, whereas there was no positive effect for the Cd content. Generally, the pig manure compost applied at high rates gave high inputs of some heavy metals, and obvious accumulation of Zn, Cu, and Pb was observed in the surface of treated soils (0–20 cm) after the second pig manure compost application.
Total concentrations of Zn, Cu, Pb, and Cd in soils treated with different rates of pig manure compost. CKC is the untreated soil; PC1 is the soil treated with 30 t pig manure-based compost ha−1 crop−1; PC2 is the soil treated with 60 t pig manure-based compost ha−1 crop−1; PC3 is the soil treated with 120 t pig manure-based compost ha−1 crop−1. The numbers indicate the replicates of each treatment. Different letters indicate statistically different numbers between treatments (p < 0.05)
However, the pig manure compost did not have any effect on the heavy metal content at 20–40 cm depth (data not shown). A high concentration of organic matter in the 0–20-cm soil layer and the low rainfall during the experiment probably blocked the mobility of heavy metals (Achiba et al. 2009). Also, the relatively high content of clay in soil may have contributed to limit the movement of heavy metals through the soil (Walker et al. 2004).
The increase in the total content of heavy metals of the surface soil layer (0–20 cm) has been previously observed after long-term applications of pig manure-based compost (Weber et al. 2007; Baldantoni et al. 2010). Our results indicated that the heavy metal limits as well as the rate and period for compost application should be established, based on scientific research, to prevent soil pollution. These limits have been set up in several countries such as Belgium, Holland, and Germany (Singh and Kalamdhad 2013), but they may be not suitable.
Compost application has been known to increase soil nutrients and crop production (Wong et al. 1999) as obtained in this study (Table s2 and Fig. s1). The highest contents of NH4 +-N, NO3 −-N, dissolved organic C (DOC), and Olsen-P were detected in the soil treated with 120 t of pig manure-based compost ha−1 crop−1. Antibiotics are commonly added to animal feed as supplements, and percentages often exceeding 70 % of administered dosage are excreted as parent compounds or metabolites in manure (Kumar et al. 2005). Heavy rates of pig manure-based compost added to soil may cause antibiotic pollution for incomplete degradation of antibiotics during the composting process (Dolliver et al. 2007). Therefore, it is necessary to evaluate the content of antibiotic in manure compost-treated soils.
Estimated number of observed sequences and bacterial diversity of each sample
Short-term changes of bacterial diversity due to different rates of pig manure-based compost applied to soil were determined by pyrosequencing of soil samples collected after the harvest of the second crop. The total number of valid sequences was 75,304 and the average read length was 400 bp; 93.44 % of these sequences were classified at the phylum level. Additionally, the mean number of quality sequences for each sample was 6275, a number similar to or greater than the bacterial sequence numbers of previous studies of soil samples analyzed by pyrosequencing (Lauber et al. 2009; Zhao et al. 2014).
As shown in Table 2, the coverage values ranged between 0.55 and 0.62, indicating that the sequencing was not sufficient to reveal the complete bacterial diversity of compost-treated soils as also confirmed by the rarefaction curves since the plateau was not reached (Fig. 3). However, the coverage values for all treatments were similar. Other indices including OTUs, ACE, Chao1, evenness, and Shannon index, that are regularly used to evaluate bacterial diversity, were also calculated using all sequences of each sample. No statistically significant differences among the respective indices were observed, suggesting that the application rates of pig manure-based compost in the short term might have no effect on the bacterial diversity. This phenomenon may be explained by the resistance and resilience of soil microbial communities subjected to these treatments (Griffiths and Philippot 2013). Innerebner et al. (2006) suggested that compost did not leave direct microbial effects in soil even after long-term applications. Poulsen et al. (2013) also considered that the soil type and particle size were the two most important determinants of bacterial diversity, rather than type of the applied fertilizer. However, Cytryn et al. (2011) showed that compost amendment affected the activity, size, and composition of soil microbial communities. Probably, the physicochemical properties of the compost and not the compost-borne microorganisms can affect the microbial diversity of the treated soil.
