Abstract
From both comparative biology and translational research perspectives, there is escalating interest in understanding how animals navigate their environments. Considerable work is being directed towards understanding the sensory transduction and neural processing of environmental stimuli that guide animals to, for example, food and shelter. While much has been learned about the spatial orientation behavior, sensory cues, and neurophysiology of champion navigators such as bees and ants, many other, often overlooked animal species possess extraordinary sensory and spatial capabilities that can broaden our understanding of the behavioral and neural mechanisms of animal navigation. For example, arachnids are predators that often return to retreats after hunting excursions. Many of these arachnid central-place foragers are large and highly conducive to scientific investigation. In this review we highlight research on three orders within the Class Arachnida: Amblypygi (whip spiders), Araneae (spiders), and Scorpiones (scorpions). For each, we describe (I) their natural history and spatial navigation, (II) how they sense the world, (III) what information they use to navigate, and (IV) how they process information for navigation. We discuss similarities and differences among the groups and highlight potential avenues for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The class Arachnida belongs to the phylum Arthropoda which includes, among others, insects and crustaceans. The approximately 98,000 described species of arachnids are divided into eleven orders, a few of which have attributes that make them outstanding research animals for navigation studies. For example, several are large, enabling the relatively easy ablation or masking of sensory organs for sensory-based navigation studies. Some can even accommodate radio transmitters. Many are long lived and easily maintained in the laboratory. Additionally, they live in a variety of habitats—from simple to complex—which opens comparative opportunities to study nuances related to navigation. Also, several species accept laboratory facsimiles of their native habitats, which allows for well-controlled behavioral studies. Some have even proven highly adaptable and accessible to electrophysiological recordings, enabling direct studies of sensory capacities that might underlie navigation.
Leveraging some subset of the above-mentioned attributes and techniques, robust spatial orientation information is now available for three arachnid orders: Amblypygi (whip spiders), Araneae (spiders), and Scorpiones (scorpions). Animals in these three orders display homing behavior at distances ranging from centimeters to hundreds of meters and different characteristics of spatial orientation have been the focus of study across these groups. Path integration and learning walks, for example, have been explored predominantly in spiders and scorpions, while the role of distinct sensory information in homing has been the primary focus of amblypygid research. Each of these groups has exceptional sensory attributes that make them important contributors to the neuroethological literature: the antenniform legs and enlarged mushroom bodies of amblypygids, the eyes and lyriform organs of spiders, and the pectines of scorpions.
This review focuses on amblypygid/spider/scorpion: (I) natural history and spatial navigation, (II) sensory abilities, (III) sensory information used for navigation, and (IV) processing of information for navigation. It builds upon recent reviews of arachnid spatial orientation (Gaffin and Curry 2020; Ortega-Escobar 2017, 2020) by incorporating important new research findings and focusing added attention on sensory systems that subserve specific navigational behaviour. We draw comparisons among the three groups while also being cognizant of the unique environmental and evolutionary forces that have selected for different neuroethological solutions to complex navigational challenges. We begin by describing and synthesizing the current literature for each group and pointing out the special behaviours and sensory attributes that have brought notice to these animals. In the end we place what we know about these animals in the context of general principles gleaned from other animal models, point out gaps in the literature, and suggest crucial avenues for further research.
Spatial orientation in Amblypygids
Natural history and spatial navigation
Amblypygids look like fictitious creatures one might see in a science fiction movie and, perhaps not surprisingly, they had a big-screen debut in the Harry Potter movie Goblet of Fire. These unusual looking arachnids are characterized by a dorso-ventrally flattened body, large raptorial pedipalps with species-specific supination (Seiter et al. 2022), and an elongate first pair of walking legs referred to as ‘antenniform legs’ that have taken on a sensory function (Weygoldt 2000; Fig. 1a). Not fictitious at all, amblypygids (sometimes called whip spiders because of their elongate antenniform legs) are nocturnal predators that can be found throughout the tropics and subtropics, with some species also inhabiting more temperate and desert zones (Weygoldt 2000; Chapin and Hebets 2016). These remarkable animals have changed little over evolutionary time, with complete fossils dating back to the Carboniferous (300 mya) and fossil fragments dating back 385 mya, to the Devonian (Dunlop 2010; Haug and Haug 2021). The order is monophyletic (Ban et al. 2022) and not particularly diverse in comparison to other arachnid orders, with just 255 currently described species compared to the spider Order Araneae with more than 50,300 currently described species (World Spider Catalog accessed 8 September 2022). But despite their strange appearance and elusive nocturnal behavior, amblypygids are quickly becoming a model study system in sensory ecology and navigation research due to their unique sensory and processing systems and their documented prowess at nocturnal homing.
The sensory structures hypothesized to be involved in navigation by amblypygids and results of displacements in the field. a Paraphrynus laevifrons fitted with a radio transmitter. b Scanning electron microscope image of the distal end of a Phrynus marginemaculatus antenniform leg and three types of sensilla from left to right: B, bristle (mechanosensory and contact chemosensory); P, multiporous sensillum (olfactory and hypothesized to be essential for amblypygid navigation); and C, club sensillum (contact chemosensory). c Trajectories of nocturnally displaced Paraphrynus laevifrons (misidentified as Phrynus pseudoparvulus in Hebets et al. 2014b; see Bingman et al. 2017), tracked with telemetry. Lines indicate the direction of movement from the release site (R) (top) or a stopover tree (middle, bottom). Numbers near arrowheads indicate the mornings after displacement that an individual was observed at a particular site. The question mark (middle) indicates that the exact morning that the subject returned to its home tree could not be determined. The catchment zone is the extension of tree buttresses in the direction an individual moved. [Results adapted from Hebets et al. 2014b]
Homing
In the field, amblypygids emerge from a home shelter—from crevices of tree buttresses or from under rocks—at night to hunt for prey in the vicinity of the shelter. An individual may be observed nightly over several weeks or months near the base of the same tree, demonstrating high site fidelity (Beck and Gorke 1974; Weygoldt 1977; Hebets 2002). Individuals have also been observed to wander distances of 30 m or more in the rainforest understory and to return to the location at which they were originally sighted several nights later (Hebets 2002).
Beck and Görke (1974) were the first to investigate amblypygid navigation in the field. In an early displacement study, nine Heterophrynus batesii were collected at night after they had emerged from the shelter of their crevices and were moved 2.5–7.5 m from their resident trees. Each individual returned to the tree from which it was removed on the same night it was displaced (Beck and Görke 1974). The team also displaced one individual 10 m, and observed that it also returned to its original tree sometime between two to five nights later. Beck and Görke (1974) noted that displaced individuals appeared to sample the air space around them with their antenniform legs, as if to orient themselves. To explore this observation further, the researchers secondarily displaced one subject after clipping the distal 30–50 articles of its antenniform-leg tarsi, which are the loci of olfactory, chemosensory and mechanosensory sensilla (Foelix 1975; Beck et al. 1977; Foelix and Hebets 2001; Santer and Hebets 2011b; Fig. 1b). They searched for this subject at the tree from which it was removed for several nights afterward, but it was never seen again. The inability of this manipulated animal to find its shelter hinted that amblypygid navigation involves odors or other cues detected by sensilla on the antenniform legs (Weygoldt 2000).
The results of Beck and Görke (1974) were corroborated and extended 40 years later by several nocturnal displacement experiments using Phrynus pseudoparvulus and Paraphrynus laevifrons. These species inhabit the rainforests of Central America and, like H. batesii, reside in tree crevices or holes at the base of a tree or in the ground. Displaced Phrynus pseudoparvulus, like H. batesii, successfully returned to their home tree when displaced up to 4.5 m. When displaced longer distances (6–8.7 m), they returned in 1–3 nights (Hebets et al. 2014b). Individuals equipped with radio transmitters demonstrated that the return route of individuals displaced longer distances typically involves a temporary residency in a crevice of one or more other trees (Hebets et al. 2014b, Fig. 1c).
In a telemetry study exploring the relative importance of different sensory inputs in nocturnal homing, the majority of Paraphrynus laevifrons individuals with all sensory systems intact returned to their home tree within 5 days after a 10 m displacement. The return routes again included multiple temporary stops (Bingman et al. 2017). Paraphrynus laevifrons have even been observed to successfully navigate back to the tree from which they were captured over the course of several nights from displacement distances as far as 25 m (1 of 2 displaced individuals returned; Wiegmann et al. 2016).
How do amblypygids sense the world?
Like all arachnids, amblypygids have eight legs, but they only use six for walking. As previously mentioned, their first pair of walking legs are modified into extraordinarily long sensory appendages (> 2.5 times the length of other walking legs) that can span more than 60 cm (Igelmund 1987; Weygoldt 2000; Foelix and Hebets 2001). These elongated legs are highly articulated, facilitating a large range of motion, and they are covered with distinct types of sensory hairs (Weygoldt 2000; Foelix and Hebets 2001; Santer and Hebets 2011b).
Based upon morphology, there are seven distinct types of sensilla on the antenniform legs of amblypygids—bristles (contact chemosensory and mechanosensory), leaflike sensilla (mechanosensory), porous sensilla (olfactory), club sensilla (chemosensory) and rod sensilla (unknown function) (reviewed in Foelix and Hebets 2001; Santer and Hebets 2011b). To date, most of the anatomical analyses of sensilla has been done on various Heterophrynus species and most of this work was done in the 1970s through early 1990s (Foelix 1975; Beck et al. 1977; Igelmund 1987; Igelmund and Wendler 1991a, 1991b). An excellent summary of sensillum type, location, length, diameter, base and pore characteristics, and presumed modality of sensation can be found in the review by Santer and Hebets (2011b, Table 1 therein). The majority of the antenniform leg sensilla are found on the distal most segments, and this, coupled with the length and numerous articulations of these legs, enable the animals to directly sample the environment in a controlled manner over a very large area relative to their body size. For example, individuals can reach into crevices with their bodies safely outside; they can sample air outside of the boundary layers of tree surfaces; and they can interact with potential rival conspecifics from a distance of several centimeters.
