Abstract
The aim of this work was to investigate the effect of engineered nanoparticles (NPs) on soil microbial biomass C (MBC) and on earthworm Lumbricus rubellus. An artificial soil was incubated for 4 weeks with earthworms fed with vegetable residues contaminated by NPs, consisting of Ag, Co, Ni and TiO2. After the treatments, soils were analysed for MBC and total and water soluble metal-NPs, whereas earthworms were purged for 28 days and then analysed for fatty acids (FAs) and total metal-NPs. Longitudinal sections of earthworms were investigated by environmental scanning electron microscopy (ESEM), equipped with energy-dispersive X-ray spectroscopy (EDS), to provide insights about the retention and localization of NPs within earthworms. The nanoparticles reduced the MBC content in the following order Ag > Co > Ni, whereas TiO2 did not affect it. The ESEM-EDS analysis confirmed NP retention in earthworm guts and tissues. The solid/water coefficient of partition suggested that NPs interfered with living organisms due to their presence in suspension. Among the 27 FAs identified in earthworm tissues, the eicosapentaenoic acid (20:5ω3) was the most abundant. The degree of unsaturation of FAs was reduced by supplying NP-contaminated food.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoparticles (NPs) can potentially cause adverse effects at any biological scale, from organ tissues, through cellular and subcellular, up to macromolecular level, due to their peculiar physico-chemical properties (Schrand et al. 2010). Their increasing use in many industrial fields must be supported by the widest scientific information on the possible risks to human health and environmental issues (Klaine et al. 2008). Many metal-based NPs, such as nanocomposite coatings, have been heavily investigated as candidates for new antimicrobial treatments alternative to antibiotic ones (Alt et al. 2004; Morones et al. 2005). Silver nanoparticles (Ag-NP) are currently added to many common home products (Jain et al. 2009; Percival et al. 2007). Metallic cobalt nanoparticles (Co-NPs) are used in medicine and biology where an induction of DNA damage in vitro was found (Ponti et al. 2009). Nickel nanoparticles (Ni-NPs) are widely used in a variety of application due to their catalytic and magnetic properties (Park et al. 2005), and toxic effect on human lung epithelial cells has been reported (Ahamed 2011). The TiO2-NPs absorb UV radiation, yielding in aqueous media hydroxyl species that may cause substantial damage to DNA (Schrand et al. 2010). NPs may reach soils through use of biosolids originating from wastewater treatment and water remediation, accidental spill during production, transport or incineration and deliberate release to environment (Barnes et al. 2010; Schlich et al. 2013). Recently, Burke et al. (2014) have demonstrated that even low concentration of TiO2-NPs may influence some important group of soil microbes such as mycorrhizal fungi.
Assessing the risks of NPs in the soil requires an understanding of their mobility, reactivity, ecotoxicity and persistency. The aggregation and/or agglomeration of NPs in soil depends on the Brownian motion, gravity force and NP concentration, while the intrinsic characteristics of NPs (e.g. electrostatic repulsion, surface charge, NP size) as well as those of soil (e.g. organic matter, ionic strength, clay content) influence their solubility in soil. Studies on the effects of NPs are rapidly increasing, above all for the aquatic environments, whereas little information is available about their effects on soil ecosystem (Tourinho et al. 2012). On the other hand, soil risk assessment cannot be based only on chemical analysis of contaminants (Sanchez-Hernandez 2006) because this approach does not provide any indication of impact on soil biota. Earthworms play an important role in the organic matter turnover in soil, by mixing and shredding organic matter, consolidating and improving the soil structure and stimulating microbial growth (Massey et al. 2013). Besides, they are sensitive indicators of environmental damage (Reinecke 1992), being also used in soil ecotoxicological tests to evaluate soil contamination (Asensio et al. 2013; Duarte et al. 2014). For example, the metabolic products of earthworms have been used as biomarkers for assessing different degree of soil contamination (Sanchez-Hernandez 2006; Calisi et al. 2012).