Bacterial diversity at the phylum and genus levels
As shown in Fig. 4, most of the observed sequences about more than 65 % for all samples were affiliated with Proteobacteria, Actinobacteria, Chloroflexi, Acidobacteria, and Bacteroidetes (relative abundance >5 %), and a minor percentage belonged to TM7, Verrucomicrobia, Planctomycetes, Firmicutes, Gemmatimonadetes, OD1, Cyanobacteria, Nitrospira, and Armatimonadetes. These bacterial groups are always found in soils and are capable of utilizing a variety of substrates (Asakawa and Kimura 2008).
The relative abundance of phyla and proteobacterial classes for each soil treatment. CKC is the untreated soil; PC1 is the soil treated with 30 t pig manure-based compost ha−1 crop−1; PC2 is the soil treated with 60 t pig manure-based compost ha−1 crop−1; PC3 is the soil treated with 120 t pig manure-based compost ha−1 crop−1. The numbers indicate the replicates of each treatment. Asterisks indicate statistically different to the control, without the applied compost (p < 0.05)
Despite the dominant phyla of the four treatments being similar, the compost application dose affected the distribution of each phylum. The relative abundance of Firmicutes only increased significantly after application of 30 t of pig manure-based compost ha−1, whereas it decreased at the rates of 60 and 120 t ha−1 crop−1 compared with the control without compost. Poulsen et al. (2013) also found a significant increase of Firmicutes in the MSW compost-amended soil. The improvement of soil structure after the addition of high amounts of compost (60 and 120 t ha−1 crop−1) significantly decreased the relative abundance of Clostridia, whose members are strictly anaerobic and belong to the phylum of Firmicutes (data not shown). The greater concentrations of heavy metals added with high rates (60 and 120 t ha−1 crop−1) of pig manure-based compost may have decreased the abundance of Firmicutes, because this group of microorganisms is not detected in heavy metal-contaminated soils (Gremion et al. 2003; Margesin et al. 2011).
Only the highest rate (120 t ha−1) of added compost significantly influenced the relative abundance of Proteobacteria phylum. The subclasses α-, β-, γ-, and δ-Proteobacteria were detected in all soil samples, and their relative abundances, except that of α-Proteobacteria, were significantly increased when 120 t ha−1 of pig manure-based compost was applied compared with the control without compost. α-Proteobacteria are capable of degrading complex organic compounds and are less competitive than other bacterial subclasses under high-nutrient concentrations (Newton et al. 2011). Sphingomonas and Pseudomonas belonging to α- and γ-Proteobacteria, respectively, were the two most abundant genera of Proteobacteria; their relative abundances significantly decreased when high amounts of pig manure-based compost were applied (Table 3). The two widespread bacterial genera are involved in various processes occurring in soil. Members of the genus Sphingomonas can degrade aromatic hydrocarbons (Aislabie et al. 2000) as well as produce sphingolipids which might contribute to the survival of Bacteroides under environmental stress (An et al. 2011). Pseudomonas strains are involved in N2 fixation, denitrification, and degradation of pollutants and can also promote plant growth and suppress plant disease (Haas and Défago 2005; Lalucat et al. 2006). Gomez-Balderas et al. (2014) demonstrated that the relative abundance of Pseudomonas significantly decreased in the rhizosphere soil by increasing Zn and Cd contaminations even though they present a high resistance to elevated Zn concentration as shown in this study. Conversely, the greatest relative abundances of four important genera belonging to Proteobacteria including Steroidobacter, Anaeromyxobacter, Phaselicystis, and Hyphomicrobium were observed in the soil treated with the highest rate (120 t ha−1 crop−1) of compost.
Actinobacteria are widely distributed in soil, water, and compost and play an import role in degrading recalcitrant compounds and suppressing pathogenic microorganisms by secreting various antibiotics (Franke-Whittle et al. 2009). This microbial phylum is dominant in heavy metal-contaminated soils, indicating high metal resistance. Margesin et al. (2011) have suggested that these Gram-positive bacteria with a high DNA G + C content could be taken as an indicator of the effects of heavy metal contamination. However, with the higher rates of pig manure-based compost incorporated and, thus, the high heavy metals accumulated in soil, the relative abundance of Actinobacteria did not change probably because the content of heavy metals in our soil was much lower than that studied by Margesin et al. (2011). In addition, the nutrients added to soil with compost may promote the growth of heavily sensitive bacteria, thus obscuring any toxic effect. Ryckeboer et al. (2003) reported that Actinobacteria are relatively ineffective competitors under high-nutrient conditions compared with many other microorganisms. Marmoricola, Nocardioides, Aciditerrimonas, Arthrobacter, and Thermoleophilum were the five most abundant genera belonging to Actinobacteria, and their relative abundances generally decreased by increasing the applied compost rate (Table 3). Among them, Arthrobacter is dominant during the thermophilic stage of composting and can take up heavy metals such as Cu, Mn, Ni, and Pb, thus decreasing heavy metal bioavailability in soil (Veglió et al. 1996; Tian et al. 2013).
