Abstract
Locomotor performance and skeletal muscle contraction are critical for animals and are susceptible to changes in the external thermal environment, especially for ectotherms. Phrynocephalus erythrurus, which is endemic to the Qinghai–Tibetan plateau, is known for living at the highest elevation among all reptiles in the world (4500–5300 m). In this study, which compares P. erythrurus with the lowland Phrynocephalus przewalskii, we evaluated the locomotor performance at different body temperatures, the effects of temperature and oxygen partial pressure (PO2) on the contractile properties of iliofibularis (IF) muscle in vitro, ATPase activity of IF muscle at different temperatures, and the fiber types of IF muscle. Lowland P. przewalskii runs significantly faster than highland P. erythrurus at all test body temperatures. Almost all contractile properties of the IF muscle of P. przewalskii were better than that of P. erythrurus under all test temperatures and PO2. However, P. erythrurus could achieve both optimal isometric (e.g., dPo/dt) and optimal isotonic (e.g., Vmax) contraction at a lower temperature compared with P. przewalskii. Multi-factor analysis further revealed that temperature has a significant effect on the contractile properties of IF muscle for both species. Although the proportion of fibers types and ATPase activities of IF muscle have no significant interspecies difference, the changing pattern of ATPase activities with temperature is consistent with certain contractile properties and locomotor performance. The interspecies differences in locomotor ability and contractile properties of skeletal muscle in high- and low-altitude lizards may be the results of long-term adaptation to the local environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Locomotion is vital for routine activities such as feeding (Miles et al. 2007), social interaction, predator avoidance (Christian and Tracy 1981; Calsbeek and Cox 2010) and survival (Gilbert and Miles 2017). Ambient temperature can markedly influence the locomotor ability of ectotherms. The high-altitude population of Qinghai toad-headed lizards (Phrynocephalus vlangalii) had lower thermal sensitivity and weaker locomotor ability than the low population (Wu et al. 2018). In addition, the optimal temperatures for initial sprinting by Liolaemus ruibali, Phymaturus extrilidus and Liolaemus parvus from different elevations were significantly different (Alés et al. 2018).
Locomotion of vertebrates relies on the contraction of skeletal muscles, whose performance is intimately associated with temperature which affects the properties of isometric and isotonic contraction of skeletal muscle (Marsh and Bennett 1985; Swoap et al. 1993). A study of the desert iguana (Dipsosaurus dorsalis) showed that the influences of temperature on contractile properties of fast-twitch glycolytic (FG) muscle were greater than on whole organism's sprint performance (Marsh and Bennett 1985). Studies on the effects of temperature on muscle contractile properties of lizards also revealed that isometric maximal twitch tension (Pt) and tetanic tensions (Po) were constant at all test temperatures, whereas time-to-peak twitch tension (TPT) increased and the maximum rate of rise in tetanic tension (dPo/dt) decreased with the descending temperature (Putnam and Bennett 1982; Bennett 1985; Marsh and Bennett 1986). These phenomena may be attributed to the reduced myosin ATPase activity and cytosolic Ca2+ concentration (Godt and Lindley 1982; Swoap et al. 1993; Ranatunga 2018). The relation between temperature and isotonic contraction of muscle was also studied. The maximum power output (P/m) of iliofibularis muscle (IF muscle) in D. dorsalis increased with temperature, from 20 W kg−1 at 15 °C to 154 W kg−1 at 42 °C (Swoap et al. 1993). A similar result was also found for the lizard Trapelus pallida (Herrel et al. 2007).
Hypoxia can alter the locomotive and respiratory muscles function in the rat (El-Khoury et al. 2003; Machiels et al. 2010; El-Khoury et al. 2012). The diaphragm muscle of rats was impaired following exposure to chronic hypoxia due to profound oxidative stress (Lewis et al. 2016). However, diaphragm muscle of mice exposed to chronic hypoxia showed increased tolerance of severe acute hypoxic stress in vitro (Lewis et al. 2015). In contrast, after rats were exposed to chronic hypobaric hypoxia for 6 weeks, the limb muscle showed an increased twitch force (El-Khoury et al. 2012). Hypoxia can not only indirectly affect muscle contraction characteristics of hypoxic acclimatized animals but also directly affect the isolated muscles. The isotonic contractile properties of diaphragm muscle in rats significantly decreased under hypoxia; but the isometric force was hardly affected (Machiels et al. 2010). The Po of hamster diaphragm muscle under hypoxia in vitro was significantly lower than that under normoxia (Esau 1989). To date, in vitro the study on the effects of hypoxia on the contractile properties of isolated skeletal muscle of reptiles has not been found.
