Abstract
Foraminiferal assemblages found at Great Meteor Seamount were studied in August 1998. Communities of living foraminifera in surface sediments from the plateau (290–325 m water depth) and from the surrounding base (2,300–4,096 m) were compared in abundances, diversity, and species composition. In this oligotrophic region, densities were very low, but diversity was high. Highest numbers were observed at the deep stations north, south, and east of the seamount and at the shallow station in the north of the plateau. Lowest densities were recorded southwest of the plateau centre and at the lee side of the seamount. We explain this distribution pattern with variable amounts of fresh organic material, caused by local oceanic currents. Generally, plateau stations indicated coarser sediments, lower organic carbon content, and higher temperatures. The foraminiferal fauna showed bathyal to abyssal characteristics and similarities to assemblages previously described from other parts of the North Atlantic and other oceans. Several dominant species extended between the two habitats, on the plateau and in the surrounding deep sediments, but other species were found exclusively at deep stations, at plateau stations, or at the abyssal reference station.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the most abundant and diverse groups in marine sediments consists of benthic foraminifera (Protista: Granuloreticulosa) (Thiel 1975; Gooday 1986; Alongi and Pichon 1988; Altenbach and Sarnthein 1989). These single-celled organisms can exist in nearly all marine environments, including deep-sea areas and extreme ecosystems (e.g. Gooday et al. 1992; Linke and Lutze 1993; Kitazato 1994; Sen Gupta and Aharon 1994). Benthic foraminifera are useful indicators of ecological variability, because their species composition, abundance, and distributional pattern mainly depend on environmental conditions. Taxa can be used as indicators of distinct sediment facies or particular hydrographic conditions (Lohmann 1978; Lutze 1980; Lutze and Coulborn 1984). Additionally, organic carbon flux is one of the main environmental factors controlling benthic foraminiferal distribution patterns (Altenbach 1988; Loubere 1996, 1997; Schmiedl et al. 1997). In oligotrophic areas some opportunistic species react very quickly to seasonal phytodetritus pulses arriving on the sediment surface (Gooday 1988, 1993; Smart and Gooday 1997). Another important parameter is the oxygen supply (Jorissen et al. 1995; Loubere 1994, 1997; Ohga and Kitazato 1997). Most of these factors are related to water depth and depend on bathymetric conditions.
Seamounts generate a habitat that has hardly been explored in research on living benthic foraminifera in deep-sea areas. Yet these unique ecosystems promise interesting findings that may explain interactions between environment and community structure. They show a high variability of environmental properties, like water depth, temperature, current systems, trophic conditions, and sediments, at a rather small scale. This might lead to the development of ecological niches and biological zoning. Seamounts can act as obstacles to current flow. They interact with ocean currents and create flow complexities that are determined by current speed, stratification, latitude, and seamount morphology (Boehlert and Genin 1987). The physical effects include local small- and mesoscale phenomena like the formation of eddies and circular currents (“Taylor column”), turbulent mixing, benthic boundary effects, and regional up- or downwelling. Some of these factors may increase local primary and secondary production, or trap particles, nutrients, or organisms. Seamounts can be biologically highly productive and highly diverse in species composition (Simpson and Heydorn 1965, Hughes 1981, Rogers 1994). They are separated from the abyssal plain and may act as underwater islands (Boehlert and Genin 1987) or “stepping-stones” for trans-oceanic dispersal (Wilson and Kaufmann 1987).
Only a few studies exist that deal with recent benthic foraminiferal assemblages at seamounts. Nienstedt and Arnold (1988) report about a direct influence of depth-related hydrographic factors on the foraminiferal community. Gradual changes were observed, except for distinct faunal shifts at the oxygen-minimum zone and at the carbonate compensation depth (CCD). In contrast, Ohkushi and Natori (2001) found very different foraminiferal populations at two seamounts in the same hydrographic structures, probably caused by varying trophic conditions. Benthic foraminifera are important tools for investigations on stratigraphy, paleoproductivity, paleoecology, and paleoceanography. Despite little knowledge about foraminiferal assemblages in seamount ecosystems, in paleoecological studies and reconstructions the occurrence of warm water and shallow water biota have been interpreted as evidence for seamounts (Buening et al. 1998). To avoid wrong interpretations, mainly caused by unknown microhabitat preferences or vital effects, it is essential to understand the biology and ecology of extant foraminiferal species. Investigations dealing with recent foraminiferal assemblages in such habitats are urgently needed.
In the present study, we document the record of foraminiferal assemblages found at the Great Meteor Seamount (eastern North Atlantic). Sediment sampling on several transects in August 1998 enabled us to compare communities of living benthic foraminifera from the plateau of Great Meteor Seamount (290–325 m water depth) and from the surrounding deep sea at the base of the slope (2,300–3,100 m). These communities were compared with one reference station at the abyssal plain (4,096 m water depth) north of the seamount. The analysis of the organic carbon content (Corg) in the sediment provided information about the trophic conditions at these sites. We had three central questions: (1) what kinds of populations can be found at the seamount? (2) Are there differences in the diversity and species composition at the various sites? (3) If so, which factors might regulate these differences?
Material and methods
Study area and sampling
Sediment samples containing living benthic foraminifera were collected at Great Meteor Seamount during R.V. “Meteor” cruise M 42/3 in August 1998. This seamount is of volcanic origin and reaches from the abyssal plain to 270 m below the water surface, with a rather flat plateau top of approximately 50 km in diameter (Hinz 1969; Thiel 1970; Ulrich 1971; Ehrich 1977). It was under investigation for several studies, and topography, morphology, and hydrography are well known (e.g. Hinz 1969; Horn et al. 1971; Ulrich 1971; Dietrich et al. 1994; Grevemeyer 1994; Mourino et al. 2001).