The aim of this work was to evaluate the effects of exposure to metal (Ag, Co, Ni) and metal oxide (TiO2) NPs, supplied by contaminated food, on the fatty acid composition of Lumbricus rubellus and on the soil microbial biomass carbon (MBC). L. rubellus was used for its key role in the soil food web and because it is among the most abundant soil earthworms (Lavelle et al. 1997). The NP effects on L. rubellus was assessed by investigating the shift of fatty acid composition of earthworm tissues. Indeed, FAs of earthworm tissues have been previously used as biomarkers for environmental stress (e.g. temperature; Petersen and Holmstrup 2000). The effects on soil microbial abundance were tested by determining the soil microbial biomass C. In addition, NP content in both soils and earthworms were measured. Finally, the environmental scanning electron microscope (ESEM), equipped with energy-dispersive X-ray spectroscopy, was used to individuate the NPs retained within the earthworm tissues. To our best knowledge, this is the first attempt to investigate the impact of NPs on soil biota through the study of metal availability, eco-physiological indicators and fatty acid earthworm profile.
Materials and methods
Nanoparticle properties
Ag-NPs were supplied as suspension of metallic silver (Ag) in deionized water (1 g L−1) with a NP size between 1 and 20 nm and purchased from Polytech (Germany, type WM 1000-c). Co- and Ni-NPs in powdered form were obtained from Nanostructured and Amorphous Materials Inc. (USA) with a size between 20 and 60 nm. TiO2-NPs in powdered form were obtained from Tal Materials Inc. (USA) with a size between 20 and 160 nm. Stable suspensions (1 g L−1) of Co-, Ni- and Ti2O-NPs were prepared with deionized water by sonication for 1 h in ultrasonic bath to have the appropriate dilution.
Experimental design
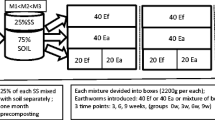
Five hundred grams of artificial soil were weighed into plastic boxes. This artificial soil, composed by fine quartz sand, carbonatic clay and peat, is recommended for acute toxicity tests according to OECD (1984). It had the following characteristics: sandy loam texture (60 % sand, 10 % clay and 30 % silt), pH 6.9, total organic C and total N 4.3 and 0.5 %, respectively. After a soil pre-incubation of 1 week at controlled constant temperature and humidity (i.e. 25 °C and 60 % of water holding capacity), ten adult earthworms with clitellum were selected from a nursery and acclimated for another week within the technological soil. The earthworms in the nursery had been fed with vegetable residues (potatoes, lettuces, carrots, apples) finely ground and homogenized. The food had been spiked with water solution containing the NP 24 h prior to feeding, to reach the 65 % of water holding capacity. After the acclimation period, 10 g of the contaminated food containing 2.5 mg (as element) of the single engineered NPs were distributed onto soil surface and no further disturbance was performed to mix food and soil. The NPs singly used to contaminate the food were Ag, Co, Ni and TiO2 singly added. The polluted food was supplied once a week for 4 weeks, thus reaching a total dose of 20 μg of NP-metals g−1 soil, and then, treated soils were incubated for 28 days at 25 °C. Each treatment was replicated three times. At the end of the experiment, the earthworms were purged by transferring them to Petri dishes for 24 h in order to empty their guts, frozen at −20 °C and lyophilized (Modulyo 4K Freeze Dryer, Edwards, UK), whereas the soils were kept moist at 4 °C. A control treatment, in which the earthworms were fed with uncontaminated food, was simultaneously arranged.