Skeletal muscles are composed of three types of muscle fibers classified on the basis of the isoforms of the contractile protein myosin heavy chain (Ishihara et al. 2000; Chaillou 2018). Slow-twitch oxidative (SO or Type I) fibers are slow in force generation and very resistant to fatigue (Bonine et al. 2001; Burke 2011). Fast-twitch glycolytic (FG or Type IIB) fibers are rapid contraction and fatigue rapidly (Burke 2011; Yan et al. 2011), while fast-twitch oxidative glycolytic (FOG or Type IIA) fibers exhibit intermediate properties. The pectoralis major muscles of bar-headed goose contain a larger proportion of oxidative fibers (Type I) compared with low-altitude geese (Scott et al. 2009). Studies on deer mice also showed that the proportion of Type I fibers in gastrocnemius muscle was higher in the high-altitude population than low-altitude counterparts (Lui et al. 2015; Scott et al. 2015). However, a high proportion (> 80%) of Type II was found in the IF muscle of Phrynosomatid lizards (Bonine et al. 2001, 2005).
The red-tailed toad-head lizard Phrynocephalus erythrurus (Lacertilia: Agamidae), which is endemic to the Tibetan plateau, is famous as the highest elevation reptile in the world (mostly 4500–5300 m above sea level) (Jin and Liu 2010; Li et al. 2017). The locomotion and skeletal muscle performance of P. erythrurus under different temperatures and/or hypoxia have not been studied yet. Here, we test the following hypotheses related to locomotor performance in the whole organism and contractile function of IF muscle in vitro for two Phrynocephalus lizards: (1) locomotor performance of P. erythrurus should be similar to the high-elevation Qinghai toad-headed lizards. (2) Compared to P. przewalskii, contraction performance of IF muscle of P. erythrurus should be better under low temperature and hypoxia. (3) The skeletal muscle of P. erythrurus should possess a higher proportion of Type II fibers compared with P. przewalskii. (4) The ATPase activities of IF muscle of P. erythrurus at low temperatures should be higher than that of P. przewalskii. Verifying these four hypotheses will help us to further understand the adaptation of high-altitude reptile to its special environment and the potential underlying mechanisms.
Materials and methods
Animals and samples
Thirty adult males of P. erythrurus and P. przewalskii were captured in August and September 2018, in Tanggula town of Qinghai province (34°13′N, 92°13′E, 4543 m asl) and Jingtai (37°18′N, 104°06′E, 1627 m asl) of Gansu province, respectively. Climatic data (1981 to 2010) for the two sampling sites (Table 1) were provided by the Chinese Climatic Data Centre (http://data.cma.cn). The mean temperature in Tanggula for August and September was lower than in Jingtai. The atmospheric pressure in Tanggula is only 58% of sea level and 70% of Jingtai. All the lizards were brought to the laboratory at Lanzhou University and raised with meal-worms and water. All experiments were finished within 30 days of the collection. The relevant morphological data of the two species are listed in Table 2. This study was carried out under the approval of the Ethics Committee of Animal Experiments at Lanzhou University and based on principles from the China Council on Animal Care.
Locomotor performance
The locomotor performance experiments were performed after one-week acclimation to the laboratory environment. Eight ambient temperatures (21, 24, 27, 30, 33, 36, 39 and 42 °C) were used to measure the locomotor performance of the two Phrynocephalus lizards (18 individuals for each species). The bottom and two walls of the raceway (length × width × height: 2 m × 4.5 cm × 12 cm) were made of wood and plexiglass, respectively. All lizards were trained to adapt to the raceway before each formal experiment, that is, we let each lizard run 2–3 times on the racetrack. For the formal experiment, the lizards were firstly put into a biochemical incubator set at the test temperature and acclimated for 2 h. The room temperature was adjusted to the corresponding test temperature. The body temperature of each lizard was measured through the cloaca using a model 925–1 channel thermometer (Testo, UK). Then, lizards were directly transferred from the incubator to the starting area of the raceway and induced to run by touching the tail with a paintbrush. The experimental lizards were given a 24 h rest between test temperatures, and each lizard ran two times at each test temperature with a minimum of 1 h rest between trials. All the running trials were video recorded using a Canon A610 digital camera (Canon, Japan) fixed vertically above the raceway. The videos were manually analyzed frame-by-frame using Corel Video Studio 2020 software (Corel, www.ulead.com) to determine the time it took for lizards to run over a distance of 25 cm. The fastest running speed over 25 cm is regarded as the sprint speed.