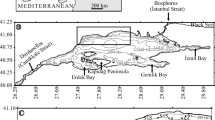
A multicorer (after Barnett et al. 1984) was used for sampling deep-sea stations at the base of the slope (2,300–3,100 m) and at the abyssal plain, where sediments were of silt and clay. Because of coarse and hard substrate at the plateau, it was not possible to get sediments with a multicorer here. Instead, a giant box corer was used (after Hessler and Jumars 1974). Directly after the arrival of the box corer on board, overlaying bottom water was gently removed. The water that covered the sediment in the box corer was always slightly cloudy, but sediment surface did not appear notably disturbed. Sediment in the box corer was subsampled by pushing Plexiglas cores into it. Sediment that was not enclosed in these tubes was removed by hand. Sampling sites are shown in Fig. 1 and a list of these together with investigated parameters is given in Table 1. The slopes of Great Meteor Seamount were too rocky and too steep to get any samples.
Benthic foraminifera and particle size
Core diameter was between 60 and 95 mm (details are given in Table 1). One core was studied for faunal analysis at each station. All cores were cut at 0.5-cm intervals until 3 cm sediment depth and in 1-cm intervals below 3 cm depth until 10 cm (if possible, because some of the box corer subsamples were shorter). Here we present results of the surface sediment layers (0–1 cm), except for one comparison of foraminiferal densities at two stations carried out to 3 cm sediment depth (Fig. 7). Deeper sediment layers are under investigation. Sampled sediment volume was between 14 and 35 cm3. For faunal analysis, each layer was preserved separately with ethanol and stained with rose bengal (1 g rose bengal per 1 l ethanol) for at least 14 days to distinguish living (containing red-stained protoplasm) from dead foraminifera (after Walton 1952). The sediment was washed and the residue was dried to make the following handling more practicable and to allow the storage of the sediments for a longer period of time. Dried sediment was fractionated in three different size classes: 30–63 μm, >63–125 μm, and >125 μm. All fractions were weighed for particle size analysis. Sediment sample splits (1/2–1/1 splits) of the two larger fractions were investigated for living foraminifera and all red-coloured individuals were picked. To minimise wrongly identified living foraminifera, specimens were individually wetted with water and only foraminifera containing well-stained dark-red protoplasm were counted. Numbers of living foraminifera in each slice were referenced to 10 cm3. The smallest fraction (30–63 μm) is under investigation.
Organic carbon
Surface sediments (0–1 cm) were taken and immediately frozen until analysis. Sediment was dried at 50°C and ground. Part of the sediment was decalcified with 1 N HCl. Percentage of total carbonate and total carbon of untreated and decalcified sediments was determined with a carbon analyser (Elementar Vario EL, Elementar Analysesysteme, Germany) and gasometric analysis. Finally, percentage of organic carbon was calculated. Accuracy was between 0.02 and 0.04 wt%.
Oxygen content measurements
At two deep stations, oxygen profiles in sediment cores were measured directly after arriving on board. Analysis was done with an oxygen microelectrode and measurement system (MasCom, Bremen, Germany). Linear calibration of the electrode signal was obtained with an oxymetre OXI 340/A (WTW, Germany), with air-saturated seawater and O2-free seawater, degassed with N2. Calibration solutions had the same temperature as measured core sediments.
Statistical analysis
For all foraminiferal community analysis procedures, the abundance data were calculated as individuals per 10 cm3. Diversity H(S) was determined according to the Shannon Wiener index (Shannon and Weaver 1963). S is the number of observed species. Equitability was calculated as e H(S)/S (Buzas and Gibson 1969). When species are totally equally distributed, the ratio reaches its maximum value of 1. Large tubular agglutinated foraminifera, such as Rhizammina algaeformis or related species, easily break into fragments during handling and are difficult to count. Three size-standardised fragments (with a fragment size of 1,000–4,000 μm) of tubular agglutinated forms were used to define a single specimen to gain a semiquantitative number (Kurbjeweit et al. 2000). Q-mode principal component analysis was carried out with SYSTAT 5.2.1. Only species constituting more than 0.5% in one of the samples were used. Factor loadings of 0.5 for the Q-mode principal component analysis were considered as significant (Backhaus et al. 1989).
Results
Particle size
Size distribution of sediment particles of the dried and sieved fractions 30–63 μm, >63–125 μm, and >125 μm are given in Fig. 2. In all cores, particles >125 μm made up the largest part of the dried sediment (after the fine fractions <30 μm were washed out and discarded). At the shallow water stations, very high percentages of the coarse fraction were recorded—between 80 and >90%—and smaller grain sizes made up only 2–12%. All deep stations contained fewer particles >125 μm (percentages of 48–70% were found), but higher masses of smaller grains.
Organic carbon
Generally, this area was very oligotrophic. Surface sediments (0–1 cm) were characterised by considerable high carbonate concentrations and low Corg content. Very small amounts of Corg were measured directly at the plateau (Fig. 3). Values were observed between 0.012 and 0.038 wt%. At the deep stations around Great Meteor Seamount, more elevated values were recorded in the surface sediments (0.067–0.103 wt%). In the northern abyssal plain (station 558, see Fig. 1), comparable amounts of Corg were measured, around 0.060 wt%. All available data points were taken to simulate area-wide gradients of Corg in this region (Fig. 3).