Soil and earthworm tissue analyses
Total organic concentration (TOC) was determined by CHN analyser (Carlo Erba, mod.1100, Italy). Microbial biomass C was determined by the fumigation-extraction (FE) method (Vance et al. 1987). Moist soil (20 g) was sampled from each box and divided into two portions. One portion (10 g soil) was fumigated for 24 h at 25 °C with ethanol-free CHCl3. After removing the chloroform by repeated evacuations, the fumigated soil was extracted with 40 mL 0.5 M K2SO4 for 30 min on a horizontal shaker and the suspension filtered through Whatman 42 filter paper (nominal pore size 2.5 μm). The equivalent non-fumigated soil portion (10 g) was similarly extracted at the time. Both fumigated and non-fumigated extracts were analysed for organic C by hot acid dichromate oxidation. MBC was calculated as EC/kEC, where EC is the organic C extracted from fumigated soil minus that extracted from non-fumigated soil and kEC is 0.38 (Jenkinson et al. 2004).
A 250-mg aliquot of soil was air dried and finely ground with an agate mortar and used to provide soil water extracts. Soils were shaken on a horizontal shaker for 16 h with deionized water (Milli-Q water, Millipore) at 1 soil:10 water (w/v) ratio. The suspensions were centrifuged for 15 min at 1200g and the supernatants filtered through Ø 0.45 μm filter (Millipore). The total metal concentration in 250 mg soil samples was determined after mineralization in aqua regia (2 mL HNO3 plus 6 mL suprapure HCl, Merck) in the microwave oven (Milestone, Start D 1200) according to Vittori Antisari et al. (2012a). The total element concentrations were determined by inductively coupled plasma (ICP-OES, Spectro Arcos Ametek). The total metal concentration in earthworms was determined on 250 mg of sub-sample (obtained by grinding eight earthworms) after mineralization in suprapure HNO3 (Merck) in the microwave oven (Milestone, Start D 1200) by ICP-OES as described by Vittori Antisari et al. (2012a). After mineralization, soil and earthworm samples were filtered through Whatman 42 filter paper (nominal pore size 2.5 μm) and the filtrate volume was adjusted to 20 mL with ultrapure water (Milli-Q water, Millipore). Calibrations were performed by using the standard solution of Bureau of Collection Recovery (BCR-909).
According to Blaser et al. (2000) equilibrium between the soil and the aqueous solution was reached at the end of the extraction. The availability of elements that derived by NP dissolution was taken into account by calculating the solid/water coefficient of partition (Kd) according to the following equation (Vittori Antisari et al. 2012a):
where [metal]soil fine earth is the total metal concentration in soil (mg kg−1) and [metal]water extract is the total metal concentration of the water extract (mg L−1). The data were expressed as log Kd.
Fatty acid methyl esters (FAMEs) were determined by the standard procedure described by Kennedy (1994). About 150 mg of sub-sample (obtained by grinding 8 earthworms) was weighed in 10-mL glass test tubes, 1 mL 4 M NaOH in 50 % methanol was added and then the mixture was heated for 30 min at 100 °C in a water bath.
After cooling at room temperature, 2 mL 6 M HCl in methanol were added for methylation of dissolved fatty acids in a water bath at 80 °C (10 min). Then, 1 mL hexane:methyl-tert-butyl ether (1:1, v/v) was added and lipids extracted by shaking for 10 min. The organic phase was transferred to a new test tube, and the extraction was repeated. The combined organic phase was washed once with 0.25 M NaOH and subsequently transferred to 2-mL vials for analysis on a gas chromatograph (Focus, Thermo Scientific) equipped with a flame ionization detector and a fused-silica capillary column Mega-10 (50 m × 0.32 mm I.D.; film thickness 0.25 μm; stationary phase 100 % cyanopropyl polysiloxane). The GC temperature progression was 115 °C for 5 min, increase at a rate of 1.5 °C per minute from 115 to 230 °C, and at 230 °C for 2 min. Both injection port and detector were set up at 250 °C, respectively, and He (grade 5.5) at 1 mL min−1 in a constant flow mode was used as carrier. The injected volume was 1 μL in a splitless mode. Nonadecanoic acid methyl ester (19:0; cat no. N-5377, Sigma-Aldrich Co.) was used as an internal standard for quantification of FAMEs. Identification of peaks was based on comparison of retention times to known standards (Supelco Bacterial Acid Methyl Esters mix cat no. 47080-U and Supelco 37 Component FAME mix cat no. 47885-U). The relative abundance of detected FAMEs was expressed as mol %. Fatty acid nomenclature used was that described by Frostegård et al. (1993). The degree of unsaturation was calculated as follows:
All soil and earthworm analyses were carried out in triplicate.
