Abstract
The survival and cast production of the tropical endogeic earthworm Pontoscolex corethrurus and the changes in chemical and physical characteristics induced by gut passage were studied over an 80-day period in soils contaminated with different levels of Pb. The soils were from a Pb mining area in the state of Paraná, SE Brazil, and ranged from clayey to sandy texture and total Pb contents from 52 to 9,716 mg kg−1. In soils with the highest total Pb contents, earthworms showed lower survival rates, reduced biomass, high Pb uptake, and negligible cast production. In soils with low to intermediate total Pb (maximum 4,278 mg kg−1), earthworm survival and cast production were higher, biomass loss was lower, and gut passage increased pH, CEC, P, K+, and Mg2+ concentrations in the casts compared to the control soil. In the sandy soil (clay <176 g kg−1), worms preferentially ingested finer soil particles, increasing organic C and silt contents in casts. However, this selective feeding also resulted in higher Pb accumulation in worm tissues. Gut passage also increased water-dispersible clay and reduced flocculation in the casts, increasing the susceptibility of the soil to erosion. Lead contamination and uptake into the tissues did not limit the ability of earthworms to select finer soil particles and to transform soil chemical and physical properties, although it affected cast production rates and survival (especially at high Pb concentrations).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The effect of earthworms on physical and chemical properties of soils under various management practices is well known (Lee 1985; Edwards and Bohlen 1996). As the soil passes through the gut, large amounts of muco-polysaccharides are added, stimulating microbial activity (Barois and Lavelle 1986; Trigo et al. 1999). Furthermore, an intense mixing of mineral and organic particles occurs, leading to soil disaggregation, reorientation of clay platelets (Barois et al. 1993), and profound changes in properties such as pH, C, N, and exchangeable cation contents and nutrient availability to plants (more mineral forms, especially N and P) (López-Hernández et al. 1993; Chapuis-Lardy et al. 1998; Barois et al. 1999; Bartz et al. 2010). As a result of microbial activity, greenhouse gases are emitted (Depkat-Jakob et al. 2012), and more simple substances transformed by the microbes are assimilated by the worm, which does not produce all enzymes needed for the breaking down the recalcitrant materials ingested with the soil (Lattaud et al. 1998).
Earthworms can also influence heavy metal mobility in soils through their feeding, burrowing, and casting activities (Zorn et al. 2005; Lukkari et al. 2006; Sizmur et al. 2011; Duarte et al. 2012). The impacts of earthworm activities on heavy metal availability in contaminated soils after gut passage have been studied by comparing the composition of casts and soil samples, and the effects appear to be variable depending on the metal, its form, and the earthworm species in question (e.g., Kizilkaya 2004; Udovic et al. 2007; Udovic and Lestan 2007). While most studies appear to report increases in metal mobility (e.g., Spurgeon and Hopkin 1996; Sizmur and Hodson 2009), others found the opposite (e.g., Li et al. 2009; Duarte et al. 2012). For instance, Li et al. (2009) found lower contents of exchangeable Cu and Zn in pig manure samples after transit through the gut (cast analysis) of compost earthworms (Eisenia fetida), and the Cu bound to organic matter increased from 60 to 75 %. These changes are particularly desirable for the reclamation of contaminated areas.
Pontoscolex corethrurus (Müller 1857) is an endogeic earthworm and a native to the Neotropics that has spread throughout the tropical and subtropical regions of the world (Gates 1973; Righi 1984). In Brazil, it is widespread and found mainly in disturbed sites such as gardens, crop fields, pastures, and secondary forests (Brown et al. 2006; Lavelle et al. 1987). Indeed 150 years ago, the German naturalist Fritz Müller described the species and stated: “the commonest of the earthworms of this country (Brazil), and which may be found in almost every clod of arable land” (Müller 1857).
The diet of P. corethrurus consists mainly of decomposed organic matter (Lavelle et al. 1987), with selective ingestion of soil particles rich in clay and C contents (Barois et al. 1999; Bartz et al. 2010). In a particular area, P. corethrurus may show preference for certain soils and vegetation types (Marichal et al. 2010). For example, it has been shown to grow better in soils with higher C, P, and K contents and in pasture soils compared with those from native forest (Marichal et al. 2012). Nevertheless, little is known of the susceptibility of P. corethrurus to contaminants such as heavy metals in soil (Buch et al. 2011; Jusselme et al. 2012) and whether these may also interfere with the process of earthworm gut passage responsible for modifying chemical and physical soil properties.
Therefore, the present study was undertaken to evaluate survival, growth, and cast production and changes in chemical and physical properties of casts of the tropical endogeic earthworm P. corethrurus kept in soils contaminated with Pb.
Materials and methods
Soil and earthworm collection
Soil samples were collected in the Ribeira River valley in Adrianópolis, Paraná State, Southern Brazil. Five soil profiles, submitted to different contamination levels, were sampled (Table 1) inside a mining and Pb metallurgy area. A georeferenced aerial photo of the study area with the location of sampling points was presented by Duarte et al. (2012). After 50 years of mining galena seams (PbS), the company shut down in 1995, leaving nearly 177,000 tons of refining residues in the open air without any protection.