The relative abundance of Acidobacteria significantly decreased at the highest compost applied rate (120 t ha−1). The ecological functions of Acidobacteria are poorly known despite being a dominant group in soil (Yang et al. 2013). The three most abundant genera of Acidobacteria, namely Gp6, Gp16, and Gp4, also found in the present study, were negatively correlated with organic C availability (Fierer et al. 2007; Jones et al. 2009). Thus, the significant decrease of Acidobacteria abundance after the addition of 120 t ha−1 crop−1 may be due to the increase of organic C availability.
The relative abundances of Chloroflexi, Nitrospira, and Armatimonadetes phyla significantly decreased in soil treated with the compost application rate of 120 t ha−1 crop−1. The three most abundant genera of Chloroflexi, namely Longilinea, Bellilinea, and Caldilinea, were detected in all compost-treated soils (Table 3), probably due to the fact that Chloroflexi is ubiquitous (Poulsen et al. 2013; Zhao et al. 2014). However, their biological functions are poorly known since most of these microbial groups are uncultured (Yamada and Sekiguchi 2009). The abundance of Nitrospira, which are involved in nitrification, was significantly affected by the higher pig manure-based compost addition rates, probably due to the elevated levels of ammonium and heavy metals of the amended soil.
Abundances of bacterial groups
The abundance of most animal, human, and plant pathogens significantly decreased in soil by increasing the applied compost rate (Table 4). This may depend on the high content of ammonium at the high rate of applied compost soil. Indeed, Tenuta and Lazarovits (2002) have detected pathogen death when ammonium was accumulated in soil.
However, it was interesting to note that after application of 30 t compost ha−1 crop−1 to soil, abundances of Clostridium, Rhodococcus, Nocardia, and Staphylococcus genera were high, which may be attributed to growth stimulation of these indigenous soil bacteria, or those of the pig manure-based compost, since an improperly managed composting process can allow the survival of these pathogens (Franke-Whittle et al. 2009). Poulsen et al. (2013) also found that the abundance of Clostridium, Rhodococcus, and Nocardia genera increased after the application of MSW compost equivalent to approximately 100 kg N ha−1 year−1. However, we have not observed the increase of Legionella genus and this discrepancy may be due to differences in soil and compost types (Bailey and Lazarovits 2003). The highest relative abundance of Streptomyces genus was detected in soil treated with 120 t ha−1 crop−1 compost, and this may be attributed to stimulation of their growth by compost nutrients and their tolerance to heavy metal pollution (Franke-Whittle et al. 2009). The human pathogens Salmonella and Escherichia were not detected in all soil samples. Plant pathogens such as Fusarium oxysporum, Rhizoctonia solani, and Verticillium biguttatum can be a problem in organic agriculture. Further studies are needed to evaluate changes in their activity and abundance. Abundance of Bradyrhizobium genus, involved in fixing atmospheric N2, was significantly decreased in soil treated with 60 and 120 t compost ha−1 crop−1, probably due to the relatively high N content compared with the other treatments. A similar result has been reported by Giller et al. (1998). However, the present study does not include N fixation as well as functions of other important bacterial groups.
