Abstract
Benthic foraminiferal analysis (315 samples, 16,271 specimens) of the shallow water (< 100 m) Maastrichtian–Thanetian rocks from the Dakhla Oasis (Western Desert, Egypt) was studied to infer the inter–relationships between species diversity, palaeooxygenation, palaeoproductivity, and palaeodepth and changes at the Cretaceous–Paleogene (K/Pg) boundary. Positive and significant correlations are noted between these proxies, suggesting a well-oxygenated oligotrophic environment. However, a brief interval (mid–lower Maastrichtian) of increased palaeoproductivity with reduced diversity and oxygenation (ventilation) is noted (a characteristic of mesotrophic–eutrophic settings) that coincides with very shallow waters during a highstand system tract (HST) and dominated by the dysoxic agglutinated species Ammobaculites khargaensis. The diversity index, Fisher’s α (< 5), and paleodepth proxy (foraminiferal wall structure types) also suggest a shallow neritic (largely littoral) depth for the entire study interval. At the bottom of the study section (Planktic Foraminiferal Zones CF8b-CF7), species diversity, palaeooxygenation, and palaeoproductivity are high. From the K/Pg boundary to the post-K/Pg period, these variables are low and fluctuate with moderate species dominance. Data suggests an overall 40% benthic foraminiferal species (38% of agglutinated and 40% of calcareous extinct species) extinction rate after the K/Pg hiatus. The period immediately following the K/Pg boundary is characterized by increased basinal ventilation and decreased palaeoproductivity, which are attributed to changes in sea level and concurrent regional subsidence. However, as stable as the community structure was at or just after the K/Pg boundary, the changes in species composition (assemblage) were dramatic and marked by a change from a pre–K/Pg agglutinated–dominated fauna (Haplophragmoides–Ammobaculites) to a post–K/Pg calcareous one (Cibicodoides–Cibicides–Anomalinoides).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The benthic foraminiferal species assemblage, distribution, and community structure are a result of the interactions between productivity and oxygenation of the bottom (Van der Zwaan et al., 1999; Jorissen et al., 2007). However, in the deep-sea environment, oxygen availability is frequently not a limiting factor, and the characteristics of individual species or assemblages (or the composition of an assemblage) are often good flux-indicators. In shallower waters, the individual effects of oxygen and organic flux are difficult to delineate (Van der Zwaan et al., 1999; Jorissen et al., 2007). In modern oceans, the quantity, quality, and periodicity of organic–flux to the sea floor (productivity) define the distribution patterns of benthic foraminifera, although, large uncertainties still remain of the relationships between the downward flux of organic carbon to the sea floor and the estimates of export production (see review by Murray, 2001).
The Upper Cretaceous-Lower Paleogene interval has been well-studied for inferring benthic foraminiferal species response (their vertical and spatial distribution, changes in community structure, and assemblages) vis-à-vis to the end Cretaceous bolide impact. Interestingly, at the end of the Cretaceous and among one of the few groups, the benthic foraminifera, experienced no major extinction above background levels (Culver, 2003), thus making them robust proxies for biota-based paleoenvironmental reconstructions. Hence, they have been used as proxies to better understand the variability in export productivity (the flux of organic matter from the surface to the seafloor), paleooxygenation (prevailing oxic and dysoxic conditions), community structure (species diversity), assemblages (species composition), and changes in their isotope signals across the K/Pg boundary (Hsü and McKenzie, 1985; Culver, 2003; d'Hondt, 2005; Alegret and Thomas, 2005; Coxall et al., 2006; Alegret, 2007; Alegret and Ortiz, 2007; Alegret and Thomas, 2009; Alegret et al., 2012). A dramatic collapse of the δ13C gradient between the surface and deep-sea carbonates across the K/Pg boundary has been documented (d'Hondt, 2005; Alegret et al., 2012) and has largely been attributed to a long-term interruption of primary productivity (the Strangelove Ocean and Living Ocean models of Hsü and McKenzie, 1985). However, this dramatic breakdown in productivity contradicts the fact that the benthic foraminifera did not experience any major extinction during this interval (Culver, 2003; Alegret et al., 2012). Hence, an alternate explanation was put forward to explain this paradox that the extinction of the carriers of this isotope signal (the Maastrichtian calcareous nannoplanktons and planktic foraminifera) was replaced in the early Danian by taxa that had a much lighter carbon isotopic signature (Alegret et al., 2012; Birch et al., 2012, 2016). Assessing this is beyond the resolution of the present study, which restricts itself in documenting changes in benthic foraminiferal inferred palaeoproductivity, palaeooxygenation, species diversity, and assemblage composition and their changes at the K/Pg boundary from the Maastrichtian–Thanetian succession exposed at the Dakhla Oasis (at Gharb El–Mawohb; 26°01′02″ N, 28°13′18″E; Western Desert, Egypt) (Fig. 1a–c). This Maastrichtian–Thanetian succession provides an excellent exposure (Figs. 1d–e) and represents a transitional area between the middle to outer neritic Farafra facies and the inner neritic Garra Al-Arbain facies (Fig. 2).
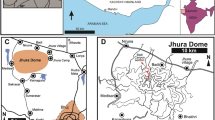
a Locality map of Egypt. The star in the center marks the location of the study area; b expanded view of the study area; c the palaeogeographic map during the Cretaceous (after Jain and Farouk, 2017); d field photograph showing the mid–lower Maastrichtian to Thanetian exposure at the Dakhla Oasis (at Gharb El-Mawohb at the main escarpment (26° 01′ 02″ N, 28° 13′ 18″E; Western Desert, Egypt); e the close of the K/Pg boundary at Gharb El-Mawohb
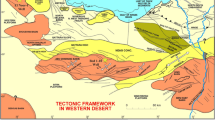
a Facies variations and rock units in Western Desert, Egypt. Three lateral and vertical facies changes in the Western Desert of Egypt are Farafra, Nile Valley and the Garra El-Arbian facies (modified from Farouk and Jain, 2016); b regional lithostratigraphy showing the extent of the Maastrichtiane–Thanetian sediments exposed at the Dakhla Oasis (modified from Jain and Farouk, 2017)
The originality of this study lies in the scarcity of comparative Upper Cretaceous-Lower Paleogene neritic benthic foraminiferal records that document changes in palaeooxygenation, palaeoproductivity, and species diversity over a longer time period, not well-documented not just from the southern Tethys margin, but also globally (Culver, 2003). Those records that deal with such changes tend to do so only for the K/Pg boundary event or are from the Tunisian regions of El Kef, Elles, and Ain Settara (Speijer and van der Zwaan, 1996; Widmark and Speijer, 1997; Li et al., 1999; Peryt et al., 2002, 2004; Coccioni and Marsili, 2007). Although these records provide a high-resolution window into changes in the benthic-pelagic coupling, they only do so across a very short K/Pg boundary interval. Rare records from Egypt are documented, but then, these only provide general trends in palaeoproductivity, palaeooxygenation, and species diversity (Farouk and Jain, 2016; Jain and Farouk, 2017), and infaunal/epifaunal changes at the K/Pg boundary interval (Orabi and Khalil, 2014). Thus, the present contribution is an attempt to bridge this gap and thereby provide a more comprehensive analysis of changes in the benthic ecosystem vis–à–vis the interplay of global eustasy and regional tectonics during the Upper Cretaceous–Lower Paleogene interval (planktic foraminifera zones CF8b–P4) in Egypt.
Geological setting
The Western Desert, part of the northeastern continental margin of Africa (Fig. 1a–c), due to the breakup of the Pangea, initiated two NW–SE trending intrashelf basins in central Egypt, namely Dakhla and Assiut; the southern and central Western Desert constitutes the Dakhla Basin (Hendriks et al., 1984). Dakhla Oasis is located in the central Western Desert of the major sedimentary basin, the Dakhla basin (Fig. 1a), and is made up of an extremely thick and well-exposed Upper Cretaceous-Lower Paleogene sequence (Fig. 2). It is distinguished by two facies types, the Nile Valley and Garra El-Arbain, and reflects lateral and vertical facies variations (Issawi, 1972; Fig. 2a–b). The Dakhla basin is located between longitudes 28°15′–29°40′ E and latitudes 25°00′–26°00′ N, 120 km west of the Kharga Oasis, about 300 km west of the Nile Valley, and roughly 300 km southeast of the Farafra Oasis (Fig. 1).
Contextually, it must be mentioned that based on data from planktonic foraminifera, benthic foraminifera, and calcareous nannofossils from Egypt, there are two competing theories—one suggesting a gap (hiatus) across the K/Pg boundary and the other advocating a continuous sedimentation pattern. Beckmann et al. (1969) noted that most sections in Egypt show a clear hiatus at the K/Pg boundary, and this has been corroborated by studies from the Western Desert (Abdel–Kireem and Samir, 1995; Tantawy et al., 2001; Khalil and Al Sawy, 2014; Orabi and Khalil, 2014; Farouk and Faris, 2012; Farouk, 2016), Nile Valley (Faris et al., 1985), Eastern Desert (Scheibner et al., 2003; Galal and Kamel, 2007), and from Sinai (Ayyad et al., 2003). However, El–Bassiouni et al. (2003) and Obaidalla (2005) have advocated a continuous sedimentation. But studies overwhelmingly suggest that the upper part of the calcareous nannofossil Micula murus subzone and the earliest Danian NP1 and NP2 zones are missing (equivalent to the interval between CF2–P1c planktonic foraminifera zones; see Farouk, 2016; Farouk and Jain, 2016). This gap is attributed to tectonic activity and irregular palaeotopography associated with low sedimentation rates (see El–Azabi and El–Araby, 2000; Jain and Farouk, 2017). Another gap is noted at the Danian/Selandian boundary and is marked by a sharp lithological break with an extensive reworking of the older Danian fauna (Farouk and El–Sorogy, 2015; Jain and Farouk, 2017). A change in the pattern of sedimentation (Velascoensis Event; Strougo, 1986) is also noted at the Selandian/Thanetian boundary and attributed to subaerial exposure (erosion) initiated by a eustatic fall, resulting in the absence of the calcareous nannofossil Zone NP6, near the base of the Tarawan Formation in different parts of Egypt (Farouk, 2016). Both these boundary events (Danian/Selandian and Selandian/Thanetian) have been dealt with in detail by Jain and Farouk (2017) and will not be dealt further.
Barren intervals
A brief mention of barren intervals (yielding no benthic and planktic foraminifera) noted in the present study is warranted. As regards the occurrence of benthic foraminifera, samples 156 to 194 (spanning Assemblages 8–10; 8 and 10 in part; with few productive samples in between, 164, 182–184) are barren (Fig. 3). This upper Maastrichtian interval is marked by an erosive HST 2 (Highstand System Tract), the near dominance of agglutinated species Trochammina rainwateri and Ammobaculites subcretaceous, and by the presence of a single calcareous species, Insculptarenula texana (see Fig. 3; for a more detailed sequence stratigraphic framework see Farouk and Jain, 2016; Jain and Farouk, 2017). Similar agglutinated taxa-dominated assemblages with an extremely small calcareous component have been documented in brackish to littoral settings (Nagy et al., 1990, 2010; Farouk and Jain, 2016; Jain and Farouk, 2017). Such shallow depths would also explain the absence of planktic foraminifera as noted in Assemblages 2 to 7 (see Fig. 3); these are also marked by highly impoverished benthic foraminiferal counts (see also Farouk and Jain, 2016; Jain and Farouk, 2017). Interestingly, a concomitant regional uplift has also been documented for this interval (see El–Azabi and El–Araby, 2000; Tantawy et al., 2001).