In addition to the previously discussed sensilla, amblypygids also possess trichobothria, or filiform sensilla capable of detecting air particle displacement (Reissland and Görner 1985; Barth 2000). Although trichobothria are notably absent on the tarsus of the antenniform legs of previously studied Heterophrynus species, a few trichobothria do occur on other antenniform leg segments and many more can be found on the walking legs (Igelmund 1987; Weygoldt 2000). These hairs enable amblypygids to detect air particle movement. They have been demonstrated to be involved in intraspecific communication in Phrynus marginemaculatus, as trichobothria on the patella of the walking legs can detect air particle movement generated through the ritualistic agonistic displays involving antenniform leg vibrations (Fowler-Finn and Hebets 2006; Santer and Hebets 2008, 2011a).
Chemo- and mechanoreception
While particular sensilla morphology suggested an olfactory function, the capacity for amblypygids to detect airborne chemicals (i.e. to smell) using their antenniform legs was confirmed with an electrophysiological study in the early 2000s (Hebets and Chapman 2000). In this study, the majority of 42 distinct chemicals from a range of chemical classes were successful in eliciting an excitatory or inhibitory response from an antenniform leg of the amblypygid Phrynus marginemaculatus, demonstrating that these arachnids can indeed detect airborne odors. No electrophysiological studies to date have explored contact chemoreception in amblypygids despite the presence of numerous presumed contact-chemosensory sensilla and the potential role of substrate-borne chemical stimuli to influence amblypygid navigation.
In terms of mechanoreception, an electrophysiological study of walking leg trichobothria demonstrated that air vibrations are sufficient to excite the trichobothria on the patella of this same species (Santer and Hebets 2008).
In addition to detecting air particle vibrations, amblypygids are also likely able to detect muscle contractions, hemolymph pressure, gravity, and vibrations on the substrate using slit sensilla, or mechanoreceptive slits in the cuticle, which are sensitive to cuticular stress (Barth 2002). The number and type of slit sensilla found on the antenniform legs of H. elaphus can be found in the review by Santer and Hebets (2011b, Table 2 therein; (based on work by Igelmund 1987)). To date, there are no electrophysiological studies exploring the sensory capacity of these sense organs, but one might expect that they are capable of proprioception; a function demonstrated by lyriform organs in spiders (see Spatial Orientation in Spiders).
Vision
Amblypygids possess eight eyes that broadly resemble the morphological characteristics of other arachnids. Their one pair of median eyes are slightly larger than their six lateral eyes and are elevated on the front region of prosoma, while one pair of three lateral eyes are found further back on the sides of the prosoma (Weygoldt 2000; Santer and Hebets 2011b; Lehmann and Melzer 2018). Like in many other arachnids, the rhabdomeres of the median eyes point towards the light and lack a tapetum while the rhabdomeres of the lateral eyes point away and possess a reflecting tapetum (Weygoldt 2000; Lehmann and Melzer 2018). Unfortunately, little is known about the neuroanatomy and physiology of the amblypygid visual system (reviewed in Lehmann and Melzer 2018; see also Sinakevitch et al. 2021). No physiological studies have been published on amblypygid vision, and thus, the capacity for visual detection remains largely unknown.
In summary, previous morphological and physiological studies on amblypygid sensory systems conclude that these nocturnal arachnids can taste (using contact chemoreceptive sensilla), smell (using multiporous sensilla), and can detect mechanoreceptive signals/cues (using trichobothria and other mechanoreceptive sensilla including slit sensilla) with their extraordinarily long sensory antenniform legs. Their vision has been much less studied as has their mechanoreceptive capacity in terms of proprioception.
What information do amblypygids use to navigate?
The amblypygid antenniform legs and their associated sensory organs are presumed to underlie their proficient nocturnal navigation (Beck and Görke 1974; Weygoldt 2000; Wiegmann et al. 2016) and numerous recent studies have provided additional support for this presumption. In this section, we summarize the evidence to date regarding the role of different sensory information in spatial orientation and navigation in amblypygids. As you will see, most navigation studies on amblypygids have thus far focused on allothetic cues for navigation. We also note that while most laboratory-based studies on amblypygid navigation and spatial orientation have employed experimental designs on a horizontal plane, spatial orientation on a vertical surface that more closely resembles the natural habitats of many species has also been established in the laboratory (Casto et al. 2020).
Chemosensory-based navigation
Two independent field studies used sensory-manipulated individuals in displacement trials to determine the importance of olfaction in nocturnal homing. In one study, zero of six Paraphrynus laevifrons individuals with the distal tips of their antenniform legs painted with nail polish, which covered the entire tarsus and thus the olfactory sensilla (see Hebets and Chapman 2000 for evidence of antenniform leg olfaction), were able to return home after a displacement of 10 m; and only one of five individuals with the tips of the antenniform legs cut to ablate olfactory inputs were able to successfully home (Bingman et al. 2017). In a study of Phrynus pseudoparvulus, individuals with the distal 1 cm of anteniform leg tarsi similarly cut were less likely to return home following a displacement of 8 m compared to olfaction-intact individuals (Hebets et al. 2014a). The same results were found when individuals were simply displaced from their shelter to the opposite side of their home tree (Hebets et al. 2014a). These field-study results strongly support a role of olfaction in amblypygid spatial orientation and navigation, but do not necessarily negate the importance of other sensory modalities.
In the laboratory, the nocturnal activity of amblypygids has been studied in arenas with artificial shelters. Results of these studies also support the hypothesis that navigation and shelter recognition, the terminal phase of a navigation route, rely on inputs from the antenniform legs with particular reliance on olfaction. For instance, Phrynus marginemaculatus wanders nightly in an arena and can be conditioned to return to a specific shelter that is cued by an odor (Graving et al. 2017). Self-deposited chemical cues have also been shown to be used for shelter recognition by P. marginemaculatus, suggesting that contact chemoreception may facilitate refuge detection at close range (Casto et al. 2019). In addition, shelter recognition by individuals that have been trained to discriminate between shelters based on odors is dramatically impaired when the tips of the antenniform legs are clipped (Wiegmann et al. 2019).
Vision-based navigation
The visual systems of both Phrynus pseudoparvulus and Paraphrynus laevifrons have been manipulated in field experiments involving displacement trials to explore the importance of vision in nocturnal homing. The results are equivocal. In one study, ten Phrynus pseudoparvulus had removable, occlusive dental resin covering all eight of their eyes and just one individual successfully homed (10%) after a displacement of 8 m. This result was not statistically distinct from the three of 10 (30%) vision-intact individuals that successfully homed (Hebets et al. 2014a). In another study, six of 10 visually deprived Paraphrynus laevifrons–all eight eyes painted with black nail polish–successfully returned home after a displacement of 10 m while eight of 10 control individuals returned (Bingman et al. 2017). These studies have small sample sizes and leave open a role for vision in nocturnal homing. The lack of statistical differences in the performance of vision-intact and vision-deprived individuals, however, suggests that vision is unlikely to be the dominant modality in amblypygid spatial orientation.
Although vision does not appear to be essential for successful navigation in the field, individuals can be trained to discriminate between shelters based on visual cues in the laboratory. Flanigan and colleagues (2021a) trained P. marginemaculatus to discriminate between two shelters based on patterns of black and white stripes, positioned on the ceiling or walls of an arena. The subjects successfully solved the discrimination problem when the visual stimuli were positioned on the ceiling of the arena, an ability that is lost when visual input to the medial eyes is obstructed. Performance on the discrimination task was less robust when stimuli were positioned on the arena walls (Flanigan et al. 2021a). These results match earlier work on ants (Oliveira and Hölldobler 1989) and suggest that, like ants, amblypygids that inhabit rainforests might use canopy orientation to navigate to a shelter.
Magnetoreception-based navigation
Magnetoreception does not appear to play a major role in amblypygid spatial orientation. When procedures similar to those used by Wiegmann and colleagues (2019) were employed to train P. laevifrons to discriminate between shelters based on a magnetic anomaly characterized by high total field intensity and 180° shift in the polarity of the ambient magnetic field, individuals failed to learn the discrimination task after 50 trials conducted over 10 daily sessions (Wiegmann et al. 2020). Similarly, in a field experiment, individual Phrynus marginemaculatus fitted with a powerful magnet exhibited return rates after displacement that were as good as individuals fitted with a similar-sized brass disc (Wiegmann et al. 2020).
Mechanoreception-based navigation
Mechanoreception-based navigation has not been studied to the same extent as chemical-based navigation in amblypygids. Nonetheless, amblypygids are clearly capable of mechanoreception-based learning, as they have been trained under two distinct experimental designs to discriminate between shelters based on tactile cues (Flanigan et al. 2021b; Santer and Hebets 2009).