For microscopic inspection of the residue remained in the earthworm gut, the purged earthworms were placed in 10 % formalin solution buffered at pH 6.8 with sodium phosphate. Then, they were incorporated in the resin, cut longitudinally, placed on an adhesive carbon disc and inserted in the chamber of the electron microscope (environmental scanning electron microscope (ESEM)), equipped with energy-dispersive X-ray spectroscopy (EDS, Quanta FEI, 250). The electron microscopy analyses were performed by catching back side electrons (BSE), in order to obtain information on the chemical nature of samples rather than their morphology.
Statistical analysis
Before performing parametric statistical analyses, normal distribution and variance homogeneity of the data were checked by Kolmogorov–Smirnov goodness-of-fit and Levene’s tests, respectively. Significant differences among treatments were tested by one-way analysis of variance (type of added NP as factor), and the least significant difference (P < 0.05) test was used as post hoc test for multiple comparisons among means. Statistical analyses were performed using SPSS 13.0.
Results
Effect of NPs on microbial biomass C
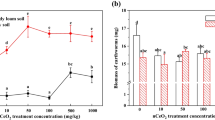
The MBC significantly (P < 0.05) decreased after exposure to NPs compared to the control soil, except for the TiO2-NP treatment (Fig. 1). In particular, the MBC decreased by more than 60 % in soil treated with Ag-NPs (P < 0.01), while a more limited reduction occurred in Co-NP- and Ni-NP- (P < 0.05) polluted soils (Fig. 1).
Metals of NPs in soil and in earthworms
The total soil concentration of each metal arising from NPs showed significant (P < 0.05) differences between the soil treated with NP-polluted food and the unpolluted control (Table 1). The significant (P < 0.05) increases in metal-NP soil concentration ranged between 3.7 (Co) and 5.2 (Ag) μg g−1, i.e. generally less than a quarter of the total theoretical dose of element arising from NPs added with the food. The absence of a significant increase for the NP-TiO2-polluted soil was likely due to the high variability ranges of the Ti data. The metal-NP body tissues were significant (P < 0.05) for all four metals, even in the case of Ti; the decreasing order was Co > Ti > Ni > Ag (Table 1).
The values of partition coefficient (Kd) were lowest (3.4 ± 0.1) in the Ag-, Ni- and Co-NP treatments and highest (5.0 ± 0.1) in the Ti2O-NP treatment.
The ESEM-EDS images showed that soil residues remained in earthworm guts even after their purging (Fig. 2) and the presence of Co-NPs (Fig. 3) inside earthworm guts, as well as isolated Co-NPs in the earthworm tissues (Fig. 4).
Fatty acids in earthworms
A total of 11 saturated, 14 mono- and poly-unsaturated, one hydroxyl and one branched FAs were detected, positively identified and quantified (Table 2) in earthworms tissues.
The most abundant FA was 20:5ω3 (eicosapentaenoic acid) followed by the saturated FA 12:0 (lauric acid). The total molar percentage amount of FAs in earthworm tissues did not show significant differences among treatments. On the contrary, the total percentage amounts of saturated FAs were slightly higher in soils treated with food polluted with TiO2- and Co-NPs than in control. Moreover, the total amount of mono-unsaturated FAs was lower in earthworms exposed to food polluted with Co- and Ni-NPs, while the percentage of poly-unsaturated FAs was lower in the TiO2-NP treatment than in the control. As a consequence, in earthworm tissues exposed at food contaminated with TiO2-, Co- and Ni-NPs, the degree of unsaturation was lower than that determined in the control soil.