Planktic foraminiferal biostratigraphy of the studied section and % Planktic. The bold numbers (1–19) are benthic foraminiferal assemblages of Jain and Farouk (2017). Sea level is inferred by using the presence of characteristic agglutinated and benthic species, the abundance values of calcareous benthic foraminifers and the presence and abundance of planktic foraminifers (also Farouk and Jain, 2016; Jain and Farouk, 2017). The sequence stratigraphy (Highstand System Tracts, HST and Transgressive System Tracts, TST) is after Farouk and Jain (2016) and Jain and Farouk (2017). Bold lines are 5–point running averages
Previous studies
Three previous studies have been conducted on the same section, Farouk and Jain (2016, 2017) and Jain and Farouk (2017). Farouk and Jain (2016, 2017) have used the benthic foraminiferal abundance patterns to build 19 assemblages (13 and 6, respectively; this is also the framework for the present study) to construct a Maastrichtian–Thanetian sea-level curve and sequence stratigraphy. Jain and Farouk (2017) extended this study and used only the distribution (abundance pattern) of agglutinated benthic foraminifera species and analyzed diversity fluctuations for the prevailing Maastrichtian–Thanetian duration. They noted that in shallow waters, the changes in the distribution patterns of agglutinated benthic foraminifera species faithfully reflect the prevailing paleoenvironmental conditions, inferred from their calcareous counterparts (Jain and Farouk, 2017). The present study differs from the aforementioned studies and is related to understanding the inter–relationships between diversity, palaeooxygenation, palaeoproductivity, and palaeodepth (incorporating both agglutinated and calcareous benthic foraminiferal species).
Methods
One gram of sediment weight was used to pick benthic foraminifers that were soaked in the Na2CO3 solution before sieving them over 630, 125, and 63 μm mesh size, respectively. The 63–125 μm fraction size was used and identified under a binocular zoom stereomicroscope. A total of 315 samples (16,271 specimens) were analyzed. Of them, 195 were productive from the 315 m thick Maastrichtian–Thanetian succession exposed at the main escarpment of Gharb El–Mawohb (26° 01′ 02″ N, 28° 13′ 18″E; Western Desert, Egypt) (see Farouk and Jain, 2016; Jain and Farouk, 2017).
Additionally, Fisher’s α is used as a proxy for species diversity, BFOI (Benthic Foraminifera Oxygen Index), and % oxiphilic taxa for estimating palaeooxygenation (ventilation) of the sea floor, are percentage abundances of High organic–flux species (% HOFS), infaunal taxa (see Fig. 4) and benthic foraminiferal morphogroups (Table 1) for palaeoproductivity (the organic–flux to the sea floor) (Figs. 5–6). These approaches are then inferred in combination with the presence of characteristic benthic foraminiferal species, genera, and paleodepth (Fig. 7).
Proxies used in the present study. Diversity indices (Fisher’s α), palaeooxygenation proxies (benthic foraminiferal oxygen index (BFOI), and % oxyphilic taxa), palaeoproductivity proxies (% high organic–flux species (% HOFS) and percent infaunal taxa), and Benthic foraminiferal mode of life preference (infaunal and epifaunal) and % planktic. The bold numbers (1–19) are benthic foraminiferal assemblages of Jain and Farouk (2017). Sea level and sequence stratigraphy is after Farouk and Jain (2016) and Jain and Farouk (2017). Bold lines are 5-point running averages
Distribution of morphogroups and sub-morphogroups identified in this study. Categories are based on Fig. 5. Morphogroups CP-A.8 and CH-B.1 constitute insignificant proportions (< 1%) and hence, have not been illustrated (see text for details)
Relationship between benthic foraminiferal morphogroups and sub-morphogroups and species diversity (Fisher’s α), palaeoxygenation (BFOI), palaeoproductivity (% infaunal), sea level and TR cycles (TST–HST). Five morphogroups (in %) (CH-A.4, CH-B.4, CH-B.5, AG-A, and AG-B.2) dominate the studied interval (see text for explanation and Appendix for actual counts). Bold lines are 5-point running averages
However, the present study does have a one undeniable flaw that the specimen size (16,271 specimens from 315 samples, 195 being productive) is small. But, considering that there is still a large representation of diverse taxa (150 species), even in only 2 grams of sediment analyzed for this study, and the fact that a large number of samples were analyzed (315) (see Appendix-Tables 1–2), we believe that simple analysis and distribution patterns will reveal trends that will not be flawed. Owning to small specimen size, no rigorous analysis can be conducted such as cluster analysis, factor analysis, and/or principal component analysis as they require a minimum of 300 specimens per sample and as is the norm in most micropaleontological studies. Hence, here, a very basic correlation analysis (see Appendix-Tables 3–6) is conducted and along with vertical distribution trends of benthic foraminiferal species and genera are considered.
The emphasis of the study, however, remains on benthic foraminiferal assemblage analysis, corroborated by the results of correlation analysis. This is also a reason why several proxies are considered in addition to filed observation for paleoenvironmental interpretation. Based on this, a tentative model is proposed for their inferred distribution (Fig. 8). The benthic foraminiferal changes at the Cretaceous/Paleogene (K/Pg) boundary are also analyzed (see Figs. 9–11, Tables 2–3; Appendix-Tables 7–8). Important benthic foraminiferal species are illustrated in Fig. 12 using scanning electron microscopy of the Geological Survey of Egypt.
The relationship between palaeoproductivity, palaeooxygenation, and species diversity for the present study duration can be explained using a combination of both the TROX model (the trophic condition and oxygen concentration of Jorissen et al., 1995) and the parabolic curve of Levin et al., (2001). Samples 1–57 and 74–315 represent the left hand-side the figure, whereas, samples 58–73 represent the top right hand-side of the figure (see text for explanation)
Benthic foraminiferal patterns for extinction, immigrant, and survivor taxa noted for long–term interval incorporating taxa from assemblages 9 to 18. Taxa that have abundance > 5% are illustrated. Complete number of taxa is given in Table 2f and a list of species for the three categories given in Appendix-Table 7. For this interval, 22% (7 species) agglutinated species (a) are absent after the K/Pg boundary, 37% (37 species) for calcareous species (b) with an overall extinction percentage of 33 (44 species; see Table 2f)
Substage calcareous benthic foraminiferal patterns for extinction, immigrant, and survivor taxa noted for long-term interval (Upper Maastrichtian–Lower Paleocene). Taxa that have abundance > 5% are illustrated. Complete number of taxa is given in Table 2g and a list of species for the three categories given in Appendix-Table 8. For this interval, 40% (37 species) calcareous species are absent after the K/Pg boundary with an overall extinction percentage of 40 (55 species; including the agglutinated ones; see Table 2g)
Substage agglutinated benthic foraminiferal patterns for extinction, immigrant, and survivor taxa noted for long-term interval (upper Maastrichtian–lower Paleocene). Taxa that have abundance > 5% are illustrated. Complete number of taxa is given in Table 2f and a list of species for the three categories given in Appendix-Table 8. For this interval, 38% (18 species) calcareous species are absent after the K/Pg boundary with an overall extinction percentage of 40 (55 species; including the agglutinated ones; see Table 2f)
Important benthic foraminiferal species identified in the present study. Bar = 200 μm, except for Figs. 12–13 = 150 μm. 1: Ammodiscus cretacea (Reuss, 1845), sample 201; 2: Ammobaculites khargaensis Nakkady, 1959, sample 195; 3: Ammobaculites fragmentarius Cushman, 1927, sample 229; 4: Ammobaculites subcretaceous Cushman and Alexander, 1930, sample 262; 5: Haplophragmoides nigeriense Petters, 1979, sample 153; 6: Trochammina deformis Grzybowski, 1898, sample 209; 7: Trochammina diagonis (Carsey, 1926), sample 183; 8: Spiroplectinella esnaensis (LeRoy, 1953), sample 320; 9: Clavulinoides trilaterus (Cushman, 1926), sample 154; 10: Coryphostoma midwayensis Cushman, 1936, sample 97; 11: Laevidentalina basiplanata (Cushman, 1938), sample 140; 12: Pyramidulina semispinosa (LeRoy, 1953), sample 253; 13: Pseudonodosaria manifesta (Reuss, 1845), sample 314; 14: Marginulinopsis tuberculata (Plummer, 1926), sample 255; 15, 16: Gyroidinoides girardanus (Reuss, 1851), sample 13; 17: Cibicidoides howelli (Toulmin, 1941), sample 312; 18: Anomalinoides affinis (Hantken, 1875), sample 144; 19: Anomalinoides zitteli (LeRoy, 1953), sample 253; 20, 21: Valvalabamina planulata (Cushman and Renz, 1941), sample 201. For placement of sample numbers see Fig. 3
Proxies used
Fisher’s α is used as a proxy for species diversity (Fig. 4). It is a within-habitat diversity index and considers both species’ evenness and richness, whereas other species abundance models consider only evenness (Buzas and Gibson, 1969).
To estimate the availability of oxygen within the sediment column, two proxies are used, benthic foraminiferal oxygen index (BFOI) and the percentage of oxyphilic taxa (Fig. 4). Kaiho (1991) developed an empirical ratio of oxic and dysoxic benthic foraminiferal morphotypes called the BFOI. Based on benthic foraminiferal test morphology, Kaiho (1991) identified three morphogroups, Oxic (O), suboxic (S), and dysoxic (D) (see Appendix–Table 1). BFOI is calculated as [O/(O+D) × 100], where O is the number of oxic species and D is the number of dysoxic species. When O = 0 and D+S > 0 (S is the number of suboxic indicators), then the BFOI is calculated using the following equation: [(S/(S+D)–1] × 50 (Fig. 2). Later, Kaiho (1994) calibrated values of the BFOI to levels of dissolved oxygen in bottom waters of modern oceans (for details also Kaminski et al., 2002). Jannink et al. (2001) proposed a transfer function (oxygen content μMol/lt = 7.9602 + 5.95 × % oxyphilic taxa) to estimate the oxygen content within the sediment column (Fig. 4). Those taxa that occur in the topmost cm of the sediment are considered as oxiphylic, and their relative abundance is used as a proxy for estimating bottom water oxygenation. The rationale is that with increasing bottom water oxygenation, the availability of oxygen within the sediment also increases, resulting in an increased volume of available niche, which the oxiphylic taxa can potentially occupy. In the present study, the epifaunal taxa (see Appendix–Table 2) are considered as oxyphylic. The transitory epifaunal to shallow infaunal taxa is not included in the calculations such as species of Lenticulina and Osangularia (see Appendix–Table 2 and references therein). In general, it must be mentioned that although, both BFOI and % oxyphilic index employ % epifaunal taxa (= the oxic (O) taxa used in BFOI; see Appendix–Table 1), however, BFOI also incorporates the presence of suboxic (S) and dysoxic (D) taxa not used in the % Oxyphilic index. Hence, the BFOI encompasses a much broader community approach.
