Abstract
Locomotion is relevant to the ecology of reptiles because of its presumed influence on an organism’s Darwinian fitness. Moreover, in ectothermic species, physiological performance capacity is affected by body temperature. We analyzed two components of locomotor performance in three species of lizards, Phymaturus extrilidus, Liolaemus parvus, and Liolaemus ruibali, in the Puna environment of Argentina. First, we estimated the thermal sensitivity of locomotion by measuring sprint speed at four different body temperatures. We included two measures of sprint speed: initial velocity and long sprint for sustained runs. Based on these data, we calculated the optimal temperature for performance and the optimal performance breadth. We also estimated endurance capacity at a single temperature. Maximum sprint speed for L. parvus was greater than L. ruibali and P. extrilidus in both initial velocity and long sprint. In contrast, L. parvus exhibited lower levels of endurance than L. ruibali and P. extrilidus. However, endurance in L. ruibali exceeded that of P. extrilidus. The species differed in the optimal temperature for the initial velocity with the lowest for L. ruibali (31.8 °C) followed by P. extrilidus (33.25 °C) and then L. parvus (36.25 °C). The optimal temperature for long sprint varied between 32 and 36 °C for all species. We found that all species attained maximum performance at body temperatures commonly experienced during daily activity, which was higher than the thermal quality of the environment. We found evidence for thermal sensitivity in locomotor performance in these species. However, we also show that the broad thermal breadth of performance suggests that the lizards are capable of sustaining near optimal levels of locomotor performance at ambient temperatures that would appear to be suboptimal. Thus, this lizard assemblage is capable of coping with the highly variable climatic conditions in the Puna region of Argentina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Locomotion is a relevant trait in the ecology of reptiles, and of animals in general, as it is linked to numerous life history characteristics and has direct implications on the fitness of an organism (Le Galliard et al. 2004; Irschick and Meyers 2007; Strobbe et al. 2009; Vanhooydonck et al. 2015; Ibargüengoytía et al. 2016). Various locomotor performance traits have been linked to predator escape (Lima and Dill 1990; Calsbeek and Cox 2010), foraging mode (Huey et al. 1984; Miles et al. 2007a, b), reproductive success (Husak et al. 2006; Byers et al. 2010), territorial defense (Garland et al. 1990; Robson and Miles 2000), and survival (Miles 2004; Gilbert and Miles 2017). In general, the estimates of locomotor performance such as sprint speed (initial velocity and long sprint; van Berkum 1988; Angilletta et al. 2002; Huey et al. 2009) and endurance (Robson and Miles 2000) have been estimated using a variety of methods. However, theory suggests that sprint speed and endurance are assumed to present conflicting demands on locomotor performance, and, consequently, cannot be simultaneously maximized (Vanhooydonck et al. 2001). For this reason, a trade-off between these performance parameters is predicted, with faster lizards exhibiting lower values for endurance and vice versa. Nevertheless, the expected trade-off between these traits has not been detected (Huey et al. 1984; Tsuji et al. 1989; Sorci et al. 1995; Vanhooydonck et al. 2001; Pinch and Claussen 2003).
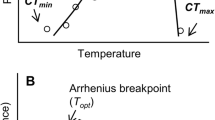
In ectotherms, performance is intimately linked with temperature (Huey and Stevenson 1979; Marsh and Bennett 1986; Huey and Kingsolver 1989; Kingsolver 2009). The relationship between temperature and performance is described by a thermal performance curve (TPC). Estimation of the optimal temperature for maximum performance (To) can be determined using TPCs (Huey and Stevenson 1979; Huey and Kingsolver 1989). In addition, TPCs allow the estimation of the thermal performance breadth (the range of temperatures that results in an ectotherm performing at or above some level, e.g., 80%), and the thermal tolerance zone. The latter describes the range of temperatures at which an ectotherm can be active. The lower end of the tolerance zone is defined as the critical thermal minimum (CTmin), whereas the upper end is defined as the critical thermal maximum (CTmax). When ambient temperatures exceed these limits, the capacity for an organism’s normal functioning ceases. Thermal performance curves typically have an asymmetrical shape, such that physiological capacity increases slowly below To; above To performance decreases rapidly (Bonino et al. 2015). However, we recognize that ectotherms are often exposed to thermal conditions below or above the optimum temperature for physiological performance, which may have deleterious consequences for survival and reproduction.
The strength of selection acting on thermal performance breadth can differ between constant or fluctuating environments (Huey and Slatkin 1976; Huey and Stevenson 1979). Ectotherms within the tropics experience low fluctuations in daily temperatures and limited seasonal variation. Therefore, species in the tropics tend to have a narrower thermal tolerance than temperate-zone lizards (Janzen 1967; Huey et al. 2009). Nevertheless, this pattern is not consistent for thermal performance breadth. For example, in tropical lizard species such as Anolis, the performance breadth is not consistently narrower than temperate-zone lizards, such as Sceloporus. Rather, the pattern suggests that the thermal performance breadth correlates with variability in field-active body temperatures (Tbs) exhibited by individuals (Crowley 1985; van Berkum 1988; Angilletta et al. 2002). Thus, lizards with a broad geographical distribution or that inhabit sites with large environmental thermal amplitudes (that is high seasonality) tend to exhibit greater variability in their Tbs, and, consequently, broader thermal performance breadth (van Berkum 1988; Huey and Kingsolver 1989; Gaston and Blackburn 2000; Cruz et al. 2005; Angilletta 2009).
On the other hand, reptiles may use behavioral thermoregulation to buffer the effects of ambient temperature on physiological performance (Huey et al. 2009). Therefore, large changes in ambient temperature have a limited effect on performance as a consequence of individual’s selecting microhabitats that minimize variation in Tb. The previous studies have shown that optimal temperature for physiological performance is at or near the preferred temperatures (Huey and Bennett 1987; Martín and Huey 2008). The tight connection between behavioral thermoregulation and thermal physiology has been used as the evidence of coadaptation. An advantage of coadaptation is the potential increase in amplitude of temperatures that maximize individual performance (Huey and Bennett 1987; Angilletta et al. 2002). Yet, to date, there is scant evidence supporting the hypothesis of coadaptation; indeed, there is evidence to the contrary (Angilletta et al. 2002).
Lizard species occupying the habitats in the Andes Mountains of Argentina provide the opportunity to examine how environmental variation in ambient temperature affects locomotor performance in ectothermic vertebrates. In this study, we focus on two genera of lizards from the family Liolaemidae, Phymaturus and Liolaemus, that often coexist along broad latitudinal and elevational gradients along the Andes mountain range in Argentina and Chile (Díaz Gómez 2009). Individuals of species from these two genera inhabit environments characterized by substantial variation in ambient temperature. Research analyzing the thermal dependence of locomotor performance is scarce for these two genera of lizards (Bonino et al. 2011, 2015; Fernández et al. 2011; Fernández and Ibargüengoytía 2012; Kubisch et al. 2011, 2016). At the southern end of the arid, Puna region in Argentina, three endemic species of Liolaemidae coexist in sympatry: P. extrilidus, L. parvus, and L. ruibali. These species present an ideal system for determining the influence of ambient temperature on locomotor performance, and whether these species exhibit similar responses to seasonal variation in the thermal environment. Given that variation in ambient temperatures can be quite large in the Puna region (Márquez et al. 2014), we predict that these species should have large thermal tolerances (i.e., the difference between critical thermal minimum and maximum temperatures), optimal temperatures for performance (To) that coincide with field-active body temperatures, and broad thermal performance breadths (defined below).