Muscle contractile properties experiments
Contractile properties of IF muscle were measured using the protocol described by Putnam and Bennett (1982). Briefly, the lizard was sacrificed by decapitation and the IF muscles were isolated from both legs (Bergmann and Hare-Drubka 2015). During the operation, a Ringer solution (145 mM NaCl, 4 mM KCl, 20 mM imidazole, 2.5 mM CaCl2 , 11 mM glucose at pH 7.2 ~ 7.4; Marsh and Bennett 1986) was continuously dropped on the muscle to prevent it from drying out.
All the contractile properties were recorded with a BL-420F biological function experimental system (Chengdu Techman, China) and stimulated by two parallel platinum electrodes with 15 mm spacing. The ilium of the IF muscle was attached to a glass rod by a ring and the fibula was connected to the transducer (isometric measurements: JH-2, Beijing Aviation Medical Institute, China; isotonic measurements: DZ100, Beijing Xinhang Xingye Electronics Co., Ltd. China) via a thin surgical suture. The glass rod was then fixed in a bath containing Ringer solution saturated with 95% O2 and 5% CO2. The temperature of the Ringer solution in the bathtub was controlled by a constant temperature water bath (HH-2, GuoHua, Changzhou, China). To ensure supramaximal stimulation, the muscle was stimulated with a voltage that is 1.25 times the voltage required for maximal activation (~ 5–15 V).
To reduce the muscle damage and fatigue caused by high temperature, we carried out experiments as much as possible at 25, 15, 10 °C on one IF muscle and at 35, 40, 45 °C on the other from the same lizard. The IF muscle of lizard was placed in 25 °C Ringer solution to equilibrate for 15 min, after which the length of the muscle was adjusted to achieve the Pt and maintained until the experiment was completed. Isometric twitch contraction, tetanic contraction and isotonic contraction were sequentially tested. The interval between every test was at least one minute to ensure that the muscle has enough time to recover. Then, the temperature was set to 15 °C and 10 °C and equilibrated for 5 min, respectively. The Ringer solution was replaced at each temperature. The other IF muscle was equilibrated for 10 min in 35 °C Ringer solution followed by the measurement of the contractile properties. Then, the contractile properties were measured at 40 °C and 45 °C after 5 min equilibrium in turn. The length of the muscle was measured with a vernier caliper after all tests were completed. Lastly, the muscles were weighed with an electronic balance after the bones at both ends were removed and the extra solution was blotted. The left or right IF muscle was randomly used at different sets of temperatures.
The experimental methods and procedures in hypoxia groups were similar to temperature groups, except that the PO2 of Ringer solution were 15, 40 and 100 Torr, and the experimental temperature was 25 °C. Different PO2 of Ringer solution were achieved by continuously pumping the mixture of nitrogen and air into the bath by manually controlling the flow rate of nitrogen or air. The PO2 was monitored by an oxygen electrode (Rank Brothers Ltd, England, accuracy: 1 Torr). The contractile properties of each sample were tested at three PO2 after 10 min equilibration in the corresponding PO2.
Muscle fibers types classification and Biochemical analyses
The dissected IF muscle used for the classification of muscle fibers types was slightly stretched relative to its normal length, then dipped in isopentane pre-cooled by liquid nitrogen for 10 s, and finally frozen in liquid nitrogen. Serial sections of 10 μm were cut in a cryostat at − 20 °C and mounted on glass slides freshly coated with 0.1% poly-L-lysine. Sections to be stained for ATPase were processed on the same day. Staining methods followed Lind and Kernell (1991). The proportion of each muscle fibers type was calculated by Image J.
The IF muscle used for the assays of ATPase activity was immediately frozen in liquid nitrogen and then stored at − 80 °C. When tested, muscle samples were homogenized in nine volumes of ice-cold normal saline. Homogenate was centrifuged at 2500 rpm min−1 for 10 min, and the supernatant was then diluted to 2% to immediately undertake the biochemical analyses. ATPase (Ca2+-Mg2+-ATPase, EC 3.6.3.8; Na+-K+-ATPase, EC 3.6.3.9) activity was assayed by measuring the increase of phosphate concentration at 660 nm using a commercial ATPase assay kit (Nanjing Jiancheng Ltd. Co., Nanjing, China). Absorbances were determined with a spectrophotometer (UV2000, Unico Instrument Co., Ltd., Shanghai, China). The activities of two kinds of ATPase were measured at 10, 25, 35 40 and 45 °C. ATPase activity was expressed as μmol Pi per milligram of protein per hour (μmolPi mgprot−1 h−1).