Discussion
Soil microbial biomass C was negatively affected by Ag-NPs, as well as by Ni- and Co-NPs. The MBC reduction following Ag-NP addition was also previously observed by Hänsch and Emmerling (2010), whereas no data exist in literature about the deleterious effect of Ni- and Co-NPs.
Currently, very little information is available on how the engineered NPs may affect soil microorganisms (Dinesh et al. 2012; Vittori Antisari et al. 2012b). The behaviour of NPs and their toxicity to soil microbial communities have been described by different mechanisms. Direct interaction or/and change in availability of nutrients and release of toxic constituents may be a possible mechanism of toxicity (Kool et al. 2011; Simonet and Valcárcel 2009). Other possible mechanisms include disruption of membranes, oxidation of proteins, genotoxicity by DNA damage and formation of reactive oxygen species (Bigorgne et al. 2011). Some authors (Du et al. 2011; Ge et al. 2011) showed that soil microbial biomass and bacterial diversity were reduced in the presence of TiO2-NPs, while in our experiment, no reduction in MBC content was observed in the TiO2-NP treatment. Hazards by NPs do not only depend on their concentration but also on the adsorption mechanisms onto inorganic and organic soil colloidal surfaces, processes which reduce their mobility and favour the coating and aggregation (Dinesh et al. 2012). Therefore, it is necessary to study the influence of dissolved element-NP species rather than the total amount added to soil (Adams et al. 2006). For this reason, the availability of elements that derived from NP dissolution, after water extraction, was taken into account, and the partition coefficient (Kd) between the solid phases and water (Vittori Antisari et al. 2012b) was calculated. The high values of Kd suggested a low NP water solubility, in particular for TiO2-NPs that showed a value of 5.0 (Vittori Antisari et al. 2012a). The low solubility of NPs suggests that their effect on living organisms may depend on their presence in suspension (Adams et al. 2006). In this way, the hazard can increase in terrestrial organisms, such as earthworms fed with NP-polluted food, both through dermal contact and ingestion; indeed, the ESEM images highlighted that the earthworm guts contaminated by NPs.
The most toxic metal-NP to MBC was Ag which showed the lowest Kd (highest water solubility), with possible formation of Ag+. Thus, the inhibition of soil enzyme activities by Ag+ may depend on the high Ag toxicity to soil microbial activity (Chaperon and Sauvé 2007).
As already mentioned, the NP impact on terrestrial organisms is poorly known (Dinesh et al. 2012). In addition, standard ecotoxicity tests with earthworms employ rather insensitive end point tests like mortality, growth and fecundity; these tests have so far only responded to engineered nanoparticles at concentrations ≥1 g kg−1 soil (Scott-Fordsmand et al. 2008). Since in our experiment neither significant earthworm death nor earthworm biomass decrease was observed, the fatty acids (FAs) composition of earthworm tissues was used as more sensitive biomarker to define the hazard of NPs contamination. Indeed, FAs in whole body or gut of earthworms have been used as indices of responses to environmental stress (Crockett et al. 2001), and the composition of fatty acids in the earthworm’s body depends on both species (Paoletti et al. 2003) and diet (Sampedro et al. 2006). The highest amount of the FA 20:5ω3, followed by the FA 12:0, partially agreed with FA data found by Petersen and Holmstrup (2000). Indeed, they identified FAs with a number of C atoms greater than 15, and no information is currently available on FAs in earthworm tissues with a number of C atoms lower than 16. Changes in the degree of unsaturation likely indicate adaptation responses of soil organisms to environmental stress. Saturated fatty acids can pack together better than unsaturated ones and, therefore, make the double lipidic layer of membranes more viscous and less permeable (Collins et al. 1990). Moreover, a low degree of unsaturation decreases the susceptibility of fatty acid to free radicals (García et al. 2005). The observed reduction in the degree of unsaturation in earthworm tissues agreed with other similar studies on eukaryotic cells (Paraszkiewicz et al. 2009; Yang et al. 2009) and could be ascribed to defence mechanisms for reducing the oxidative stress on the lipid layers generated by heavy metals (Howlett and Avery 1997; Yang et al. 2009).