Soil samples were digested in a microwave oven, by placing approximately 0.2 g in concentrated 4 mL HNO3, 3 mL HF, and 1 mL of H2O2 30 % (v/v) (Lim and Jackson 1986) and the total contents of Pb determined by individually coupled plasma atomic emission spectroscopy (ICP-AES). Total Pb contents (Lim and Jackson 1986), texture (Gee and Bauder 1986), and pH (Hue and Evans 1986) of the soils are presented in Table 1. Further data on the soils and sample sites are provided by Duarte et al. (2012).
Soil 3 had the highest total Pb contents (Table 1), due to its proximity to the factory. The suspended material (fly ash) from the chimneys deposited large amounts of Pb in this area, as the emitted particles fell close to the chimneys. The high concentrations of Pb in soil 2 are due to its position at mid slope (Table 1), which favored the addition of sediments rich in coarse and dark metallurgy residues eroding from the higher mining areas (author’s field observations). Soil 8, despite its sandy texture, had high amounts of Pb due to runoff water with high Pb concentrations flowing from the factory through soil 8 en route to the Ribeira River. Total Pb concentration of the ash on the deactivated factory floor was in the order of 200,000 mg kg−1 (author’s unpublished data).
Around 5 kg of each soil was collected at 0–10 and 20–40 cm depth for the earthworm incubation study. The soil was air-dried, macerated, and sieved at 2 mm to obtain air-dried fine earth (ADFE). Adult earthworms (P. corethrurus) were collected from surface soil (0–15 cm) at the Federal University of Paraná Experimental Farm, in a farmed plot without heavy metal contamination, approximately 150 km from the Pb mining area studied. Some 220 individuals were collected of which 150 were immediately used in the experiment. The remainders were maintained in trays with their source soil for the replacement of dead earthworms along the experiment.
Earthworm incubation
The experimental units consisted of a lidded transparent plastic box, with approximately 300 cm3 of soil. The experimental design was completely randomized and consisted of 5 soils × 2 depths × 2 earthworm population densities (with and without earthworms) × 3 replicates, totaling 60 experimental units. The ADFE was placed in the plastic boxes, in a fine layer (approximately 1.5 cm thick), to facilitate the collection of casts on the surface and to visualize any dead earthworms in the boxes.
Source soil from the collected earthworms was eliminated by (i) placing the earthworms to be used in the experiment in plastic boxes with a damp paper towel for 48 h and (ii) discarding the two first cast collections at the beginning of the experiment.
The soil was moistened to 70 % of its field capacity (and thus maintained throughout the experiment), and five individuals were added per box with an area of 0.03 m2 (166 individuals m−2, a common value of abundance for this species in Brazil, Brown et al. 2006). The boxes were kept in the dark and at room temperature (approx. 20 °C). Casts were collected from the soil surface every 48 h using tweezers. The casts were easily identified because they were oblong and compact and had a smooth external surface compared to natural sieved soil aggregates (smaller than 2 mm ADFE). The castings collected over each 20-day period were dried in an oven for 48 h at 40 °C and weighed. The total period of cast collection was 80 days (March–May 2008).
The earthworms that died during this period were replaced with living earthworms that had undergone the same preparatory process as described. At 80 days, control soil samples (without earthworms, n = 30 boxes) were dried for 48 h at 40 °C and sieved at 2 mm. The soil from the boxes with earthworms was manually sorted for cocoons, and the remaining soil was discarded, and only the casts previously collected and dried were analyzed.
Surviving earthworms in each treatment were counted and placed in plastic pots with moist paper towel for 48 h to void their guts (P. corethrurus gut passage time is approx. 2–4 h; Barois and Lavelle 1986). After that, they were rinsed in tap water and dried with a paper towel, weighed (fresh biomass), killed in a water + ethanol solution (20 % v/v), dried (105 °C, 24 h), reweighed (dry biomass), and ground to fine powder to determine tissue Pb contents. Earthworm tissues (0.2 g of dry sample) were digested at 105 °C for 24 h with 25 mL of concentrated HNO3 in a microwave oven (Milestone Ethon Plus). After 30 min of cooling, the suspension was filtered, and Pb concentration was determined by ICP-AES.
Control soil and casting analyses
Casts and control soil samples were chemically analyzed to determine the following soil fertility properties (Hue and Evans 1986): pH in water (soil/solution relation, 1:2.5); non-exchangeable potential acidity (H) extracted with pH 7 0.5 mol L−1 Ca acetate; exchangeable Ca2+, Mg2+, and Al3+ extracted with 1 mol L−1 KCl; and available P and exchangeable K+ in 0.05 mol L−1 H2SO4 and 0.025 mol L−1 HCl (Mehlich-1).
Total organic C and N concentrations were determined on 0.015 g samples of soil sieved at 0.25 mm and measured by combustion in the elementary CNHS analyzer, model Vario EL III (Elementar, Hanau, Germany).