Data collection and analysis
The maximal twitch tension (Pt), time-to-peak twitch tension (TPT), time of half relaxation from peak twitch tension (1/2 RT), tetanic tension (Po), maximum rate of rise in tetanic tension (dPo/dt) and maximal velocity of shortening (Vmax) were all directly obtained from BL-420F system. The force applied to muscles in isotonic contraction is constant. Power (P) was equal to the constant force multiplied by Vmax and normalized by the mass of the IF muscle i.e. P/m (Marsh and Bennett 1985; Josephson 1993). Moreover, Pt, Po, dPo/dt and Vmax were normalized by the cross-sectional area of the muscle. The cross-sectional area was calculated by the mass of the muscle divided by the product of the length of the muscle and 1.056 g/cm3 (El-Khoury et al. 2012).
Data analyses were performed using SPSS version 24.0 (SPSS, Inc., Chicago, Illinois, USA). All the data were tested for homogeneity of variance. Then, one-way analysis of variance (ANOVA) was used to determine the significance of all parameters between two species of lizards. The post hoc Bonferroni test was used for analyzing the significance of all contraction parameters in different temperature/PO2 of the same species. Differences in muscle contractile properties were analyzed using multi-factor analysis of variance with temperature * species or oxygen partial pressure * species as the fixed factors. In addition, we used univariate analysis of general linear model to evaluate the effect of hind limb length on sprint speed with temperature as a fixed factor and hind limb length as a covariate. The values were presented as mean ± standard error (SE). The graphs were made with Origin 2018 (OriginLab Corporation, USA). The significance level was p < 0.05.
Results
Lizard sprint speed at different body temperatures
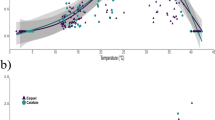
The body temperature of lizards was not exactly equal to the experiment temperature, so the temperature shown in results refers to the body temperature of lizards. The sprint speed at different body temperatures (Fig. 1) shows that overall P. przewalskii ran significantly faster than P. erythrurus at each body temperature (p < 0.001 for each temperature). In both species, sprint speed continuously increased when body temperature rose from 21 °C to 30 °C, then kept stable when temperature increased to 36 °C. It is worth noting that the hind limb length of P. przewalskii is significantly larger than that of P. erythrurus (Table 2). Moreover, the results of univariate analysis showed that hind limb length has a significant effect on sprint speed of these two species (p < 0.001).
Sprint speed (mean ± SE) at different body temperatures and the number of individuals used in each test. Black symbols for P. przewalskii; open symbols for P. erythrurus. Uppercase letters indicate the significance of differences for sprint speeds of P. przewalskii at different temperatures. Lowercase letters indicate the significance of differences for sprint speeds of P. erythrurus between different temperatures. No significant intraspecies differences are indicated once there are same letters between any two conditions (p > 0.05). Sprint speeds are significantly different between the two species under each test temperature (p < 0.001)
Effect of temperature and hypoxia on the contractile properties
We first used multi-factor analysis of variance to investigate the effects of temperature and oxygen partial pressure on the contraction characteristics of IF muscle for the two Phrynocephalus lizards (Tables 3, 4).
Temperature shows significant effects on all contractile properties, and species has a significant effect on Pt, TPT, dPo/dt and Vmax, but only TPT is significantly affected by both temperature and species (Table 3). The TPT and 1/2 RT of the two species decreased with the increase of temperature; the figures showing the changes of Pt, Po, dPo/dt, Vmax and P/m with temperature are inverted bell curve (Fig. 2). Pt and Po showed little change over a broad temperature range (as much as 40 °C) for both species. The Vmax and P/m of isotonic contraction showed similar thermal dependence. Surprisingly, Vmax of P. erythrurus tends to be stable from 25 °C to 40 °C, while P. przewalskii continuously increased. As for the interspecies comparisons, the contraction performances of P. przewalskil (except for 1/2 RT) are better than P. erythrurus although significant differences were only found in some properties at certain temperatures (Fig. 2).