Prior field experiments that used clipping of the antenniform leg segments to demonstrate decreased homing success were interpreted as supporting a primary role of olfaction in homing (Hebets et al. 2014a; Bingman et al. 2017), but these manipulations affected more than just olfactory sensilla (Santer 2019). Amblypygid antenniform legs uniquely possess an array of at least seven giant sensory afferents with cell bodies located in the distal-most segments of the antenniform leg (Igelmund and Wendler 1991a, 1991b; Spence and Hebets 2006; Santer and Hebets 2011b). At least four of these giant neurons (GNs) are known to have a mechanosensory function, with two (GN1 and GN2) known to receive overlapping fields of inputs from sensilla at the tip of the antenniform leg. Two additional GNs (GN6 and GN7) are stimulated with slit sensilla sensitive to movement between the 21st and 22nd articulation of the antenniform leg distal-tip (Igelmund and Wendler 1991a, 1991b; Spence and Hebets 2006; Santer and Hebets 2011b). Finally, Santer and Hebets (2009, 2011b) describe the distinctive movements of the antenniform legs when amblypygids sample tactile stimuli. These movements are suggested to facilitate the coding of tactile information like shape and texture. Thus, based upon the sensory and processing system alone, there appears strong potential for a role of mechanosensation in amblypygid navigation (Santer 2019).
Multisensory-based navigation
The olfactory and tactile experiments conducted in the laboratory suggest, like the field studies, that the navigational abilities of amblypygids may be primarily mediated by sensory inputs to their antenniform legs and that olfactory cues, which could in principle function at long distances, may be critical. However, overcoming the sensory and cognitive challenges associated with navigating distances of 10 m or more while embedded in the sensory noise in the understory of a dense tropical rain forest would seem to benefit from a protective redundancy that could come with a spatial representation derived from the integration of multisensory inputs (Wiegmann et al. 2016). Therefore, the experimental demonstration that vision is not necessary for Paraphrynus laevifrons to navigate back to its home refuge (Bingman et al. 2017) does mean that vision does not contribute to navigational success when visual cues are available (see Flanigan et al. 2021a). But is there any evidence of multisensory control of amblypygid spatial behavior, and if so, what nervous system structures might be important for any multisensory control?
In a revealing study, Flanigan et al. (2021b) successfully trained amblypygids to recognize a home shelter characterized by both a distinctive olfactory and tactile cue (Fig. 2). The surprising result was that when individuals were tested for shelter recognition with either of the two stimulus elements alone, they were unable to locate their home shelter (Fig. 2). The authors concluded that the amblypygids learned a configural (see Pearce 2002), multisensory and odor-tactile representation of the home shelter. In other words, the home shelter was uniquely recognized by the integrated association of the odor and tactile stimuli such that neither of the stimuli alone could control behavior. It was proposed that such a configural and integrated multisensory representation would support navigation by reducing ambiguity in encoding a shelter’s defining sensory characteristics, as predicted by Wiegmann et al. (2016).
Amblypygids are able to use the configuration of multimodal cues to recognize a shelter (Flanigan et al. 2021b). Experimental design of experiments (left panel) in which subjects were trained to discriminate between accessible (CS+) and inaccessible (CS–) shelters (plastic cylinders) cued by tactile (T) or olfactory (O) stimuli, where T (sandpaper that varied in coarseness, blue parallelograms) and O (odorants geraniol or 1-hexanol, red and blue clouds in cylinders) stimuli were conditioned either singly (top left panel) or as pairs (bottom left panel; modified from Flanigan et al. 2021b Fig. 1). In tests, subjects were given a choice between two inaccessible shelters cued by conditioned stimuli. Individuals trained on a single cue readily discriminated between shelters in tests (top right panel), whereas subjects trained on TO pairs of stimuli failed to discriminate between shelters that were cued by only the tactile or olfactory element of the CS+ and CS– (bottom right panel; modified from Flanigan et al. 2021b Fig. 2). Filled circles in boxplots are group means; lines in IQR boxes are medians; whiskers indicate the lowest and highest values within 1.5 IQR of the upper and lower quartiles; and open circles are outliers. An asterisk indicates a stronger association with the inaccessible shelter cued by T, O or OT in tests than the chance expectation of 0.5
In a follow-up study, the same experimental design was employed to test for the ability of multisensory configural learning within a sensory modality, where the paired stimuli were now two distinct olfactory cues (Bostelman et al. in prep). Although open to alternative interpretations and in need of clarifying experiments, the results suggest that the whip spiders did not learn a within-modality, configural representation to recognize their home shelters. When only one of the two olfactory stimuli was present, the amblypygids were just as good in recognizing their home shelter compared to when both stimuli were presented together (Bostelman et al. in prep). Thus, current data suggest that amblypygids are capable of multimodal configural learning, but not unimodal multicomponent configural learning.
Using a different experimental setting not involving navigation, Lehmann et al. (2022) similarly offered evidence, albeit without statistical verification, that transfer of concept learning was easier when the stimuli used were of different sensory modalities. Specifically, the research team used delayed tactile matching and nonmatching tasks to determine if Phrynus marginemaculatus and Paraphrynus laevifrons could learn the concept of same/different. Following this same/different training, the team then tested whether individuals could transfer this learning to a novel cross-modal odor stimulus. Though results were not significant, there was an intriguing trend towards increased learning capacity in the cross-modal tests (Lehmann et al 2022).
Taken together, these results point to the capacity of amblypygids to learn complex, configural representations of multimodal environmental stimuli, which can be used at least for the home refuge recognition phase of navigation. We hypothesize that the same multimodal associative learning can be employed for approximating the direction home from farther distances.
How do amblypygids process information for navigation?
The capacity for integrated, multisensory configural learning necessarily raises the question of how the amblypygid nervous system supports such an ability. The integration of sensory information in amblypygids is presumed to take place in the mushroom bodies, a brain neuropil hypothesized to underlie complex behavior (Strausfeld 1998). Although the likely homology between the mushroom bodies of different arthropod groups has not been completely resolved (Strausfeld et al. 1998, 2020; Loesel and Heuer 2010; Farris 2005; Wolff and Strausfeld 2015; Wolff et al. 2017), it is notable that in insects, the mushroom bodies are known to support complex learning based on the integration of multimodal sensory inputs (Menzel 2001; Strausfeld and Reisenman 2009; Strausfeld et al. 2009; Avarguès-Weber and Giurfa 2013; Giurfa 2013). Indeed, in honeybees the mushroom bodies are required for configural, but not elemental olfactory learning (Devaud et al. 2015).
In the context of navigation, the importance of the insect mushroom bodies for integrated, multisensory guidance of spatial behaviour has been well described (e.g., Mizunami et al. 1998; Wessnitzer and Webb 2006; Cruse and Wehner 2011; Webb and Wystrach 2016). It is therefore noteworthy that the mushroom bodies of amblypygids are among the largest found in arthropods (Strausfeld et al. 1998; Wiegmann et al. 2016; Sinakevitch, et al. 2021), suggesting that the amblypygid mushroom bodies support the type of multimodal sensory learning described in insects. Consistent with this hypothesis is the organization of multimodal sensory inputs into the amblypygid mushroom bodies as described in the seminal study of Sinakevitch et al. (2021).
Mushroom body integration
The mushroom bodies of amblypygids are notably large and complex (Sinakevitch et al. 2021), as are the mushroom bodies of Thelyphonida (vinegaroons) (Lehmann and Melzer 2019); especially as compared to some other arachnid orders including Araneae (true spiders) (Steinhoff et al. 2020), Solifugae (camel spiders or sun scorpions) (Sombke et al. 2019) and Pseudoscorpiones (Stemme and Pfeffer 2022). Among arachnids, multimodal inputs into the mushroom bodies have only been recently described in amblypygids (Sinakevitch et al. 2021).
The glomeruli of the mushroom body calyces in amblypygids are substantially larger than their insect equivalents (Lehmann and Melzer 2018; Sinakevitch al. 2021). In amblypygids, distinct mushroom body calyx subdivisions are characterized by large and small glomeruli, which receive olfactory and visual inputs, respectively (Sinakevitch et al. 2021; Fig. 3). Although anatomically dissociated at the level of the calyx, which appears to be an input region for ascending olfactory information from the antenniform leg neuromere and visual information from the lateral and medial eye medulla in the brain (Fig. 3), the organization of the mushroom bodies would still enable integration of olfactory and visual inputs in the mushroom body lobes or within the matrix of the large and diverse number of axonal inputs to the neighboring reticulate body. There is also a shared region of visual input from the median and lateral eyes—the second lateral eye visual neuropil (Lehmann and Melzer 2018). This shared region of overlap between the median and lateral eye terminals is also seen in Xiphosura (horseshoe crabs) and Scorpiones. Finally, the organization of the reticulate body (a paired structure near the ventral mushroom body lobes and calyces) appears particularly well suited to support associative memory processes reminiscent of the vertebrate hippocampus (Wolff and Strausfeld 2016; Sinakevitch et al. 2021).
a Photograph of Phrynus marginemaculatus; left (l1–l4) and right legs (r1–r4) indicated; note the elongated antenniform leg l1 (distal part of r1 omitted); cyan line indicates vertical plane of section as shown in b, c, e and f; red trapezoid indicates horizontal view as shown in (d). b Summarizing sketch (vertical section) of mushroom body MB input pathways. Left side shows visual, right side olfactory input. Olfactory afferents oAf originating from sensilla on the antenniform leg terminate in the primary olfactory glomeruli oG; from there, olfactory projection neurons (oPN) connect to the large calycal glomeruli (lG). Visual afferents vAf originating from the lateral eyes supply the lateral medulla (pink; not labelled) from where visual projection neurons vPN supply the small calycal glomeruli sG; esophagus Es; mushroom body lobes MBL. c Photomicrograph of a vertical section through the central nervous system; ventral primary olfactory glomeruli oG and the corresponding secondary olfactory glomeruli located dorsally in the mushroom body calyx MBC color-coded in cyan; smaller visual MBC glomeruli color coded in magenta. d Three-dimensional reconstruction of the mushroom body (dorsal view) showing the lobes MBL and the calyx MBC; visual and olfactory calycal regions color-coded as in (b, c); arcuate body AC. e Photomicrograph of the approximate area boxed in (d). Small (sG; visual) and large (oG; olfactory) calycal glomeruli color coded as in previous panels. f Section from tracer-filled preparation with color-coded small and large glomeruli; large glomeruli with input fibers from ventral olfactory glomeruli; small glomeruli supplied by fine axons of visual interneurons; approximate area boxed in (e). Arrows indicate directions dorsal do, anterior an, and medial me; scale bars 5 mm (a), 200 μm (b – e), 20 μm (f)
The neuroanatomical revelations that emerge from Sinakevitch et al. (2021) and Lehmann and Melzer (2018) have profound implications for understanding multicomponent and multimodal sensory, associative learning in the context of amblypygid navigation. To date, only olfactory-mechanosensory multisensory (configural) learning has been described in amblypygids (Flanigan et al. 2021b). Sinakevitch et al. (2021) note that olfactory and mechanosensory inputs from the antenniform legs are segregated at the level of the antenniform leg neuromere but did not investigate possible mechanosensory projections to the mushroom bodies. If our hypothesis is correct that the mushroom bodies are necessary for learning based on multimodal sensory integration, then we would expect that mechanosensory inputs should also reach the mushroom bodies.