Soil texture was assessed using the pipette method, after the removal of organic matter with H2O2 30 % (v/v), with the first sampling of silt + clay at 10 cm by using a pipette and the second at 5 cm after 3 h and 28 min to collect clay (Gee and Bauder 1986).
Water-dispersed clay (DC) and degree of flocculation (DF) were determined also following the difference in velocity of sedimentation of soil fractions (Gee and Bauder 1986): 5 g of soil was placed into plastic recipients, and 12.5 mL of water was added. The suspension was stirred with a glass rod and was left resting overnight. Next, the suspension was mixed using an electric stirrer for 2.5 min. The contents were sieved at 0.053 mm, and the clay and silt fractions collected were placed into a 250-mL graduated cylinder. The volume collected was completed to 100 mL and stirred for 20 s, and the suspension was left resting for 3 h and 28 min, when a 20-mL fraction was removed using a pipette at 5 cm depth to collect the clay fraction. The contents were dried (105 °C, 24 h) and weighed. The DF was calculated according to the formula
Statistical analysis
All the data reported (except for Table 1) are means of three replicates. ANOVA and significant differences between means were compared with Tukey’s test (p < 0.05) using Statgraphics 4.0 for Windows. Depending on the significance of interactions of the factorial ANOVA (F test), soil properties were either analyzed separately or divided into groups. When three-way interactions were significant, soil type, soil depth, and cast vs. control soils were compared separately; when two-way interactions were significant, depths were combined, and only soil types and casts vs. control soils were compared; when interactions were not significant, soil types and depths were combined, and only castings vs. control soils were compared. The data distributions were always normal, and no data transformations were necessary.
Analysis of Pearson correlation was done between mortality, biomass, cast production, and Pb uptake for earthworms and various Pb forms (soluble, exchangeable, carbonate organic matter, Fe and Mn oxides, Al oxides and aluminosilicates, residual) in the topmost soil layer (0–10 cm) obtained through sequential analysis by Duarte et al. (2012).
Results and discussion
Earthworm survival, cast production, and Pb concentration in tissues
Total Pb concentrations in all used soils, except those in the uncontaminated reference forest (soil 1) and in the deeper layer (20–40 cm) of soil 6, were much higher than those established by Brazil’s environmental agency for residential (300 mg kg−1) uses, and in soils 3 and 6 (20–40 cm), they were higher than those established for industrial uses (900 mg kg−1). Furthermore, even in soil 1, at the greatest distant from the factory and under native forest (Table 1), used as a control (reference value) of natural soil heavy metal content in the region, Pb concentration in the surface layer was higher than those considered safe for agricultural uses (180 mg kg−1). This high natural concentration is due to the occurrence of galena veins (PbS) in the parent material in the region (Duarte et al. 2012).
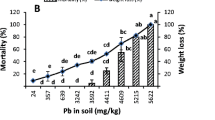
Lead content in earthworm tissues (Table 2) was positively correlated with Pb in the topmost layer of soil determined in different forms such as total, carbonates and Al oxides, as can be seen for the carbonate fraction in Fig. 1. The meaning of seven soil Pb pools obtained in sequential extraction was fully discussed by Duarte et al. (2012). Due to a higher stability of Pb precipitated as carbonate and strongly adsorbed (inner sphere) in the Al oxides in relation to soluble forms, the intestine is likely the most important Pb uptake route into worm tissues. The linear regression coefficient between soluble Pb forms (data from Duarte et al. 2012) and earthworm tissues was low and not significant (R 2 = 0.2), suggesting a low dermal exposure route (Hobbelen et al. 2006). Individuals selectively follow existing galleries rather than building new galleries (Caro et al. 2012), which can reduce the dermal contact of worms with new portions of contaminated soils.
Relationship between various earthworm characteristics (mortality, biomass, cast production, and Pb uptake) and Pb carbonate form in the topmost soil layer (0–10 cm) from a Pb-contaminated site. The Pb carbonate contents extracted with 1.0 M sodium acetate at pH 5 in a sequential analysis of the 0–10 cm layer are presented in detail in Duarte et al. (2012). *p < 0.05 (significant)
In general, earthworms accumulate heavy metals from soil leading to compartmentalization, storage, or excretion of these elements by the more sensitive tissues (Morgan and Morgan 1998; Kizilkaya 2004). Leveque et al. (2013) showed that even after depuration, Pb remains localized in the gut wall and, above all, in the longitudinal muscles of exposed earthworms. The chloragosomes (intracellular vesicles in the posterior alimentary canal) are the major foci for Pb accumulation, and such compartmentalization appears to prevent the dissemination of this contaminant into other worm tissues (Morgan and Morgan 1998). Jusselme et al. (2012) reported high tolerance of P. corethrurus to Pb uptake (up to 1,000 mg kg−1 year−1), and Pb accumulation by worms in the present study (Table 2) was similar to that observed by Sanderson (2008) for the epigeic E. fetida in soil contaminated with 10,000 mg Pb kg−1.