The thermal dependence of contraction parameters in P. erythrurus and P. przewalskii. A maximal twitch tension, Pt; B time-to-peak twitch tension, TPT; C time of half relaxation from peak twitch tension, 1/2 RT; D tetanic contraction, Po; E maximum rate of rise in tetanic tension, dPo/dt; F maximal power output, P/m; G maximal velocity of shortening, Vmax. Values are presented as mean ± SE. Uppercase letters indicate the significance of differences for sprint speeds of P. przewalskii at different temperatures. Lowercase letters indicate the significance of differences for sprint speeds of P. erythrurus between different temperatures. No significant intraspecies differences are indicated once there are same letters between any two conditions (p > 0.05). Significant interspecies differences are indicated by asterisks (*p < 0.05; **p < 0.01)
PO2 had no significant effect on any contractile properties for P. erythrurus (Table 4 and Fig. 3). For P. przewalskii, a significant difference was observed only between 40 and 15 Torr (p = 0.009) for the 1/2 RT. Pt, TPT, Po, Vmax, and P/m for P. przewalskii were significantly higher than for P. erythrurus at all test PO2 (p = 0.001, p = 0.021, p < 0.001, p < 0.001, p < 0.001, respectively), while there was no significant difference in 1/2 RT and dPo/dt between the two species (p = 0.096, p = 0.269, respectively).
The influences of hypoxia on the contraction parameters in P. erythrurus and P. przewalskii. A Maximal twitch tension, Pt; B time-to-peak twitch tension, TPT; C time of half relaxation from peak twitch tension, 1/2 RT; D tetanic contraction, Po; E maximum rate of rise in tetanic tension, dPo/dt; F maximal power output, P/m; G maximal velocity of shortening, Vmax. Values are presented as mean ± SE. Uppercase letters indicate the significance of differences for sprint speeds of P. przewalskii at different oxygen partial pressure, and no significant intraspecies differences are indicated once there are same letters between any two conditions (p > 0.05). Contraction parameters of P. erythrurus have no significant differences with the three different oxygen partial pressure. Significant interspecies differences are indicated by asterisks (*p < 0.05; **p < 0.01; ***p < 0.001)
Muscle fibers types and ATPase activity
A representative section ATPase stained section to identify the types of fibers in IF muscle in both species is shown in Fig. 4. Because Type IIB and Type IIA were not well recognized in sections, they were combined and counted as Type II. There was no significant difference in the proportion of two types of fibers between species (Table 5).
Temperature had a significant effect on the ATPase activity for both species, and the changing pattern of ATPase activity was similar to dPo/dt. It increased from 10 °C to 35 °C and decreased from 35 °C to 45 °C (Fig. 2 and Fig. 5). The Na+–K+–ATPase activity of P. erythrurus was significantly higher than that of P. przewalskii at 40 °C (p < 0.001). Ca2+–Mg2+–ATPase activity of P. erythrurus is significantly lower at 35 °C but significantly higher at 40 °C and 45 °C compared with P. przewalskii (p = 0.002, p = 0.002, p = 0.014, respectively).
The thermal dependence of Ca2+–Mg2+–ATPase (A) and Na+–K+–ATPase (B) in P. erythrurus and P. przewalskii. Data are presented as mean ± SE with n = 6–8 per individual. Uppercase and lowercase letters indicate a statistical intraspecific difference, no significant intraspecies differences are indicated once there are same letters between any two conditions (p > 0.05). Significant interspecies differences are indicated by asterisks (*p < 0.05; **p < 0.01; ***p < 0.001)
Discussion
Thermal effects on locomotor performance
Locomotor performance showed a significant thermal dependence for both P. erythrurus and P. przewalskii, as reported for other lizard species (Crowley, 1985; Du et al., 2007; Lin et al., 2008; Bonino et al., 2015; Zamora-Camacho et al., 2015). We used body temperature rather than ambient temperature in our analysis of thermal dependence of locomotion. Our results indicated that P. przewalskii always ran significantly faster than P. erythrurus at all body temperatures. One possible explanation is that the hind limb of P. przewalskii was significantly longer than for P. erythrurus. In general, after accounting for the effect of body size, animals with longer hind limbs sprinted faster (Losos 1990; Winchell et al. 2018). This may affect the distance through which the animals accelerated. Sprint speed of urban Anolis was greater than their forest counterparts, and the urban lizards had longer hind-limbs (Winchell et al. 2018). Another reason is that the phenomenon may be most related to their habitats and genetic difference. Compared to P. przewalskii, P. erythrurus living at high-altitude may be less affected by predators, and it is not necessary to run fast. Further, a slower sprint speed is beneficial for saving energy under hypoxia.
Effects of temperature and hypoxia on contractile properties
No uniform pattern of thermal dependence underlies all these parameters of isometric contraction of IF muscle. Our results showed that Pt and Po were temperature independent from 10 °C to 40 °C for both species. This aligns with results obtained from studies for other lizards (Putnam and Bennett 1982; Marsh and Bennett 1985). This enables the lizards to evade predators and forage at low temperatures. At temperatures above 40 °C, the decrease of Pt and Po suggested that IF muscle was subjected to irreversible heat damage. The rates of force generation and relaxation (TPT and 1/2 RT) increased with higher temperature for both species. This pattern is also observed for other lizards (Marsh and Bennett 1985; Swoap et al. 1993). The mechanism could be attributed to that myofibrillar ATPase activity and the rate of calcium dissociation from parvalbumin increased with rising temperature (Bárány 1967; Stein et al. 1982; Brenner and Eisenberg 1986; Hou et al. 1992). The results of ATPase activity in present study were consistent with the rates of force generation and relaxation. The dPo/dt was strongly temperature dependent for both species.