Phymaturus extrilidus is an herbivorous species that is found in habitats at high elevations. It is dorsoventrally flattened, which is presumably an adaptation to occupy narrow cracks in rocks as a refuge from predators. The other two species, L. parvus and L. ruibali, are morphologically similar, despite inhabiting different habitats. Liolaemus parvus is insectivorous and lives in sites with rocks and meadows, whereas L. ruibali is omnivorous and mainly inhabits flat areas characterized by open shrubland consisting of widely scattered, low growing shrubs (Lycium chanar, Artemisia mendozana, Adesmia horrida, among others; Márquez et al. 2014). This partitioning of trophic resources and microhabitats may facilitate coexistence among the species, lead to morphological differentiation; favor the evolution of differences in their thermal biology, or a combination of these. In addition to morphological and ecological differences between these genera, we expect that locomotor performance and thermal tolerance should be more similar between species in the genus Liolaemus, than between these and P. extrilidus. This is because the two Liolaemus species are more closely related to each other than to Phymaturus (Abdala and Quinteros 2014).
The objectives of this study were to (1) quantify variation in the initial velocity and long sprint at different temperatures, (2) measure endurance and test for a trade-off with running speed, (3) measure critical thermal limits (CTmin and CTmax), and (4) explore the relationship between optimal temperature for performance in relation to field activity body temperatures (Tb), preferred temperatures (Tpref), and environmental operative temperatures (Te) for P. extrilidus, L. parvus, and L. ruibali.
Materials and methods
Fieldwork
Fieldwork was carried out at the “Don Carmelo” Reserve located in the western part of the Ullum Department (30°56.99′S, 69°04.83′W; 3166 m amsl), in the foothills of the Precordillera of the Andes Mountains in the province of San Juan, Argentina. The reserve is situated in the mountainous terrain east of the Andes. This area is within the Puna desert ecoregion and lies between 2700 and 3400 m above mean sea level (amsl) (Roig Juñent et al. 2003). The climate is cold and dry, with a median annual temperature below 8 °C. Daily temperatures exhibit large fluctuations from spring to fall (nocturnal temperatures below 0 °C and diurnal temperatures exceed 30 °C). The region also experiences substantial seasonal temperature fluctuations, with snow from June to October (Cabrera 1994; Martínez Carretero 1995; Roig and Martínez Carretero 1998; Roig Juñent et al. 2003) and hot and dry conditions during the austral summer.
We captured individuals of P. extrilidus, L. parvus, and L. ruibali using nooses or by hand between 10:00 h and 19:00 h during November 2014. This corresponds with the diurnal activity period for the species and within the breeding season. For each individual, we recorded field body temperature using a digital thermometer with a K-type thermocouple (TES Electronic Corp., Taipei, Taiwán 1303 digital thermometer, accuracy ± 0.01 °C; TP-K01 thermocouples, 1.62 mm diameter). We also measured snout-vent length (SVL) using a Vernier caliper to the nearest 0.02 mm, and body mass (BM) with a Pesola® spring scale ± 0.05 g. We also determined sex by the presence of precloacal pores and the eversion of the hemipenis in males. Females were identified using coloration and presence of embryos and follicles (by palpation). The research was authorized by Secretaría de Medio Ambiente, Dirección de Conservación y Áreas Protegidas, Provincia de San Juan (exp. no. 13004047, J.C.A.).
Husbandry
After capture, the lizards were brought to the laboratory and held in 100 × 30 × 40 cm terraria. Each individual was held in a separate terrarium. We held lizards for a period of 5 days, which time required for estimating the performance traits. The terraria were kept in a room at an ambient temperature of 24 °C and lizards were provided with ad libitum water. We did not feed the lizards prior to the experiments.
Locomotor performance
Velocity
Sprint performance was measured between 10:00 and 19:00 h during the normal activity period of lizards. We used a linear track measuring 0.08 m wide and 1.2 m long to quantify sprint speed. A series of infrared sensors were placed along the walls of the track at intervals of 0.15 m to measure speed. The sensors were connected to a computer, which calculated the speed between each 0.15 m split. We used the difference in time between successive sensors to estimate velocity. Based on the data from the sensors, we calculate: the initial velocity (IV) determined by the difference time between the first and second sensors (0.15 m) and can be considered an estimate of acceleration; and long sprint (LS) that was calculated by the difference in time registered between the first and last sensors (1.05 m). We consider this estimate to best represent the locomotor capacity of lizards to carry out activities such as foraging, territorial defense, and courtship (sensu Cabezas Cartes et al. 2014).
We induced lizards (P. extrilidus N = 19, L. parvus N = 16, and L. ruibali N = 15) to run at four temperatures within the range of thermal tolerance for each species (23, 38, 28, and 33 ± 1 °C, respectively) in random order. Prior to running a lizard, we kept it in a terrarium at the desired temperature for 1 h.
We ran a lizard three consecutive times at each temperature. The fastest run was used as estimate of maximum performance for both IV and LS. Lizards were run at one temperature per day and rested for 24 h between temperature treatments. As a control, we repeated a run at the initial temperature to determine if individual performance had exhibited a decrease in speed over the course of the study (Huey et al. 1990; Angilletta et al. 2002).
We used the sprint trials to calculate the initial velocity and long sprint for each lizard (Vi max) and the maximum speed for the species (Vmax; Fernández et al. 2011; Kubisch et al. 2011). We also used the change in sprint performance among the temperatures to estimate the optimal sprint temperature (To) for each species. We used the average body temperatures of each lizard at which speed was the highest. We estimated the thermal performance breadth (B80) by calculating the range of body temperatures where performance was equal to or higher than 80% of Vmax (see Hertz et al. 1983).
Endurance capacity
Endurance measurements were taken following the methodology used by Miles et al. (2000), Robson and Miles (2000), Vanhooydonck et al. (2001), and Angilletta et al. (2002). Lizards were placed on a motorized treadmill and a mild stimulus was applied to their hind legs to make them run. Endurance was measured as the time (in seconds) the lizard ran before becoming exhausted at a constant speed of 0.5 km/h (following Miles 1994). The lizards were considered to be fatigued when they did not demonstrate a righting response when placed on their backs (Huey et al. 1990; Robson and Miles 2000). Only one trial was carried out for each lizard, at 32 °C (P. extrilidus) and 33 °C (Liolaemus). We selected these temperatures, because they correspond to the field-active body temperature for the species (Gómez Alés et al. 2017, Gómez Alés unpublished data).
Thermal tolerance: CT min and CT max
Minimum critical temperature (CTmin) and maximum critical temperature (CTmax) were recorded for each individual (P. extrilidus N = 20, L. parvus N = 15, and L. ruibali N = 15). In both cases, the critical temperature was determined when the individual lost its righting response after being placed on its back. In addition, we used the signs of muscular spasms as a cue for CTmax (Jacobson and Whitford 1970; Pough and Gans 1982; Cruz et al. 2005).