Pore water oxygen
Direct sediment oxygen concentrations were only available at two deep stations, 506 and 459 (Fig. 4). Profiles measured in 1-mm steps showed highest oxygen content at the surface (about 6.7–6.8 ml l−1) and decreasing values with increasing sediment depth. At station 506, oxygen concentration seemed to diminish faster than at station 459. Nevertheless, both cores contained high amounts of oxygen in the pore water and showed a good aeration of the sediment in the upper first 3 cm.
Foraminiferal abundance
Densities of living benthic foraminifera in surface sediments (0–1 cm) are shown in Figs. 5 and 6. Total numbers and counts separated into the fractions >63–125 μm and >125 μm are given in Fig. 5. All available data points were taken to simulate area-wide gradients of foraminiferal densities in this region (Fig. 6). Generally, abundances were very low, but stations showed variability. On average, we found more living individuals at the deeper stations in the first centimetre. Highest densities were observed at the reference station 556 at the abyssal plain north of the seamount (47 individuals in 10 cm3) and at the deep stations south and east of the seamount plateau, at station 566 (28 individuals in 10 cm3) and station 548 (37 individuals in 10 cm3). At the other two deep stations, distinctly lower numbers of living foraminifera were counted (10–12 individuals/10 cm3), even lower than in most of the shallow-water sediments. At the plateau, generally elevated quantities were observed in the north (22 individuals/10 cm3 at station 467) and the lowest densities with only 4 individuals/10 cm3 southwest of the plateau centre at station 456. Dominant size extent in most of the surface sediments was the fraction >63–125 μm.
At two stations, deeper sediment layers were also investigated for living foraminifera. A comparison of the vertical distribution down to 3-cm sediment depth at one plateau station (station 467) and one deep station (station 548) is given in Fig. 7. Living foraminifera were still found in deeper sediment layers at station 548; in particular, bigger foraminifera increased between 0.5 and 2.0 cm sediment depth.
Species composition
A comparison of the number of species, diversity, and equitability at single stations is given in Fig. 8. At the bank, most stations showed an elevated species diversity, between 2.5 and 3.2. Only station 456 southwest of the plateau centre had a strikingly lower diversity. Equitability is mainly high, between 0.5 and 0.9. The reference station 558 presented lower diversity and equitability compared to seamount sediments.
In total, 93 taxa were identified in the surface sediments of the nine stations (raw data sets can be found in the Appendix), but only 43 species were found at more than one station. Table 2 compares these species. Taxa in Table 2 can be classified into four groups: (I) species exclusively recorded on the plateau; (II) species observed in both habitats, shallow and deep stations; (III) species found only at the deep stations (including the abyssal reference station 558); (IV) species solely counted at the deep stations of the seamount base. Species of group I were dominated by taxa that form calcareous tests. In groups III and IV, the number of agglutinated species increased.
A principal component analysis (not shown) demonstrated that every station was characterised by its own faunal community, with low numbers of individuals of many species. Dominant species of single stations were often observed at other stations, too. At the reference station 558, Eponides tumidulus (calcareous species, C) dominated the community. Major taxa at station 459 were Trochammina conglobata (agglutinated species, A) and Rhizammina algaeformis (A), and at station 566 Hoeglundina elegans (C) and Glomospira gordialis (A). Cassidulina grassa (C) mainly influenced station 548. Epistominella exigua (C) and Ammobacculites filiformis (A) controlled station 506. Except for H. elegans and C. grassa, all dominant species at the deep stations were only found in deep station sediments. On the plateau, all main characteristic taxa were also found at deep stations. Station 516 was dominated by T. inflata (A), station 456 again by H. elegans (C), and station 489 and 467 by Eggerelloides sp. (A).
Discussion and conclusions
Habitat characterisation
Great Meteor Seamount represents an isolated barrier in the abyssal plain that surely influences local ocean currents. The seamount lies within the southwestwards-flowing cold Canary current system, and a weak northeasterly directed bottom current includes small amounts of Antarctic Bottom Water (AABW; von Stackelberg et al. 1979). Sediment particle size distribution at the shallow plateau stations and in surrounding deep stations displays a more coarse sediment at the plateau (Fig. 2). One reason for this may be faster bottom water currents above the plateau that remove and wash out clayey and silty small material from these sediments. In March 1992, Mourino et al. (2001) observed that water and its components was flushed off the bank rather than removed by local eddy diffusion from an anticyclonic vortex. The more elevated organic carbon content in sediments of the deep sites, compared to plateau sediments, supports this thesis. Higher levels of organic material in the abyssal plain may be induced by an accumulation of material during lateral and vertical transport and resuspension processes. Calculated organic carbon data are very low, and interpretations have to be made carefully because of the limitation of the precision of measurements. Still, considerably higher values can be found at all deeper stations, compared to the plateau.
Generally, high oxygen content and very low organic carbon content in the surface sediments indicate an oligotrophic environment above the seamount and in its surroundings (Figs. 3, 4). This agrees well with other published observations. Great Meteor Seamount is located in the biogeochemical province of the subtropical eastern Gyre (Sathyendranath et al. 1995). This province has a low estimated primary production, between 88 and 140 g C m−2 year−1 (Yentsch 1990; Platt et al. 1995; Sathyendranath et al. 1995). Nutrients measured during cruise M 42-3 (August 1998) showed low values of nitrate, ammonia, phosphate, and silica in the upper 100–150 m water column and increasing concentrations with increasing water depth (Pfannkuche et al. 2000). Weak seasonal fluctuations and local increases of chlorophyll a can be observed during the year, associated with the seamount (Mourino et al. 2001). Relatively high values of primary production (36 mg C m−2 h−1, integrated over the upper 130 m), and chlorophyll a (46 mg m−2, integrated over the upper 150 m) were found in August 1998, compared to other seasons and years (Mourino et al. 2001). In oligotrophic areas, seasonal changes of food availability may influence foraminiferal densities, species composition, vertical distribution in the sediment, population dynamics, and reproduction cycles of benthic foraminifera. Some opportunistic species (like Epistominella exigua) react very quickly to phytodetritus blooms arriving on the sediment surface; they respond with a high increase in abundance due to reproduction and rapidly colonize freshly deposited phytodetrital aggregates (Gooday 1988, 1993; Gooday and Lambshead 1989; Gooday and Turley 1990; Smart and Gooday 1997). E. exigua can be observed in our samples (Table 2), but only at deep stations. They are common but do not dominate these sediments in high amounts. Probably no strong phytodetritus pulse recently arrived on the sea floor here.