The numbers of FAs of each NP treatment significantly (P < 0.05) differed from those of control soil and the decreasing order at which NPs affect the FA distribution of earthworm tissues was Ni > TiO2 = Co > Ag.
Conclusions
Silver, cobalt and nickel nanoparticles, added to soil through food (fruit and vegetables) for L. rubellus, negatively affected the soil microbial biomass C. Also, earthworm lipid composition was altered by decreasing the degree of unsaturation of fatty acids of L. rubellus tissues. Notably, the most toxic metal-NP to soil microbial biomass (Ag-NP) was the least affecting FAs of L. rubellus, whereas the opposite occurred for TiO2-NP. This raises the need for monitoring both the soil microbial communities and fauna when the soil interacts with NPs of any origin.
The low NP solubility suggests that their effects on soil microbial biomass mainly depend on their presence in suspension. The metal-based NPs were retained by earthworms and found in their guts in aggregated particles of soil and increased the Ag, Co, and Ni contents of earthworm tissues. The ESEM-EDS investigation showed a direct interaction between isolated and non-aggregated nanoparticle and cells in the earthworm tissues. Further studies are needed to better assess the ecotoxicity of NPs on soil microbial composition and activity and on earthworm health. To find out indices that could be used as early warning of stress linked to soil food web is also important.
References
Adams L, Lyon DY, Alvarez PJJ (2006) Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res 40:3527–3532
Ahamed M (2011) Toxic response of nickel nanoparticles in human lung epithelial A549 cells. Toxicol In Vitro 25:930–936
Alt V, Bechert T, Steinrucke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R (2004) An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 25:4383–4391
Asensio V, Rodríguez-Ruiz A, Garmendia L, Andre J, Kille P, Morgan AJ, Soto M, Marigómez I (2013) Towards an integrative soil health assessment strategy: a three tier (integrative biomarker response) approach with Eisenia fetida applied to soils subjected to chronic metal pollution. Sci Total Environ 442:344–365
Barnes RJ, Riba O, Gardner MN, Scott TB, Jackman SA, Thompson IP (2010) Optimization of nano-scale nickel/iron particles for the reduction of high concentration chlorinated aliphatic hydrocarbon solutions. Chemosphere 79:448–454
Bigorgne E, Foucaud L, Lapied E, Labille J, Botta C, Sirguey C, Falla J, Rose J, Joner EJ, Rodius F, Nahmani J (2011) Ecotoxicological assessment of TiO2 byproducts on the earthworm Eisenia fetida. Environ Pollut 159:2698–2705
Blaser P, Zimmermann S, Luster J, Shotyk W (2000) Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb, and Zn in Swiss forest soils. Sci Total Environ 249:257–280
Burke DJ, Zhu S, Pablico-Lansigan MP, Hewins CR, Samia ACS (2014) Titanium oxide nanoparticle effects on composition of soil microbial communities and plant performance. Biol Fertil Soils. doi:10.1007/s00374-014-0938-3
Calisi A, Zaccarelli N, Lionetto MG, Schettino T (2012) Integrated biomarker analysis in the earthworm Lumbricus terrestris: application to the monitoring of soil heavy metal pollution. Chemosphere 90:2637–2644
Chaperon S, Sauvé S (2007) Toxicity interaction of metals (Ag, Cu, Hg, Zn) to urease and dehydrogenase activities in soils. Soil Biol Biochem 39:2329–2338
Collins JM, Dominey RN, Grogan WM (1990) Shape of the fluidity gradient in the plasma membrane of living HeLa cells. J Lipid Res 31:261–270