Two palaeoproductivity proxies used in this study are the relative abundances of high organic–flux species (%HOFS) and infaunal taxa (Fig. 4). The following species are considered as high–organic–flux species—Anomalinoides aegyptiacus, Bolivina cretosa, Bulimina prolixa, Nonionella africana, N. insect, Praebulimina kikapoensis, P. russi, Pyramidulina affinis, P. distans, P. semispinosa, P. vertebralis, P. zippei, and Reussella aegyptiaca (see also Sen Gupta and Machain–Castillo, 1993; Sen Gupta, 1999; Fontanier et al., 2002; Gebhardt et al., 2004; Friedrich and Erbacher, 2006; Jorissen et al., 2007; Friedrich et al., 2009; Alegret and Thomas, 2013; Sprong et al., 2013) (see Fig. 4). The species grouped under infaunal taxa include those categorized as shallow and deep infaunal; the epifaunal to shallow infaunal transitory forms are not included in calculations (see Appendix-Table 2) (Fig. 4). Reference data from various studies (Sliter, 1968; Sliter and Baker, 1972; Corliss, 1985; Jones and Charnock, 1985; Corliss and Chen, 1988; Bernhard, 1986; Langer, 1993; Rathburn and Corliss, 1994; Severin and Erskian, 1981; Jorissen, 1988; Corliss and Emerson, 1990; Linke, 1992; Sjoerdsma and Van der Zwaan, 1992; Jorissen et al., 1992; Linke and Lutze, 1993; Jorissen, 1988; Kaminski and Gradstein, 2005) have been used to categorize the microhabitat preference of the identfied species in the present study (see also Appendix-Table 2). Regardless of taxonomy, the groupings of similar shapes or growth patterns of benthic foraminiferal tests reflect a particular type of environment, and hence, are good proxies for assessing the prevailing palaeoenvironment, particularly for well-oxygenated temperate environments (Chamney, 1976; Jones and Charnock, 1985; Nagy, 1992; Kaminski et al., 1995; Nagy et al., 1995; Jones, 1999; Preece et al., 1999; van der Akker et al., 2000; Jones et al., 2005; Kender et al., 2008a, b; Cetean et al., 2011, Murray et al., 2011). In the present study, the benthic foraminiferal morphogroup assignment (Figs. 6–7; Table 1) follows the characterization done by Koutsoukos and Hart (1990), as more recent schemes provide somewhat less differentiation (Nagy, 1992; Tyszka, 1994; Nagy et al., 1995; van der Akker et al., 2000; Reolid et al., 2008; Nagy et al., 2009; Cetean et al., 2011; Chan et al., 2017). Additionally, most of these aforementioned studies are either from the Jurassic (Nagy, 1992; Tyszka, 1994; Nagy et al., 1995; Nagy et al., 2009) or from a different time period (Cetean et al., 2011, Santonian–Campanian; Chan et al., 2017, Tertiary) and thus have fewer common species to correctly interpret their morphotypes. Those that are from a similar period (van der Akker et al., 2000, Campanian–Maastrichtian) are largely from bathyal settings, contrary to the neritic one in the present study, and hence also have fewer common species for comparison. The closest comparative account is either that of Tyszka (1994) or of Koutsoukos and Hart (1990). The latter has the maximum number of common species with a detailed explanation of their habitat preferences. Thus, based on this latter dataset, the species from the present study are interpreted for their microhabitat assignment.
To infer bathymetry, the agglutinated foraminiferal dataset is grouped based on their preference for palaeodepth, i.e., into simple-walled arenaceous agglutinated foraminifera assemblage (representing a littoral environment with fresh water supply), complex-walled arenaceous agglutinated foraminifera assemblage (representing deeper littoral environment with normal marine water), and calcareous agglutinated foraminifera assemblage (representing shelf environment) (see also Berggren, 1974; Luger, 1985, 1988; Cherif and Hewaidy, 1986; Orabi, 1995, 2000) (Fig. 7). This is further corroborated with the bathymetry inferred from %Planktic foraminifera (%P) and total calcareous benthic foraminifera (see Fig. 7).
All the above-mentioned proxies are subjected to basic statistical analysis (Pearson correlation) (Appendices-Tables 3–6) and are evaluated based on the framework of 19 benthic foraminiferal assemblages as established by Farouk and Jain (2016, 2017) and Jain and Farouk (2017) (see Fig. 7).
Results
Species diversity
The correlation between diversity indices, Fisher’s α and Shannon H, yielded positive and statistically significant correlation (0.949, 0.000, significant at the 0.01 level, 2–tailed); hence, Fisher’s α is used as a proxy for species diversity (Fig. 4). Fisher’s α displays high values at the bottom of the studied section (planktic foraminiferal zones CF8b–CF7; samples 1–17), low and fluctuating values until the K/Pg boundary (samples 18–200) and then moderate to high values for the post K/Pg interval (samples 201–315) (Fig. 4). Diversity is low just prior to the K/Pg boundary but gradually increases for the post K/Pg planktic foraminiferal zone P1c (see Fig. 4). The beginning of planktic foraminiferal Zone P2 registers high values as also the interval between P2–P3a zones, whereas the post-P3a–P4 zone interval has consistently low values, coincident with low %P (Fig. 4).
Palaeoproductivity proxies
High organic–flux species (% HOFS)
The high organic–flux species (% HOFS), on average, do not constitute a major fraction of the assemblage throughout the studied section; ranging between 0 to 12%, except in assemblage 19 where they make up 27% of the total population (Fig. 4). Individually, there are four data points that register appreciable higher values: sample 97 (35.3%), 112 (42.9%), 240 (44.4%), and 306 (41.7%) belonging to assemblages 5, 6, 13, and 19, respectively (see Fig. 8 for assemblages). At the K/Pg boundary (between assemblages 10 and 11; see Fig. 7), there is no appreciable change, and the proxy remains low (< 5%) (Fig. 4). Higher values are only noted at the lower part of planktic foraminiferal zones P3a (sample 238–254) and in P4 (sample 306–315), and moderate values for assemblages 5–8 (see Fig. 4).
Percent infaunal
The infaunal species make up 35% of the total benthic foraminiferal dataset (6,351 specimens), epifaunal makeup 52% (8419 specimens), and the transitory epifaunal to shallow infaunal make up 11% (1789 specimens) (Fig. 4). There is a dominance of infaunal taxa throughout the pre–K/Pg boundary interval and particularly during the middle part of lower Maastrichtian (i.e., between samples 58–73) (Fig. 4). A dominance of epifaunal forms is noted at the bottom of the section in planktic foraminiferal zones CF8b–CF7 (sample 1–17), just after the K/Pg boundary (samples 201–226), and in planktic foraminiferal zones P3a–P4 (sample 238–315) (Fig. 4). As regards to sequence stratigraphy (as established by Farouk and Jain, 2016, 2017 and Jain and Farouk, 2017), the transgressive system tracts (TST) displays higher abundances of both epifaunal taxa and %P, and in the high system tracts (HST) of infaunal forms (Fig. 4).
Oxygenation proxies
% Oxyphilic taxa
As with species diversity and BFOI, the % oxyphilic taxa also follows the same trend; high values at the bottom of the section (planktic foraminiferal zones CF8b–CF7; samples 1–17), fluctuating values until the K/Pg boundary (samples 18–200), and then consistently high values for the post K/Pg interval (samples 201–315) (Fig. 4).
Morphogroups and subgroups
Following Koutsoukos and Hart (1990), for the agglutinated (AG) morphotypes, three morphogroups (AG-A, AG-B, and AG-C) and two sub-morphogroups of AG-B (AG-B.1 and AG-B.2) are identified (see Figs. 5–6; Table 1; Appendix-Table 1). For the calcareous-hyaline (CH) morphotypes, two morphogroups (CH-A and CH-B) with six sub-morphogroups of CH-A (CH-A.1, CH-A.2, CH-A.3, CH-A.4, CH-A.5, and CH-A.6) and five sub-morphogroups of CH-B (CH-B.1, CH-B.2, CH-B.3, CH-B.4, and CH-B.5) are identified (see Figs. 5–6; Table 1; Appendix–Table 1). Two morohogroups, CH-B.1 and CP–A8 constitutes only one species (and in negligible numbers), Globulina lacrima and Quinquloculina gussensis, respectively. The former occurs in sample 154 only (assemblage 8) whereas the latter occurs in two samples (246 and 299; assemblages 13 and 18, respectively) and hence, are not shown (Fig. 5).
Of the 16 identified sub-morphogroups, only five (CH-A.4, CH-B.4, CH-B.5, AG-A and AG-B.2; 34%, 12%, 12%, 9%, and 8%, respectively) dominate (Fig. 6–7). For the pre-K/Pg interval, for the calcareous forms, CH-A4 and for the agglutinated, AG-A and AG-B.2 dominate (Fig. 6) and for the post-K/Pg interval, CH-A4 and AG-B.2 dominate, respectively (Fig. 6). The deeper portions of the section are largely characterized by the dominance of CH-A4 (coincident with high %P values; see Fig. 6), whereas the shallower ones are either dominated by the agglutinated morphotypes AG-A and AG-B.2 (Fig. 6).
Wall structure
Of the palaeodepth indicators (littoral, deeper littoral, and shelf), the littoral setting dominates the section (Fig. 7); the deeper littoral proxy dominates at the base of the section (CF8b–CF7 planktic foraminiferal zone) and shelf forms dominate within the CF3 and at the end of P4 planktic foraminiferal zones (Fig. 7). The abundance of planktic foraminifera (%P; Fig. 3) correlates well with higher values of the deeper littoral and shelf indicators (Fig. 8; see also Appendix-Table 4).
Statistical analysis
All aforementioned proxies (Fischer’s α, BFOI, % Oxiphylic, % HOFS, and % Infaunal) are significantly and positively correlated (see Appendix-Table 3). The brief interval of high palaeoproductivity and low diversity and oxygenation, during the mid–lower Maastrichtian (between samples 58 to 73), was also subjected to statistical analysis (Pearson correlation) and has returned with negative and statistically significant values for the palaeoproductivity proxies, especially the % infaunal taxa (Appendix-Table 3b). A more high-resolution data set is likely to yield the same for the other palaeoproductivity proxy, % HOFS (Appendix-Table 3b). Statistical analysis was again performed after deleting the samples for this brief interval (16 samples). The results have yielded the same positive and significant relationship between all proxies (Appendix–Table 3c). The relationship between the % P and test wall type (palaeodepth indicators; Fig. 7) was also analyzed; the shelf indicators correlate positively and statistically significantly with % P (Appendix-Table 4). Statistical analysis was also performed between the dominant morphogroups and all proxies and yielded positive and statistically significant relationships (Appendix-Table 5), corroborating the relationships noted for oligotrophic settings (Jain et al., 2007).
Changes at the K/Pg boundary
Comparative accounts
Most benthic foraminiferal records that address changes at the K/Pg boundary or for the intervening interval (Late Maastrichtian–Early Paleogene) are from the deep sea (Early Maastrichtian: Friedrich et al., 2005; Friedrich and Hemleben, 2007; Late Maastrichtian: Widmark and Speijer, 1997; Widmark, 2000; K/Pg boundary: Keller, 1988; Speijer and Van der Zwaan, 1996; Culver, 2003; Palaeocene/Eocene boundary, PETM: Miller et al., 1987, Thomas and Shackleton, 1996; Alegret and Ortiz, 2006, Giusberti et al., 2009). There are very few records (see Culver, 2003 for a review) that address changes at shallow water settings (i.e., neritic, < 200 m water depth) (Sikora, 1984; Huber, 1988; Keller, 1992; Schmitz et al., 1992; Olsson et al., 1996; Keller et al., 1998). The classic records are by Plummer (1927) on midway (Palaeocene) and Cretaceous foraminifera from Texas (North America), and by Olsson (1960) on the latest Cretaceous–earliest Paleogene foraminifera from New Jersey (North America).
At shallow neritic waters, for benthic foraminiferal species that do not cross the K/Pg boundary, the data is variable: 16% from Texas (North America) (Plummer, 1927), ~50% disappear/emigrate (but temporarily) at the Brazos River (Texas) (Keller, 1992), 32% in New Jersey (North America) (Olsson, 1960), 64% at Seymour Island, Antarctica (Huber, 1988) and 13% “common” species that are restricted to the upper Maastrichtian at Seldja (Tunisia) (Keller et al., 1998). Culver (2003) while summarizing published data, noted that at shallow depths, 40% of benthic foraminiferal species disappeared, at intermediate depths, 35% (using maximum figures), or 29% (using minimum figures), whereas for deep waters, 32% (maximum) or 22% (minimum). He further noted that there is a (a) weak trend of decreasing values with increasing depth, (b) the pattern of one-third disappearance/extinction/turnover, is regardless of bathymetry, and (c) available data do not support the suggestion that that shallow-water benthic foraminifera were more severely affected by environmental events across the K/Pg boundary than the intermediate or deep-water faunas.