Unfortunately, oxygen profiles of sediments were only available for two deep stations (Fig. 4), and no measurements were possible on the plateau, since the coarse sediment would had damaged glass microelectrodes. We assume that the other deep stations had similarly high pore water oxygen content, because only low amounts of organic material were deposited on the sediment (even lower compared to stations with existing oxygen profiles) and were decomposed by oxygen-consuming degradation processes. Mourino et al. (2001) measured high oxygen concentrations in the water column near the seafloor on the summit, about 4.7 ml l−1. This bottom-water concentration indicates high oxygen content in the plateau sediment surface. Additionally, very low amounts of organic matter and coarse sediments support this.
Salinity of bottom water on the plateau of Great Meteor Seamount was between 36.0 and 36.2 (Pfannkuche et al. 2000; Mourino et al. 2001). At the deep stations around the seamount, in water depths below 2,000 m, values between 34.9 and 35.1 were measured in conductivity-temperature-depth (CTD) profiles (Pfannkuche et al. 2000). Water temperature near the sediment floor at the shallow plateau stations was about 14–16°C, and 2–4°C in water depths deeper than 2,000 m (Pfannkuche et al. 2000; Mourino et al. 2001).
Total foraminiferal densities
In general, living benthic foraminifera were found in low amounts on and in the surroundings of Great Meteor Seamount, which is typical of oligotrophic regions. Comparison between foraminiferal abundances of plateau and deep station sediments showed reduced densities southwest of the plateau centre, in the lee side of the seamount, and considerable increased numbers north, east, and south of the seamount (Figs. 5, 6). All other stations showed more gradual differences.
Benthic foraminiferal densities reported from depth transect studies at basins or at the continental slope also included sediments from different water depths. In contrast to our results, they showed significantly higher foraminiferal abundances in the upper stations but comparable low numbers at deep stations. However, a direct comparison is sometimes difficult because of diverse investigated size fractions and sediment depths and different environmental conditions influencing the benthos in these regions. Rathburn and Corliss (1994) found comparable low foraminiferal numbers (size >63 μm) at depth transects in the Zulu Sea in water depths deeper than 3,000 m. In other depth transect studies, similar low densities were detected at shallower depths, depending, for example, on food or oxygen availability (e.g. De Stigter et al. 1998; Jannink et al. 1998; Fontanier et al. 2002).
Foraminiferal communities of only one sediment core were studied at each station of Great Meteor Seamount. Analysing one multicorer core for each station is usual and acceptable for field investigations on living benthic foraminifera, which are very time consuming, especially when they include important small size fractions such as the fraction >63–125 μm included in this study. A compromise has to be found between what is practicable, realisable, and utilisable. Small-scale patchiness cannot be disregarded and some of the differences between sites could be due to small-scale variability. Small-scale patch structures were noted for benthic foraminifera in the abyssal realm (Bernstein et al. 1978; Bernstein and Meador 1979), probably due to habitat selection, reproductive patterns, and small-scale patchiness of food supply and sampling procedure. Additionally, different gear was used for sampling deep stations and plateau stations. Box corers can never sample sediments totally undisturbed, as can multicorer sampling. Epifaunal to shallow infaunal taxa, like Adercotryma glomerata, Hoeglundina elegans, and Spiroplectammina species, can be found in the first sediment layer in both multicorer and box corer samples. Additionally, infaunal species were present, such as Trochammina inflata. Therefore, it is not possible to assess with certainty if surface sediments were lost in the box corers. Nevertheless, we believe that strong differences in foraminiferal densities, as observed for stations 558, 548, and 456, indicate enhanced or decreased populations in these regions.
Bottom waters and measured pore waters in the sediments show high available oxygen concentrations for benthic organism, that surely do not limit foraminiferal densities at Great Meteor Seamount. Other environmental factors, such as temperature, water depth, salinity, and sediment composition, were constant between single stations inside the two habitats, “plateau” and “surrounding deep stations”, and were probably not the cause of the differences in foraminiferal densities inside these habitats. Generally, benthic foraminifera are strongly influenced by surface ocean productivity and organic carbon flux to the sea bed (e.g. Gooday 1988; Altenbach and Sarnthein 1989). We assume that organic carbon fluxes and distribution of fresh arriving phytodetritus at the sea floor, controlled by water currents, is one important factor controlling foraminiferal populations inside and even between the two habitats at this seamount. In our study, increased and decreased foraminiferal densities did not always directly match with elevated or alleviated organic carbon content in the sediment surface, but trends can be recognised. Lowest numbers of individuals correlated with lowest Corg values at station 456 southwest of the plateau centre. Station 516 and 489 in the east and west of the plateau showed very similar foraminiferal abundances, in agreement with comparable Corg concentrations. But in the north, at station 467, very low amounts of organic material were measured, whereas the highest densities of foraminifera at the plateau were recorded here. At the same time, the next northern station 452 indicated again increased Corg data. At the deep stations, more elevated Corg content was found in surface layers, compared to the plateau sediments. Highest concentrations were observed northeast and southwest of the seamount. But these stations showed low foraminiferal densities. We should keep in mind that analysed Corg data can only roughly mirror nutrient conditions for benthic organisms, because these data contain the sum of all organic material arrived, produced, and decomposed at the seafloor and do not reveal anything about the decomposition state or phytodetritus arriving at the floor, which is the intrinsic food source. We can speculate that arriving food material at some stations was enough to trigger an increase of foraminiferal densities but not intense enough to be preserved in high amounts in surface sediments. Asymmetric sedimentation around Great Meteor Seamount was found by von Stackelberg et al. (1979). They explained higher sedimentation rates east of the bank with decreased bottom-water flow velocities in this area. Increased sedimentation of nutrient particles east of the bank would justify higher foraminiferal densities here. It is well known that in deep-sea areas, some species can react very quickly to phytodetritus arriving on the sediment surface (Gooday 1988, 1993; Smart and Gooday 1997). The biomass of benthic foraminifera has been reported to correlate well with the estimated organic carbon flux (Altenbach and Sarnthein 1989; Altenbach et al. 1999). In laboratory experiments, the addition of organic material resulted in a rise of biomass, a higher number of food vacuoles (Altenbach 1992; Linke et al. 1995), and increased foraminiferal densities (Heinz et al. 2001, 2002).