The regression coefficient between mortality and Pb carbonate contents was positive (R 2 = 0.69), but not significant (n = 5). On the other hand, total earthworm biomass and cast production at the end of the experiment were significantly negatively correlated (with R 2 = 0.95 and 0.87, respectively) with Pb carbonate (Fig. 1). These results imply that negative sublethal effects of Pb are acting upon P. corethrurus, as observed for other earthworm species (e.g., Leveque et al. 2013), despite high tolerance to uptake (Jusselme et al. 2012).
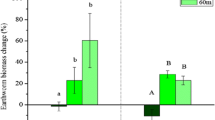
Total cast production after 80 days was highest in soil 1 and the top layer of soil 6 and lowest in soils 3 and 8 (Fig. 2). Mean cast production per worm reached a maximum of around 0.8–0.9 g dry weight soil worm−1 day−1 in soil 1 to 1.0 g dry weight soil worm−1 day−1 in the top layer of soil 6. When an annual rate (considering the total production of five worms in a box area of 0.03 m2 and 365 days activity) was calculated, cast production in topsoil-subsoil layers would be 34–38 kg m−2 in soil 1, 5–7 kg m−2 in soil 2, 0.5–0.3 kg m−2 in soil 3, 36–8 kg m−2 in soil 6, and 1.6–0.7 kg m−2 in soil 8. The values for soil 1 and the 0–10 cm layer of soil 6 are within the range of estimated annual cast production for this species (20–40 kg m−2) reported by Lavelle et al. (1983) for pasture soils in Mexico and under laboratory conditions (Bartz et al. 2010).
Mean cumulative cast production (dry mass in grams per container) by earthworms after 80 days of incubation in different soils from a lead mining area. Numbers represent soils. Bars represent standard deviations. See Table 1 for information on site and soil characteristics
Soil 3, with the highest total Pb concentration in earthworms compared with other soils, had the highest mortality rate, lowest end total biomass (Table 2), lowest cast production (Fig. 2), as well as visible signs of toxicity (Fig. 3), probably from Pb. At low bioavailable concentrations, earthworms may regulate Pb uptake (Davies et al. 2003), but at higher concentrations, this mechanism becomes insufficient and toxicity is observed. Wu et al. (2012) analyzed the combined toxicity of Cd and Pb on E. fetida and concluded that a single Pb exposure could increase the cellulase activity and the DNA damage to earthworm coelomocytes.
In soil 8, the worms were submitted to multiple stress conditions: total Pb contents >3,600 mg kg−1, a weak degree of soil development (fluvial sediments) and low clay and soil organic carbon contents (Table 1). These conditions inhibited earthworm growth and activity, leading to the smallest cast production (Fig. 2). Although it is known that the impact of pollutants on earthworm physiology and ecotoxicity varies both with earthworm species and soil characteristics (e.g., clay, organic matter contents, and pH; Ernst et al. 2008; Nannoni et al. 2011; Leveque et al. 2013); these results appear to confirm those of Nahmani et al. (2007), who reported that the most Pb toxic soils to E. fetida were organic poor (1–10 g C kg−1) and sandy soils (ca. 74 % sand).
In the deeper layer (20–40 cm) of soil 6, the earthworms assimilated high quantities of Pb, reaching a value of about 2,000 mg kg−1 (Table 2), even though total Pb concentrations were relatively low, compared to the other contaminated soils (Table 1), probably because the percentage of exchangeable Pb associated with negatively charged OC and finer soil particles were much higher than those in other soils (data in Duarte et al. 2012). Therefore, the high tissue concentrations were probably due to selective feeding of organic and silt-rich particles by the earthworm (see cast texture with mean for both layers in Table 3) in this coarse-textured soil (Table 1), which was poor in nutrients. It has been reported that this species tends to select finer particles in coarser textured soils (Barois et al. 1999). In the deeper layer of soil 2, with higher clay and OC contents than the respective layer of soil 6 (Table 1), the earthworms probably did not have to select the colloidal fractions to feed (no difference between cast and control soil texture using the mean of both depths in Table 3), as observed in soil 6. This behavior resulted in less Pb content in the worm’s tissues in the more contaminated soil 2 (Table 2).
In the reference soil 1, the earthworms displayed behavior typical of uncontaminated soils (Figs. 2 and 3) with an increase in biomass (Fig. 4), low Pb uptake (Table 2), and proper conditions of reproduction (this was the only soil in which cocoons were produced). Cocoon production and hatching rate are more sensitive to soil contamination than survival or weight change (Nahmani et al. 2007). As discussed earlier, conditions of the 20–40-cm layer of soil 6 and of soils 2, 3, and 8, with high Pb contents and low clay and OC contents, decreased worm biomass after 80 days of incubation (Fig. 4).
Chemical and physical characteristics of soils and casts
In soils 3 and 8, the cast production after 80 days of incubation was insufficient (maximum 10.8 g; Fig. 2) to perform soil physical and chemical analysis proposed in this work; so, the statistical analyses of soil properties were performed only for soils 1, 2, and 6 (Tables 3, 4, and 5).