The parallel inputs of olfaction and vision into the mushroom body calyces is somewhat paradoxical, especially with respect to how much of amblypygid behaviour is controlled by vision. On the one hand, the anatomical finding is consistent with the robust visual associative learning described in amblypygids (Flanigan et al. 2021b), and the suggestion that despite their underdeveloped eyes a considerable amount of the amblypygid brain is involved in visual processing (Sinakevitch et al. 2021; Fig. 3). On the other hand, at the very least, vision is unnecessary for amblypygids to navigate home after being displaced in the field (Bingman et al. 2017). It would be informative if the demonstrated multisensory integration of olfactory and mechanosensory inputs in support of shelter recognition could be replicated with vision as one of the sensory inputs.
Finally, recent behavioural data suggest that the integration of multisensory inputs in support of spatial-associative learning is successful when inputs are of a different modality (Flanigan et al 2021b) but not when they are of the same modality, or at least two distinct odors (Bostelman et al. in prep). Acknowledging that experimental confirmation for the absence of within-olfaction associative/configural learning is still needed, it is nonetheless tantalizing to speculate that successful between sensory-modality associative integration combined with failed within sensory-modality associative integration may suggest much about the anatomical organization of the mushroom bodies. For example, given the segregation of the calyx glomeruli associated with olfaction and vision (Fig. 3), it would appear unlikely that the actual integration of multisensory inputs takes place at the level of the calyces. It would seem that the mushroom body lobes or reticulate body are likelier candidates as the site(s) of integration. Also, the suggestion that amblypygids are seemingly unable to integrate holistically separate olfactory inputs suggests that different olfactory processing streams lack the anatomical connectivity to support such integration. Further behavioral experiments, with designs like those used to study similar olfactory discrimination problems in insects, are needed to confirm this (Devaud et al. 2015).
Spatial orientation in Araneae (i.e. spiders)
Natural history and spatial navigation
Unlike their nocturnal hunting amblypygid relatives that reside in crevices or holes during the day and come out at night to forage, the more than 50,300 species of spiders (World Spider Catalog, accessed 08 September 2022) demonstrate a variety of lifestyles and hunting strategies. In general, spiders have three primary approaches for getting their prey: (1) web-building; (2) sit and wait in a burrow or retreat and walking out for passing prey; or (3) active pursuit (Nentwig 1987; Barth 2002; Foelix 2011). Similar to amblypygids, spiders using this third strategy sometimes also have home burrows or shelters. Given these varied hunting strategies, many spiders must have spatial orientation mechanisms for returning to their burrow or shelter following foraging, or other, excursions.
There is an impressive range of distances that various spiders travel before returning to a home site. These distances range from a few centimeters (funnel-web spider, Agelena labyrinthica, Görner and Claas 1985) to about a half meter (Drassodes cupreus, Dacke et al. 1999; Central American spider, Cupiennius salei, Seyfarth et al. 1982; wolf spider, Lycosa tarantula, Ortega-Escobar and Munoz-Cuevas 1999), to hundreds of meters (the Namib Desert spider, Leucorchestris arenicola, Nørgaard 2005). Given some of these extraordinarily navigational feats, it is no wonder that scientists have spent decades exploring the mechanisms underlying spider navigation.
One of the earliest studies of spider spatial orientation was carried out on the Lycosidae spider, Arctosa perita, that exhibits active pursuit of the prey. This species was shown to exhibit zonal orientation, or movement at right angles from one zone such as a river or lake, to the shore where the spider lives. Papi (1955) determined that the escape direction of each A. perita population varied according to the zone of the river it inhabited. Papi found that (a) the spiders used the sun as a cue to navigate their way to their shore; (b) the spider’s escape direction remained constant throughout the day; and (c) the spiders had the capacity of perceiving polarized light and using it for orientation. This study laid the foundation for future work exploring the role of sensory inputs in spider navigation.
Path integration
Studies of the mechanisms of spider navigation have been leveraged by the impressive amount of knowledge that has been accumulated in insects with regards to mechanisms underlying navigation (Collett 2019; Heinze et al. 2018). As such, path integration has been a focal mechanism of study for spider navigation researchers.
Path integration is a form of route-based homing in which the animal continuously updates its position relative to its departure point. During its outbound journey, the animal integrates the direction and distance of each route fragment to calculate a home-bound vector for its return (Papi 1992a, b). The vector’s distance can be measured using idiothetic information, or information obtained by the animal via proprioceptors when it moves, such as stimulation of lyriform organs or by the optic flow across the eyes. Simultaneously, the vector’s direction can be derived from either idiothetic or allothetic information, or information used to calculate the angle of turn relative to stable external reference cues, such as polarized light patterns, visual landmarks, etc.
Path integration has been demonstrated to be important in several types of spiders that emerge from retreats to capture prey. Using foraging trials, for example, Cupiennius salei, a spider that sits and waits, was shown to immediately return to the location of a previously encountered prey (Barth and Seyfarth 1971; Seyfarth et al. 1982). In the experiment, the spiders were induced to drop their prey item (a fly) and were then chased into a nearby semicircular corridor. Upon emerging from the corridor’s exit, the spider moved in the direction of the prey item that it dropped (Fig. 4a). Similarly, when the wolf spider Lycosa tarantula, a spider that sits and waits, was coaxed to move along adjoining walls of a rectangular terrarium and then displaced to the center of a large circular arena, it walked in a direction parallel to the one it would have taken to return to the burrow had it not been displaced (Ortega-Escobar and Munoz-Cuevas 1999) (Fig. 4b). Finally, it has been suggested that path integration is involved in the return of male Namib Desert spiders, Leucorchestris arenicola, a sit-and-wait spider, to their burrows after a night of long-distance (up to 810 m) searching (NØrgaard 2005) for females (Fig. 4c).
Path integration in Cupiennius salei (a), Lycosa tarantula (b), and Leucorchestris arenicola (c). a The spiders were chased away from previously captured prey into and through a semicircular corridor. After emerging from the end of the corridor, they walked in the direction (mean ± standard deviation) of the yellow arrow; 0º would mean a straight line from the exit point to the fly. b Path integration in Lycosa tarantula. A female was displaced (blue arrows) in a 60 × 30 cm terrarium placed in the laboratory. When the spider reached the end of the short leg, it was removed in a glass and placed in a 90 cm diameter arena oriented in the same direction; the inward path searching for the virtual burrow (black solid line) was approx. 40 cm in length and after this the systematic search (loops) began. c The longest path, 810 m, registered for a spider, a male of L. arenicola living in the Namib Desert. [c is adapted from NØrgaard (2005)]
In addition to the above-mentioned non-web building spiders, the web-building black widow, Latrodectus hesperus, has been shown to use path integration for homing (Sergi et al. 2021). Most spiders that took circuitous outbound paths from retreats on the edge of their web sheets took shorter, more directed inbound paths. Furthermore, displaced spiders moved in a direction parallel to putative homebound vectors. Finally, spiders whose webs were rotated in their absence made navigational errors and engaged in systematic searching movements such as moving around the web, plucking, or tugging on its lines. These studies all suggest that multiple spider species use path integration for navigation.
Learning walks
In both walking and flying hymenopterans (bees, ants, bumblebees, and wasps), individuals will engage in locomotory patterns that include moving in circles or arcs as they leave their nest. During these movements they look back to face the nest, gathering nest-directed visual information from different viewpoints. Such locomotory patterns have been termed learning walks (Collett and Zeil 2018; Zeil and Fleischmann 2019) and spiders have also been shown to engage in them.
Naïve male Leucorchestris arenicola perform sinusoidal movement patterns when departing their burrows (NØrgaard et al. 2012). These movement patterns are limited to the area near the burrows, and they change shape and become less pronounced as the spiders gain experience. It is hypothesized that these sinusoidal movement patterns are analogous to learning walks and learning flights observed in some hymenopterans. Leucorchestris arenicola does not walk in circles or arcs near the burrow, but the sinusoidal movements may similarly allow the animals to gather burrow-related visual information from various directions.
How do spiders sense the world?
Mechanoreception and vision have been the two prominent sensory systems studied in association with spider spatial orientation.
Mechanoreception
Mechanoreceptors are particularly well developed in spiders, especially the compound slit sense organs or lyriform organs (Barth 2002, 2020). These organs consist of a variable number of up to 30 parallel, closely spaced cuticular slits. The dendritic tips of two mechanosensitive neurons attach to specialized cuticular structures within the slits (French et al. 2002). It is proposed that mechanotransduction takes place at the dendritic tips and that the structure only responds when the stimulus is a compressive strain (French et al. 2002).In the most-studied spider from a mechanical sense point of view, Cupiennius salei, the lyriform organs are situated on the extremities close to the leg joints (Barth 2002) where forces are strong and transmitted from one leg segment to the next (Barth 2002).