Vertical distribution
The comparison of vertical distribution patterns of living foraminifera at the two stations 467 and 548 (Fig. 7) shows higher densities in deep sediment layers at the deep station 548, where we found high oxygen content and more elevated Corg values in the sediment, compared to the plateau station. This observation agrees with the predictions of the trophic-oxygen-microhabitat-reaction model of foraminiferal microhabitats (TROX model, after Jorissen et al. 1995; Fontanier et al. 2002). According to this model, the vertical occurrence of benthic foraminifera in oligotrophic areas is limited by low food supply. The oxygen concentration is high in deeper sediment layers and will not limit foraminiferal distribution, but the lack of organic matter causes the foraminifera to stay near the sediment surface. At station 467, live foraminifera are restricted to the surface because of nutrient limitation. At station 548, increased food supply can improve foraminiferal numbers in deeper sediment layers. Probably, living foraminifera can even be found deeper than 3 cm in the sediment here. Deeper sediment layers from this and other stations are currently under investigation. Other environmental factors, such as sediment physical characteristics, shear stress, porosity, or different pore water chemistry may additionally influence and limit vertical distribution.
Species composition
At all stations, comparable high diversity was observed with relatively balanced species distribution in each core, except for station 456, where only a few individuals were found, and the reference station 588, where Eponides tumidulus very strongly dominates the foraminiferal community (Fig. 8). This high diversity is typical of oligotrophic deep-sea regions. We did not determine endemic species at Great Meteor Seamount. The foraminiferal fauna showed bathyal characteristics and was very similar to assemblages previously described from other parts of the North Atlantic (e.g. Lutze 1980; Murray 1991; Timm 1992; Gooday 1993), and other oceans (e.g. Kurbjeweit et al. 2000; Timm 1992). Every station was characterised by its own faunal community, with low numbers of individuals of many species, and only 43 of the 93 identified taxa were found at more than one station (Table 2). No species appeared at all sampled sediments. The most common species were found in both habitats, on the plateau and in the surrounding deep sediments of the Great Meteor Seamount (group II), but we found a large number of taxa that appeared only at the deep stations and established a typical deep-sea assemblage (groups III and IV), and a few species that formed a characteristic plateau faunal community that was found exclusively on the plateau stations and separated these stations from the deep sea (group I). Environmental factors such as temperature, water depth, salinity, and sediment composition were constant between single stations inside the two habitats but showed variations between the habitats, which may explain the restriction of some taxa to one habitat. Substrate types can influence foraminiferal assemblages at the shelf and the spread of single species can be correlated with specific sediment grain size (Haake 1977; Lutze 1980; Lutze and Coulbourn 1984; Hempel 1985; Schiebel 1992; Levy et al. 1993). This can be observed, for example, for attached epifaunal surface-dwelling species and coarsely agglutinated foraminifera (Schmiedl 1995; Schmiedl et al. 1997). In our investigations, species forming a characteristic plateau faunal community do not belong to these taxa. Some of the plateau taxa have been described as living in shelf to upper bathyal water depths, for example, Trifarina species (Murray 1991), found at station 489, or Bolivina pygmaea (Jones 1994; see Table 2) and probably are restricted to more shallow water depths. Spiroplectinella sagittula was found in the shelf edge association at the southern Portuguese continental margin (Schönfeld 1997). Foraminifera, particularly small individuals such as juveniles and small taxa, may easily be dispersed by water currents (Alve 1999). It is conceivable that shelf and slope foraminifera can be transported in resuspended sediment materials driven by water currents from the coast of western Europe or western Africa to the Great Meteor Seamount. There have been observations of foraminifera living on submarine plants that were uprooted during storms or other impacts and were transported offshore to float in the open sea. As an example, T. carinata living along the Argentina coast on the seaweed Macrocystis pyrifera was transported to the north by the Malvin Current (Boltovskoy and Lena 1969).