To determine CTmin, individuals were placed separately in a plastic terrarium within a refrigerator set at a constant temperature (− 15 °C). Body temperature was measured every 20 s as we tested the righting response of the individual. We recorded the temperature when a lizard failed to right itself. To determine CTmax, individuals were placed in cylindrical vessel (25 cm diameter and 35 cm height), with sterile sand as substrate. A 150-W incandescent light bulb was placed above the cylinder at a distance of 50 cm from the surface of the sand. As with CTmin, we checked Tb every 10 s until the lizard lost its righting ability. We immediately removed the lizard from the cylinder and allowed it to recover. We inserted ultrathin thermocouples (1 mm) within the cloaca (10 mm) held in place by hypoallergenic adhesive tape to record CTmin and CTmax. The thermocouples were connected to a data acquisition module (OMEGA®, USB TC-08), which allowed us to monitor the Tb of lizards. This method prevents manipulating the lizard every time that cloacal temperature is taken. None of the individuals died during the trials.
Thermal tolerance for each species was calculated as the difference between the critical thermal limits, \({\overline {{{\text{CT}}}} _{\hbox{max} }} - {\overline {{{\text{CT}}}} _{\hbox{min} }}\) (Cruz et al. 2005). We also computed the thermal safety margin, which is estimated as the difference between To and CTmax, and following Deutsch et al. (2008) as the difference between To and mean Te. Critical thermal limits were used to compute the thermal sensitivity curves for the initial velocity and long sprint (Angilletta et al. 2002).
Statistical analyses
The relationship among variables was assessed using ordinary and multiple regression analyses. For comparisons between means, we used paired and unpaired t tests and one-way repeated-measures analysis of variance (R-ANOVA). We also conducted post hoc tests to compare means among different groups. Sprint speed and endurance were compared among species with an ANCOVA, using SVL as a covariate. To test for correlations among the performance traits (long sprint vs. endurance, and initial velocity vs. endurance), we log-transformed the data and calculated Pearson product–moment correlation coefficients. Prior to conducting any statistical procedure, we evaluated whether the data followed a normal distribution and homogeneity of variance among groups using Kolmogorov–Smirnov and Shapiro–Wilk tests, respectively. In cases where data did not comply with the assumptions for parametric statistics, we used the equivalent non-parametric test (e.g., Mann–Whitney U test, Wilcoxon and Friedman; Sokal and Rohlf 1969). Tests were performed in Sigma Stat 3.5® and Statistica version 7.0 (Statsoft Inc., Tulsa, OK). Descriptive statistics are represented as mean ± standard error or median. Significance level was fixed at α = 0.05. For multiple tests, significance levels were corrected using a sequential Bonferroni adjustment (Rice 1989; Chandler 1995). We estimated the thermal performance curves using a Kumaraswamy equation based on a custom R script (Sheth and Angert 2014, MW Sears unpublished). In addition, we compared the shape of the thermal performance curve between species using a generalized additive mixed model (GAMM; Zajitschek et al. 2012). The latter two analyses used the computing environment R and the nlsLM function from the minpack.lm package (Kumaraswamy fitting) and function gamm in the mgcv package (Wood 2017).
Results
Relationship between maximum individual speed (V i max) and snout–vent length and body mass
The average values of snout-vent length and body mass for each species and sex are given in Table 1. The snout-vent length and body mass of P. extrilidus and L. parvus, did not differ between the sexes (t test, SVL: P. extrilidus P = 0.98, L. parvus P = 0.56, BM: P. extrilidus P = 0.94, L. parvus P = 0.83). However, SVL in L. ruibali males was significantly greater than females (Mann–Whitney, U = 37.5, P = 0.03), but there was no significant difference in body mass (Mann–Whitney, P = 0.70).
In all species, long sprint was uncorrelated with SVL or body mass (multiple regression: P. extrilidus F2,17 = 0.89; L. parvus F2,14 = 1.62; L. ruibali F2,13 = 0.453; all P > 0.05). Similarly, the initial velocity was not significantly correlated with SVL or body mass (Multiple regression: P. extrilidus F2,17 = 0.36; L. parvus F2,14 = 0.84; L. ruibali F2,13 = 0.55; all P > 0.05).
Effect of temperature on running speed (initial velocity and long sprint)
Thermal performance curves showed a significant effect of temperature on speed for all species in the initial and long sprint (GAMM: FP. extrilidus = 2.62; FL. parvus = 2.2; FL.ruibali = 0.93; P < 0.001; Fig. 1). The long sprint of P. extrilidus increased with temperature, with its minimum speed at 23 °C, maximum speed at 33 °C, and a decreased speed at 38 °C. However, we found significant differences between trials at 23–33 °C and 33–38 °C (repeated-measures ANOVA on ranks: F3,71 = 5.46; Bonferroni corrected P33°–23° = 0.002; P33°–38° = 0.044). For L. parvus, there were no differences between trials (repeated-measures ANOVA on ranks: F3,60 = 1.53; P = 0.20), suggesting a broad thermal performance breadth. However, its maximum speed was also attained at 33 °C. Finally, long sprint of L. ruibali increased with temperature, with the highest value occurring at 38 °C, though significant differences were only found between trials at 23–33 and 23–38 °C (Friedman repeated-measures ANOVA on ranks: χ23,56 = 10.10; P = 0.018; Dunn’s method: Q33°–23° = 2.73, Q38°–23° = 2.73; P < 0.05).
Thermal performance curves showing the relationship between body temperature (°C) and speed (m/s) of the initial velocity and long sprint for Phymaturus extrilidus, Liolaemus parvus, and Liolaemus ruibali. Black triangles designate the optimum temperature for performance (To) and white triangles shows the mean field-active body temperature (Tb). Broken vertical lines represent the set-point (interquartile) range of selected temperatures in a laboratory thermal gradient. Continuous vertical lines are the average operative environmental temperature Te, i.e., the distribution of potential Tb’s registered by a non-thermoregulating animals in a specific microhabitat (see Gómez Alés et al. 2017). The minimum and maximum critical temperatures were used as thermal limits to anchor the curves
The initial velocity in P. extrilidus increased with temperature, with minimum initial speed occurring at 23 °C and maximum value at 33 °C, which subsequently decreased at warmer temperatures (i.e., 38 °C). Significant differences were only found between 23 and 33 °C (repeated-measures ANOVA on ranks: F3,71 = 3.99; Bonferroni corrected P33°–23° = 0.007). For L. parvus, there were no differences between trials (Friedman repeated-measures ANOVA on ranks: χ23,60 = 6.37; P = 0.09). The initial velocity of L. ruibali increased with temperature, reaching its maximum at 33 °C, though significant differences were only found between trials at 23 and 33 °C (Friedman repeated-measures ANOVA on ranks: χ23,52 = 9.64; P = 0.022; Dunn’s method: Q33°–23° = 2.88; P < 0.05).
There was no significant relationship between maximum performance (initial velocity and long sprint) and body temperatures for any of the species (linear regression: initial velocity: FP. extrilidus 2,17 = 0.15; FL. parvus 2,14 = 0.36; FL. ruibali 2,13 = 0.88; all P > 0.05; long sprint: FP. extrilidus 2,17 = 1.48; FL. parvus 2,14 = 0.01; FL. ruibali 2,13 = 1.93; all P > 0.05).
Comparison between the initial velocity and long sprint for each temperature trial
The mean values of Viv max and Vls max for the three species are shown in Table 2. For P. extrilidus, the mean value for initial velocity was significantly greater than the mean value for long sprint (Wilcoxon test: W = 33; P = 0.012). However, when comparing among temperature trials, there were no significant differences between the two measures of velocity (P > 0.05; Table 3). Mean initial velocity of L. parvus was significantly greater than long sprint (Wilcoxon Test: W = 29; P = 0.043). When we compared running speed for each temperature trial, the IV speed was greater than the LS speed at 23 °C and 28 °C (P < 0.05; Table 3), whereas there were no significant differences for the remaining trials (P > 0.05; Table 3). For L. ruibali, differences were only found on trials at 38 °C (Table 3), with long sprint exceeding the value for the initial velocity.