The P and K+ contents as well as CEC were significantly greater in casts than those in control soil (Table 4), showing a positive effect of gut passage through P. corethrurus. Elevated concentrations of available P in the casts of this species have been already reported (López-Hernández et al. 1993; Bartz et al. 2010) and have been attributed to selective ingestion of finer particles and stimulation of mineralization of organic P and K+ into exchangeable forms in the gut (Chapuis-Lardy et al. 1998).
In the soils richer in Ca2+ (soils 1 and 2), concentration of this nutrient decreased in the casts (Table 3). The percentage of reduction was similar in both the reference soil 1 and contaminated soil 2 (13 and 15 %, respectively). However, in soils 2 and 6, the Ca2+ + Mg2+ contents were higher in casts than those in control soil (Table 5), due to an increase in Mg2+ concentration after gut passage.
Soil pH is frequently altered by gut passage, due to the presence of calciferous glands in many earthworms, including P. corethrurus (Barois et al. 1999). In the present case, pH increased in casts of soils 1, 2, and 6 in all depths (Table 5). This internal regulation of pH by the earthworm may be due both to excretion of NH3 and the secretion of CaCO3 from calciferous glands into the intestines (Kale and Krishnamoorthy 1980; Bartz et al. 2010) as mentioned above. In soils 1 and 2, with high Ca2+ contents, the earthworms probably absorbed greater amounts of Ca2+ as supported by the decrease in the Ca2+ concentration in casts (Table 3) and produced CaCO3 which raised the cast pH (Table 5). The largest increases in pH observed in casts were observed in the cast produced in acid soil 6 (13 % for 0–10 cm and 21 % for 20–40 cm), which had the lowest acid buffering capacity, due to its low OC and clay contents.
The reduction in Ca2+ contents and the increase in pH values in casts were not affected by the intensity of soil Pb contamination as the responses were similar in reference soil 1 and in soils 2 and 6. Even when subjected to different levels of stress due to soil contamination, earthworms maintained similar chemical interactions with the soil by gut passage.
The few significant differences found in total soil N (TN) concentration were due to a decrease in casts compared with the control soil, independent of Pb contamination (soils 1 and 2; Table 5). We are unable to explain these differences, and other authors have found either higher or similar TN contents in P. corethrurus casts compared with control soils (Barois et al. 1987; Bartz et al. 2010). Earthworms increase the mineralization of N from organic matter through direct and indirect effects on the microbial community (Li et al. 2013). Organic N is important for earthworm growth and reproduction (Huerta et al. 2005), but the weight gain by worms and their reproduction in the present experiment cannot account for the observed differences in N contents. More likely, these are due to selective ingestion of soil particles richer in C than in N, as the C/N ratio of the casts was higher than that of soil.
The only significant difference in total soil organic C (TOC) was observed for the surface layer of sandy soil 6, with highest TOC in casts (Table 5), a sample also characterized by a high cast production (as much as in uncontaminated soil; Fig. 2). In the 20- to 40-cm layer of this soil, even though TOC contents were slightly higher in castings, the difference was not significant. Brown (2010, personal communication) also found an inverse relationship between cast production and TOC contents in three soil types, with the highest cast production in sandy soils with lowest TOC. This is because the earthworm must ingest more soil to obtain the food needed for growth.
Fine sand and silt contents were also higher in earthworm casts than those in the respective control soil 6 (Table 3). The ingestion of fine sand grains facilitates the assimilation of nutrients from organic matter and the grinding function of the gizzard (Marhan and Scheu 2005). This process can also contribute to the breaking up of ingested mineral particles (Suzuki et al. 2003). This selective ingestion also reduced coarse and total sand contents in the casts, as this sandy soil had low clay content, and no significant differences were observed in clay contents of casts and control soil (Table 3).
Therefore, even when accumulating large amounts of Pb in the tissues, the worms continued a selective ingestion of organic and finer mineral particles, leading to particularly high cast production in soil 6 to compensate for its sandy texture and poverty in nutrients. The apparent inability of the worms to chemically identify and avoid feeding on colloidal particles with high Pb contents may be one of the reasons for the high accumulation rate as well as for the usefulness of these organisms in toxicity testing and as bioindicators of heavy metal pollution. The sensibility of worms of soil contaminated with heavy metals (dermal and intestine uptakes) is normally estimated by LC50 values (the concentration at which half of the test species are killed) (Nahmani et al. 2007; Sanderson 2008; Leveque et al. 2013).