Similarity analysis of different samples
An unweighted heat map of bacterial communities was generated based on Bray-Curtis distance indices that were calculated by OTUs at a distance of 3 %. This approach is an additional comparison between different treatments (Fig. 5a). The hierarchical cluster plot showed that different bacterial communities were detected after application of different rates of pig manure-based compost. It has been shown that different fertilization regimes can influence bacterial community composition (Crecchio et al. 2004; Ding et al. 2013; Zhao et al. 2014). The three replicates of each soil treated with the same rate of pig manure-based compost almost clustered together, indicating similar bacterial communities among replicates. However, one of the replicates (PC21) of the 60 t compost ha−1 crop−1 treated soil was close to replicates of the 120 t ha−1 crop−1 compost-treated soil (PC31). Only 2430 valid sequences were obtained from PC21 for technical reason, and this number was much lower than those of the other two replicates (6286 and 6376 for PC23 and PC23, respectively). There was a positive linear correlation between the number of sequences and predicted OTUs with Chao1 (R 2 = 0.99, Poulsen et al. 2013). PCA of the relative abundance of bacterial species (OTUs) further confirmed the results determined by cluster analysis (Fig. 5b).
Heat map and principal component analysis (PCA) of bacterial communities based on Bray-Curtis distance indices and OTUs at a distance of 3 %, respectively. a Heat map analysis, clustering of samples based on Bray-Curtis distance indices calculated by OTUs at a distance of 3 %. Color from black to red indicates increasing similarity. b PCA analysis: the first two components are 39.6 and 22.0 %. CKC is the untreated soil; PC1 is the soil treated with 30 t pig manure-based compost ha−1 crop−1; PC2 is the soil treated with 60 t pig manure-based compost ha−1 crop−1; PC3 is the soil treated with 120 t pig manure-based compost ha−1 crop−1
Conclusion
Application of heavy rates of pig manure-based compost increased the total amounts of each heavy metal (Zn, Cu, Pb, and Cd) in the short term. The diversity indices did not reveal significant changes in the bacterial diversity, but some detailed variations in the abundance of the bacterial community composition occurred in soils treated with different rates of pig manure-based compost. The joint cluster and PCA analysis demonstrated that heavy rates of compost applied in a short term did not increase bacterial diversity. Short-term composition and functional changes in microbial community composition under other soil conditions should be conducted and the optimum rate and period for compost application should be evaluated in future studies.
References
Achiba WB, Gabteni N, Lakhdar A, Laing GD, Verloo M, Jedidi N, Gallali T (2009) Effects of 5-year application of municipal solid waste compost on the distribution and mobility of heavy metals in a Tunisian calcareous soil. Agr Ecosyst Environ 130:156–163
Achiba WB, Lakhdar A, Gabteni N, Laing GD, Verloo M, Boeckx P, Cleemput OV, Jedidi N, Gallali T (2010) Accumulation and fractionation of trace metals in a Tunisian calcareous soil amended with farmyard manure and municipal solid waste compost. J Hazard Mater 176:99–108
Aislabie J, Foght J, Saul D (2000) Aromatic hydrocarbon-degrading bacteria from soil near Scott Base, Antarctica. Polar Biol 23:183–188
An D, Na CZ, Bielawski J, Hannun YA, Kasper DL (2011) Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. PANS 108:4666–4671
Asakawa S, Kimura M (2008) Comparison of bacterial community structures at main habitats in paddy field ecosystem based on DGGE analysis. Soil Biol Biochem 40:322–1329
Bailey KL, Lazarovits G (2003) Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res 72:169–180
Bakken LR, Frostegård Å (2006) Nucleic acid extraction from soil. In: Nannipieri P, Smalla K (eds) Nucleic acids and proteins in soil. Springer, Berlin, pp 49–73
Baldantoni D, Leone A, Iovieno P, Morra L, Zaccardelli M, Alfani A (2010) Total and available soil trace element concentrations in two Mediterranean agricultural systems treated with municipal waste compost or conventional mineral fertilizers. Chemosphere 80:1006–1013
Bibby K, Viau E, Peccia J (2010) Pyrosequencing of the 16S rRNA gene to reveal bacterial pathogen diversity in biosolids. Water Res 44:4252–4260