Differences in the thermal sensitivity of speed between sexes for each species
For P. extrilidus, no significant differences between males and females were found for any of the temperature trials, both for IV and LS (P > 0.05; Tables 2, 3). For L. parvus, the IV was greater for males (Table 2); likewise, the IV speed for trials at 38 °C was also greater for males (Table 3) and there were no significant differences for the remaining trials (P > 0.05; Table 3). Male and female L. ruibali did not differ in speed at any temperature for IV and LS (P > 0.05; Tables 2, 3).
Comparison of running speed between species
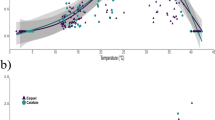
Snout–vent length was significantly different between P. extrilidus and the two Liolaemus species, but not between L. parvus and L. ruibali (Kruskal–Wallis: H2,48 = 35.43; P = 0.0005; Dunn’s Method: QL. parvus–L. ruibali = 4.26; P > 0.05; QP. extrilidus–L. parvus = 4.63; QP. extrilidus–L. ruibali = 5.4; P < 0.05; Table 1). Thus, we used ANCOVA with SVL as a covariate to evaluate differences in performance between species. The Vi max for LS and IV for L. parvus was higher than that of L. ruibali and P. extrilidus (ANCOVAs: FLS (2,48) = 11.2; Bonferroni corrected PL. parvus–L. ruibali < 0.001; PL. parvus–P. extrilidus < 0.001; FIV (2,48) = 11.54; Bonferroni corrected PL. parvus–L. ruibali < 0.001; PL. parvus–P. extrilidus = 0.004; Fig. 2). The three species also differed in the shape of the thermal performance curves (GAMM: tP. extrilidus = − 2.95; tL. parvus = 0.20; tL. ruibali = − 7.43; P < 0.01; Fig. 1).
Endurance capacity
P. extrilidus exhibits a wide range of endurance capacity from 45 to 161 s (94.44 ± 9.32) at 32 °C. However, endurance was not associated with either SVL or BM (multiple regression: FSVL 2,14 = 1.08; FBM 2,14 = 3.21; P > 0.05). The range of endurance for L. parvus varied from 38 to 104 s (52.44 ± 4.29) at 33 °C, and was not associated with SVL, but did exhibit a positive and significant relation to body mass (multiple regression: FSVL 2,14 = 1.79; P > 0.05; FBM 2,14 = 4.93; P = 0.044). For L. ruibali, endurance capacity varied from 89 to 178 s (125.0 ± 6.97) at 33 °C, and was not correlated with either SVL or BM (multiple regression: FSVL 2,11 = 2.22; FBM 2,11 = 3.73; P > 0.05). None of the species showed differences in endurance between males and females (t test: tP. extrilidus, 14 = − 0.22; tL. ruibali, 11 = − 0.14; P > 0.05; Mann–Whitney: UL. parvus = 31.5; P = 0.885).
Correlation analyses between long sprint vs. endurance and initial velocity vs. endurance, at the same temperature, were not significant for any of the species (LS: Pearson: rP. extrilidus = − 0.01; rL. parvus = − 0.39; rL. ruibali = − 0.12; P > 0.05; IV: Pearson: rP. extrilidus = 0.22; rL. parvus = − 0.29; rL. ruibali = − 0.02; P > 0.05).
Liolaemus parvus had lower endurance than L. ruibali and P. extrilidus. At the same time, differences were found between these two species, with endurance being greater for L. ruibali (ANCOVA: F (2,46) = 23.77; Bonferroni corrected PL. ruibali–L. parvus < 0.0001; PL. ruibali–P. extrilidus = 0.018; PP. extrilidus–L. parvus < 0.001; Fig. 2).
Thermal tolerance: CT min and CT max
The critical thermal minimum (CTmin) was higher for L. parvus than the other two species in the assemblage (ANCOVA: F(2,46) = 11.03; Bonferroni corrected PL. parvus–L. ruibali < 0.001; PL. parvus–P. extrilidus = 0.017; Table 4). In contrast, the critical thermal maximum was similar among the species (ANCOVA: F(2, 46) = 2.98; P = 0.104, Table 4). Phymaturus extrilidus, Liolaemus parvus, and L. ruibali showed a wide thermal tolerance (above 30 °C), and wide thermal safety margins (7–12 °C) for both types of runs. Thermal tolerance and thermal safety margins for long sprint and initial velocity of the three species are shown in Table 4.
The minimum and maximum critical temperatures showed no relationship with SVL and body mass for any of the species (multiple regression: P. extrilidus: FCTmin (2,19) = 1.24; FCTmax (2,19) = 0.28; L. parvus: FCTmin (2,13) = 2.57; FCTmax (2,13) = 0.98; L. ruibali: FCTmin (2,13) = 0.81; FCTmax (2,13) = 0.35; P > 0.05).
Optimum temperature at running speed (T o), performance range (B 80) and its relation to field body temperatures (T b), preferred body temperatures (T pref), and operative body temperatures (T e)
Field-active body temperature, preferred body temperature, and set-point range of the three species, are given in Table 4. For P. extrilidus, the thermal performance breadth (B80) was not reached in any of the temperature trials for both runs (LS and IV). To was similar to field body temperature, but below the set-point range (Fig. 1; Table 4). The thermal performance breadth (B80) for L. parvus was between 28 and 38 °C for long sprint and between 23 and 38 °C for the initial velocity. To for LS was similar to Tb and not within the set-point range, while To for IV was greater to Tb and was included in the set-point range (Fig. 1; Table 4). For L. ruibali, the B80 range was only reached at 33 °C for LS and 28 °C for IV. To for IV was less to Tb and was not included in the set-point range of Tpref, while To for LS was greater to Tb and was included in the set-point range (Fig. 1; Table 4). Finally, To of the three species of the assemblage were markedly greater than the operative environmental temperature (Te; 24.55 ± 5.41 °C; Gómez Alés et al. 2017; Fig. 1).
Discussion
Morphological differences between lizard species are often associated with different performance capacities, such as running speed and endurance, and may generate variation in the locomotor capacity of individuals in different ecological contexts (Arnold 1983; Miles 1994; Macrini and Irschick 1998; Calsbeek and Irschick 2007). Running speed was not related to body length or body mass in any of the species. In contrast, we found that body mass was positively related to endurance in L. parvus. The absence of a significant relationship between SVL, body mass, and speed has been reported for other Liolaemus species such as L. sarmientoi, L. magellanicus, and L. pictus (Fernández et al. 2011; Kubisch et al. 2011) and for some species of the group L. lineomaculatus (Bonino et al. 2011). The absence of an association between body size (either SVL or mass) and speed (mainly used for the evasion of predators) can be interpreted as an advantage for these species. For example, they could reduce a trade-off between gaining energy and capacity to escape (Pérez Tris et al. 2004; Zamora Camacho et al. 2014), as observed in Psammodromus algirus, where mass losses induced by evasion of predation did not improve the escape performance during predator attacks, and fat lizards did not show variation in escape performance (Martín 1996; Pérez Tris et al. 2004).