All taxa of group II, living in both habitats, seamount plateau and surrounding deep sea, must show high flexibility and no specialisation. They are very tolerant and proliferate in regions that are very different in hydrographic factors such as temperature, water depth, pressure, grain size distribution, and age and degradation state of the arriving organic material. All these genera have been described from other marine regions showing a broad bathymetric range with distribution patterns between shelf and bathyal to abyssal water depths (e.g. Murray 1991; De Stigter et al. 1998). Taxa observed exclusively at the deep stations of Great Meteor Seamount probably prefer environmental conditions related to deeper water conditions. They can be divided into groups III (taxa of all deep stations, including the abyssal reference station 558) and IV (species found solely at the base of the seamount). Group IV cannot be interpreted as a particular seamount assemblage that generally is able to characterise seamount sediments. Most of the taxa of groups II–IV were reported as well in other deep-sea field studies carried out in different oceans, for example the Arabian Sea (Kurbjeweit et al. 2000; Heinz and Hemleben 2003), and show global distribution. Much accordance in species spectra (but not in dominance) can be observed between Great Meteor Seamount taxa of groups II–IV and seamount studies near the East Pacific Rise (Nienstedt and Arnold 1988) or central North Pacific (Ohkushi and Natori 2001), in which sediments were analysed below 788 m water depth. Several corresponding taxa (Adercotryma glomerata, Epistominella exigua, Globocassidulina subglobosa, Lagenammina (Reophax) difflugiformis, Pullenia bulloides, Pyrgo murrhyna, Reophax scorpiurus) can be found in all of these studies. Yet, again, these species are not restricted to seamount areas but can live in many marine bathyal to abyssal sediments.
Conclusions
Living benthic foraminiferal assemblages collected on several transects at the Great Meteor Seamount (eastern North Atlantic) were examined. Abundances, diversity, and species composition in surface sediments were compared between the plateau of Great Meteor Seamount (290–325 m water depth), the surrounding deep sea at the base of the slope (2,300–3,100 m), and one reference station at the abyssal plain (4,096 m) north of the seamount. Environmental factors (sediment particles, organic carbon content, oxygen) were included in our investigations. Low foraminiferal densities, high diversities, and low vertical distribution were recorded, which mirrors oligotrophic conditions. Local variations in abundances (reduced numbers southwest of the plateau centre and on the lee side of the seamount, increased values north, east, and south of the seamount) were probably due to differences in the distribution of fresh organic material, caused by local oceanic currents around Great Meteor Seamount. Generally, plateau stations indicated more coarse sediments and lower organic carbon content, which suggest faster bottom water currents above the plateau that flushed off the bank water and its components. No endemic species were observed at Great Meteor Seamount. The foraminiferal fauna showed bathyal to abyssal characteristics and similarities to assemblages previously described from other parts of the North Atlantic and other oceans. Taxa can be classified into four groups, establishing a plateau fauna (probably restricted to more shallow water depths), two deep-sea assemblages (probably preferring environmental conditions related to deeper water conditions), and a group that can be observed in both habitats (showing high flexibility and no specialisation). Identified taxa have been reported from other marine environments and are not restricted to seamount areas.
References
Alongi DM, Pichon M (1988) Bathyal meiobenthos of the western Coral Sea: distribution and abundance in relation to microbial standing stocks and environment factors. Deep-Sea Res 35:755–790
Altenbach AV (1988) Deep-sea benthic foraminifera and flux rates of organic carbon. Rev Paléobiol 2:719–720
Altenbach AV (1992) Short term processes and patterns in the foraminiferal response to organic flux rates. Mar Micropaleontol 19:119–129
Altenbach AV, Sarnthein M (1989) Productivity record in benthic foraminifera. In: Berger WH, Smetacek VS, Wefer G (eds) Productivity of the ocean: present and past. Wiley, Chichester, pp 255–269
Altenbach AV, Pflaumann U, Schiebel R, Thies A, Timm S, Trauth M (1999) Scaling percentages and distributional patterns of benthic foraminifera with flux rates of organic carbon. J Foram Res 29:173–185
Alve E (1999) Colonization of new habitats by benthic foraminifera: a review. Earth Sci Rev 46:167–185
Backhaus K, Erichson B, Plinke W, Schuchard-Ficher C, Weiber R (1989) Multivariate Analysenmethoden, 5th edn. Springer, Berlin Heidelberg New York
Barnett PRO, Watson J, Connelly D (1984) A multiple corer for taking virtually undisturbed samples from shelf, bathyal and abyssal sediments. Oceanol Acta 7:399–408
Bernstein BB, Meador JP (1979) Temporal persistence of biological patch structure in an abyssal benthic community. Mar Biol 51:179–183
Bernstein BB, Hessler RR, Smith R, Jumars PA (1978) Spatial dispersion of benthic foraminifera in the central North Pacific. Limnol Oceanogr 23:401–416