The changing patterns of Vmax and P/m with temperature for both species agreed with previous studies (Hill 1938; Gleeson and Johnston 1987; Rome et al. 1990). Vmax of P. erythrurus remains stable, while P. przewalskii continues to rise from 25 °C to 40 °C. This indicated that skeletal muscles of P. erythrurus could achieve fast isotonic contraction at relatively low temperatures. In the summer, individuals of both species were observed to begin running at around 8 o’clock in the morning; however, at 8:00, the body temperature of P. erythrurus is about 25 °C, while P. przewalskii is about 35 °C (unpublished data). The P/m of P. przewalskii was significantly higher than that of P. erythrurus at 35 °C and 40 °C. Muscle with higher Vmax most likely produces more power than muscle with lower Vmax (Hill 1938; James 2013; Lieber and Fridén 2015). In addition, the Vmax of P. przewalskii was higher than for P. erythrurus at certain test temperatures. Similar results were also found in the hypoxic experiment and for locomotor performance. This may be due to a larger proportion of Type IIB fibers in P. przewalskii compared with P. erythrurus.
The influence of hypoxia on the contractile properties of isolated skeletal muscle has not been reported in reptiles. Our results showed that there was no significant effect on contraction parameters caused by hypoxia except for 1/2 RT in the two species of lizard (Fig. 3 and Table 5). It was reported that PO2 in skeletal muscles was only approximately 3 Torr (Richardson et al. 1995). So, 15 Torr in hypoxia treatment used in our experiment may be not low enough to cause significant changes. Also, the oxygen consumption rate of IF muscle at 25 °C may be relatively low so that the diffusion of O2 can match the requirement, resulting in an adequate supply of O2 in the IF muscle at 25 °C even at a PO2 of 15 Torr.
Muscle fibers types
Muscles comprised of mainly Type IIB fibers contract quickly, but also fatigue quickly, while muscles composed of primarily Type I fibers have high endurance but slow speed. Type IIA fibers combine speed and endurance (Brooks et al. 1996; Saltin and Gollnick 1983). In the present study, the Type II fibers are predominant in the IF muscle of the two species. This may be the characteristic of IF muscle itself. There was no significant difference in muscle fibers types between the two species. In addition, Phrynocephalus lizards run intermittently according to our observations in the field, and in our locomotor experiments, which might indicate that the proportion of Type IIB fibers is higher than that of Type IIA fibers in the IF muscle of both species.
In summary, our results showed that the locomotor performance of P. przewalskii was significantly better than that of P. erythrurus at all body temperatures, reflecting the better contractile properties of P. przewalskii IF muscle at different temperatures and PO2. Temperature has a significant effect on the contractile properties of IF muscle for the two species. P. erythrurus achieved both optimal isometric (dPo/dt) and optimal isotonic (Vmax) contractile properties at a lower temperature compared to P. przewalskii, which probably results from the long-term adaption to the cold environment of high altitudes. Although the proportion of fibers types and ATPase activities of IF muscle have no significant interspecies difference, the changing trend of ATPase activities with temperature is congruous with certain contractile properties and locomotor performance. These findings may provide new insights into the adaptative mechanisms of locomotor performance in P. erythrurus endmic to Tibetan plateau. However, in-depth studies are needed to further investigate the mechanism of locomotor performance in the terms of the calcium pathway and muscle contraction related genes.