Globally, at shallow water neritic depths, there are only four data points available for comparison. At Brazos River (Texas, North America), infaunal benthic foraminiferal species dominate on both sides of the K/Pg boundary (Keller, 1992). At Millers Ferry (Alabama, North America), the Maastrichtian infaunal species give way to Danian epifaunal ones (Olsson et al., 1996). At Aïn Settara (Tunisia), low-diversity epifaunal species dominate in the Danian, reflecting extreme oligotrophic conditions (Peyrt et al., 2002). At El Kef, 50 km to the north, similar low diversity and low dominance Danian assemblages are noted, reflecting low-oxygen conditions (Keller, 1988; Speijer and van der Zwaan, 1996). However, Peyrt et al. (2002) pointed out that there is no geochemical indication to suggest that low-oxygen conditions prevailed (see also Tribovillard et al., 2000) and that the lack of burrowing within the laminated sediments may simply be related to the K/Pg mass extinction of burrowing invertebrates. Peyrt et al. (2002) also argued that the low-diversity assemblages immediately above the K/Pg boundary are a product of environmental stress resulting from changes in the nature of the phytoplankton flux to the seafloor (see also Culver, 2003). The calcareous nannofossils, a major Late Cretaceous primary producer, suffered a major extinction (Romein and Smit, 1981), and so also the early Danian benthic foraminifera that were left with a changed food supply composed mainly of dinofagellates, a group that did not experience any mass extinction (Brinkhuis et al., 1998).
Thus, the disappearance of infaunal species and the dominance of epifaunal species in the early Danian has been attributed to the sudden decline in primary productivity at the K/Pg boundary, coupled with the cessation of organic carbon flux to the seafloor (Thomas, 1990; Widmark and Malmgren, 1992; Alegret et al., 2001; Peyrt et al., 2002). This would have led to the eventual decline in abundance of the infaunal taxa that were typically adapted to high food supply and/or low-oxygen conditions. Therefore, food and oxygen availability are seen as prime ecological factors in influencing changes in benthic foraminiferal assemblages across the K/Pg boundary (see also Culver, 2003). In the context of the present study, this interplay of food and oxygen is elaborated further under the “Discussion” section.
Present data
Seven scenarios (Table 2) are explored to access neritic benthic foraminiferal changes at the K/Pg boundary ranging from the analysis of an assemblage below and above the K/Pg boundary (scenario “a,” assemblages 10 and 11, 26 samples; the boundary is drawn between samples 200 and 201; see Farouk and Jain, 2016) to a more extended interval (scenarios “f” and “g”; scenario “f”—incorporating data from assemblages 9 to 18, 294 samples; Upper Maastrichtian to the Thanetian interval and scenario “g”—substage data, Upper Maastrichtian–Lower Paleocene). A sample-by-sample approach analysis is not undertaken as at the top of assemblage 10 and following the K/Pg boundary, is marked by a regional hiatus (of missing CF2 to P1b planktic foraminiferal zones; see Farouk, 2016; Farouk and Jain, 2016). Hence, an assemblage approach is used as it gives a broader and better picture of the prevailing benthic ecosystem. The logic for using an extended time interval (scenario f; Table 2) is to make sure that the temporary disappearance/reappearance of species does not affect the dataset and the interpretation, thereof (see Culver, 2003). Hence, for all analysis (scenarios a–e; Table 2), the term “disappearance” is used, and “extinction” is used only for scenarios “f” and “g” (Table 2; see also Figs. 9–11), as the latter two analyses incorporate an extended time period (“extinction” is used with caution; only three Lazarus taxa are noted in this study, namely Cibicides beaumontianus, Praebulimina kikapoensis and Praebulimina russi). Scenario “f” (Fig. 9 with abundances >5%) spans from Assemblages 9 to 18 (see Fig. 7) and scenario “g” (Table 2; see also Figs. 10–11 with abundances > 5%) deals with the comparison between substages, Upper Maastrichtian and Lower Palaeocene. Table 3 summarizes the changes of proxies (before and after the K/Pg boundary) both at the level of individual samples and assemblages (10 and 11) (see also Fig. 7).
Discussion
In the present study, six points are noted:
-
1.
Species diversity, palaeoproductivity, and palaeooxygenation are correlated positively and significantly, a characteristic feature of an oligotrophic environment. But a very brief interval (the mid–lower Maastrichtian; samples 58–73) of increased palaeoproductivity with reduced diversity and oxygenation (reduced ventilation) yields a negative and significant correlation characteristic of a mesotrophic–eutrophic setting.
-
2.
Test wall-based palaeodepth indicators suggest littoral depths throughout the studied interval, punctuated briefly by deeper littoral depths at the base of the section (planktic foraminifera zones CF8b–CF7), and shelf environment for planktic foraminiferal zones CF3 and at the end of P4. The abundance of planktic foraminifera (%P) correlates with higher values for the deeper littoral and shelf indicators, being positive and significant with the shelf indicator.
-
3.
Seven (a–g) scenarios are explored to access changes at the K/Pg boundary. The “long term” data for the substage (Upper Maastrichtian–Lower Palaeocene) suggests that 38% of the agglutinated and 40% of the calcareous species became extinct after the K/Pg boundary with an overall 40% “extinction.” The data for the short term (i.e., samples before and after the K/Pg; termed “disappearances”) are 10% (agglutinated), 36% (calcareous), and 25% (overall).
-
4.
As regards benthic foraminiferal changes across the K/Pg boundary, in this study as with other global ones, the dominance of epifaunal taxa in the early Danian is marked.
-
5.
In an oligotrophic setting like this, the effects noted across the K/Pg are more dramatic with respect to a shift in species composition (assemblage), a shift from a pre-K/Pg agglutinated–dominated assemblage (Haplophragmoides-Ammobaculites) to a post-K/Pg calcareous one (Cibicodoides-Cibicides-Anomalinoides), rather than in the community structure, itself (i.e., species diversity—Fisher’s α).
This contribution reaffirms that palaeoproductivity, palaeooxygenation, and species diversity are closely interlinked (Fig. 4; Appendix-Table 5) (see also Mackensen et al., 1990, 1995; Thomas and Gooday, 1996; Schmiedl et al., 1997; van der Zwaan et al., 1999; Levin et al., 2001; Gooday, 2003; Jorissen et al., 2007; Jain et al., 2007; Alegret and Thomas, 2009). In oligotrophic settings, benthic foraminiferal diversity and palaeoproductivity display a positive relationship as noted in examples from the Arctic (Wollenburg and Mackensen, 1998; Wollenburg and Kuhnt, 2000), Eastern Central Atlantic (Heinz et al., 2004), South China Sea (Hess and Kuhnt, 2005), and the Caribbean (Jain et al., 2007), whereas a negative relationship is noted in mesotrophic–eutrophic environments such as in the Arabian Sea (Den Dulk et al., 1988; Gooday et al., 1998). Wollenburg and Mackensen (1998), in the ice-covered Arctic (an oligotrophic setting), noted that food availability and competition for it largely controls benthic foraminiferal species composition. In seasonally ice-free periods, high abundance and high benthic foraminiferal diversities increase with increased food supply (Wollenburg and Mackensen, 1998). This positive relationship is also noted for low latitude sites in the Neogene Caribbean (Jain et al., 2007; Jain and Collins, 2007; Jain, 2011) and also in the present study (Fig. 8).
However, a very brief interval within the mid–lower Maastrichtian (samples 58–73 only; Assemblage 3) reflects a period of increased palaeoproductivity with reduced species diversity and ventilation (reduced oxygenation), consistent with mesotrophic–eutrophic settings (Fig. 4). This interval coincides with shallow littoral depths (HST1; Figs. 4 and 8), high % of infaunal taxa (Fig. 4), and the dominance of the agglutinated morphogroups AG-A (infaunal) and AG-B.2 (infaunal to shallow infaunal) (Figs. 5–6). This brief interval (Assemblage 3; see Fig. 8) is also marked by the abundance of Ammobaculites khargaensis, a species that disappeared after the K/Pg boundary when the environment became more oxygenated (Figs. 4 and 9a) suggesting a preference of this species for low oxygen conditions in shallow to deep infaunal microhabitats. However, for this brief interval, moderate diversity values and moderate dominance (Fig. 4) suggests that oxygen was not a limiting factor, in spite of increased palaeoproductivity (food availability), that was possibly made available due to increased runoff in a brackish/lagoonal setting (as evidenced by reduced values of the diversity index Fisher’s α; < 5) (see Fig. 4).
The samples 58–73 (Assemblage 3) are barren of planktic foraminifers but most likely encompass the middle part of lower Maastrichtian, the planktic foraminifera Zone CF6 (see also Tantawy et al., 2001; Farouk and Jain, 2016) (se Fig. 3). For this interval, cooler climates and lower sea level (the latter is also noted in the present study; see Fig. 4) are well-documented (Li et al., 1999, 2000; Haq, 2014). It is likely that in such a shallow setting, possibly there was an upwelling of cooler waters (and aided by increased runoff) resulted in increased food supply, but not beyond a threshold so as to result in oxygen being a limiting factor.
The mid Maastrichtian is also an interval that is globally marked by a positive δ13C values (a plateau-like high) called the Mid Maastrichtian Event (MME) (Voigt et al., 2012) and has recently been identified (in part) at Gebel Matulla (west-central Sinai, Egypt; Farouk, 2014) encompassing the top of planktic foraminiferal zone CF5 and broadly corresponds to the top of HST1 (= present work Assemblages 5 and 6; samples 86–127; see Fig. 4). Since the duration is barren of planktic foraminifera (Fig. 3), the placement of MME is tentative but follows the same pattern - HST, marked by low species diversity, neritic depth, higher percentages of infaunal taxa and % HOFS (Fig. 4) as noted in the Gebel Matulla section in Sinai, Egypt (Farouk, 2014: CF7–CF5 = Coryphostoma incrassata, Gavelinella limbata, Nonionella cretacea, N. robusta, and Osangularia expansa; = middle to outer neritic). As in the present study, the influence of sea level coupled with tectonic uplift (to shallower depths) is the main causal mechanism. Assemblage 5 and 6 in the present work (see Fig. 7) are also marked by, but, with somewhat more shallow-water taxa (inner to ?middle neritic depths) including the calcareous species, Discorbis pseudoscopus and Valvalbamina depressa and the agglutinated, Ammobaculites khargaensis and Trochammina rainwateri (Fig. 7).
The distribution of morphogroups, in the present study, also reflects the influence of the availability of food (palaeoproductivity), oxygenation and palaeodepth (Fig. 6; Appendix-Table 5). The TST’s are marked by the dominance of the epifaunal calcareous morphogroup CH-A4 and the HST’s by the agglutinated morphogroups of AG-A and AG-B.2 (and % infaunal taxa) (Fig. 6). Palaeoproductivity never reached the threshold, where one would expect negative relationship, characteristic of meso– to eutrophic environment (Appendix-Table 5). The influence of palaeodepth (Fig. 7) seems remarkable as the calcareous morphogroups CH-A4, CH-B4 and CH-A5 do not correlate with the shallower depth proxies (littoral and deeper littoral) but positively and significantly with the deeper shelf indicator (Appendix-Table 5).