In general, performance increased with temperature up to the thermal optimum, and then decreased beyond this value (Fig. 1). This pattern has been reported for Liolaemini lizards (Bonino et al. 2011; Fernández et al. 2011; Kubisch et al. 2011, 2016) and for lizards in general (Hertz et al. 1983; Bennett 1990; Ibargüengoytía et al. 2007; Gaby et al. 2011; Beal et al. 2014; McElroy 2014; among others), indicating that locomotor performance is sensitive to temperature. Nevertheless, the thermal performance breadth of running speeds (IV and LS) was wide for the three species of the assemblage. This pattern is similar to the world’s southernmost species, L. magellanicus and L. sarmientoi, which attain high speeds at a wide range of temperatures. The pattern of locomotor efficiency at low temperatures may be interpreted as an adaptation to cold and variable environments (Fernández et al. 2011). The climate in the Puna region is cold, dry, and characterized by large thermal amplitudes and strong winds (Roig Juñent et al. 2003). Consequently, the ability to achieve high speeds across a wide range of temperatures provides an enhanced ability of these lizards to escape from predators, optimize food acquisition, and affect social interactions with conspecifics (e.g., territorial defense and reproduction), relative to lizards that have a narrow performance breadth (B80), as in some tropical forest lizards (Hertz et al. 1983; van Berkum 1988; Huey et al. 2009).
Our measurement of long sprint over a longer distance has implications for foraging, territorial defense, and courtship, whereas the initial velocity may have a greater impact on the ability to flee from predators (Huey et al. 2009; Cabezas Cartes et al. 2014). Given the differences in the functional consequences of IV and LS, we expected to observe a trade-off in performance. We note that, in P. extrilidus and L. parvus, the initial velocity is higher than long sprint over 1 m, which has been observed in the other species of Liolaemus (Fernández et al. 2011). In L. ruibali, although there are no differences between LS and IV, we found higher values of long sprint at 38 °C, which coincides with To.
The observed differences in performance could be related to the type of habitat utilized; P. extrilidus and L. parvus inhabit primarily rocky areas and meadows with vegetation nearby, making it unnecessary to travel great distances to obtain food, but optimizing flight speeds (IV) should be favored by selection as these species are more exposed to predators in rocky areas (Corbalán and Debandi 2013; Gómez Alés et al. 2017; Acosta et al. 2018). On the other hand, L. ruibali inhabits flat areas with low vegetation cover and open shrubland (Villavicencio et al. 2005; Acosta et al. 2018), so higher performance capacities in both estimates of speed would be beneficial for both foraging and fleeing from predators (Pietrek et al. 2009; Fuller et al. 2011).
No correlation was found between speed and endurance, which suggests a decoupling between these two measures of locomotor performance (Tsuji et al. 1989; Sorci et al. 1995). Other differences observed between the species include running speeds and endurance, where a trade-off is evident as was found by Vanhooydonck et al. (2001). Liolaemus parvus has greater running speed and lower endurance, while L. ruibali has lower running speeds and greater endurance than of the other species (Fig. 2), which could be due to differences in foraging behavior between species (Miles et al. 2007a, b). Huey et al. (1984) proposed that differences in locomotor capacity coevolve with feeding behavior. These authors found that widely-foraging lizards of the genus Eremias have greater endurance, whereas “sit-and-wait” species have greater running speeds. In this sense, the higher endurance of L. ruibali could be related to its feeding habits, as it is an omnivorous species characterized by a mixed foraging strategy (Villavicencio et al. 2005; Pérez unpublished data). Similarly, P. extrilidus is herbivorous, as are other species in the genus, and moves frequently among edible plants, which would explain its intermediate endurance. On the other hand, given that L. parvus feeds mainly on insects using a mixed foraging strategy (Acosta et al. 2018; Pérez unpublished data), maximizing its running speeds could also be advantageous.
In addition, the type of microhabitat could influence locomotion. Greater endurance could be linked to lizards that inhabit open areas with a little vegetation coverage and must, therefore, travel greater distances to find shelter (Jayne and Bennett 1990; Miles et al. 2001), which could explain greater endurance for L. ruibali. The saxicolous P. extrilidus and L. parvus reached greater speeds than L. ruibali, which is found in flat substrates. This differs from the pattern reported by Tulli et al. (2012), who found that saxicolous species were the slowest of the Liolaemus group studied. Moreover, many authors have established that differences in the utilization of microhabitats could lead to morphological differences (Arnold 1983; Lauder and Reilly 1991; Vanhooydonck and Van Damme 1999; Schulte et al. 2004; Goodman et al. 2008; Tulli et al. 2009) and that differences in body shape and limbs could have implications on locomotor capacity (Tulli et al. 2012). Although we did not find a link between SVL and performance, we did not include other morphological variables, such as forelimb and hind-limb length, in our analyses. These traits are known to positively influence locomotor performance (Miles 1994; Bonine and Garland Jr. 1999; Vanhooydonck and Van Damme 1999; Gifford et al. 2008; Kohlsdorf and Navas 2012). Therefore, we hypothesize that different lengths of the limbs among the species (longer limbs in P. extrilidus and shorter in L. ruibali; Castro and Castillo unpublished data), could explain, in part, the interspecific differences in locomotor performance.
Thermal sensitivity in performance among ectotherms can be represented by an asymmetrical, nonlinear curve, thermal performance curve (TPC) that describes the effect of temperature on fitness (Huey and Stevenson 1979; Angilletta et al. 2002). Thermal tolerance (CTmax − CTmin) describes the range of temperatures where performance is greater than 0 and can be quite broad. However, the thermal performance breadth, defined as the range of temperatures at which performance is at 80% of maximum, is often narrower. If the thermal performance breadth is broad, then thermal limits will probably not play an important role in defining locomotor efficiency. However, if the thermal performance breadth is narrow, any change in the critical temperatures for acclimation will affect a lizard’s locomotor capacity, at least at extreme temperatures (Kaufmann and Bennett 1989). In the context of global climate change, the studied species could cope with an increase in ambient temperatures, at least in locomotor performance, as the difference in temperatures between the performance extremes evaluated and the thermal limits obtained is wide (Fig. 1). Puna lizards are thermal generalists and have a wide thermal tolerance zone as was evident in the broad thermal safety margin (TSM; Table 4) for all three species, compared to the low TSM of Liolaemus pictus (0.43–0.64 °C) a lizard from the cold temperate climate (Kubisch et al. 2016) or tropical species of Anolis (A. lemurinus 1.5 °C, A bicaorum 2.5 °C, A. roatanensis 3.4 °C, A. allisoni 4–5.5 °C; Logan et al. 2013). The thermal safety margins of Puna lizards suggest that, in the event of an increase in temperature, these species may not be as affected by impact of global warming on performance. Nevertheless, to test this hypothesis, it would be important to assess the flexibility of thermal parameters and performance for these species by conducting studies on acclimation to different temperatures.