Abbreviations
- IF muscle:
-
Iliofibularis muscle
- P t :
-
Maximal twitch tension
- TPT:
-
Time-to-peak twitch tension
- 1/2 RT :
-
Time of half relaxation from peak twitch tension
- P o :
-
Tetanic tension
- dP o/dt :
-
Maximum rate of rise in tetanic tension
- P/m :
-
Maximal power output
- V max :
-
Maximal velocity of shortening
- PO2 :
-
Oxygen partial pressure
References
Alés RG, Acosta JC, Astudillo V, Córdoba M, Blanco GM, Miles D (2018) Effect of temperature on the locomotor performance of species in a lizard assemblage in the Puna region of Argentina. J Comp Physiol B 188:977–990
Bárány M (1967) ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol 50:197–218
Bennett AF (1985) Temperature and muscle. J Exp Biol 115:333–344
Bergmann PJ, Hare-Drubka M (2015) Hindlimb muscle anatomical mechanical advantage differs among joints and stride phases in basilisk lizards. Zoology 118:291–298
Bonine KE, Gleeson TT, Garland T Jr (2001) Comparative analysis of fiber-type composition in the iliofibularis muscle of phrynosomatid lizards (Squamata). J Morphol 250:265–280
Bonine KE, Gleeson TT, Garland T (2005) Muscle fiber-type variation in lizards (Squamata) and phylogenetic reconstruction of hypothesized ancestral states. J Exp Biol 208:4529–4547
Brenner B, Eisenberg E (1986) Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci USA 83:3542–3546
Brooks GA, Fahey TD, White TP (1996) Exercise physiology: human bioenergetics and its applications. vol Ed. 2. Mayfield publishing company, Mountain View, CA
Burke R (2011) Motor units: anatomy, physiology, and functional organization. Comprehensive Physiology:345–422
Calsbeek R, Cox RM (2010) Experimentally assessing the relative importance of predation and competition as agents of selection. Nature 465:613–616
Chaillou T (2018) Skeletal muscle fiber type in hypoxia: adaptation to high-altitude exposure and under conditions of pathological hypoxia. Front Physiol 9:1450
Christian KA, Tracy CR (1981) The effect of the thermal environment on the ability of hatchling Galapagos land iguanas to avoid predation during dispersal. Oecologia 49:218–223
El-Khoury R, O’Halloran K, Bradford A (2003) Effects of chronic hypobaric hypoxia on contractile properties of rat sternohyoid and diaphragm muscles. Clin Exp Pharmacol Physiol 30:551–554
El-Khoury R, Bradford A, O’Halloran KD (2012) Chronic hypobaric hypoxia increases isolated rat fast-twitch and slow-twitch limb muscle force and fatigue. Physiol Res 61:195–201
Esau SA (1989) Hypoxic, hypercapnic acidosis decreases tension and increases fatigue in hamster diaphragm muscle in vitro. Am Rev Respir Dis 6:1410–1417
Gilbert AL, Miles DB (2017) Natural selection on thermal preference, critical thermal maxima and locomotor performance. Proceedings of the Royal Society b: Biological Sciences 284:20170536
Gleeson TT, Johnston IA (1987) Reptilian skeletal muscle: contractile properties of identified, single fast-twitch and slow fibers from the lizard Dipsosaurus dorsalis. J Exp Zool 242:283–290
Godt RE, Lindley BD (1982) Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol 80:279–297
Herrel A, James RS, Van Damme R (2007) Fight versus flight: physiological basis for temperature-dependent behavioral shifts in lizards. J Exp Biol 210:1762–1767
Hill AV (1938) The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond 126:136–195
Hou TT, Johnson JD, Rall JA (1992) Effect of temperature on relaxation rate and Ca2+, Mg2+ dissociation rates from parvalbumin of frog muscle fibres. J Physiol 449:399–410
Ishihara A, Itoh K, Itoh M, Hirofuji C (2000) Effect of hypobaric hypoxia on rat soleus muscle fibers and their innervating motoneurons: a review. Jpn J Physiol 50:561–568
James RS (2013) A review of the thermal sensitivity of the mechanics of vertebrate skeletal muscle. J Comp Physiol B Biochem Syst Environ Physiol 183:723–733
Jin YT, Liu NF (2010) Phylogeography of Phrynocephalus erythrurus from the Qiangtang Plateau of the Tibetan Plateau. Mol PhyloGenet Evol 54:933–940
Josephson RK (1993) Contraction dynamics and power output of skeletal muscle. Annu Rev Physiol 55:527–546
Lewis P, McMorrow C, Bradford A, O’Halloran KD (2015) Improved tolerance of acute severe hypoxic stress in chronic hypoxic diaphragm is nitric oxide-dependent. J Physiol Sci 65:427–433
Lewis P, Sheehan D, Soares R, Coelho AV, O’Halloran KD (2016) Redox remodeling is pivotal in murine diaphragm muscle adaptation to chronic sustained hypoxia. Am J Respir Cell Mol Biol 55:12–23
Li XT et al (2017) The Cold Hardiness of Phrynocephalus erythrurus, the Lizard Living at Highest Altitude in the World. CryoLett 38:216–227