Earthworms are able to change soil structural stability (Chapuis and Brossard 1995) by increasing the amounts of water-dispersible clay in casts (Table 5) and thus decreasing the degree of flocculation (Table 3). This may have negative effects on soil structure since small particles can fill soil macropores, facilitating erosion (Blanchart et al. 2004), as observed in pastures with high P. corethrurus populations in Manaus (Chauvel et al. 1999). Soil structure destruction occurs by soil grinding in the gizzard and, subsequently, in the anterior gut, with increasing water and polysaccharide contents that function as diluting agents (Barois et al. 1993). Together with this physical effect in the gut, chemical clay dispersion must be also considered. The increase in pH and the reduction in Ca2+ concentration in relation to Mg2+ (Tables 3 and 5) favor the reduction of clay flocculation in the casts. Due to its lesser hydrated ionic radius, Ca2+ has greater flocculating power than Mg2+ (Emerson and Chi 1977), and the pH increase favors the formation of negative charges in the colloids, favoring soil dispersion. Clay dispersion, as observed for chemical properties, was also not affected by total Pb concentration of soils since the correlation between the percentage increase of water-dispersed clay and the total soil Pb concentration was low and not significant (r = 0.33).
Conclusion
In the natural reference soil with low Pb concentrations, P. corethrurus exhibited behavior typical of this species, with high survival rates, biomass increase, and highest cast production, as well as being the only soil where reproduction occurred. In the contaminated soils, the earthworm response was related to Pb contamination level; earthworms were more sensitive to the extremely adverse conditions of soils 3 and 8 (group 1), with high levels of total Pb (maximum 9,716 mg kg−1), where cast production was insignificant (less than 1.6 kg m−2 year−1) and lost more weight, survival was low, and the worms accumulated high Pb contents. In the other two contaminated soils (2 and 6) with intermediate to high levels of total Pb (191 to 4,278 mg kg−1) (group 2), cast production was higher, while mortality and mass loss were lower. In the sandy soil 6 (clay < 176 g kg−1), selective ingestion of soil particles by the worms was evident, increasing TOC and silt contents and reducing total sand contents in casts. However, this selective ingestion also resulted in high Pb accumulation in worm tissues. Increases in the concentration of several plant nutrients were observed in earthworm casts, even in the Pb-contaminated soils. The only undesirable change in soil properties was an increase of water-dispersible clay in casts due to the increase in pH values and the decrease in Ca/Mg ratios of these casts. Therefore, Pb contamination and uptake into the tissues did not limit the ability of earthworms to select finer soil particles and to transform soil chemical and physical properties, although it affected cast production rates and survival (at high Pb concentrations).
References
Barois I, Lavelle P (1986) Changes in respiration rate and some physicochemical properties of a tropical soil during transit through Pontoscolex corethrurus (Glossoscolecidae, Oligochaeta). Soil Biol Biochem 18:539–541
Barois I, Verdier B, Kaiser P, Mariotti A, Rangel P, Lavelle P (1987) Influence of the tropical earthworm Pontoscolex corethrurus (Glossoscolecidae) on the fixation and mineralization of nitrogen. In: Pagliai AMB, Omodeo P (eds) On earthworms. Mucchi Editore, Modena, pp 151–158
Barois I, Villemin G, Lavelle P, Toutain F (1993) Transformation of the soil structure through Pontoscolex corethrurus (Oligochaeta) intestinal tract. Geoderma 56:57–66
Barois I, Lavelle P, Brossard M, Tondoh J, Martinez M, Rossi J, Senapati B, Angeles A, Fragoso C, Jimenez J, Decaëns T, Lattaud C, Kanonyo J, Blanchart E, Chapuis L, Brown GG, Moreno AG (1999) Ecology of earthworm species with large environmental tolerance and or extended distributions. In: Lavelle P, Brussaard L, Hendrix P (eds) Earthworms management in tropical agroecosystems. CABI International, Wallingford, pp 57–85
Bartz MLC, Costa ACS, Tormena CA, Souza IG Jr, Brown GG (2010) Sobrevivência, produção e atributos químicos de coprólitos de minhocas em um Latossolo vermelho distroférrico (Oxisol) sob diferentes sistemas de manejo. Acta Zool Mex 26:261–280
Blanchart E, Albrecht A, Brown GG, Decäens T, Duboisset A, Lavelle P, Mariani L, Roose E (2004) Effects of tropical endogeic earthworms on soil erosion: a review. Agric Ecosyst Environ 104:303–315
Brown GG, James SW, Pasini A, Nunes DH, Benito N, Martins PT, Sautter KD (2006) Exotic, peregrine, and invasive earthworms in Brazil: diversity, distribution, and effects on soils and plants. Carib J Sci 42:339–358