We have shown how adjustments in thermal physiology are favorable for lizards living in cold and variable environments. In particular, a low thermal sensitivity is advantageous, because it allows the ability to achieve near maximal levels of locomotor performance (broader thermal performance breadth) at low Tb’s as in Liolaemus pictus from the Patagonian region of Argentina (Kubisch et al. 2016) and high elevation populations of Phrynocephalus vlangalii from Qinghai-Tibetan Plateau (Wu et al. 2018). Similarly, according to our predictions, L. parvus and L. ruibali, and to a lesser extent P. extrilidus, show a broad thermal performance breadth; this may be related to pronounced variation of environment temperature in the Puna region and to high variability on Tbs that these species experience in the field (25–38 °C; Gómez Alés et al. 2017). Apparently, L. parvus has the lowest thermal sensitivity in running speed. Several studies have shown that the physiological traits of species are related to its geographical distribution range (Buckley 2010; Kearney and Porter 2009; Bonino et al. 2015; Artacho et al. 2017). Thus, the narrow thermal performance breadth in P. extrilidus with respect to L. parvus, could be linked to the endemic character of P. extrilidus, whose distribution is restricted to our study site, whereas L. parvus and L. ruibali exhibit a broad latitudinal and altitudinal distribution (Lobo et al. 2012; Acosta et al. 2018). These two latter species may be thermal generalists. Similar results were found by Bonino et al. (2015), who observed in a group of Liolaemini species broader thermal performance breadth in species of wide distribution, suggesting a higher physiological plasticity in comparison with Phymaturus tenebrosus, a species with a highly restricted geographic distribution. Alternatively, differences in thermal performance breadth between syntopic species could reflect adaptations to divergent selective agents, e.g., variation in antipredator behavior, prey selection, or thermoregulation (Tepler et al. 2011; Yuan et al. 2016; Gómez Alés et al. 2017). Likewise, spatial differences in microclimates within a habitat type may confer greater tolerance to environmental variability in a species (Yuan et al. 2016). In this sense, L. parvus is an efficient thermoregulator and generalist in use of trophic and spatial resources, exploiting a wide range of thermally different microhabitats (Gómez Alés et al. 2017; Acosta et al. 2018), which would favor a broader thermal performance breadth with respect to L. ruibali and P. extrilidus.
The optimal temperature for locomotor performance has been evaluated for a limited number of species in the genus Phymaturus. Bonino et al. (2015) reported a To of 35.4 °C for P. tenebrosus in Patagonia, which is higher than the To that we found for P. extrilidus. However, our To results for the three species are within the range of temperatures reported for Liolaemini lizards (30.4–36.1 °C; Bonino et al. 2011, 2015; Fernández et al. 2011). One exception is the To for L. pictus, which has one of the lowest To values of the group (26 °C; Kubisch et al. 2011). Some authors have found a close correspondence between thermal optimum and preferred temperatures, and that maximum speeds often fall within the set-point range (Martín and Huey 2008; Bonino et al. 2011; Gaby et al. 2011). Such a pattern suggests coadaptation between preferred temperature and thermal optimum (Huey and Bennett 1987). We observed an overlap between To and Tpref for only Liolaemus parvus in IV and L. ruibali in LS. In contrast, Tpref for P. extrilidus was higher than the To (Fig. 1). We hypothesize that Tpref is selected to optimize other physiological functions such as digestion or endurance capacity (Angilletta 2009). Although we did not measure locomotor performance at each species Tpref (Table 4), we endeavored to select temperatures that bracketed the observed field body temperatures and potentially warmer ambient temperatures expected with climate change. Lizard species occupying environments at high altitude and latitude, e.g., the Puna region, have limited availability of thermally suitable microhabitats and must engage in daily activities at low temperatures. Our results demonstrate the three species in our sample which achieved maximum performance at body temperatures commonly experienced during daily activity. Moreover, the field-active body temperatures are even higher than operative environmental temperatures (Fig. 1). Contrary to expectations, L. parvus and L. ruibali exhibited clear differences in running speeds and endurance, and, at the same time, differed from P. extrilidus. Consequently, despite their phylogenetic relatedness, and living in similar environmental conditions, differences in microhabitats use and trophic habits may explain the differences observed in speed and endurance.
We conclude that locomotor performance is sensitive to temperature in the studied species; nevertheless, there is a wide range of temperatures, mainly in L. parvus, in which the performance of species attain 80% or greater of the maximum speed, which allows them to face the highly variable climatic conditions of the Puna region.
References
Abdala CS, Quinteros AS (2014) Los últimos 30 años de estudios de la familia de lagartijas más diversa de Argentina: Actualización taxonómica y sistemática de Liolaemidae. Cuad Herpetol 28:55–82
Acosta JC, Blanco GM, Gómez Alés R, Acosta R, Piaggio Kokot L, Victorica AE, Villavicencio HJ, Fava GA (2018) Los Reptiles de San Juan. Editorial Brujas, Córdoba
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford
Angilletta MJ, Hill T, Robson MA (2002) Is physiological performance optimized by thermoregulatory behavior?: a case study of the eastern fence lizard, Sceloporus undulatus. J Therm Biol 27:199–204
Arnold SJ (1983) Morphology, performance and fitness. Am Zool 23:347–361
Artacho P, Saravia J, Perret S, Bartheld JL, Le Galliard JF (2017) Geographic variation and acclimation effects on thermoregulation behavior in the widespread lizard Liolaemus pictus. J Therm Biol 63:78–87
Beal MS, Lattanzio MS, Miles DB (2014) Differences in the thermal physiology of adult Yarrow’s spiny lizards (Sceloporus jarrovii) in relation to sex and body size. Ecol Evol 4:4220–4229
Bennett AF (1990) Thermal dependence of locomotor capacity. Am J Physiol Regul Integr Comp Physiol 259:253–258
Bonine KE, Garland T Jr (1999) Sprint performance of phrynosomatid lizards, measured on a high-speed treadmill, correlates with hind limb length. J Zool 248:255–265
Bonino MF, Azócar DLM, Tulli MJ, Abdala CS, Perotti MG, Cruz FB (2011) Running in cold weather: morphology, thermal biology, and performance in the southernmost lizard clade in the world (Liolaemus lineomaculatus section: Liolaemini: Iguania). J Exp Zool A Ecol Gen Physiol 315:495–503
Bonino MF, Azócar DLM, Schulte JA, Abdala CS, Cruz FB (2015) Thermal sensitivity of cold climate lizards and the importance of distributional ranges. Zoology 118:281–290
Buckley LB (2010) The range implications of lizard traits in changing environments. Global Ecol Biogeogr 19:452–464
Byers J, Hebets E, Podos J (2010) Female mate choice based upon male motor performance. Anim Behav 79:771–778