Boehlert GW, Genin A (1987) A review of the effects of seamounts on biological processes. In: Keating B, Fryer P, Batiza R, Boehlert G (eds) Seamounts, islands and atolls. Geophys Monogr 43:319–334
Boltovskoy E, Lena H (1969) Les epibiontes de “Macrocystis” flotante como indicadores hidrologicos. Neotropica 15:135–137
Buening N, Carlson SJ, Spero HJ, Lee DE (1998) Evidence for the Early Oligocene formation of a proto-subtropical convergence from oxygen isotope records of New Zealand Paleogene brachiopods. Palaeogeogr Palaeoclimatol Palaeoecol 138:43–68
Buzas, MA, Gibson TG (1969) Species diversity: benthonic foraminifera in western North Atlantic. Science 163:72–75
De Stigter HC, Jorissen FJ, Zwaan van der GJ (1998) Bathymetric distribution and microhabitat partitioning of live (rose bengal stained) benthicforaminifera along a shelf to bathyal transect in the southern Adriatic Sea. J Foram Res 28:40–65
Dietrich G, Kalle K, Kraus W, Siedler G (1994) Allgemeine Meereskunde. Gebr Bornträger, Berlin
Ehrich S (1977) Die Fischfauna der Großen Meteorbank. Meteor Forsch Ergebn D 25:1–23
Fontanier C, Jorissen FJ, Licari L, Alexandre A, Anschutz P, Carbonel P (2002) Live benthic foraminiferal faunas from the Bay of Biscay: faunal density, composition, and microhabitats. Deep-Sea Res I 49:751–785
Gooday AJ (1986) Meiofaunal foraminiferas from the bathyal Porcupine Seabight (northeast Atlantic): size, structure, taxonomic composition, species diversity and vertical distribution in the sediment. Deep-Sea Res 33:1345–1373
Gooday AJ (1988) A response by benthic foraminifera to the deposition of phytodetritus in the deep-sea. Nature 332:70–73
Gooday AJ (1993) Deep-sea benthic foraminiferal species which exploit phytodetritus: characteristic features and controls on distribution. Mar Micropaleontol 22:187–205
Gooday AJ, Lambshead PJD (1989) Influence of seasonally deposited phytodetritus on benthic foraminiferal populations in the bathyal northeast Atlantic: the species response. Mar Ecol Prog Ser 58:53–67
Gooday AJ, Turley C (1990) Response by benthic organism to inputs of organic material to the ocean floor: a review. Philos Trans R Soc Lond A 331:119–138
Gooday AJ, Levin LA, Linke P, Heeger T (1992) The role of benthic foraminifera in deep-sea food webs and carbon cycling. In: Rowe GT, Pariente V (eds) Deep-sea food chains and the global carbon cycle. NATO ASI series C 360. Kluwer, Dordrecht, Netherlands, pp 63–91
Grevemeyer I (1994) Der Atlantis-Meteor-Seamount Komplex. Diss Zent Meeres- Klimaforsch C 5:1–126
Haake FW (1977) Living benthic foraminifera in the Adriatic Sea: influence of water depth and sediment. J Foram Res 7:62–75
Heinz P, Hemleben C (2003) Regional and seasonal variations of recent benthic deep-sea foraminifera in the Arabian Sea. Deep-Sea Res I 50:435–447
Heinz P, Kitazato H, Schmiedl G, Hemleben C (2001) Response of deep-sea benthic foraminifera from the Mediterranean Sea to simulated phytoplankton pulses under laboratory conditions. J Foram Res 31:210–227
Heinz P, Hemleben C, Kitazato H (2002) Time-response of cultured deep-sea benthic foraminifera to different algal diets. Deep-Sea Res I 49:517–737
Hempel S (1985) Zur Verbreitung rezenter Foraminiferen auf dem südlichen südafrikanischen Schelf. Diploma thesis, University of Kiel
Hessler RR, Jumars PA (1974) Abyssal community analysis from replicate box cores in the central North Pacific. Deep-Sea Res 21:185–209
Hinz K (1969) The “Great Meteor Seamount”. Results of seismic reflection measurements with a pneumatic sound source, and their geological interpretation. Meteor Forsch Ergebn C 2:63–77
Horn W, Hussels W, Meincke J (1971) Schichtungs- und Strömungsmessungen im Bereich der Großen Meteorbank. Meteor Forsch Ergebn A 9:31–46
Hughes SE (1981) Initial U.S. exploration of nine Gulf of Alaska seamounts and their associated fish and shellfish resources. Mar Fish Rev 43:26–33
Jannink NT, Zachariasse WJ, Zwaan van der GJ (1998) Living (rose bengal stained) benthic foraminifera from the Pakistan continental margin (northern Arabian Sea). Deep-Sea Res I 45:1483–1513
Jones RW (1994) The challenger foraminifera. Oxford University Press, Oxford
Jorissen FJ, De Stigter HC, Widmark JGV (1995) A conceptual model explaining benthic foraminiferal microhabitats. Mar Micropaleontol 26:3–15
Kitazato H (1994) Diversity and characteristics of benthic foraminiferal microhabitats in four marine environments around Japan. Mar Micropaleontol 24:29–41
Kurbjeweit F, Hemleben C, Schmiedl G, Schiebel R, Pfannkuche O, Wallmann K, Schäfer P (2000) Distribution, biomass and diversity of benthic foraminifera in relation to sediment geochemistry in the Arabian Sea. Deep-Sea Res II 47:2913–2955
Levy A, Mathieu R, Poignant A, Rosset-Moulinier M, Ubaldo ML, Ambroise D (1993) Recent foraminifera from the continental margin of Portugal. Micropaleontology 39:75–87
Linke P, Lutze GF (1993) Microhabitat preferences of benthic foraminifera—a static concept or a dynamic adaption to optimize food acquisition? Mar Micropaleontol 20:215–234
Linke P, Altenbach AV, Graf G, Heeger T (1995) Response of deep-sea benthic foraminifera to a simulated sedimentation event. J Foram Res 25:75–82
Lohmann GP (1978) Abyssal benthonic foraminifera as hydrologic indicators in the western South Atlantic Ocean. J Foram Res 8:6–34
Loubere P (1994) Quantitative estimation of surface ocean productivity and bottom water oxygen concentration using benthic foraminifera. Paleoceanography 99:723–737