Cole JR, Chai B, Farris RJ, Wang RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Bandela AM, Cardenas E, Garrity GM, Tiedje JM (2007) The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res 35:169–172
Convertini G, De Giorgio D, Ferri D, La Cava P, Giglio L (1999) Sugar beet and durum wheat quality characteristics as affected by composted urban waste. In: Anac D, Prevel M (eds) Improved crop quality by nutrient management. Kluwer, Dordrecht, pp 241–244
Crecchio C, Curci M, Pizzigallo MDR, Ricciuti P, Ruggiero P (2004) Effects of municipal solid waste compost amendments on soil enzyme activities and bacterial genetic diversity. Soil Biol Biochem 36:1595–1605
Cytryn E, Kautsky L, Ofek M, Mandelbaum RT, Minz D (2011) Short-term structure and functional changes in bacterial community composition following amendment with biosolids compost. Appl Soil Ecol 48:160–167
Ding XL, Han XZ, Zhang XD (2013) Long-term impacts of manure, straw, and fertilizer on amino sugars in a silty clay loam soil under temperate conditions. Biol Fertil Soils 49:949–954
Dolliver H, Gupta S, Noll S (2007) Antibiotic degradation during manure composting. J Environ Qual 37:1245–1253
Fierer N, Bradford M, Jackson R (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Franke-Whittle IH, Knapp BA, Fuchs J, Kaufmann R, Insam H (2009) Application of COMPOCHIP microarray to investigate the bacterial communities of different composts. Microb Ecol 57:510–521
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30:1389–1414
Gomez-Balderas CDC, Cochet N, Bert V, Tarnaud E, Sarde C (2014) 16S rDNA analysis of bacterial communities associated with the hyper accumulator Arabidopsis halleri grown on a Zn and Cd polluted soil. Eur J Soil Biol 60:16–23
Gremion F, Chatzinotas A, Harms H (2003) Comparative 16S rDNA and 16S rRNA sequence analysis indicates that Actinobacteria might be a dominant part of the metabolically active bacteria in heavy metal-contaminated bulk and rhizosphere soil. Environ Microbiol 5:896–907
Griffiths BS, Philippot L (2013) Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol Rev 37:112–129
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Horrigan L, Lawrence RS, Walker P (2002) How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ Health Perspect 110:445–456
Innerebner G, Knapp B, Vasara T, Romantschuk M, Insam H (2006) Traceability of ammonia-oxidizing bacteria in compost-treated soils. Soil Biol Biochem 38:1092–1100
Jones RT, Robeson MS, Lauber CL, Hamady M, Knight R, Fierer N (2009) A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J 3:442–453
Ju XT, Kou CL, Christie P, Dou ZX, Zhang FS (2007) Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Environ Pollut 145:497–506
Kumar K, Gupta SC, Chander Y, Singh AK (2005) Antibiotic use in agriculture and its impact on the terrestrial environment. Adv Agron 87:1–54
Lalucat J, Bennasar A, Bosch R, Garcia-Valdes E, Palleroni NJ (2006) Biology of Pseudomonas stutzeri. Microbiol Mol Biol Rev 70:510–547
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120
Margesin R, Płaza GA, Kasenbacher S (2011) Characterization of bacterial communities at heavy-metal-contaminated sites. Chemosphere 82:1583–1588
Myrold DD, Nannipieri P (2014) Classical techniques versus omics approaches. In: Nannipieri P, Pietramellara G, Renella G (eds) Omics in soil science. Caster Academic Press, Norfolk, pp 179–187
Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49
Poulsen PHB, Abu Al-Soud W, Bergmark L, Magid J, Hansen LH, Sørensen SJ (2013) Effects of fertilization with urban and agricultural organic wastes in a field trial—prokaryotic diversity investigated by pyrosequencing. Soil Biol Biochem 57:784–793
Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196
Quince C, Lanzen A, Davenport RJ, Turnbaugh PJ (2011) Removing noise from pyrosequenced amplicons. BMC Bioinformatics 12:38
Ros M, Klammer S, Knapp B, Aichberger K, Insam H (2006) Long-term effects of compost amendment of soil on functional and structural diversity and microbial activity. Soil Use Manag 22:209–218
Ryckeboer J, Mergaert J, Coosemans J, Deprins K, Swings J (2003) Microbiological aspects of biowaste during composting in a monitored compost bin. J Appl Microbiol 94:127–137
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Singh J, Kalamdhad AS (2013) Chemical speciation of heavy metals in compost and compost amended soil—a review. Int J Environ Res 2:27–37