This study also demonstrates (both qualitatively and quantitatively; Figs. 4, 5–7, Table 1; Appendix-Tables 3–6, respectively) that in a shallow water setting, the distribution of benthic foraminifera is not just governed by the availability of organic-flux to the sea floor (palaeoproductivity) and oxygen content (palaeooxygenation) but also by palaeodepth. But, for now, the individual influence of these three to the distribution of benthic foraminifera are hard to delineate but it appears that in a well-oxygenated setting, the availability of food is a function of depth and its downward movement is governed by the availability of oxygen. Diversity clearly shows depth-dependency, with significant positive correlations increasing in strength with increasing depth (least for littoral depths; see Appendix-Table 6).
The relationship of palaeoproductivity, palaeooxygenation and species diversity for the present study can be explained using a combination of the TROX model of Jorissen et al. (1995) and Jorissen (1999) and the parabolic curve of Levin et al. (2001) (see also Gooday, 2003; Jain et al., 2007) (Fig. 8). Species diversity is low in highly oligotrophic settings as food supply (flux of organic carbon to the seafloor = productivity) is very low; diversity is maximized in well-oxygenated settings (even at bathyal and abyssal depths; see also Gooday et al., 1998) (the left hand-side of Fig. 8). In highly mesotrophic–eutrophic settings (such as in oxygen minimum zones), species diversity is also low, as the stress caused by the abundance of species leads to reduced diversity (the right hand-ide of Fig. 8). The abundance of food and reduced predation facilitates increased species diversity. However, when oxygen is a limiting factor, species diversity falls (see Fig. 8). In the present study, samples 1–57 and 74–315 represents the left hand-side of Fig. 8, whereas, samples 58–73 represent the top right hand-side of Fig. 8.
As regards benthic foraminiferal changes across the K/Pg boundary, it is apparent from the present data that depending upon the calculated interval, the “disappearance” and “extinctions” vary considerably (see Figs. 9–11; Table 2-3). But, by only considering the short-term interval data (i.e., across the K/Pg boundary; samples 200/201; scenario “a”; Table 3), the picture may not be complete due to the hiatus that is ubiquitous across Egypt, from CF2 to P1c planktic foraminiferal zones (see Farouk, 2016; Jain and Farouk, 2017). Hence, scenarios “b–e” (the Assemblage approach) gives a somewhat better picture but on a coarser timescale (see Table 2). However, the latter is the best option for understanding changes at the K/Pg boundary in Egypt, for now. Interestingly, the results of this “long-term” approach (see Figs. 9–11; Table 2–3) is also consistent with the “pattern of approximately one third disappearance/extinction/turnover” noted in other exposures, globally (Culver, 2003) (see also Table 2).
The other point of commonality in the present study with other global ones (Culver, 2003) is the general pattern of the dominance of epifaunal taxa in the early Danian (see Fig. 4). This shift is also corroborated by the morphogroup analysis where a sudden increase in the epifaunal morphogroup CH-A4 is noted (see Fig. 4 and 6). Both these changes (dominance of epifaunal taxa and epifaunal morphogroup) also coincide with improved ventilation (higher BFOI and % Oxiphylic values; see Fig. 4) suggesting that in shallow largely oligotrophic waters, as this one (< 100 m), the breakdown of productivity (surface and bottom) and the subsequent decrease in food supply due to the bolide impact, may not have had a dramatic effect on the larger benthic environment (represented by the cumulative proxies such as species diversity, palaeoproductivity and palaeooxygenation) that were already well-adapted to a lowered food supply (oligotrophy). Hence, in such oligotrophic settings, the effects noted are more dramatic with respect to a shift in species composition (assemblage) (see Figs. 9–11), i.e., a shift from a pre–K/Pg agglutinated-dominated assemblage (Haplophragmoides–Ammobaculites) to a post-K/Pg calcareous one (Cibicodoides–Cibicides–Anomalinoides), rather than in the community structure (species diversity), itself (see also Fig. 4). Even, from the prospective of the morphogroup analysis, the shift occurs from a largely agglutinated AG-A morphogroup (infaunal, deposit feeders) to a dominantly CH-A4 morphogroup (epifaunal, deposit feeders and passive herbivores; browsers), across the K/Pg boundary (see Figs. 5–6). The other ubiquitous agglutinated morphogroup, AG-B.2 (epifaunal/shallow infaunal, deposit feeders), successfully transgresses, albeit a bit diminished, across the boundary event (see Figs. 5–6); both emphasizing the preferential survivorship of epifaunal forms or that the neritic species may have been more adapted to environmental variability, as compared to bathyal forms (Alegret and Thomas, 2013).
Globally, the shift in the early Danian, towards the dominance of the epifaunal taxa is also reflected (directly or indirectly) by other proxies, such as the (a) collapse in δ13C values, (b) interruption in primary productivity, and (c) by its geographical heterogeneity. Globally, the K/Pg boundary displays a collapse in the δ13C gradient between surface and deep-sea carbonates (Alegret et al., 2003; d'Hondt, 2005; Alegret et al., 2012; Alegret and Thomas, 2013) and has been interpreted as the result of a long-term interruption of primary productivity (the Strangelove Ocean and Living Ocean models; Hsü and McKenzie, 1985). But the lack of correspondingly significant extinction in benthic foraminifera (Alegret et al., 2012), whose species abundance and diversity are a function of the export of organic matter from surface down to the sea floor, the benthic-pelagic coupling, suggests otherwise. Probable explanations for this decoupling include: (a) the oceanic primary productivity recovered very quickly after the K/Pg event, (b) the collapse in δ13C values (highly negative) reflects the effects of the extinction of the carriers of the isotope signal, i.e., the calcareous nannoplankton and planktic foraminifera, that were eventually replaced by taxa with a much lighter carbon isotope signature than their Maastrichtian predecessors (Alegret et al., 2012; Birch et al., 2012), and (c) there exists geographical heterogeneity with increased primary productivity in some areas (Pacific Ocean, New Zealand), and decreased in other areas (in Indian Ocean, Tethys, and in some Atlantic sections; see Hollis et al., 2003; Alegret and Thomas, 2005, 2009; Alegret, 2007; Hull and Norris, 2011; Alegret et al., 2012; Sibert et al., 2014; Esmeray–Senlet et al., 2015; Birch et al., 2016). Recently it has been documented that the last scenario (“c”) is a more viable option (Birch et al., 2016) and operated in three stages. These include Stage 1 (i.e., from the K/Pg boundary to ~300 k.y.): this is marked by planktic to benthic δ13C values that are close to zero or negative with very low bulk δ13C and carbonate accumulation rates. Stage 2: the planktic–benthic Δδ13C returns to pre–extinction levels and the bulk δ13C and carbonate accumulation rates also increase. Stage 3 is the final recovery stage 3 that occurs ~2.5 m.y. after the event. This is marked by the return of differences between mixed-layer and thermocline dwelling planktic foraminifera. Our data is too coarse to corroborate or evaluate this heterogeneity model but rather points towards a much larger role of sea level. The rising sea level near the end of the Maastrichtian and across the K/Pg boundary (see Li et al., 1999, 2000; Haq, 2014; Farouk and Jain, 2016), would have brought in warm waters to the largely shallow (deeper littoral; see Figs. 4 and 7) oligotrophic setting (Fig. 4), thereby providing an already thriving epifaunal community newer niches to exploit further and diversify. A gradually increasing diversity indices with consistently moderate to high values after the K/Pg boundary (Fig. 4) would have also meant that oxygen was not limiting, which is what one would expect from a transgressive event. This marine incursion also possibly coincided with subsidence (regional tectonic) as noted by a sudden increase in %P, from 0 to 63 (see Table 3) as well as by changes in benthic foraminiferal assemblage and paleodepth proxies (see Fig. 7).
Conclusions
This multi-proxy approach enables to better understand the palaeoenvironmental changes and the inter-relationships between palaeooxygenation, palaeoproductivity, species diversity, and palaeodepth for the shallow water Maastrichtian–Thanetian rocks exposed at the Dakhla Oasis (Western Desert, Egypt).
-
Data suggests a positive and statistically significant correlation between species diversity, palaeooxygenation, and palaeoproductivity consistent with a well-oxygenated oligotrophic setting. A brief interval (mid–lower Maastrichtian) of increased palaeoproductivity with reduced diversity and oxygenation shows a negative and significant correlation, consistent with mesotrophic–eutrophic settings. This interval also coincides with very shallow waters during a Highstand System Tract (HST) and is dominated by the dysoxic agglutinated taxa, Ammobaculites khargaensis.
-
Species diversity (Fisher’s α) displays high values at the bottom of the studied section (planktic foraminiferal zones CF8b–CF7), low and fluctuating values until the K/Pg boundary, and then moderate to high values for the post K/Pg interval.
-
On average, the high organic-flux species (% HOFS) do not constitute a major fraction of the assemblage except in assemblage 19 where they make up 27%. At the K/Pg boundary (between assemblages 10 and 11) there is no appreciable change, and the proxy remains low (< 5%).
-
The infaunal species make up 35% of the total benthic foraminiferal dataset. A dominance of epifaunal forms is noted at the bottom of the section in planktic foraminiferal zones CF8b–CF7, then, just after the K/Pg boundary and somewhat for planktic foraminiferal zones P3a–P4. The transgressive system tracts (TST) display higher abundances of the epifaunal taxa (and of %P) whereas the high system tracts (HST) are marked by an increase in infaunal taxa.
-
The BFOI, the % Oxyphilic taxa follow the diversity pattern (Fisher’s α); high values at the bottom of the section, fluctuating values until the K/Pg boundary and then consistently high values for the post K/Pg interval.
-
Of the 16 identified sub-morphogroups only five (CH-A.4, CH-B.4, CH-B.5, AG-A, and AG-B.2) dominate. For the pre-K/Pg interval, for the calcareous forms, CH-A4 and for the agglutinated one, AG-A and AG-B.2 dominate, and for the post-K/Pg interval, CH-A4 and AG-B.2 dominate, respectively. The deeper portions of the section are largely characterized by the dominance of CH-A4 (coincident with high %P values), whereas the shallower ones are dominated by the agglutinated morphotypes AG-A and AG-B.2.
-
Of the palaeodepth indicators (littoral, deeper littoral, and shelf), the littoral dominates the entire studied section; the deeper littoral proxy dominates at the base of the section (CF8b–CF7 planktic foraminiferal zone) and shelf forms dominate within the CF3 and at the end of P4 planktic foraminiferal zones.
-
Seven scenarios were explored to access neritic benthic foraminiferal changes at the K/Pg boundary ranging from an analysis of an assemblage below and above the boundary (assemblages 9 and 10) to a more extended interval incorporating data from assemblages 9 to 18 (Upper Maastrichtian–Thanetian interval). The “long term” data (Upper Maastrichtian–Lower Palaeocene) suggests 38% agglutinated and 40% calcareous species became extinct after the K/Pg boundary with an overall 40% “extinction.” The data for the short term (i.e., samples before and after K/Pg; termed “disappearances”) is 10% (agglutinated), 36% (calcareous), and 25% (overall).
-
In an oligotrophic setting like this, the effects noted across the K/Pg are more dramatic with respect to a shift in species composition (assemblage), a shift from a pre-K/Pg agglutinated-dominated assemblage (Haplophragmoides-Ammobaculites) to a post- K/Pg calcareous one (Cibicodoides-Cibicides-Anomalinoides), rather than in the community structure, itself (represented by Fisher’s α).
-
All change in benthic foraminifera (proxies, diversity, morphogroup, and assemblage) is a function of changes in the sea level exacerbated by regional tectonics, whenever they work in tandem such as during the K/Pg boundary interval where sudden deepening provided newer niches to the already thriving (oligotrophic) epifaunal community.