Loubere P (1996) The surface ocean productivity and bottom water oxygen signals in deep water benthic foraminiferal assemblages. Mar Micropaleontol 28:247–261
Loubere P (1997) Benthic foraminiferal assemblage formation, organic carbon flux and oxygen concentrations on the outer continental shelf and slope. J Foram Res 27:93–100
Lutze GF (1980) Depth distribution of benthic foraminifera on the continental margin off NW Africa. Meteor Forsch Ergebn C 32:31–80
Lutze GF, Coulborn WT (1984) Recent benthic foraminifera from the continental margin of northwest Africa: community structures and distribution. Mar Micropaleontol 8:361–401
Mourino B, Fernandez E, Serret P, Harbour D, Sinha B, Pingree R (2001) Variability and seasonality of physical and biological fields at the Great Meteor Tablemount (subtropical NE Atlantic). Oceanol Acta 24:167–185
Murray JW (1991) Ecology and palaeontology of benthic foraminifera. Longman Scientific, New York, pp 1–377
Nienstedt JC, Arnold AJ (1988) The distribution of benthic foraminifera on seamounts near the East Pacific Rise. J Foram Res 18:237–249
Ohga T, Kitazato H (1997) Seasonal changes in bathyal foraminiferal populations in response to the flux of organic matter (Sagami Bay, Japan). Ter Nov 9:33–37
Ohkushi K, Natori H (2001) Living benthic foraminifera of the Hess Rise and Suiko Seamount, central North Pacific. Deep-Sea Res I 48:1309–1324
Pfannkuche O, Müller TJ, Nellen W, Wefer G (2000) Meteorberichte 00-1 Ostatlantik 1998. Cruise No. 42, 16 June–26 October 1998. Leitstelle METEOR. Institut für Meereskunde der Universität Hamburg, Hamburg, pp 1–254
Platt T, Sathyendranath RS, Longhurst A (1995) Remote sensing of ocean colour: a vehicle for estimation of marine primary production at a regional scale. Proc Philos Trans R Soc Lond 348:191–202
Rathburn AE, Corliss BH (1994) The ecology of living (stained) deep-sea benthic foraminifera from the Sulu Sea. Paleoceanography 9:87–150
Rogers AD (1994) The biology of seamounts. Adv Mar Biol 30:305–350
Sathyendranath RS, Longhurst A, Caverhill C, Platt T (1995) Regionally and seasonally differentiated primary production in the North Atlantic. Deep-Sea Res I 42:1773–1802
Schiebel R (1992) Rezente benthische Foraminiferen in Sedimenten des Schelfes und oberen Kontinentalhanges im Golf von Guinea (Westafrika). Report 51. Institute of Geology and Palaeontology, University of Kiel, Germany
Schmiedl G (1995) Rekonstruktion der spätquartären Tiefenwasserzirkulation und Produktivität im östlichen Südatlantik anhand von benthischen Foraminifeenvergesellschaftungen. Ber Polarforsch 160: pp 1–207
Schmiedl G, Mackensen A, Müller PJ (1997) Recent benthic foraminifera from the eastern South Atlantic Ocean: dependence on food supply and water masses. Mar Micropaleontol 32:249–287
Schönfeld J (1997) The impact of the Mediterranean outflow water (MOW) on benthic foraminiferal assemblages and surface sediments at the southern Portuguese continental margin. Mar Micropaleontol 29:211–236
Sen Gupta BK, Aharon P (1994) Benthic foraminifera of bathyal hydrocarbon vents of the Gulf of Mexico: initial report on communities and stable isotopes. Geo-Mar Lett 14:88–96
Shannon CE, Weaver W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Simpson ESW, Heydorn AEF (1965) Vema Seamount. Nature 207:249–251
Smart CW, Gooday A (1997) Recent benthic foraminifera in the abyssal northeast Atlantic ocean: relation to phytodetrital inputs. J Foram Res 27:85–92
Stackelberg U von, Rad U von, Zobel B (1979) Asymmetric sedimentation around Great Meteor Seamount (North Atlantic). Mar Geol 33:117–132
Thiel H (1970) Bericht über die Benthosuntersuchungen während der “Atlantischen Kuppenfahrt 1967” von F.S. “Meteor”. Meteor Forsch Ergebn D 7:23–42
Thiel H (1975) The size structure of the deep-sea benthos. Int Rev Ges Hydrobiol 60:575–606
Timm S (1992) Rezente Tiefsee-Benthosforaminiferen aus Oberflächensedimenten des Golfes von Guinea (Westafrika)—Taxonomie, Verbreitung, Ökologie und Korngrößenfraktionen. Report 59. Geol-Paläont Inst Univ Kiel
Ulrich J (1971) Zur Topographie und Morphologie der Großen Meteorbank. Meteor Forsch Ergebn C 6:48–68
Walton WR (1952) Techniques for recognition of living foraminifera. Contr Cushman Found Foram Res 3:56–60
Wilson RR, Kaufmann RS (1987) Seamount biota and biography. In: Keating B, Fryer P, Batiza R, Boehlert G (eds) Seamounts, islands and atolls. Geophys Monogr 43:355–377
Yentsch CS (1990) Estimates of “new production” in the mid-North Atlantic. J Plankton Res 12:717–734
Acknowledgements
We thank Wiebke Ruschmeier, Ulrike Honnert, and Tesfey Wubet for helpful support during sample collection and processing and the crew of F.S. “Meteor” for good collaboration during the cruises. This work received financial support from the Bundesministerium für Bildung und Forschung (BMBF), BIGSET program (Biogeochemische Stoff- und Energietransporte in der Tiefsee), No. 03F0177C, and the “Deutsche Forschungsgemeinschaft” (Grant No. HE 3460/1-1), which are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Appendix
Appendix
Raw data sets of taxa identified in the surface sediments of the nine stations are given in Tables 3, 4, 5, 6, 7, 8, 9, 10, and 11.
Rights and permissions
About this article
Cite this article
Heinz, P., Ruepp, D. & Hemleben, C. Benthic foraminifera assemblages at Great Meteor Seamount. Marine Biology 144, 985–998 (2004). https://doi.org/10.1007/s00227-003-1257-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1257-7