Vision
In terms of vision, most spider species have eight eyes placed on the anterior part of the prosoma. From a frontal perspective they appear to be arranged roughly in two rows (Foelix 2011; Morehouse 2020), although salticids have four eyes arranged on the sides of the carapace and four eyes facing forward (Harland et al. 2012). Spider eyes are of a camera-type composed of a cuticular lens, a cellular vitreous body, and a retina with rhabdomeric photoreceptors (Blest 1985). If we consider the two-row arrangement, the first row contains the antero-median (AME) and the antero-lateral (ALE) eyes, and the second row contains the postero-median (PME) and postero-lateral (PLE) eyes (Land 1985; Foelix 2011; Morehouse 2020). The AMEs are usually called the principal eyes, while the others are referred to as the secondary eyes. Both principal (AMEs) and secondary eyes (ALEs, PMEs, and PLEs) project to first-order optic neuropil (ON1) which then projects to the second-order optic neuropil (ON2). However, the AME neuropils are different from those of secondary eyes, which also are different among them. The AME second-order neuropil projects to the “central body” or “arcuate body” while the second order neuropil of the secondary eyes projects to the “mushroom bodies”.
There are important functional differences between principal (AME) and secondary eyes: the rhabdoms of the AME photoreceptors are close to the vitreous body while the rhabdoms of all the other eyes are inverted such that the receptor cell nuclei lie between the rhabdoms and the vitreous body. Also, in the families in which it has been studied, only the AMEs have a variable number of muscles to facilitate retina movement and only the secondary eyes have a tapetum, a guanine-based reflective surface immediately behind the rhabdoms (Eakin and Brandenburger 1971; Homann 1971; Kovoor et al. 1993; Land 1985; Mueller and Labhart 2010; Schröer 2017).
A significant factor regarding spider spatial orientation is also the visual field of the eyes. For example, the field of view of the AMEs of the wolf spider Lycosa tarantula and the funnel-web spider Agelena labyrinthica is directed towards the sky while their ALEs face towards the substratum (Kovoor et al. 1993; Schröer 2017). Therefore, for these spiders, the AMEs appear to perceive the position of the sun or the pattern of polarized light, and ALEs the ground structure.
The detection of polarized light has shown to be critical for many navigating arthropods. In insects, polarized light analyzers consist of two sets of photoreceptors with orthogonally oriented microvilli. The receptors in this POL area (POL for polarization) are arranged in the superior region of the compound eye called the dorsal rim area (Wehner and Strasser 1985; Labhart and Meyer 1999; Wehner and Labhart 2006; Mathejczyk and Wernet 2017). Are there orthogonally oriented microvilli in spiders?
Histological studies of lycosid (Lycosa tarantula, Kovoor et al. 1993; Geolycosa godeffroyi, Geolycosa sp. and Pardosa prativaga, Dacke et al. 2001) and agelenid (Agelena gracilens, Schröer 1974, 1976; Agelena labyrinthica, Schröer 2017) retinas have shown that the AME retina is asymmetrical. The inferior part of the retina has a striped-like region, which has photoreceptor cells with rhabdomeres arranged on two parallel sides with two groups of cells orthogonal to each other (A. labyrinthica, Fig. 5a; L. tarantula, Fig. 5b). However, rhabdomeres are located on all sides in the central photoreceptor cells. Therefore, the ventral part of the retina in lycosids and agelenids could be a POL area, looking towards the sky (the optic axis of the L. tarantula AMEs is oriented 20º upwards and 15º lateral to the sagittal plane, Kovoor et al. 1993; whereas the optic axis of A. labyrinthica is tilted by 45º in relation to the horizontal plane, Schröer 2017). This could explain behavioural (Görner 1958; Ortega-Escobar and Munoz-Cuevas 1999; Dacke et al. 2001) and physiological (Magni et al. 1965) results related to polarized light detection.
Organization of spider photoreceptors. a Central and ventral parts of the AME retina in Agelena gracilens. left: Arrangement of the rhabdoms in the two ventral populations (magenta double-headed arrows) orthogonal between them. right: Arrangement of the rhabdoms (red lines) in three or four sides of the cells. b Central and ventral parts of the AME retina in Lycosa tarantula. left: Light microscope photograph of the AME retina; the central part shows vitreous body cells; a: central retina; b: ventral retina. right superior: Cells of the central retina with rhabdomeres (red lines) on all faces. right inferior: Cells of the ventral retina with rhabdomeres in two parallel faces; there are two cell populations according to the rhabdom orientation (magenta double-head arrows). c Retina of the PME of Drassodes cupreus. left superior: Horizontal section through the PME retina showing a regular rhabdomere arrangement; white zones: photoreceptor cell somata; gray lines: rhabdomeres. right superior: Enlargement of the box in left superior showing the parallel microvillar arrangement. inferior: Schema showing the V-shaped tapetum (black and gray) and the arrangement of microvilli (yellow); arrow: one possible path of light through the retina by double reflection. [a is adapted from Arthropod Structure & Development (2017), 46(2), 196–214. c is adapted from The Journal of Experimental Biology (2001), 204, 2481–2490.]
The AME retina of L. tarantula is moved by the contraction of two antagonistic muscles (Kovoor et al. 1993) attached to its lateral external surface. Ophthalmoscopic observations reveal that the retina can move both up and down. The AME retina of A. labyrinthica only possesses one muscle that can produce a rapid trembling that can also be observed ophthalmoscopically (Schröer 2017). The movements of the retina caused by muscle contraction could serve to simultaneously detect all the possible e-vector angles at each point in the sky in a manner analogous to the fan-like array of the ommatidia in the dorsal rim area of some insect species (Zeil et al. 2014).
A completely different mechanism for analyzing skylight polarization is found in the gnaphosid spider Drassodes cupreus (Dacke et al. 1999, 2001; Mueller and Labhart 2010). Drassodes cupreus use a completely different pair of eyes—the posterior median eyes (PMEs)—to analyze skylight polarization (Dacke et al. 1999, 2001). The PMEs of D. cupreus are arranged with their long axis perpendicular to each other. In each eye, the photoreceptor microvilli are oriented parallel to each other and the eye long axis (Fig. 5c); therefore, the microvilli of each eye could perceive polarized light parallel to the eye long axis. In this way, the pair of PMEs in D. cupreus is analogous to the ventral retina of lycosid and agelenid AMEs with one eye detecting polarized light in one plane, and the other eye detecting it in a plane orthogonal to the first. Dacke et al. (1999) discovered that this spider has a reflecting tapetum consisting of two flat mirrors with an angle of approximately 95º between them (see also Mueller and Labhart 2010). The angled tapetum strongly polarizes the reflected light with an e-vector parallel to the microvilli of the photoreceptor cells (Fig. 5c) (Dacke et al. 1999; Mueller and Labhart 2010).
Most photoreceptors of the Drassodes cupreus PME are called “main receptors” (Dacke et al. 1999), which have microvilli oriented parallel to the eye's long axis. There are two receptors with microvilli aligned predominantly parallel to the eye's short axis called “shallow receptors” (Dacke et al 1999) or “central receptors” (Mueller and Labhart 2010). The disposition of the main receptors can be found in all the secondary eyes. Dacke et al. (1999) made intracellular recordings of two main receptors to measure their polarization sensitivity ratios and obtained values of 7.6 and 9.1, higher than those measured in the dorsal rim area of the desert ant Cataglyphis, 6.3 (Labhart 1986), or the honeybee, 6.6 (Labhart 1980). One of the cells was recorded for long enough to measure its spectral sensitivity which peaked in the ultraviolet (350 nm). While the ALEs and PLEs could also provide input to the polarized-light compass, as these eyes are polarized similarly to the PMEs, Dacke et al. (2001) stated that an overlap of the field of view of each pair of ALEs or PLEs, would need to be greater to be able to compare the signals from cells with different polarization axes.
What information do spiders use to navigate?
Mechanoreception-based navigation
Cleverly designed experimental studies have explored the role of mechanoreceptive cues from the lyriform organs in guiding spider movement. Using Cupiennius salei, spiders were allowed to capture a tethered fly which was suspended above the arena floor. Upon capture, an electric current was passed through the fly, whereupon the spider left the prey and was chased off through the semi-circular corridor (Seyfarth and Barth 1972; Seyfarth et al. 1982). After a period of immobility, the spider searched for the prey at the original capture site with little error (Fig. 4a). Next, animals with various lyriform organs ablated were tested using the same design. Intact and control animals (with small holes in the cuticle near the lyriform organs) had a 95% success rate while the success rate for all the groups with mechanically ablated lyriform organs was 33% or less. The experimental procedure used in C. salei ruled out the possibility of the spider using cues (e.g., visual, gravitational, substrate, or olfactory) other than mechanical information coming from its lyriform organs (Seyfarth and Barth 1972; Seyfarth et al. 1982). This behavioural study then, suggested that the lyriform organs are necessary for correct evaluation of travel direction. Furthermore, in addition to the difference in successful returns, the intact and control groups differed significantly from the experimental groups in the mean starting direction, the correct direction, and in their mean angular deviation. The experimental groups with all tibia lyriform organs ablated or with all femur lyriform organs ablated showed uniform distributions in the mean starting direction. These studies highlight the importance of mechanosensory idiothetic information gathered during the spider’s outbound journey for calculating a goal-directed vector for its return trip.