Data availability
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
References
Abdel-Kireem MR, Samir AM (1995) Biostratigraphic implications of the Maastrichtian–Lower Eocene sequence at the North Gunna section, Farafra Oasis, Western Desert, Egypt. Marine Micropalaeontology 26:329–340
Alegret L (2007) Recovery of the deep-sea floor after the Cretaceous/Paleogene boundary event: the benthic foraminiferal record in the Basque–Cantabrian basin and in South-eastern Spain. Palaeogeogr Palaeoclimatol Palaeoecol 255:181–194
Alegret L, Ortiz S (2006) Global extinction event in benthic foraminifera across the Paleocene/Eocene boundary at the Dababiya Stratotype section. Micropaleontology 52:433–447
Alegret L, Ortiz S (2007) Global extinction event in benthic foraminifera, Paleocene/Eocene boundary, Dababiya Stratotype section. Micropaleontology 52:433–447
Alegret L, Thomas E (2005) Cretaceous/Paleogene boundary bathyal palaeo–environments in the central North Pacific (DSDP Site 465), the Northwestern Atlantic (ODP Site 1049), the Gulf of Mexico and the Tethys: the benthic foraminiferal record. Palaeogeogr Palaeoclimatol Palaeoecol 224:53–82
Alegret L, Thomas E (2009) Food supply to the seafloor in the Pacific Ocean after the Cretaceous/Paleogene boundary event. Marine Micropalaeontology 73:105–116
Alegret L, Thomas E (2013) Benthic foraminifera across the Cretaceous/Paleogene boundary in the Southern Ocean (ODP Site 690): Diversity, food and carbonate saturation. Marine Micropalaeontology 105:40–51
Alegret L, Molina E, Thomas E (2001) Benthic foraminifera at the Cretaceous/tertiary boundary around the Gulf of Mexico. Geology 29:891–894
Alegret L, Molina E, Thomas E (2003) Benthic foraminiferal turnover across the Cretaceous/Paleogene boundary at Agost (southeastern Spain): palaeoenvironmental inferences. Marine Micropalaeontology 48:251–279
Alegret L, Thomas E, Lohmann KC (2012) End–Cretaceous marine mass extinction not caused by productivity collapse. Proc Natl Acad Sci 109:728–732
Ayyad SN, Faris M, El Nahass HA, Saad KAA (2003) Planktonic Foraminiferal and Calcareous Nannofossil biostratigraphy from the upper Cretaceous–Lower Eocene successions in Northeast Sinai, Egypt. The 3rd International Conference on the Geology of Africa, pp 649–683
Beckmann, J. P., El Heiny, I., Kerdany, M. T., Said, R., Viotti, L. 1969. Standard planktonic zones in Egypt. In: Proc. 1st International Conference on Planktic Fossils (Geneva, 1967) 1, 92–103.
Berggren WA (1974) Late Palaeocene–early Eocene benthonic foraminiferal biostratigraphy and palaeoecology of Rockall Bank. Micropalaeontology 20:426–448
Bernhard RP (1986) Late quaternary benthic foraminifera of the Colombia Basin and Cayman Trough, Caribbean Sea. M.S. thesis. Louisiana State University, Baton Rouge, USA, p 185
Birch H, Coxall H, Pearson P (2012) Evolutionary ecology of early Palaeocene planktonic foraminifera: size, depth habitat and symbiosis. Palaeobiology 38:374–390
Birch HS, Coxall HK, Pearson PN, Kroon D, Schmidt DN (2016) Partial collapse of the marine carbon pump after the Cretaceous–Paleogene boundary. Geology. https://doi.org/10.1130/G37581.1
Brinkhuis H, Bujak JP, Smit J, Versteegh GJM, Visscher H (1998) Dinoflagellate–based sea surface temperature reconstructions across the Cretaceous–Tertiary boundary. Palaeogeogr Palaeoclimatol Palaeoecol 141:67–83
Buzas MA, Gibson TG (1969) Species diversity: benthonic foraminifera in western North Atlantic. Science 163:72–75
Cetean CG, Ramona Bălc R, Kaminski MA, Filipescu S (2011) Integrated biostratigraphy and palaeoenvironments of an upper Santonian – upper Campanian succession from the southern part of the Eastern Carpathians, Romania. Cretac Res 32:575–590
Chamney TP (1976) Foraminiferal morphogroup symbol for palaeoenvironmental interpretation of drill cutting samples: arctic America. Maritime Sediments, Special Publication 1B:585–624
Chan SA, Kaminski MA, Al Ramadan K, Babalola LO (2017) Foraminiferal biofacies and depositional environments of the Burdigalian mixed carbonate and siliciclastic Dam Formation, Al–Lidam area, Eastern Province of Saudi Arabia. Palaeogeogr Palaeoclimatol Palaeoecol 469:122–137
Cherif OH, Hewaidy AA (1986) The Maastrichtian planktic foraminiferal fauna of the Abu Tartur area, Western Desert. Egypt Egyptian Journal Geology 31:217–231
Coccioni R, Marsili A (2007) The response of benthic foraminifera to the K/Pg boundary biotic crisis at Elles (northwestern Tunisia). Palaeogeogr Palaeoclimatol Palaeoecol 255:157–180
Corliss BH (1985) Microhabitats of benthic foraminifera within the deep–sea sediments. Nature 314:435–438
Corliss BH, Chen C (1988) Morphotype patterns of Norwegian Sea deep sea benthic foraminifera and ecological implications. Geology 16:716–719
Corliss BH, Emerson S (1990) Distribution of Rose bengal stained deep–sea benthic foraminifera from the Nova Scotian continental margin and Gulf of Maine. Deep-Sea Res 37:381–400
Coxall, H.K., d’Hondt, S., Zachos, J.C. 2006. Pelagic evolution and environmental recovery after the Cretaceous–Paleogene mass extinction. Geology 34, 297–300.
Culver SJ (2003) Benthic foraminifera across the Cretaceous–Tertiary (K–T) boundary: a review. Marine Micropalaeontology 47:177–226
d'Hondt S (2005) Consequences of the Cretaceous/Paleogene mass extinction for marine ecosystems. Annu Rev Ecol Syst 36:295–317
Den Dulk M, Reichart GJ, Memon GM, Roelofs EMP, Zachariasse WJ, van der Zwaan GJ (1988) Benthic foraminiferal response to variations in surface water productivity and oxygenation in the northern Arabian Sea. Marine Micropalaeontology 35:43–66
El-Azabi MH, El-Araby A (2000) Depositional cycles: an approach to the sequence stratigraphy of the Dakhla Formation, west Dakhla–Farafra stretch, Western Desert, Egypt. J Afr Earth Sci 30:971–996
El-Bassiouni A, Ayyad SN, Shahin AM, Shahin SM (2003) Planktonic foraminiferal bio–and– chronostratigraphy of the upper Maastrichtian–middle Eocene succession in northeastern Sinai. Egypt Journal of Palaeontology 3:109–140
Esmeray-Senlet S, Wright J, Olsson R, Miller K, Browning J, Quan T (2015) Evidence for reduced export productivity following the Cretaceous/Paleogene mass extinction. Palaeoceanography 30:718–738
Faris M, Allam A, Marzouk AM (1985) Biostratigraphy of the Late Cretaceous –early tertiary rocks in the Nile Valley (Qena region). Egypt Ann Geological Survey of Egypt 15:278–300
Farouk S (2014) Maastrichtian carbon cycle changes and planktonic foraminiferal bioevents at Gebel Matulla, westecentral Sinai, Eqypt. Cretac Res 50:238–251
Farouk S (2016) Paleocene stratigraphy in Egypt. J Afr Earth Sci 113:126–152
Farouk S, EL-Sorogy E (2015) Danian / Selandian unconformity in the central and southern Western Desert of Egypt. J Afr Earth Sci 103:42–53
Farouk S, Faris M (2012) Late Cretaceous calcareous nannofossil and planktonic foraminiferal bioevents of the shallow–marine carbonate platform in the Mitla Pass, west central Sinai, and Egypt. Cretac Res 33:50–65
Farouk S, Jain S (2016) Benthic foraminiferal response to relative sea–level changes in the Maastrichtian–Danian succession at the Dakhla Oasis, Western Desert, Egypt. Geol Mag 2016:1–18
Farouk S, Jain S (2017) Sea–level changes in the Palaeocene (Danian–Thanetian) succession at the Dakhla Oasis, Western Desert, Egypt: implications from benthic foraminifera. Proc Geol Assoc 128:764–778
Fontanier C, Jorissen FJ, Licari L, Alexandre A, Anschutz P, Carbonel P (2002) Live benthic foraminiferal faunas from the Bay of Biscay: faunal density, composition and microhabitats. Deep–Sea Research I 49:751–785
Friedrich O, Erbacher J (2006) Benthic foraminiferal assemblages from Demerara Rise (ODP Leg 207, western Tropical Atlantic): possible evidence for a progressive opening of the Equatorial Atlantic Gateway. Cretac Res 27:377–397
Friedrich O, Hemleben C (2007) Early Maastrichtian benthic foraminiferal assemblages from the western North Atlantic (Blake Nose) and their relation to palaeoenvironmental changes. Marine Micropalaeontology 62:31–44
Friedrich O, Nishi H, Pross J, Schmiedl G, Hemleben C (2005) Interruptions of the Oceanic Anoxic Event 1b (Lower Albian, Middle Cretaceous): evidence from benthic foraminiferal repopulation events. Palaios 20:64–77
Friedrich O, Erbacher J, Wilson PA, Moriya K, Mutterlose J (2009) Paleoenvironmental changes across the Mid Cenomanian Event in the tropical Atlantic Ocean (Demerara Rise, ODP Leg 207) inferred from benthic foraminiferal assemblages. Mar Micropaleontol 71:28–40
Galal G, Kamel S (2007) Early Paleogene Planktonic foraminiferal biostratigraphy at the Monastery of Saint Paul, Southern Galala, Eastern Desert, Egypt. Rev Paléobiol 26:391–402
Gebhardt H, Kuhnt W, Holbourn A (2004) Foraminiferal response to sea level change, organic flux and oxygen deficiency in the Cenomanian of the Tarfaya Basin, southern Morocco. Mar Micropaleontol 53:133–157
Giusberti L, Coccioni R, Sprovieri M, Tateo F (2009) Perturbation at the sea floor during the Paleocene–Eocene thermal maximum: evidence from benthic foraminifera at Contessa Road, Italy. Mar Micropaleontol 70:102–119
Gooday AJ (2003) Benthic foraminifera (Protista) as tools in deepwater palaeoceanography: environmental influences on faunal characteristics. Adv Mar Biol 46:1–90
Gooday AJ, Bett BJ, Shires R, Lambshead PJD (1998) Deep–sea benthic foraminiferal diversity in the NE Atlantic and NW Arabian sea: a synthesis. Deep–Sea Research II 45:165–201
Haq BU (2014) Cretaceous eustasy revisited. Global Planet. Change 113:44–58
Heinz P, Ruepp D, Hemleben C (2004) Benthic foraminifera assemblages at Great Meteor Seamount. Mar Biol 144:985–998
Hendriks F, Luger P, Kallenbach H, Schroeder JH (1984) Stratigraphical and sedimentological framework of the Kharga–Sinn El–Kaddab stretch (western and southern part of the Upper Nile Basin), Western Desert, Egypt. Berliner Geowiss Abh, (A) 50:117–151
Hess S, Kuhnt W (2005) Neogene and Quaternary palaeoceanographic changes in the southern South China Sea (Site 1143): the benthic foraminiferal record. Marine Micropalaeontology 54:63–87
Hollis CJ, Strong CP, Rodgers KA, Rogers KM (2003) Palaeoenvironmental changes across the Cretaceous/Tertiary boundary at Flaxbourne River and Woodside Creek, Eastern Marlborough New Zealand. N Z J Geol Geophys 46:177–197
Hsü KJ, McKenzie J (1985) A “Strangelove Ocean” in the earliest Tertiary. Geophys Monogr 32:487–492
Huber BT (1988) Upper Campanian–Paleocene foraminifera from the James Ross Island region, Antarctic Peninsula. Geological Society of America, Memoir 169:163–252