Lieber RL, Fridén J (2015) Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 23:1647–1666
Lind A, Kernell D (1991) Myofibrillar ATPase histochemistry of rat skeletal muscles: a “two-dimensional” quantitative approach. J Histochem Cytochem 39:589–597
Losos J (1990) The evolution of form and function: morphology and locomotor performance in West Indian Anolis lizards. Evolution 44:1189–1203
Lui MA et al (2015) High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. Am J Physiol Regul Integr Comp Physiol 308:R779–R791
Machiels HA, Hf VDH, Heunks LM, Dekhuijzen PN (2010) The effect of hypoxia on shortening contractions in rat diaphragm muscle. Acta Physiol 173:313–321
Marsh RL, Bennett AF (1985) Thermal dependence of isotonic contractile properties of skeletal muscle and sprint performance of the lizard Dipsosaurus dorsalis. J Comp Physiol B 155:541
Marsh RL, Bennett AF (1986) Thermal dependence of contractile properties of skeletal muscle from the lizard Sceloporus occidentalis with comments on methods for fitting and comparing force-velocity curves. J Exp Biol 126:63–77
Miles DB, Calsbeek R, Sinervo B (2007) Corticosterone, locomotor performance, and metabolism in side-blotched lizards (Uta stansburiana). Horm Behav 51:548–554
Putnam RW, Bennett AF (1982) Thermal dependence of isometric contractile properties of lizard muscle. J Comp Physiol 147:11–20
Ranatunga KW (2018) Temperature effects on force and actin–myosin interaction in muscle: a look back on some experimental findings. Int J Mol Sci 19:1538
Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD (1995) Myoglobin O2 desaturation during exercise. evidence of limited O2 transport. Jclininvest 96:1916–1926
Rome LC, Sosnicki AA, Goble DO (1990) Maximum velocity of shortening of three fibre types from horse soleus muscle: implications for scaling with body size. J Physiol 431:173–185
Saltin B, Gollnick PD (1983) Skeletal muscle adaptability: significance for metabolism and performance. In: Peach LO (ed) Handbook of physiology. American Physiological Society, Bethesda, pp 555–631
Scott GR, Egginton S, Richards JG, Milsom WK (2009) Evolution of muscle phenotype for extreme high altitude flight in the bar-headed goose. Proc R Soc B Biol Sci 276:3645–3653
Scott GR, Elogio TS, Lui MA, Storz JF, Cheviron ZA (2015) Adaptive modifications of muscle phenotype in high-altitude deer mice are associated with evolved changes in gene regulation. Mol Biol Evol 32:1962–1976
Stein RB, Gordon T, Shriver J (1982) Temperature dependence of mammalian muscle contractions and ATPase activities. Biophys J 40:97–107
Swoap SJ, Johnson TP, Josephson RK, Bennett AF (1993) Temperature, muscle power output and limitations on burst locomotor performance of the lizard Dipsosaurus dorsalis. J Exp Biol 174:185–197
Winchell K, Maayan I, Fredette J, Revell L (2018) Linking locomotor performance to morphological shifts in urban lizards. Proc R Soc B 285:20180229
Wu Q, Dang W, Hu YC, Lu HL (2018) Altitude influences thermal ecology and thermal sensitivity of locomotor performance in a toad-headed lizard. J Therm Biol 71:136–141
Yan Z, Okutsu M, Akhtar YN, Lira VA (2011) Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol 110:264–274
Acknowledgements
The authors gratefully thank Wangjie Cao, Xingwen Yang and Yaofeng Zhao for their support and help in this study.
Funding
Research funding was supported by the National Natural Science Foundation of China (NO. 31971416 and NO. 31472005 to Q. Chen) and the Fundamental Research Funds for the Central Universities (lzujbky-2017-150 to X. Tang) and Project funded by China Postdoctoral Science Foundation (2021M691380 to H. Wang).
Author information
Authors and Affiliations
Contributions
Conceptualization: QCh, ZhN, ML; methodology: ZhN, ML, PP; software: ZhN, PP, TZh; formal analysis: ZhN, PP, TZh, HW; investigation: QCh, ZhN, XT, TZh; resources: QCh, XT; data curation: QCh, YZh, ML; writing-original draft: ZhN, ML, PP; writing review and editing: QCh, XT, PP, ZhN, ML; visualization: PP, ZhN, ML; supervision: QCh, XT; project administration: QCh; funding acquisition: QCh, XT.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by P. Withers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Niu, Z., Li, M., Pu, P. et al. Effects of temperature on the locomotor performance and contraction properties of skeletal muscle from two Phrynocephalus lizards at high and low altitude. J Comp Physiol B 191, 907–916 (2021). https://doi.org/10.1007/s00360-021-01391-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-021-01391-9