Seyfarth et al. (1982) carried out another study in C. salei that focused on distance assessment rather than direction. Using a similar design as discussed previously, they made rectilinear chases after the spider left the fly, displacing the spiders 20 to > 40 cm in intervals of 5 cm from the capture point. They also made curvilinear chases, in which the spiders were displaced by a mean distance of 38 cm. There were similar intact, control, and ablated groups as in the study carried out by Seyfarth and Barth (1972), and they measured the distance walked from the point where it had been displaced to where it made the first sharp turn searching for the lost prey. In the rectilinear chases, successful returns depended on the distance chased and the functioning of the lyriform organs. When intact spiders were chased 20 cm, all returns were successful, but for > 41 cm chases, success rate fell to 60%. The operated spiders failed to get to the goal (the lost prey) in two-thirds or more of the returns except for the 20 cm group in which the success was around 50%. Similarly, in curvilinear chases, only the intact (with eyes covered) and control (with small holes in the cuticle near the lyriform organs) walked a mean distance that was similar to the mean distance traversed through the semi-circular corridor, and this was independent of the chase direction through the corridor (the animal turning to left or to right to enter it). However, the sensory ablated spiders (with all tibia lyriform organs ablated or with all femur lyriform organs ablated) had successful returns of less than 50% and therefore could not adequately measure the distance walked. These studies highlight the importance of mechanoreceptive information from lyriform organs in distance, as well as direction, detection.
The capacity to measure distance walked by proprioceptive cues was also tested in Lycosa tarantula. Ortega-Escobar and Ruiz (2014) displaced spiders linearly a distance of 30 cm from their burrow through a longitudinal corridor and transferred them to a parallel corridor in which the burrow was absent. The distance walked by spiders was measured when they had all their eyes uncovered (the control test) and with all their eyes covered (the experimental test; Fig. 6, 1st experiment). Spiders with all their eyes uncovered searched for the burrow mainly before arriving to the virtual burrow while spiders with all their eyes covered searched for the virtual burrow along the entire corridor. In conclusion, it appears that L. tarantula does not use proprioceptive cues during its diurnal walks to measure the distance walked; however, this kind of study has not been carried out during the night.
Distance measurement in Lycosa tarantula. A spider was placed in a channel 52 cm long and 9.5 cm wide. Spiders lived in this channel (burrow: black circle) for three days prior to the beginning of the study. The procedure consisted of gently pushing the spider 30 cm from its burrow, removing it in a transparent glass cup, and transferring it to the same point in the test channel. The burrow was absent in the test channel but its position is marked by a red circle. In all the experiments, the grey boxes and the black line inside them show the interquartiles and median, respectively. The red dashed line shows the spider’s position after walking 30 cm; the red continuous line shows the position of the burrow. In the first experiment, the test channel was as the same length as the control, i.e. 52 cm; in the second and third experiments, the test channel was longer, 90 cm (this greater length is indicated by two parallel slanted lines). In all the experiments, the covered eyes appear green. Given the visual field of the anterior median eyes (AMEs), it was not necessary to cover them. [Adapted from Ortega-Escobar and Ruiz (2017)]
Vision-based navigation
Sky-based—Vision is important in the navigation of the wolf spider L. tarantula. In studies conducted under a natural sky and with the sunlight blocked by an opaque screen, the spiders showed burrow orientation if the sky was not overcast, or when a polarizer sheet was absent (Ortega-Escobar and Munoz-Cuevas 1999). If the sky was overcast or a polarizer sheet was present, they showed a systematic search, that is they searched in ever increasing loops “trying” to find their burrows. This demonstrated the importance of polarized light and further demonstrated that it was detected through the AMEs. In a follow-up laboratory-based study, Ortega-Escobar (2006) described the role of the anterior lateral eyes (ALEs) in spatial orientation. The rationale for this study was that the visual field of the ALEs is directed towards the substratum (Land 1985) and therefore the spiders could observe changes that could be used for path integration. When only ALEs were uncovered, spiders took an inbound path, as we shall describe later (Substrate-based), in which visual and proprioceptive information was added.
Vision also appears involved in the nocturnal homing of the Namib Desert spider Leucorchestris arenicola (Fig. 4c). It is unlikely that the spiders use the sun or the moon (or their patterns of polarized light) since these spiders are strictly nocturnal, and even prefer moonless nights (NØrgaard et al. 2006). Gravity (as measured by the slope of the substrate) has also been dismissed (NØrgaard et al. 2003). Vision appears to be important because those spiders with all their eyes covered moved very short distances of 50 cm or less; or, if they moved farther than 50 cm they were not able to return to their burrows (NØrgaard et al. 2008). Probably, males did not return because their sexual motivation induced them to navigate, while most females and immature spiders, without sexual motivation, managed to return to their burrows from those short distances. The role of vision in the L. arenicola learning walks is also suggested by NØrgaard et al. (2012) who hypothesized that during the sinusoidal outbound journey the spider could scan the panorama around the burrow using the ALEs and PLEs.
Following the discovery that L. arenicola spiders need visual information to carry out their long-distance homing, NØrgaard et al. (2008) selectively covered various groups of eyes and measured the resulting homing success. The spiders in the group with only their PMEs uncovered exhibited the lowest homing success, even less than the spiders with all their eyes covered. The group with only their AMEs uncovered showed the highest homing success, which did not differ from the eyes-intact control group. The group with only their ALEs uncovered also showed high homing success. Therefore, L. arenicola spiders appear to use their AME and ALE eyes for navigation.
The potential role of vision in homing was also studied in the gnaphosid Drassodes cupreus, a spider using strategy two. The study used a 1.5 m diameter circular arena that contained four symmetrically placed shelters that the spiders could use to spin their web (Dacke et al. 1999, 2001). After one day of habituation and construction of the webs, the experiments began in the presence or absence of a polarizing sheet over the arena using animals with various eyes covered. The rate of return of the spiders to their home shelters served as the dependent variable. Among animals with their eyes uncovered, three of the ten spiders returned to their shelters after the first foraging trip when the light was unpolarized, while nine of the ten spiders returned to their shelters in the presence of the polarizing sheet. Only three of ten spiders with their secondary eyes covered returned to their shelters under polarized light. Based on morphological and physiological characteristics, the PMEs were implicated as the polarized light detectors important in visual homing.
Substrate-based—Numerous studies have suggested a role of the ALEs of Lycosa tarantula in successful homing. In an initial study (Ortega-Escobar 2006), spiders were placed in a rectangular terrarium with an artificial burrow placed in the middle of a long side of the terrarium. After five days of habituation, each spider was displaced along the terrarium wall, to one of the corners on the wall opposite its burrow. They were then caught and transferred to the center of an arena 90 cm in diameter. Two groups of spiders were used: one group in which all the eyes were uncovered (control phase) and afterward all eyes but ALEs were covered (experimental phase) and a second group with a similar control phase but in which all eyes except the ALEs were uncovered (experimental phase). The results showed that only spiders with their ALEs uncovered traveled in the correct direction to a virtual burrow. In a later study, Ortega-Escobar (2011) showed that after being displaced on a black-and-white grating in the outward path, the spiders placed on the same grating but rotated by 90° showed trajectories that were less linear, initially directed towards the virtual burrow and followed by a change of direction of nearly 90°. The grating rotation made the searching directions very scattered, suggesting that the rotation of the substrate had been perceived by the spider, and that the inward path was an integration of both proprioceptive and visual information. A further set of experiments, in which either ALEs or AMEs/PMEs/PLEs were masked, showed that only the ALEs are used to perceive substrate structure. The studies carried out on circular arenas (Ortega-Escobar 2006, 2011) excluded the use of silk or olfactory information for home orientation, although these stimuli could play a role in natural conditions (for example, the male L. tarantula follows female silk threads when looking for the female burrow, J. O.-E observation). Thus, in L. tarantula, the ALEs appear to be important for learning substrate patterns used in homing.
Ortega-Escobar and Ruiz (2017) also performed a study like the previous one, but the substratum was a grating of black-and-white stripes (λ = 1 cm) that allowed them to test for a role of external visual cues on distance assessment. Following a training phase (learning walks), the distance walked by two groups of spiders was measured—group 1 had their ALEs covered while group 2 had their PLEs and PMEs covered. (Fig. 6, 2nd and 3rd experiments, respectively). While all manipulated spiders (with some eyes covered) walked significantly less than the control spiders when searching for the burrow, the highest effect was due to the absence of function of the ALEs. This study provides evidence for visual cues on the substrate to play a role in distance determination during navigation.
Multisensory-based navigation
The funnel web spider, Agelena labyrinthica, uses multimodal sensory cues to move from their burrows to regions of interest (Görner 1958; Mittelstaedt 1983; Görner and Claas 1985), akin to other arachnids (Hebets et al. 2014a). In this case, the central nervous system brings together both visual and proprioceptive information. As for vision, when the spider was studied outside under a polarization filter whose direction of maximal transmission was either parallel or perpendicular to the e-vector of the sky polarization, only spiders under the parallel filter moved directly home (Görner and Claas 1985). The position of the sun was not important since Santschi’s mirror experiments (an experiment where direct sun is screened from the animal and reflected with a mirror to the other side of the animal’s trajectory; Santschi 1911; Wehner 2016) showed the spiders deviated by only a few degrees from the correct direction to their retreat. The spiders also use proprioceptors on their legs to glean information about the elasticity pattern of the web as well as gravity (Görner 1958).