Buch AC, Brown GG, Niva CC, Sautter KD, Lourençato LF (2011) Life cycle of Pontoscolex corethrurus (Glossoscolecidae) in tropical artificial soil. Pedobiologia 53S:19–25
Caro G, Abourachid A, Decaëns T, Buono L, Mathieu J (2012) Is earthworms’ dispersal facilitated by the ecosystem engineering activities of conspecifics? Biol Fertil Soils 48:961–965
Chapuis L, Brossard M (1995) Modifications et stabilité du phosphore échangeable d’un ferralsol ingéré par un ver géophage. CR Acad Sci Paris 320:587–592
Chapuis-Lardy L, Brossard M, Lavelle P, Schouller E (1998) Phosphorus transformations in a ferralsol through ingestion by Pontoscolex corethrurus, a geophagous earthworm. Eur J Soil Biol 34:61–67
Chauvel A, Grimaldi M, Barros E, Blanchart E, Sarrazin M, Lavelle P (1999) Pasture degradation by an Amazonian earthworm. Nature 389:32–33
Davies NA, Hodson ME, Black S (2003) The influence of time on lead toxicity and bioaccumulation determined by the OECD earthworm toxicity test. Environ Pollut 121:55–61
Depkat-Jakob PS, Hunger S, Schulz K, Brown GG, Tsai SM, Drake HL (2012) Emission of methane by Eudrilus eugeniae and other earthworms from Brazil. Appl Environ Microbiol 7949:1–6
Duarte AP, Melo VF, Brown GG, Pauletti V (2012) Changes in the forms of lead and manganese in soils by passage through the gut of the tropical endogeic earthworm (Pontoscolex corethrurus). Eur J Soil Biol 53:32–39
Edwards CA, Bohlen PJ (1996) Biology and ecology of earthworms, 3rd edn. Chapman & Hall, London
Emerson WW, Chi CL (1977) Exchangeable calcium, magnesium and sodium and the dispersion of illites in water. II. Dispersion of illites in water. Aust J Soil Res 15:255–262
Ernst G, Zimmermann S, Christie P, Frey B (2008) Mercury, cadmium and lead concentrations in different ecophysiological groups of earthworms in forest soils. Environ Pollut 156:1304–1313
Gates GE (1973) Contributions to North American earthworms (Annelida), no. 6. Contributions to a revision of the earthworm family Glossoscolecidae I. Pontoscolex corethrurus (Müller, 1857). Bull Tall Timbers Res Station 14:1–12
Gee G, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis: part 1—physical and mineralogical methods. Soil Science Society of America, Madison, pp 383–411
Hobbelen PHF, Koolhaas JE, van Gestel CAM (2006) Bioaccumulation of heavy metals in the earthworms Lumbricus rubellus and Aporrectodea caliginosa in relation to total and available metal concentrations in field soils. Environ Pollut 144:639–646
Hue NV, Evans CE (1986) Procedures used for soil and plant analysis by the Auburn University soil testing laboratory no. 106. Department of Agronomy and Soils, Auburn
Huerta EL, Fragoso C, Barois I, Lavelle P (2005) Enhancement of growth and reproduction of the tropical earthworm Polypheretima elongata (Megascolecidae) by addition of Zea mays and Mucuna pruriens var. utilis litter to the soil. Eur J Soil Biol 41:45–53
Jusselme MD, Poly F, Miambi E, Mora P, Blouin M, Pando A, Rouland-Lefèvre C (2012) Effect of earthworms on plant Lantana camara Pb-uptake and on bacterial communities in root-adhering soil. Sci Total Environ 416:200–207
Kale RD, Krishnamoorthy RV (1980) The calcium content of the body tissues and castings of earthworm Pontoscolex corethrurus. Pedobiologia 20:309–315
Kizilkaya R (2004) Cu and Zn accumulation in earthworm Lumbricus terrestris L. in sewage sludge amended soil and fractions of Cu and Zn in casts and surrounding soil. Ecol Eng 22:141–151
Lattaud C, Locati S, Mora P, Rouland C, Lavelle P (1998) The diversity of digestive systems in tropical geophagous earthworms. Appl Soil Ecol 9:189–195
Lavelle P, Rangel P, Kanyonyo J (1983) Intestinal mucus production by two species of tropical earthworms: Millsonia lamtoiana (Megascolecidae) and Pontoscolex corethrurus (Glossoscolecidae). In: Lebrun P, Medts A, Grégoire-Wibo C, Wauthy G (eds) New trends in soil biology. Louvain la Neuve, Dieu-Brichart, pp 405–410
Lavelle P, Barois I, Cruz I, Fragoso C, Hernandez A, Pineda A, Rangel P (1987) Adaptive estrategies of Pontoscolex corethrurus (Glossoscolicidae, Oligochaeta), a peregrine geophagous earthworm of the humid tropics. Biol Fertil Soils 5:188–194
Lee KE (1985) Earthworms: their ecology and relationships with soils and land use. Academic Press, Sydney
Leveque T, Capowiez Y, Schreck E, Mazzia C, Auffan M, Foucault Y, Austruy A, Dumat C (2013) Assessing ecotoxicity and uptake of metals and metalloids in relation to two different earthworm species (Eiseina hortensis and Lumbricus terrestris). Environ Pollut 179:232–241
Li L, Wu J, Tian G, Xu Z (2009) Effect of the transit through the gut of earthworm (Eisenia fetida) on fractionation of Cu and Zn in pig manure. J Hazard Mater 167:634–640