Cabezas Cartes F, Kubisch EL, Ibargüengoytía NR (2014) Consequences of volcanic ash deposition on the locomotor performance of the Phymaturus spectabilis lizard from Patagonia, Argentina. J Exp Zool A Ecol Gen Physiol 321:164–172
Cabrera AL (1994) Enciclopedia Argentina de agricultura y jardinería, Tomo II, Fascículo 1: regiones fitogeográficas Argentinas. ACME, Buenos Aires
Calsbeek R, Cox RM (2010) Experimentally assessing the relative importance of predation and competition as agents of selection. Nature 465:613–616
Calsbeek R, Irschick DJ (2007) The quick and the dead: correlational selection on morphology, performance, and habitat use in island lizards. Evolution 61:2493–2503
Chandler CR (1995) Practical considerations in the use of simultaneous inference for multiple tests. Anim Behav 49:524–527
Corbalán V, Debandi G (2013) Basking behaviour in two sympatric herbivorous lizards (Liolaemidae: Phymaturus) from the Payunia volcanic region of Argentina. J Nat Hist 23:56–63
Crowley SR (1985) Thermal sensitivity of sprint-running in the lizard Sceloporus undulatus: support for a conservative view of thermal physiology. Oecologia 66:219–225
Cruz FB, Fitzgerald LA, Espinoza RE, Schulte IIJA (2005) The importance of phylogenetic scale in tests of Bergmann’s and Rapoport’s rules: lessons from a clade of South American lizards. J Evol Biol 18:1559–1574
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci 105:6668–6672
Díaz Gómez JM (2009) Historical biogeography of Phymaturus (Iguania: Liolaemidae) from Andean and Patagonian South America. Zool Scr 28:1–7
Fernández JB, Ibargüengoytía NR (2012) Does acclimation at higher temperatures affect the locomotor performance of one of the southernmost reptiles in the world? Acta Herpetol 7:281–296
Fernández JB, Smith J, Scolaro A, Ibargüengoytía NR (2011) Performance and thermal sensitivity of the southernmost lizards in the world, Liolaemus sarmientoi and Liolaemus magellanicus. J Therm Biol 36:15–22
Fuller PO, Higham TE, Clark AJ (2011) Posture, speed, and habitat structure: three-dimensional hindlimb kinematics of two species of padless geckos. Zoology 114:104–112
Gaby MJ, Besson AA, Bezzina CN, Caldwell AJ, Cosgrove S, Cree A, Hare KM (2011) Thermal dependence of locomotor performance in two cool-temperate lizards. J Comp Physiol A 197:869–875
Garland T Jr, Hankins E, Huey RB (1990) Locomotor capacity and social dominance in male lizards. Funct Ecol 4:243–250
Gaston KJ, Blackburn TM (2000) Pattern and process in macroecology. Blackwell Science, Malden
Gifford ME, Herrel A, Mahler DL (2008) The evolution of locomotor morphology, performance, and anti-predator behaviour among populations of Leiocephalus lizards from the Dominican Republic. Biol J Linn Soc 93:445–456
Gilbert AL, Miles DB (2017) Natural selection on thermal preference, critical thermal maxima and locomotor performance. Proc R Soc B 284:20170536
Gómez Alés R, Acosta JC, Laspiur A (2017) Thermal biology in two syntopic lizards, Phymaturus extrilidus and Liolaemus parvus, in the Puna region of Argentina. J Therm Biol 68:73–82
Goodman BA, Miles DB, Schwarzkopf L (2008) Life on the rocks: habitat use drives morphological and performance evolution in lizards. Ecology 89:3462–3471
Hertz PE, Huey RB, Nevo E (1983) Homage to Santa Anita: thermal sensitivity of sprint speed in agamid lizards. Evolution 37:1075–1084
Huey RB, Bennett AF (1987) Phylogenetic studies of coadaptation: preferred temperatures versus optimal performance temperatures of lizards. Evolution 41:1098–1115
Huey RB, Kingsolver JG (1989) Evolution of thermal sensitivity of ectotherm performance. Trends Ecol Evol 4:131–135
Huey RB, Slatkin M (1976) Cost and benefits of lizard thermoregulation. Q Rev Biol 51:363–384
Huey RB, Stevenson RD (1979) Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Am Zool 19:357–366
Huey RB, Bennett AF, John Alder H, Nagy KA (1984) Locomotor capacity and foraging behaviour of Kalahari lacertid lizards. Anim Behav 32:41–50
Huey RB, Dunham AE, Overall KL, Newman RA (1990) Variation in locomotor performance in demographically known populations of the lizard Sceloporus merriami. Physiol Zool 63:845–872
Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Pérez HJÁ, Garland T (2009) Why tropical forest lizards are vulnerable to climate warming. Proc R Soc Lond B Biol Sci 276:1939–1948
Husak JF, Fox SF, Lovern MB, Bussche RA (2006) Faster lizards sire more offspring: sexual selection on whole-animal performance. Evolution 60:2122–2130
Ibargüengoytía NR, Renner ML, Boretto JM, Piantoni C, Cussac VE (2007) Thermal effects on locomotion in the nocturnal gecko Homonota darwini (Gekkonidae). Amphib Reptil 28:235–246
Ibargüengoytía NR, Cabezas Cartes F, Boretto JM, Piantoni C, Kubisch EL, Fernández MS, Lara Resendiz RA, Méndez De La Cruz FR, Scolaro A, Sinervo B (2016) Volcanic ash from Puyehue-Cordón Caulle eruptions affects running performance and body condition of Phymaturus lizards in Patagonia, Argentina. Biol J Linn Soc 118:842–851
Irschick DJ, Meyers JJ (2007) An analysis of the relative roles of plasticity and natural selection in the morphology and performance of a lizard (Urosaurus ornatus). Oecologia 153:489–499
Jacobson ER, Whitford WG (1970) The effect of acclimation on physiological responses to temperature in the snakes, Thamnophis proximus and Natrix rhombifera. Comp Biochem Physiol 35:439–449
Janzen DH (1967) Why mountain passes are higher in the tropics. Am Nat 101:233–249
Jayne BC, Bennett AF (1990) Selection on locomotor performance capacity in a natural population of garter snakes. Evolution 44:1204–1229
Kaufmann JS, Bennett AF (1989) The effect of temperature and thermal acclimation on locomotor performance in Xantusia vigilis, the desert night lizard. Physiol Zool 62:1047–1058
Kearney M, Porter W (2009) Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol Lett 12:334–350
Kingsolver JG (2009) The well-temperatured biologist. Am Nat 174:755–768
Kohlsdorf T, Navas C (2012) Evolution of form and function: morphophysiological relationships and locomotor performance in Tropidurine lizards. J Zool 288:41–49
Kubisch EL, Fernández JB, Ibargüengoytía NR (2011) Is locomotor performance optimized at preferred body temperature? A study of Liolaemus pictus argentinus from northern Patagonia, Argentina. J Therm Biol 36:328–333
Kubisch EL, Fernández JB, Ibargüengoytía NR (2016) Vulnerability to climate warming of Liolaemus pictus (Squamata, Liolaemidae), a lizard from the cold temperate climate in Patagonia, Argentina. J Comp Physiol B 186:243–253
Lauder GV, Reilly SM (1991) Behavior, morphology, and muscle function-the physiological bases of behavioral evolution. Am Zool 31:1041
Le Galliard JF, Clobert J, Ferrière R (2004) Physical performance and Darwinian fitness in lizards. Nature 432:502–505
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lobo F, Espinoza RE, Sanabria EA, Quiroga LB (2012) A new Phymaturus (Iguania: Liolaemidae) from the southern extreme of the Argentine Puna. Copeia 1:12–22
Logan ML, Huynh RK, Precious RA, Calsbeek RG (2013) The impact of climate change measured at relevant spatial scales: new hope for tropical lizards. Glob Change Biol 19(10):3093–3102
Macrini TE, Irschick DJ (1998) An intraspecific analysis of trade-offs in sprinting performance in a West Indian lizard species (Anolis lineatopus). Biol J Linn Soc 63:579–591
Márquez J, Ripoll Y, Dalmasso A, Ariza M, Jordan M (2014) Árboles Nativos de la provincia de San Juan. Universidad Nacional de San Juan, San Juan