The situation is a little different in laboratory-based studies of Agelena labyrinthica. Scientists examined the spider’s use of light cues for homing using an experimental design in which the spider’s web is placed in a circular frame in a darkened laboratory with two light sources whose beams intersect at the center of the web. In one test the spider was lured while one of the lights was on. When she took the prey and was homing, the first light was turned off and the second light turned on (somewhat like the mirror experiment). The spider’s response was to run in an intermediate direction derived from optical information from the new light and stored idiothetic information from its outbound movements (Görner and Claas 1985). In this experiment, where the spider did not have polarized-light pattern information, it used artificial light sources and web structure for orientation.
Considering both laboratory and outdoor experiments, Agelena labyrinthica navigates by using multisensory information, coming from the web structure and from either polarized light patterns or artificial light sources. This statement does not imply that other species cited in this review do not rely on multimodal sensory information, but rather that most studies have only taken one sense into consideration.
How do spiders process information for navigation?
Light detection and processing
Of the 50,000 plus of described spider species, relatively few have been studied with respect to visual system processing and navigation. In those species, however, we do find some differences. In Lycosa tarantula and Cuppienius salei, each secondary eye has its own ON1 and ON2. In Marpissa muscosa, the connectivity of the AME is similar to that of L. tarantula and C. salei (ON1, ON2 and arcuate body) while that of the secondary eyes is different. In M. muscosa, there is a different ON1 for each eye, but there is a shared ON2 between the anterior lateral and posterior lateral eyes (L. tarantula, Kovoor et al. 1993, 2005; C. salei, Strausfeld and Barth 1993, Strausfeld et al. 1993; jumping spider Marpissa muscosa Steinhoff et al. 2020). Any navigational relevance of these centers and different connection patterns remains mostly unknown.
Unlike some insects in which polarization neuron projections have been described (cricket optic lobe: Labhart 1988; fruit-fly optic lobe: Sancer et al. 2019; locust anterior optic tubercle and central complex: Homberg 2004; Heinze et al. 2009; Heinze 2014), we only know the visual pathway from polarized-light receptors to the central brain in Lycosa tarantula. In this species these cells project to the AME first-optic neuropil in an area separated from that which receives axons from non-polarized-light receptors (Kovoor et al. 1993). In the AME optic nerve, axons from central and inferior areas of the retina could be differentiated by their diameter, with the thinner fibers corresponding to the polarized-light receptors. The visual pathway of Agelena has not yet been described, and no record has been made of polarization neurons in any spider’s visual center.
Spatial orientation in scorpions
Natural history and spatial navigation
Nearly all scorpions are nocturnal (Warburg and Polis 1990; Warburg 2013), emerging in the early evening to hunt using exquisite seismic sensors on their legs that guide them to prey vibrations (Brownell and Farley 1979a, b, c). Scorpions feed mostly on other arthropods, such as crickets, beetles, spiders, and moths, but in some cases, they consume other scorpions (Polis 1979; Polis and Farley 1979). Many scorpions dig burrows or enhance pre-existing retreats (Eastwood 1978; Polis et al. 1986; Polis 1990; Adams et al. 2016) that offer protection not only from predators such as birds, bats, grasshopper mice, and other scorpions (Polis 1979; Polis and Farley 1979, 1980; McCormick and Polis 1990) but also from harsh environmental conditions (Bradley 1988; Brownell 2001; Kaltsas et al. 2009; Becker and Brown 2016).
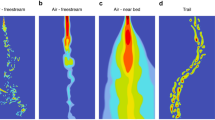
Most scorpion field studies have been concerned with population distributions relative to various environmental factors. A spate of papers on the desert sand scorpion Paruroctonus mesaensis (now Smeringerus mesaensis) from the Mojave Desert chronicled surface densities, sex and age demographics, intraguild predation, cannibalism, etc. (Polis 1980; Polis and Farley 1980; Polis et al. 1985, 1986). These studies also took advantage of the remarkable phenomenon of scorpion fluorescence (Fig. 7a) (Stachel et al. 1999; Frost et al. 2001; Stahnke 1972). A long-term study of S. mesaensis showed that burrow fidelity and home-range geometry varied among age classes and sexes (Polis et al. 1985). While all individuals showed some degree of homing, mature males moved longer distances and did not reuse the same burrow for extended periods. Mature females and immatures were highly faithful to their burrows and their movements were mostly concentrated to a circular pattern within 1 m of the burrow.
Tracking scorpion excursions in the field. a A female scorpion (P. uthahensis), photographed under UV light, is shown emerging from its sand burrow [photo by M. Hoefnagels]. b A long pole is impaled into the sand and supports an IR camera trained on the area around a scorpion burrow. The camera feed runs to a nearby trailer for video storage and processing. c Tracing of a scorpion (red lines) in response to cricket stimuli (black dashed lines). The black dots indicate stimulus position every 2 s the numbers adjacent red lines indicate time in seconds after the beginning of the cricket movements. On the left side (Night 1) an 11 s cricket excursion took it near to the scorpion burrow (B). The scorpion emerged from its burrow about 10 s after the cricket passed and made two short loops before returning to its burrow at 41 s. On the right side (Night 2) a cricket was dropped within 5 cm of the burrow and made a large counterclockwise loop before jumping ~ 11 s later. The scorpion emerged quickly from its burrow as the cricket circled close to the burrow and nearly caught the insect (at point marked 11*). d A different scorpion is lured from its burrow by tossing and dragging a 2 × 2 cm piece of duct tape folded over the end of a strand of dental floss past the scorpion’s burrow. In this case, the animal emerged immediately after the lure hit the sand (point 2*). The scorpion made a few short loops before returning to its burrow 38 s after emerging. [Plots adapted from Gaffin 2011]
These natural history accounts have inspired detailed spatial–temporal tracking of scorpions (Kaltsas and Mylonas 2010; Gaffin 2011). Some examples of home burrow navigation in the field were captured in a study of P. utahensis in a sandy region of the Northern Chihuahuan Desert (Gaffin 2011). UV flashlights were used to spot adult scorpions at their burrow thresholds (Fig. 7a), and IR cameras were positioned to capture video of scorpion surface movements (Fig. 7b). The animals emerged from their burrow retreats within seconds of a passing vibrational stimulus, such as insects walking near the burrow (Fig. 7c) or artificial lures pulled across the sand (Fig. 7d) and returned to their burrows soon after (less than 2 min). Importantly, the animals did not retrace their outbound paths for their returns, which argues against the animals using their own chemical cues or mechanosensory detection of their own footprint patterns to return home. The animals also tended to make additional looping forays, ostensibly in search of the elusive prey, before making meandering returns to their burrows.
Scorpions lend themselves well to laboratory investigations. The animals are readily collected using blacklights, are long-lived (several years depending on species (Polis and Sissom 1990)), and easily maintained. Sand scorpions are particularly adaptable navigation subjects because the simplicity of their habitat allows facsimiles of their environment in the laboratory (Camp and Gaffin 1999; Bost and Gaffin 2004; Vinnedge and Gaffin 2015; Prévost and Stemme 2020; Gaffin et al. 2022). Well-controlled lab studies can mitigate variables that confound field investigations, such as fluctuations in temperature, wind, rain, light levels (including moon phase), and insects. Furthermore, in the field, scorpions often go for days without emerging, especially if they have recently captured prey (Bradley 1982; Polis 1990). In the lab, scorpion activity can be enhanced by restricting food.
Path integration
A clever, lab-based navigation study produced evidence of path integration in scorpions (Prévost and Stemme 2020). The study used a sizeable circular (150 cm diam, 38 cm ht), sand-filled arena equipped with overhead lights and cameras. First, each scorpion, Mesobuthus eupeus, was maintained for at least four months in a small box containing a protective shelter to serve as a home refuge. Next, the box was placed in the center of the arena, and a trial was initiated by gently opening a side panel to allow the animal access to the large arena. The animals’ departures and return paths were monitored under various light conditions using animals with eyes intact or covered. The departure paths meandered more than the return paths and, concordant with the field observations described earlier, the departure and return angles were dissimilar. The shapes of return paths were analyzed (e.g., perpendicular distance from home vector, indices of straightness, distributions of deviations from a direct path) after the animals left the arena wall and crossed a fictive circular border line (Fig. 8a left). The researchers predicted that in a true homing bout, the deviations from a direct path would be normally distributed. Interestingly, in support of their predictions for path integration, for both sighted and blinded scorpions under white light, the path deviations from home-directed vectors were all normally distributed. However, only about 50% of the deviations were normally distributed for sighted animals under red light (Fig. 8a right).
Laboratory assays show signs of path integration and learning walks. a Evidence of path integration in M. eupeus. Left: A box containing an acclimated scorpion is placed in the middle of a large circular sand-lined arena under various light conditions and monitored via an overhead camera. A hinged panel in the box opens to allow the animal access to the arena. The animal is allowed 3 h to reach the wall (phase 1 = short-dashed line) and up 3 more hours (phase 2 = long-dashed line) to pass a fictive border line (white circle perimeter) and return to the box. The animal is tracked every 2 s (dots) after it crosses the border line until it reaches the box, and its path is compared to a home-directed vector (wide gray arrow). Several measures were extracted from these homing bouts, including whether the deviations of the home path from the home vector are normally distributed (upper left graph) or not (upper right graph). The lower graph at right shows the percentage of normal and non-normal paths for sighted animals under white and red light and blinded animals under white light (adapted from Prévost and Stemme 2020). b Evidence of learning walks in P. utahensis. An animal is introduced into a circular sand-lined arena that contains a small mound of moistened sand. The animal is monitored through the evening via an IR camera. Once the animal shows its initial sign of burrow digging in the mound, its subsequent movements are plotted every 2 s until it resumes walking along the arena perimeter. In this example, about 200 s of movement arranged in four looping excursions of various sizes are plotted. The data are transformed linear distance to the burrow vs the cumulative path length (top graph) and the individual loops are delineated based on each return to the burrow (vertical gray dashed lines). The walks are then superimposed in the bottom graph. [Adapted from Gaffin et al. 2022]
The above study yielded several interesting observations, e.g., differential homing activity under white, red, and IR light, but the directed home-bound paths of the blinded scorpions strongly suggested the animals were using path integration to calculate home-bound vectors. The researchers suggested that the homing bouts were less direct in sighted animals under red light because the relatively safe low light environment induced them to explore more before returning home. Moving forward, displacement studies (Ortega-Escobar 2002; Wolf 2011) are crucial for further exploring path integration and determining the environmental, sensory (visual and chemo-tactile), and idiothetic (self-motion cues) factors involved in navigation.