Li H, Wang C, Li X, Christie P, Dou Z, Zhang J, Xiang D (2013) Impact of the earthworm Aporrectodea trapezoides and the arbuscular mycorrhizal fungus Glomus intraradices on 15N uptake by maize from wheat straw. Biol Fertil Soils 49:263–271
Lim CH, Jackson ML (1986) Dissolution for total elemental analysis. In: Page AL (ed) Methods of soil analysis, part 2: chemical and microbiological properties. American Society of Agronomy, Madison, pp 1–12
López-Hernández D, Lavelle P, Fardeau JC, Niño M (1993) Phosphorous transformations in two P-sorption contrasting tropical soils during transit through Pontoscolex corethrurus (Glossoscolecidae, Oligochaeta). Soil Biol Biochem 25:789–792
Lukkari T, Teno S, Väisänen A, Haimi J (2006) Effects of earthworms on decomposition and metal availability in contaminated soil: microcosm studies of populations with different exposure histories. Soil Biol Biochem 38:359–370
Marhan S, Scheu S (2005) Effects of sand and litter availability on organic matter decomposition in soil and in casts of Lumbricus terrestris L. Geoderma 128:155–166
Marichal R, Martinez AF, Praxedes C, Ruiz D, Carvajal AF, Oszwald J, Pilar Hurtado M, Brown GG, Grimaldi M, Desjardins T, Sarrazin M, Decaëns T, Velázquez E, Lavelle P (2010) Invasion of Pontoscolex corethrurus (Glossoscolecidae, Oligochaeta) in landscapes of the Amazonian deforestation. Appl Soil Ecol 46:443–449
Marichal R, Grimaldi M, Mathieu J, Brown GG, Desjardins T, Silva ML Jr, Praxedes C, Martins M, Velázquez E, Lavelle P (2012) Is invasion of deforested Amazonia by the earthworm Pontoscolex corethrurus driven by soil properties? Pedobiologia 55:233–240
Morgan JE, Morgan AJ (1998) The distribution and intracellular compartmentation of metals in the endogeic earthworm Aporrectodea caliginosa sampled from an unpolluted and metal-contaminated site. Environ Pollut 99:167–175
Müller F (1857) Lumbricus corethrurus, Burstenschwanz. Archiv fur Naturg 23:6–113
Nahmani J, Hodson ME, Black S (2007) Effects of metals on life cycle parameters of the earthworm Eisenia fetida exposed to field-contaminated, metal-polluted soils. Environ Pollut 149:44–58
Nannoni F, Protano G, Riccobono F (2011) Uptake and bioaccumulation of heavy elements by two earthworm species from a smelter contaminated area in northern Kosovo. Soil Biol Biochem 43:2359–2367
Righi G (1984) Pontoscolex (Oligochaeta, Glossoscolecidae) a new evaluation. Stud Neotropical Fauna Environ 19:159–177
Sanderson P (2008) Ecotoxicological testing of lead contaminated soil using a multispecies soil system. Division of Information Technology, Engineering and the Environment, University of South Australia, Mawson Lakes Campus (Bachelors of Applied Science Honours)
Sizmur T, Hodson ME (2009) Do earthworms impact metal mobility and availability in soil?—A review. Environ Pollut 157:1981–1989
Sizmur T, Palumbo-Roe B, Watts MJ, Hodson ME (2011) Impact of the earthworm Lumbricus terrestris (L.) on As, Cu, Pb and Zn mobility and speciation in contaminated soils. Environ Pollut 159:742–748
Soil Taxonomy (1999) A basic system of soil classification for making and interpreting soil surveys. United States Department of Agriculture, Agriculture Handbook No 436, Washington
Spurgeon DJ, Hopkin SP (1996) Effects of variations of the organic matter content and pH of soils on the availability and toxicity of zinc on the earthworm Eisenia fetida. Pedobiologia 40:80–96
Suzuki Y, Matsubara T, Hoshino M (2003) Breakdown of mineral grains by earthworms and beetle larvae. Geoderma 112:131–142
Trigo D, Barois I, Garvin MH, Huerta E, Irisson S, Lavelle P (1999) Mutualism between earthworms and soil microflora. Pedobiologia 43:866–873
Udovic M, Lestan D (2007) The effect of earthworms on the fractionation and bioavailability of heavy metals before and after soil remediation. Environ Pollut 148:663–668
Udovic M, Plavc Z, Lestan D (2007) The effect of earthworms on the fractionation, mobility and bioavailability of Pb, Zn and Cd before and after soil leaching with EDTA. Chemosphere 70:126–134
Wu B, Liu Z, Xu Y, Li D, Li M (2012) Combined toxicity of cadmium and lead on the earthworm Eisenia fetida (Annelida, Oligochaeta). Ecotoxicol Environ Saf 81:122–126
Zorn MI, van Gestel CAM, Eijsackers H (2005) The effect of two endogeic earthworm species on zinc distribution and availability in artificial soil columns. Soil Biol Biochem 37:917–925
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duarte, A.P., Melo, V.F., Brown, G.G. et al. Earthworm (Pontoscolex corethrurus) survival and impacts on properties of soils from a lead mining site in Southern Brazil. Biol Fertil Soils 50, 851–860 (2014). https://doi.org/10.1007/s00374-014-0906-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-014-0906-y