Sun J, Zhang Q, Zhou J, Wei QP (2014) Pyrosequencing technology reveals the impact of different manure doses on the bacterial community in apple rhizosphere soil. Appl Soil Ecol 78:28–36
Tenuta M, Lazarovits G (2002) Ammonia and nitrous acid from nitrogenous amendments kill microsclerotia of Verticillium dahliae. Phytopathology 92:255–264
Tian W, Sun Q, Xu DB, Zhang ZH, Chen D, Li CY, Shen QR, Shen B (2013) Succession of bacterial communities during composting process as detected by 16S rRNA clone libraries analysis. Int Biodeter Biodegr 78:58–66
Trewavas A (2004) A critical assessment of organic farming-and-food assertions with particular respect to the UK and the potential environmental benefits of no-till agriculture. Crop Prot 23:757–781
Tu C, Louws FJ, Creamer NG, Mueller JP, Brownie C, Fager K, Bell M, Hu SJ (2006) Responses of soil microbial biomass and N availability to transition strategies from conventional to organic farming systems. Agr Ecosyst Environ 113:206–215
Van Elsas JD, Cretoiu MS, Kielak AM, Dini-Andreote F (2014) Soil metagenomics, potential applications and methodological problems. In: Nannipieri P, Pietramellara G, Renella G (eds) Omics in soil science. Caster Academic Press, Norfolk, pp 31–44
Veglió F, Beolchini F, Gasbarro A (1996) Biosorption of toxic metals: an equilibrium study using free cells of Actinobacteria sp. Process Biochem 32:95–107
Walker DJ, Clemente R, Bernal P (2004) Contrasting effects of manure and compost on soil pH, heavy metals availability and growth of Chenopodium album in a soil contaminated by pyritic mine waste. Chemosphere 57:215–224
Weber J, Karczewska A, Drozd J, Licznar M, Licznar S, Jamroz E, Kocowicz A (2007) Agricultural and ecological aspects of a sandy soil as affected by the application of municipal solid waste composts. Soil Biol Biochem 39:1294–1302
Widmer F, Rasche F, Hartmann M, Fliessbach A (2006) Community structures and substrate utilization of bacteria in soils from organic and conventional farming systems of the DOK long-term field experiment. Appl Soil Ecol 33:294–307
Wong JWC, Ma KK, Fang KM, Cheung C (1999) Utilization of a manure compost for organic farming in Hong Kong. Bioresour Technol 67:43–46
Yamada T, Sekiguchi Y (2009) Cultivation of uncultured Chloroflexi subphyla: significance and ecophysiology of formerly uncultured Chloroflexi ‘Subphylum I’ with natural and biotechnological relevance. Microbes Environ 24:205–216
Yang C, Hamel C, Gan Y, Vujanovic V (2013) Pyrosequencing reveals how pulses influence rhizobacterial communities with feedback on wheat growth in the semiarid prairie. Plant Soil 367:493–505
Zhao J, Zhang RF, Xue C, Xun WB, Sun L, Xu YC, Shen QR (2014) Pyrosequencing reveals contrasting soil bacterial diversity and community structure of two main winter wheat cropping systems in China. Microbiol Ecol 67:443–453
Zhou DM, Hao XZ, Wang YJ, Dong YH, Cang L (2005) Copper and Zn uptake by radish and pakchoi as affected by application of livestock and poultry manures. Chemosphere 59:167–175
Acknowledgments
This work was supported by the Youth Science Foundation of Jiangsu Province (BK20130101), the Specialized Public Welfare Fund of Environmental Protection (201209036 and 201309036), and the Major Project of National Science and Technology (2014ZX07206001).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table s1
(DOCX 18 kb)
Table s2
(DOCX 19 kb)
Fig. s1
Dry weights of Brassica chinensis and Cichorium endivia L. from different treatments. CKC is the untreated soil; PC1 is the soil treated with 30 t pig manure-based compost ha−1 crop−1; PC2 is the soil treated with 60 t pig manure-based compost ha−1 crop−1; PC3 is the soil treated with 120 t pig manure-based compost ha−1 crop−1. The numbers indicate the replicates of each treatment. Different letters indicate statistically different numbers between treatments (p < 0.05). (GIF 7 kb)
Rights and permissions
About this article
Cite this article
Tian, W., Zhang, Z., Hu, X. et al. Short-term changes in total heavy metal concentration and bacterial community composition after replicated and heavy application of pig manure-based compost in an organic vegetable production system. Biol Fertil Soils 51, 593–603 (2015). https://doi.org/10.1007/s00374-015-1005-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-015-1005-4