Marsh RL, Bennett AF (1986) Thermal dependence of sprint performance of the lizard Sceloporus occidentalis. J Exp Biol 126:79–87
Martín J (1996) Effects of recent feeding on locomotor performance of juvenile Psammodromus algirus lizards. Funct Ecol 10:390–395
Martín TL, Huey RB (2008) Why “suboptimal” is optimal: Jensen’s inequality and ectotherm thermal preferences. Am Nat 171:102–118
Martínez Carretero E (1995) La Puna Argentina: Delimitación general y división en distritos florísticos. Bol Soc Argent Bot 31:27–40
McElroy MT (2014) Countergradient variation in locomotor performance of two sympatric Polynesian skinks (Emoia impar, Emoia cyanura). Physiol Biochem Zool 87:222–230
Miles DB (1994) Population differentiation in locomotor performance and the potential response of a terrestrial organism to global environmental change. Am Zool 34:422–436
Miles DB (2004) The race goes to the swift: fitness consequences of variation in sprint performance in juvenile lizards. Evol Ecol Res 6:63–75
Miles DB, Sinervo B, Frankino WA (2000) Reproductive burden, locomotor performance, and the cost of reproduction in free ranging lizards. Evolution 54:1386–1395
Miles DB, Snell HL, Snell HM (2001) Intrapopulation variation in endurance of Galapagos lava lizards (Microlophus albemarlensis): evidence for an interaction between natural and sexual selection. Evol Ecol Res 3:795–804
Miles DB, Calsbeek R, Sinervo B (2007a) Corticosterone, locomotor performance, and metabolism in side-blotched lizards (Uta stansburiana). Horm Behav 51:548–554
Miles DB, Losos JB, Irschick DJ (2007b) Morphology, performance, and foraging mode. In: Reilly LB, McBrayer LB, Miles DB (eds) Lizard ecology: the evolutionary consequences of foraging mode. Cambridge University Press, Cambridge, pp 49–93
Pérez Tris J, Díaz JA, Tellería JL (2004) Loss of body mass under predation risk: cost of antipredatory behaviour or adaptive fit-for-escape? Anim Behav 67:511–521
Pietrek AG, Walker RS, Novaro AJ (2009) Susceptibility of lizards to predation under two levels of vegetative cover. J Arid Environ 73:574–577
Pinch FC, Claussen DL (2003) Effects of temperature and slope on the sprint speed and stamina of the Eastern Fence Lizard, Sceloporus undulatus. J Herpetol 37:671–679
Pough FH, Gans C (1982) The vocabulary of reptilian thermoregulation. Biol Reptil 12:17–23
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Robson MA, Miles DB (2000) Locomotor performance and dominance in male tree lizards, Urosaurus ornatus. Funct Ecol 14:338–344
Roig F, Martínez Carretero E (1998) La vegetación puneña de la provincia de Mendoza, Argentina. Phitocoenologia 28:565–608
Roig Juñent S, Flores GE, Mattoni C (2003) Consideraciones biogeográficas de la Precordillera (Argentina), con base en artrópodos epigeos. In: Morrone JJ, Llorente Bousquets J (eds) Una perspectiva latinoamericana de la Biogeografía. Las prensas de Ciencias, Facultad de Ciencias. Universidad Nacional de México, México, pp 275–288
Schulte JA, Losos JB, Cruz FB, Núñez H (2004) The relationship between morphology, escape behaviour and microhabitat occupation in the lizard clade Liolaemus (Iguanidae: Tropidurinae: Liolaemini). J Evol Biol 17:408–420
Sheth SN, Angert AL (2014) The evolution of environmental tolerance and range size: a comparison of geographically restricted and widespread Mimulus. Evolution 68:2917–2931
Sokal RR, Rohlf FJ (1969) Biometry: the principles and practice of statistics in biological research. WH Freeman and Company, San Francisco
Sorci G, Swallow JG, Garland T Jr, Clobert J (1995) Quantitative genetics of locomotor speed and endurance in the lizard Lacerta vivipara. Physiol Zool 68:698–720
Strobbe F, McPeek MA, De Block M, De Meester L, Stoks R (2009) Survival selection on escape performance and its underlying phenotypic traits: a case of many-to-one mapping. J Evol Biol 22:1172–1182
Tepler S, Mach K, Denny M (2011) Preference versus performance: body temperature of the intertidal snail Chlorostoma funebralis. Biol Bull 220:107–117
Tsuji JS, Huey RB, Van Berkum FH, Garland T Jr, Shaw RG (1989) Locomotor performance of hatchling fence lizards (Sceloporus occidentalis): quantitative genetics and morphometric correlates. Evol Ecol 3:240–252
Tulli MJ, Cruz FB, Herrel A, Vanhooydonck B, Abdala V (2009) The interplay between claw morphology and microhabitat use in neotropical iguanian lizards. Zoology 112:379–392
Tulli MJ, Abdala V, Cruz FB (2012) Effects of different substrates on the sprint performance of lizards. J Exp Biol 215:774–784
van Berkum FH (1988) Latitudinal patterns of the thermal sensitivity of sprint speed in lizards. Am Nat 132:327–343
Vanhooydonck B, Van Damme R (1999) Evolutionary relationships between body shape and habitat use in lacertid lizards. Evol Ecol Res 1:785–805
Vanhooydonck B, Van Damme R, Aerts P (2001) Speed and stamina trade-off in lacertid lizards. Evolution 55:1040–1048
Vanhooydonck B, Measey J, Edwards S, Makhubo B, Tolley KA, Herrel A (2015) The effects of substratum on locomotor performance in lacertid lizards. Biol J Linn Soc 115:869–881
Villavicencio HJ, Acosta JC, Cánovas MG (2005) Dieta de Liolaemus ruibali Donoso Barros (Iguania: liolaeminae) en la reserva de usos múltiples Don Carmelo, San Juan, Argentina. Multequina 14:47–52
Wood SN (2017) Generalized additive models: an introduction with R, 2nd edn. Chapman and Hall/CRC, Boca Raton
Wu Q, Dang W, Hu YC, Lu HL (2018) Altitude influences thermal ecology and thermal sensitivity of locomotor performance in a toad-headed lizard. J Therm Biol 71:136–141
Yuan FL, Pickett EJ, Bonebrake TC (2016) Cooler performance breadth in a viviparous skink relative to its oviparous congener. J Therm Biol 61:106–114
Zajitschek SR, Zajitschek F, Miles DB, Clobert J (2012) The effect of coloration and temperature on sprint performance in male and female wall lizards. Biol J Linn Soc 107:573–582
Zamora Camacho FJ, Reguera S, Rubiño Hispán MV, Moreno Rueda G (2014) Effects of limb length, body mass, gender, gravidity, and elevation on escape speed in the lizard Psammodromus algirus. Evol Biol 41:509–517
Acknowledgements
We thank Arturo Curatola and Andres Calderon for permission to work in Reserva “Don Carmelo”; Nora Ibargüengoytía for lend us the track to trials sprint speed and Alyson Nuñez for assisting us with the English version. Thanks also to Secretaría de Medio Ambiente y Dirección de Conservación y Áreas Protegidas, Provincia de San Juan for research permits. Financial support was received from Beca CICITCA (Res. 1767/14-R, RGA) and project CICITCA 881 (JCA). This research was partially supported by the Universidad Nacional de San Juan and Consejo Nacional de Investigaciones Científicas y Técnicas (beca doctoral CONICET, Res. 2358/14, RGA). Miles was supported by NSF Grant (EF128428).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm there are no known conflicts of interest associated with this publication.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was not required.
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Gómez Alés, R., Acosta, J.C., Astudillo, V. et al. Effect of temperature on the locomotor performance of species in a lizard assemblage in the Puna region of Argentina. J Comp Physiol B 188, 977–990 (2018). https://doi.org/10.1007/s00360-018-1185-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-018-1185-y