Hull PM, Norris RD (2011) Diverse patterns of ocean export productivity change across the Cretaceous–Paleogene boundary: New insights from biogenic barium. Palaeoceanography 26(3):PA 3205. https://doi.org/10.1029/2010PA002082
Issawi B (1972) Review of Upper Cretaceous–Lower Tertiary stratigraphy in central and southern Egypt. Am Assoc Pet Geol Bull 56:1448–1473
Jain S (2011) Changes in Late Neogene Caribbean Benthic Foraminifers: Palaeoproductivity. Diversity and Test Size, Lap Lambert Academic Publishing Gmbh and Co. Kg, Germany, p 150
Jain S, Collins LS (2007) Trends in Caribbean palaeoproductivity related to the Neogene closure of the Central American seaway. Marine Micropalaeontology 63:57–74
Jain S, Farouk S (2017) Shallow Water Agglutinated Foraminiferal response to Late Cretaceous – early Palaeocene sea–level changes in the Dakhla Oasis, Western Desert, Egypt. Cretac Res 78:240–257
Jain S, Collins LS, Hayek L-AC (2007) Relationship of benthic foraminiferal diversity to palaeoproductivity in the Neogene Caribbean. Palaeogeogr Palaeoclimatol Palaeoecol 255:223–245
Jannink NT, Van der Zwaan GJ, Almogi-Labin A, Duijnstee I, Jorissen FJ (2001) A transfer function for the quantitative reconstruction of oxygen contents in marine palaeoenvironments. Geol Ultraiect 203:161–169
Jones RW (1999) Forties Field (North Sea) revisited; a demonstration of the value of historical micropalaeontontological data. In: Jones RW, Simmons MD (eds) Biostratigraphy in Production and Development Geology. Geological Society. Special Publication 152, London, pp 185–200
Jones RW, Charnock MA (1985) Morphogroups of agglutinated foraminifera. Their life positions and feeding habits and potential applicability in (palaeo) ecological studies. Rev Paléobiol 4:311–320
Jones RW, Pickering KT, BouDagher-Fadel M, Matthews S (2005) Preliminary observations on the micropalaeontological characterisation of submarine fan / channel sub–environments, Ainsa system, south–central Pyrenees, Spain. In: Powell AJ, Riding JB (eds) Recent Developments in Applied Biostratigraphy: The Micropalaeontological Society. Special Publication 1, pp 55–68
Jorissen FJ (1988) Benthic foraminifera from the Adriatic Sea: principles of phenotypic variation. Utrecht Micropalaeontological Bulletin 37:174
Jorissen FJ (1999) Benthic foraminiferal microhabitats below the sediment–water interface. In: Sen Gupta BK (ed) Modern Foraminifera. Kluwer Academic Press, Dordrecht, pp 161–179
Jorissen FJ, Barmawidjaja DM, Puskaric S, Van der Zwaan GJ (1992) Vertical distribution of benthic foraminifera in the northern Adriatic Sea; the relation with the organic flux. Marine Micropalaeontology 19:1–2
Jorissen FJ, De Stigter HC, Widmark JG (1995) A conceptual model explaining benthic foraminiferal microhabitats. Marine Micropalaeontology 22:3–15
Jorissen FJ, Fontanier C, Thomas E (2007) Palaeoceanographical proxies based on deep–sea benthic foraminiferal assemblage characteristics. In: Hillaire-Marcel C, De Vernal A (eds) Proxies in Late Cenozoic Palaeoceanography, Volume 1. Developments in Marine Geology, pp 263–325
Kaiho K (1991) Global changes of Paleogene aerobic/anaerobic benthic foraminifera and deep–sea circulation. Palaeogeogr Palaeoclimatol Palaeoecol 83:65–85
Kaiho K (1994) Benthic foraminiferal dissolved–oxygen index and dissolved–oxygen levels in the modern ocean. Geology 22:719–722
Kaminski MA, Gradstein FM (2005) Atlas of Paleogene cosmopolitan deepwater agglutinated foraminifera. Grzybowski Foundation Special. Publication 10:574
Kaminski MA, Boersma A, Tyszka J, Holbourn A (1995) Response of deep–water agglutinated foraminifera to dysoxic conditions in the California borderland basins. In: Kaminski MA, Geroch S, Gasinski MA (eds) Proceedings of the Fourth International Workshop on Agglutinated Foraminifera: Grzybowski Foundation Special Publication 3, pp 131–140
Kaminski MA, Aksu A, Box M, Hiscott RN, Filipescu S, Al-Salameen M (2002) Late Glacial to Holocene benthic foraminifera in the Marmara Sea: implications for Black Sea–Mediterranean Sea connections following the last deglaciation. Mar Geol 190:165–202
Keller G (1988) Biotic turnover in benthic foraminifera across the Cretaceous–Tertiary boundary at El Kef, Tunisia. Palaeogeogr Palaeoclimatol Palaeoecol 66:153–171
Keller G (1992) Paleoecologic response of Tethyan benthic foraminifera to the Cretaceous–Tertiary boundary transition. In: Takayanagi Y, Saito T (eds) Studies in Benthic Foraminifera. Tokai University Press, Tokyo, pp 77–91
Keller G, Adatte T, Stinnesbeck W, Stueben D, Kramar U, Berner Z, Li L, Von Salis Perch-Neilsen K (1998) The Cretaceous–Tertiary transition on the shallow Saharan Platform of southern Tunisia. Geobios 30:951–975
Kender S, Kaminski MA, Jones RW (2008a) Oligocene deep–water agglutinated foraminifera from the Congo Fan, offshore Angola: palaeoenvironments and assemblage distributions. In: Kaminski MA, Coccioni R (eds) Proceedings of the Seventh International Workshop on Agglutinated Foraminifera: Grzybowski Foundation Special Publication 13, pp 107–156
Kender S, Kaminski MA, Jones RW (2008b) Early to middle Miocene foraminifera from the deep–sea Congo Fan, offshore Angola. Micropaleontology 54:477–568
Khalil H, Al Sawy S (2014) Integrated biostratigraphy, stage boundaries and Paleoclimatology of the Upper Cretaceous–Lower Eocene successions in Kharga and Dakhala Oases, Western Desert, Egypt. J Afr Earth Sci 96:220–242
Koutsoukos AM, Hart MB (1990) Cretaceous foraminiferal morphogroup distribution patterns, palaeocommunities and trophic structures: a case of study from the Sergipe Basin, Brazil. Trans R Soc Edinb Earth Sci 81:221–246
Langer MR (1993) Epiphytic foraminifera. Mar Micropaleontol 20:235–265
Levin LA, Etter RJ, Rex MA, Gooday AJ, Smith CR, Pineda J, Stuart CT, Hassler RR, Pawson D (2001) Environmental influences on regional deep–sea species diversity. Annu Rev Ecol Syst 32:51–93
Li L, Keller G, Stinnesbeck W (1999) The late Campanian and Maastrichtian in northwestern Tunisia: palaeoenvironmental inferences from lithology, macrofauna and benthic foraminifera. Cretac Res 20:231–252
Li L, Keller G, Adatte G, Stinnesbeck W (2000) Late Cretaceous sea level changes in Tunisia: a multi–disciplinary approach. Geological Society of London 157:447–458
Linke P (1992) Metabolic adoptions of deep–sea benthic foraminifera to seasonally varying food input. Mar Ecol Prog Ser 81:51–63
Linke P, Lutze GF (1993) Microhabitat preferences of benthic foraminifera ^ a static concept or a dynamic adaptation to optimize food acquisition? Marine Micropalaeontology 20:215–234
Luger P (1985) Stratigraphie der marinen Oberkreide und des Alttertiars im Sudwestlichen Obernil–Becken (SW–Aegypten) unterbesonderer berucksichtigung der mikropaläontologie, paläoekologie und paläogeographie. Berliner geowiss. Abh. (A) 63, Berlin, pp 1–150
Luger P (1988) Maastrichtian to Paleocene facies evolution and Cretaceous/Tertiary boundary in middle and southern Egypt. Rev Espan Micropaleontol, Numero Extraord:83–90
Mackensen A, Grobe H, Kuhn G, Fütterer DK (1990) Benthic foraminiferal assemblages from the eastern Weddell Sea between 68° and 73°S: distribution, ecology and fossilization potential. Marine Micropalaeontology 16:241–283
Mackensen A, Schmiedl G, Harloff J, Giese M (1995) Deep–sea foraminifera in the South Atlantic Ocean: ecology and assemblage generation. Micropalaeontology 41:342–358
Miller KG, Janecek TR, Katz ME, Keil DJ (1987) Stable carbon and oxygen isotope record of benthic foraminifera from Paleocene–Eocene sediments. Paleoceanography 2:741–761
Murray JW (2001) The niche of benthic foraminifera, critical thresholds and proxies. Marine Micropalaeontology 41:1–8
Murray JW, Alve E, Jones BW (2011) A new look at modern agglutinated benthic foraminiferal morphogroups: their value in palaeoecological interpretation. Palaeogeography, Palaeoclimatology, Palaeoecology 309:229–241
Nagy J (1992) Environmental significance of foraminiferal morphogroups in Jurassic North Sea deltas. Palaeogeography, Palaeoecology, Palaeoclimatology 95:111–134
Nagy J, Pilskog B, Wilhelmsen R (1990) Facies controlled distribution of foraminifera in the Jurassic North Sea Basin. In: Hemleben C, Kaminski MA, Kuhnt W, Scott DB (eds) Papleoecology, Biostratigraphy, Paleoceanography and Taxonomy of Agglutinated Foraminifera. Kluwer Academic Press, Dordrecht, the Netherlands, pp 621–657
Nagy J, Gradstein FM, Kaminski MA, Holbourn AEL (1995) Late Jurassic to Early Cretaceous foraminifera of Thakkhola, Nepal: Palaeoenvironments and description of new taxa. In: Kaminski MA, Geroch S, Gasinski MA (eds) Proceedings of the Fourth International Workshop on Agglutinated Foraminifera. Grzybowski Foundation Special Publication 3, pp 181–209
Nagy J, Reolid M, Rodríguez-Tovar FJ (2009) Foraminiferal morphogroups in dysoxic shelf deposits from the Jurassic of Spitsbergen. Polar Res 28:214–221
Nagy J, Hess S, Alve E (2010) Environmental significance of foraminiferal assemblages dominated by small–sized Ammodiscus and Trochammina in Triassic and Jurassic delta–influenced deposits. Earth Sci Rev 99:31–49
Obaidalla NA (2005) Complete Cretaceous/Paleogene (K/P) boundary section at Wadi Nukhul, southwestern Sinai, Egypt: inference from planktic foraminiferal biostratigraphy. Rev Paléobiol 24:201–224
Olsson RK (1960) Foraminifera of latest Cretaceous and earliest Tertiary age in the New Jersey coastal plain. J Paleontol 34:1–58
Olsson RK, Chengjie L, van Fossen M (1996) The Cretaceous–Tertiary catastrophic event at Millers Ferry, Alabama. In: Ryder G, Fastovsky D, Gartner S (eds) The Cretaceous–Tertiary Event and Other Catastrophes in Earth History. Geological Society of America, Special Paper 307, pp 263–277
Orabi HO (1995) Biostratigraphy and paleoecology of the Campanian–Paleocene agglutinated foraminifera from Gebel Um El Ghanayim Kharga Oasis, Egypt. Science Journal Faculty of Science, Menoufia University 11:25–68
Orabi OH (2000) Foraminiferal morphogroups and paleoenvironments of some Upper Cretaceous exposures in Egypt. Egypt J Geol 44:359–397
Orabi OH, Khalil HM (2014) Calcareous benthonic foraminifera across the Cretaceous / Palaeocene transition of Gebel Um El–Ghanayem, Kharga Oasis, Egypt. J Afr Earth Sci 96:110–121
Peryt, D., Alegret, L., Molina, E. 2002. The Cretaceous/Paleogene (K/P) boundary at Aìn Settara, Tunisia: restructuring of benthic foraminiferal assemblages. Terra Nova 14, 101–107.