Crockett EL, Dougherty BE, McNamer AN (2001) Effects of acclimation temperature on enzymatic capacities and mitochondrial membranes from the body wall of the earthworm Lumbricus terrestris. Comp Biochem Phys B 130:419–426
Dinesh R, Anandaraj M, Srinivasan V, Hamza S (2012) Engineered nanoparticles in the soil and their potential implications to microbial activity. Geoderma 173–174:19–27
Du W, Sun Y, Ji R, Zhu J, Wu J, Guo H (2011) TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit 13:822–828
Duarte AP, Freitas Melo V, Brown GG, Pauletti V (2014) Earthworm (Pontoscolex corethrurus) survival and impacts on properties of soils from a lead mining site in Southern Brazil. Biol Fertil Soils 50:851–860
Frostegård Å, Tunlid A, Bååth E (1993) Phospholipid fatty acid composition, biomass and activity of microbial communities from two soil types experimentally exposed to different heavy metals. App Environ Microb 59:3605–3617
García JJ, Martinez-Ballariin E, Millan-Plano S, Allué JL, Albendea C, Fuentes L, Escanero JF (2005) Effects of trace elements on membrane fluidity. J Trace Elem Med Bio 19:19–22
Ge Y, Schimel JP, Holden PA (2011) Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ Sci Technol 45:1659–1664
Hänsch M, Emmerling C (2010) Effects of silver nanoparticles on the microbiota and enzyme activity in soil. J Plant NutrSoil Sci 173:554–558
Howlett NG, Avery SV (1997) Relationship between cadmium sensitivity and degree of plasma membrane fatty acid unsaturation in Saccharomyces cerevisiae. App Microbiol Biotechnol 48:539–545
Jain D, Daima HK, Kachhwaha S, Kothari SL (2009) Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti-microbial activities. Dig J Nanomater Bios 4:557–563
Jenkinson DS, Brookes PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36:5–7
Kennedy AC (1994) Carbon utilization and fatty acid profiles for characterization of bacteria. In Weaver RW, Angle S, Bottomley P, Bezdicek D, Smith S, Tabatabai A, Wollum A (Eds), Methods of soil analysis. Part 2: microbiological and biochemical properties, Soil Science Society of America, Madison, WI, pp. 543–556
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behaviour, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851
Kool PL, Diez Ortiz M, van Gestel CAM (2011) Chronic toxicity of ZnO nanoparticles, non-nano ZnO and ZnCl2 to Folsomia candida (Collembola) in relation to bioavailability in soil. Environ Pollut 159:2713–2719
Lavelle P, Bignell D, Lepage M, Wolters V, Roger P, Ineson P, Heal OW, Dhillion S (1997) Soil function in a changing world: the role of invertebrate ecosystem engineers. Eur J Soil Biol 33:159–193
Massey PA, Creamer RE, Schulte RPO, Whelan MJ, Ritz K (2013) The effects of earthworms, botanical diversity and fertiliser type on the vertical distribution of soil nutrients and plant nutrient acquisition. Biol Fertil Soils 49:1189–1201
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez TJ, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
OECD (1984) Guideline for testing of chemicals No. 207, Earthworm acute toxicity test. Organization for Economic Co-operation and Development. Paris, France
Paoletti MG, Buscardo E, VanderJagt DJ, Pastuszyn A, Pizzoferrato L, Huang YS, Chuang LT, Millson M, Cerda H, Torres F, Glew RH (2003) Nutrient content of earthworms consumed by Ye’Kuana Amerindians of the Alto Orinoco of Venezuela. P Roy Soc B-Biol Sci 270:249–257