Peryt D, Alegret L, Molina E (2004) Agglutinated foraminifers and their response to the Cretaceous/Paleogene (K/P) boundary event at Ain Settara, Tunisia. In: Bubik M, Kaminski MA (eds) Proceedings of the Sixth International Workshop on Agglutinated Foraminifera. Grzybowski Foundation Special Publication 8, pp 393–412
Plummer HJ (1927) Foraminiferal of the Midway Formation in Texas. University of Texas Bulletin 2644:3–206
Preece RC, Kaminski MA, Dignes TW (1999) Miocene benthonic foraminiferal morphogroups in an oxygen minimum zone, offshore Cabinda. In: Cameron NR, Bate RH, Clure VS (eds) The oil and gas habitats of the South Atlantic: Geological Society. Special Publications 153, London, pp 267–282
Rathburn AE, Corliss BH (1994) The ecology of living (stained) deep–sea benthic foraminifera from the Sulu Sea. Palaeoceanography 9:87–150
Reolid M, Rodríguez-Tovar FJ, Nagy J, Olóriz F (2008) Benthic foraminiferal morphogroups of mid to outer shelf environments of the Late Jurassic (Prebetic Zone, Southern Spain): Characterisation of biofacies and environmental significance. Palaeogeogr Palaeoclimatol Palaeoecol 261:280–299
Romein AJT, Smit J (1981) The Cretaceous/Tertiary boundary: calcareous nannofossils and stable isotopes. Proc K Ned Akad Wet Ser B 84:295–314
Scheibner C, Kuss J, Speijer RP (2003) Stratigraphic modeling of carbonate plateform–to–basin sediments (Maastrichtian to Palaeocene) in the Eastern Desert, Egypt. Palaeogeogr Palaeoclimatol Palaeoecol 3136:1–23
Schmiedl G, Mackensen A, Müller PJ (1997) Recent benthic foraminifera from the eastern South Atlantic Ocean: dependence on food supply and water masses. Marine Micropalaeontology 32:249–287
Schmitz B, Keller G, Stenvall O (1992) Stable isotope and foraminiferal changes across the Cretaceous–Tertiary boundary at Stevns Klint, Denmark: arguments for long–term oceanic instability before and after bolide–impact event. Palaeogeogr Palaeoclimatol Palaeoecol 96:233–260
Sen Gupta BK (1999) Introduction to modern Foraminifera. In: Sen Gupta, B.K. (Ed.), Systematics of Modern Foraminifera. Kluwer Academic Publishers, Dordrecht, Netherlands, pp 7–36
Sen Gupta BK, Machain-Castillo ML (1993) Benthic foraminifera in oxygen–poor habitats. Marine Micropalaeontology 20:3–4
Severin KP, Erskian MG (1981) Laboratory experiments on the vertical movement of Quinqueloculina impressa Reuss through sand. J Foraminifer Res 11:133–136
Sibert E, Hull P, Norris D (2014) Resilience of Pacific pelagic fish across the Cretaceous/Paleogene mass extinction. Nat Geosci 7:667–670
Sikora PJ (1984) Quantitative analysis of Foraminifera from the Kemp Clay, north–central Texas. MS Thesis,. University of Texas
Sjoerdsma PG, Van der Zwaan GJ (1992) Simulating the effect of changing organic flux and oxygen content on the distribution of benthic foraminifers. Marine Micropalaeontology 19:1–2
Sliter WV (1968) Upper Cretaceous foraminifera from southern California and northwestern Baja California. The University of Kansas Paleontoogical Contribution, Mexico, pp 1–141 Art. 7
Sliter WV, Baker A (1972) Cretaceous bathymetric distribution of benthic foraminifera. J Foraminifer Res 2:167–183
Speijer RP, van der Zwaan GJ (1996) Extinction and survivorship of southern Tethyan benthic foraminifera across the Cretaceous/Paleogene boundary. In: Hart MB (ed) Biotic Recovery from Mass Extinction Events. Geological Society Special Publication 102, pp 343–371
Sprong J, Kouwenhoven TJ, Bornemann A, Dupuis C, Speijer RP, Stassen P, Steurbaut E (2013) In search of the Latest Danian Event in a paleobathymetric transect off Kasserine Island, north–central Tunisia. Palaeogeogr Palaeoclimatol Palaeoecol 379–380:1–16
Strougo A (1986) The Velascoensis event: A significant episode of tectonic activity in the Egyptian Paleogene. Neues Jahrb Geol Palaontol Abh 173:253–269
Tantawy AA, Keller G, Adatte T, Stinnesbeck W, Kassab A, Schulte P (2001) Maastrichtian to Paleocene depositional environment of the Dakhla Formation, Western Desert, Egypt: Sedimentology, mineralogy, and integrated micro– and macrofossil biostratigraphies. Cretac Res 22:795–827
Thomas E (1990) Late Cretaceous–early Eocene mass extinctions in the deep sea. In: Sharpton VL, Ward PD (eds) Global Catastrophes in Earth history. Geological Society of America. Special Publication 247, pp 481–495
Thomas E, Gooday AJ (1996) Cenozoic deep–sea benthic foraminifers: tracers for changes in oceanic productivity? Geology 24:355–358
Thomas E, Shackleton NJ (1996) The Paleocene–Eocene benthic foraminiferal extinction and stable isotope anomalies. In: Knox RW, Corfield RM, Dunay RE (eds) Correlation of the Early Paleogene in Northwest Europe. Geological Society of London Special Publication 101, pp 401–441
Tribovillard N, Dupuis C, Robin E (2000) Sedimentological and diagenetical conditions of the impact level of the Cretaceous/Tertiary boundary in Tunisia: no anoxia required. Bulletin de la Société géologique de France 171:629–636
Tyszka J (1994) Response of Middle Jurassic benthic foraminiferal morphogroups to dysoxic/anoxic conditions in the Pieniny Klippen Basin, Polish Carpathians. Palaeogeography, Palaeoecology, Palaeoclimatology 110:55–81
van der Akker TJHA, Kaminski MA, Gradstein FM, Wood J (2000) Campanian to Palaeocene biostratigraphy and palaeoenvironments in the Foula Sub–basin, west of the Shetland Islands, UK. J Micropalaeontol 19:23–43
Van der Zwaan GJ, Duijnstee IAP, Den Dulk M, Ernst SR, Jannink NT, Kouwenhoven TJ (1999) Benthic foraminifers: proxies or problems? A review of palaeoecological concepts. Earth Sci Rev 46:213–236
Voigt S, Gale AS, Jung C, Jenkyns HC (2012) Global correlation of Upper Campanian–Maastrichtian successions using carbon-isotope stratigraphy: development of a new Maastrichtian timescale. Newsl Stratigr 45:25–53
Widmark JGV (2000) Biogeography of terminal Cretaceous benthic foraminifera: deep–water circulation and trophic gradients in the deep South Atlantic. Cretac Res 21:367–379
Widmark JGV, Malmgren B (1992) Benthic foraminiferal changes across the Cretaceous–Tertiary boundary in the deep sea; DSDP sites 525, 527 and 465. J Foraminifer Res 22:81–113
Widmark JGV, Speijer RP (1997) Benthic foraminiferal ecomarker species of the terminal Cretaceous (late Maastrichtian) deepsea Tethys. Mar Micropaleontol 31:135–155
Wollenburg JE, Kuhnt W (2000) The response of benthic foraminifers to carbon flux and primary production in the Arctic Ocean. Marine Micropalaeontology 40:189–231
Wollenburg JE, Mackensen A (1998) Living benthic foraminifers from the central Arctic Ocean: faunal composition, standing stock and diversity. Marine Micropalaeontology 34:153–185
Acknowledgements
We would like to thank Maria-Angela Bassetti, editor of Geo-Marine Letters, and anonymous reviewers for their useful comments that improved this paper. Research Supporting Project Number (RSP-2023/139), King Saud University, Riyadh, Saudi Arabia.
Funding
Filed study and laboratory work funding is provided by King Saud University.
Author information
Authors and Affiliations
Contributions
Sherif Farouk: ideas; filed works; formulation and evolution of overarching research goals and aims, preparation and writing the initial draft. Management and coordination responsibility for the research activity planning and execution.
Sreepat Jain: writing and idea of the initial paper, preparation, creation, and presentation the data.
Khaled Al-Kahtany, design the idea of the initial paper, preparation, creation, foraminiferal examination.
Fayez Ahmad: performing the experiments and data collection.
Ahmed Abdeldaim: discussion, preparation, and creation.
Corresponding author
Ethics declarations
Ethics approval
No human participants and/or animals were involved in the making of this manuscript. Hence, no ethics approval is required.
Consent to participate
All authors have given their consent for this publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
Appendix. Appendix-Table 1. Percentages of calcareous and agglutinated benthic foraminiferal morphogroups and sub-morphogroups identified in this study with sample numbers (on the left). Appendix-Table 2. Habitat preference of the studied species based on ecology and literature survey. See text for explanation. Appendix-Table 3. Pearson correlation between proxies used in the present study. Diversity indices (Fisher’s α), Palaeooxygenation proxies (Benthic Foraminiferal Oxygen Index (BFOI), and % Oxyphilic taxa), Palaeoproductivity proxies (% High organic–flux species (% HOFS) and Percent Infaunal taxa. Appendix-Table 4. Pearson correlation between % planktic foraminifera (%P) and agglutinated palaeodepth proxies (Littoral represented by simple-walled arenaceous agglutinated foraminifera; Deep littoral represented by complex-walled arenaceous agglutinated foraminifera and Shelf represented by calcareous agglutinated foraminifera). See text for explanation. Appendix-Table 5. Pearson correlation between dominant morohogroups identified in the present study (calcareous benthic foraminiferal morphogroups, CH-A.4, CH-B.4 and CH-B.5; agglutinated benthic foraminiferal morphogroups, AG-A and AG-B.2), Palaeooxygenation proxies (BFOI, and % Oxyphilic taxa), Palaeoproductivity proxies (% HOFS and Infaunal taxa) and palaeodepth proxies (Littoral and Deep littoral; the latter is Deeplit). See text for explanation. Appendix-Table 6. Pearson correlation between species diversity index (Fisher’s α) and palaeodepth proxies (Littoral and Deep littoral). See text for explanation. Appendix-Table 7. List (in order of abundance) of agglutinated (a) and calcareous benthic foraminiferal (b) patterns for Extinction, Immigrant and Survivor taxa noted for long–term interval incorporating taxa from Assemblages 9 to 18. See text for explanation. Appendix-Table 8. Substage (Upper Maastrichtian–Lower Paleocene) list (in order of abundance) of calcareous benthic foraminiferal patterns for Extinction, Immigrant and Survivor taxa noted for long-term. See text for explanation. Appendix-Table 9. Substage (Upper Maastrichtian–Lower Paleocene) list (in order of abundance) of benthic foraminiferal species patterns for Extinction, Immigrant and Survivor taxa noted for long–term. See text for explanation. (DOCX 77 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farouk, S., Jain, S., Al-Kahtany, K. et al. Relationship of Maastrichtian–Thanetian benthic foraminiferal species diversity, palaeooxygenation, and palaeoproductivity in shallow waters of the Western Desert, Egypt. Geo-Mar Lett 43, 4 (2023). https://doi.org/10.1007/s00367-023-00745-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00367-023-00745-2