Paraszkiewicz K, Bernat P, Dlugonski J (2009) Effect of nickel, copper, and zinc on emulsifier production and saturation of cellular fatty acids in the filamentous fungus Curvularia lunata. Int Biodeter Biodegr 63:100–105
Park J, Kang E, Son SU, Park HM, Lee MK, Kim J, Kim KW, Noh HJ, Park JH, Bae CJ, Park JG, Hyeon T (2005) Monodisperse nanoparticles of Ni and NiO: synthesis, characterization, self-assembled superlattices, and catalytic applications in the Suzuki coupling reaction. Adv Mater 17:429–434
Percival SL, Bowler PG, Dolman J (2007) Antimicrobial activity of silver-containing dressings on wound microorganisms using an in vitro biofilm model. Int Wound J 4:186–191
Petersen SO, Holmstrup M (2000) Temperature effects on lipid composition of the earthworms Lumbricus rubellus and Eisenia nordenskioeldi. Soil Biol Biochem 32:1787–1791
Ponti J, Sabbioni E, Munaro B, Broggi F, Marmorato P, Franchini F, Colognato R, Rossi F (2009) Genotoxicity and morphological transformation induced by cobalt nanoparticles and cobalt chloride: an in vitro study in Balb/3T3 mouse fibroblasts. Mutagenesis 24:439–445
Reinecke AJ (1992) A review of ecotoxicological test methods using earthworms. In Greig-Smith PW, Becker H, Edwards PJ, Heimbach F (Eds.), Ecotoxicology of Earthworms. Intercept, Andover, MA, (pp. 7–19)
Sampedro L, Jeannotte R, Whalen JK (2006) Trophic transfer of fatty acids from gut microbiota to the earthworm Lumbricus terrestris L. Soil Biol Biochem 38:2188–2198
Sanchez-Hernandez JC (2006) Earthworm biomarkers in ecological risk assessment. Rev Environ Contam T 188:85–126
Schlich K, Klawonn T, Terytze K, Hund-Rinke K (2013) Effects of silver nanoparticles and silver nitrate in the earthworm reproduction test. Environ Toxicol Chem 32:181–188
Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF (2010) Metal-based nanoparticles and their toxicity assessment. Wiley interdisciplinary reviews. Nanomed Nanobiotechnol 2:544–568
Scott-Fordsmand JJ, Krogh PH, Schaefer M, Johansen A (2008) The toxicity testing of double-walled nanotubes-contaminated food to Eisenia veneta earthworms. Ecotox Environ Safe 71:616–619
Simonet BM, Valcárcel M (2009) Monitoring nanoparticles in the environment. Anal Bioanal Chem 393:7–21
Tourinho PS, Cornelis AM, Van Gestel AM, Lofts S, Svnedsen C, Soares AMVM, Loureiro S (2012) Metal-based nanoparticles in soil: fate, behaviour, and effects on soil invertebrates. Environ Toxicol Chem 31:1679–1692
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vittori Antisari L, Carbone S, Gatti A, Fabrizi A, Vianello G (2012a) Toxicological effects of engineered nanoparticles on earthworms (Lumbricus rubellus) in short exposure. Environ Qual 8:51–60
Vittori Antisari L, Carbone S, Gatti A, Vianello G, Nannipieri P (2012b) Toxicity of metal oxide (CeO2, Fe3O4, SnO2) engineered nanoparticles on soil microbial biomass and their distribution in soil. Soil Biol Biochem 60:87–94
Yang H, Wu Q, Tang M, Kong L, Lu Z (2009) Cell membrane injury induced by silica nanoparticles in mouse macrophage. J Biomed Nanotechnol 5:528–535
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antisari, L.V., Laudicina, V.A., Gatti, A. et al. Soil microbial biomass carbon and fatty acid composition of earthworm Lumbricus rubellus after exposure to engineered nanoparticles. Biol Fertil Soils 51, 261–269 (2015). https://doi.org/10.1007/s00374-014-0972-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-014-0972-1