Learning walks
Another lab-based study revealed evidence of putative learning walks in scorpions (Gaffin et al. 2022). In this case, the animals were allowed to establish their own homes in lightly moistened sand mounds in the middle of circular arenas (76 cm diam) while being monitored through the day and night via overhead IR cameras. The scorpions, adult Paruroctonus utahensis, found and readily dug burrows in the mounds. Immediately after their first digging behavior, the animals made variously sized looping excursions away from and back to the burrow site (Fig. 8b). These loops were also inducible by encouraging animals to find and accept a small partially buried paper disc as a place to begin a burrow (i.e., without a mound). These paths have elements in common with learning walks in various ant species that make looping excursions immediately after emerging from their nests for their initial foraging bouts (Wehner et al. 2004; Narendra et al. 2013; Fleischmann et al. 2016; Collett and Zeil 2018; Jayatilaka et al. 2018; Zeil and Fleischmann 2019; Deeti and Cheng 2021).
How do scorpions sense the world?
Scorpions have several well-developed senses for transducing allothetic (environmental) information. For example, basitarsal slit sensilla (arced grooves on the distal end of each basitarsus) allow scorpions to detect the seismic waves of their arthropod prey up to a half meter away in loose, unconsolidated sand (Brownell 1977, 2001; Brownell and Farley 1979a, b, c). Similar to those discussed in amblypygids, pedipalpal trichobothria respond to minute air currents (Meßlinger 1987; Ashford et al. 2018; Murayama and Willemart 2019), and tarsal organs respond to near-range humidity (Foelix and Schabronath 1983; Gaffin et al. 1992). Additional tarsal structures include mechanosensory sensilla that detect surface compressional waves (Brownell and Farley 1979a) and curved, pore-tipped sensilla that taste ground-based chemicals (Foelix and Schabronath 1983). There are even photosensitive elements in the metasoma (Zwicky 1968, 1970a, b; Geethabali and Rao 1973) and behavioural studies suggest a putative olfactory function for the pedipalps (Nisani et al. 2018), perhaps within the enigmatic constellation array sensilla (Fet et al. 2006). However, when it comes to spatial navigation, the two most salient organs are the pectines and median eyes, which we address in the next sections.
Chemo- and mechanoreception—pectines
Of all impressive scorpion assets—fluorescent epicuticle, neurotoxic tail, extreme seismic and visual sensitivity—perhaps the most impressive are the pectines. These paired midventral organs extend from the second mesosomal segment of all scorpions (Wolf 2017). Each pecten is composed of a flexible spine and a species-specific number of teeth (Fig. 9a) adorned on their distal, ground-facing surfaces with arrays of minute, peg-shaped sensilla (Fig. 9a1) of various densities (Gaffin and Brownell 2001; Ali et al. 2001). The peg sensilla arise from flexible cuticular bases (Fig. 9a2) and vary in shape from short paddle forms to taller thinner structures (Prendini et al. 2006; Booncham et al. 2007). A slit-shaped pore at the tip of each peg communicates with a fluid-filled chamber that receives the dendritic outer segments of at least 10 bipolar, chemosensory neurons (Ivanov and Balashov 1979; Foelix and Müller-Vorholt 1983; Wolf 2017). In addition, the dendrite of a mechanosensory neuron with a characteristic tubular body terminates near the base of each peg (Fig. 9a3) (Foelix and Müller-Vorholt 1983; Melville 2000; Wolf 2008).
Anatomy and physiology of pectines and median eyes. a Each of the paired pectines extends laterally from the ventral mesosoma and is composed of a spine (consisting of marginal and medial lamellae and fulcra) and a series of ground-directed teeth. a1 Expansion of several distal teeth reveal fields of peg sensilla (ps) on distal surfaces of each tooth (to). Mechanosensory hair sensilla (hs) extend from various parts of the pecten spine. a2 Expansion of a patch of peg sensilla (ps) shows their close spacing and regular arrangement of their slit-shaped terminal pores (tp). a3 A longitudinal cut through a peg sensillum reveals dendritic outer segments of several chemosensory neurons (red) terminating in a fluid-filled chamber and a single mechanosensory neuron (blue) terminating near the peg base (cu = cuticle). a4 Top shows a stimulant-containing pipette moving every 10 s between 0 and 20 microns of a recorded peg tip that induces high-frequency barrages of peg neural activity. Middle trace shows sample extracellular electrophysiological recording of spontaneous spiking from a peg of P. utahensis that contains A1 and A2 (red) and B (black) cell activity; the spike types have characteristic waveforms as shown in the superimposed expansions at right. Lower panel is a cross-correlogram of near-temporal A cell activity relative to B cell firings (upper is a raster plot, lower graph sums activity over the entire record). Note the clear inhibition of A cell activity immediately after B cell firing along with an inhibitory rebound ~ 50 ms later. There is also an increase in A cell activity immediately prior to B cell firing (asterisk). At right is a proposed feedback circuit to account for this behaviour. a5 Sensory projections of pecten neurons to posterior portion of CNS (PN = pecten nerve; LMPN = lateral mechanosensory pecten neuropil; PPN1-3 = posterior pecten neuropils1-3; APN = anterior pecten neuropil; VLST, CLST, DLST = ventral, central, dorsal lateral sensory tracts). b The median eyes are centrally located on the dorsal prosoma. b1 Each of the paired median eyes consists of a cuticularly derived lens, a cellular vitreous body, and a retina. b2 upper: Expanded region of the retina shows the lens, vitreous body (vb), and receptor cells (rc). Each receptor cell contains a rhabdomere (rm) formed of arrays of microvilli that abut rhabdomeres of adjacent receptor cells. The dendrites of arhabdomeric cells (ac) are synaptically connected to the receptor cells just proximal to the rhabdomeric region. Pigment cells (pc) photo-isolate retinular cell clusters. b2 lower: A cross section of a retinular unit (dashed line) shows five receptor cells with their rhabdomeres abutted at large angles to each other and that together form a star-shaped rhabdom (rh). The pigment granules of dark-adapted eyes migrate to the peripheral and proximal regions of the receptor cells (left) while pigment granules of light-adapted eyes migrate distally and closer to the rhabdomeres (right). b3 Receptor cells (rc) project to the first median eye visual neuropil (M1) while arhabdomeric cells (ac) project to the second median eye visual neuropil (M2) in the protocerebrum. b4 A horizontal cut through the protocerebrum shows the putative relay of arhabdomeric cell information through median optic nerve (MON) to M2 to the arcuate body (ab) and/or the mushroom bodies (mb). Input from the lateral eyes courses through the lateral eye neuropil (LON) to the first lateral eye visual neuropil (L1) and second lateral eye visual neuropil (L2) which adjoins M2 (M/L2). Center bottom shows the relationship of the pectinal and visual projections (pd = pedipalp nerve; 1–4 = walking leg nerves 1–4; pn = pecten nerve; vn = ventral nerve cord). [Images adapted from Drozd et al. 2020, 2022; Gaffin and Walvoord 2004; Gaffin and Shakir 2021; Gaffin and Brownell 2001; Locket 2001; Fleissner and Fleissner 2001b; Lehmann and Melzer 2013]
Electrophysiological responses of bimodal peg sensilla to chemicals and mechanical stimuli are easily obtained by impaling the bases of individual sensilla with either tungsten or saline-filled glass electrodes. Baseline extracellular recordings typically have a few spontaneously active units with circadian rhythmicity, increasing in activity during evening hours (Gaffin and Brownell 1997a). Chemosensory responses obtained by blowing vapors of volatile organic compounds (typically > 10–2 M) across the peg tip has shown that peg neurons are broadly responsive to a variety of chemicals in the 5–9 carbon chain length (e.g., alcohols, aldehydes, esters, ketones, etc.) and to a variety of aromatics (Gaffin and Brownell 1997a). Instead of blowing chemicals, a more realistic approach involves filling a glass pipette tip with a pure volatile substance and using a micromanipulator to maneuver the tip very near to a recorded peg, allowing the chemicals to diffuse out (Fig. 9a4 upper panel) (Gaffin and Walvoord 2004; Gaffin and Shakir 2021). This technique yields precise, repeatable neural responses, but only when the stimulant is very near to the peg (< 20 microns). Robust chemical responses can also be obtained by flooding the peg fields with oil and touching the peg tip with the open end of a saline-filled glass electrode which also contains a water-miscible stimulant (Knowlton and Gaffin 2009, 2011a). This technique isolates stimulants to individual pegs; it can also be used to quickly gather responses from patches of adjacent pegs (Knowlton and Gaffin 2010). Importantly, this work has shown that while the pegs have a rich repertoire of responsivity, each peg essentially responds the same to similar stimulants and that multiple pegs responding in concert are required to discern the difference between two chemicals (Knowlton and Gaffin 2011b).
Mechanosensory responses can often be induced by tapping on the recording electrode or moving it from side to side (Gaffin and Brownell 1997a; Gaffin 2001). These mechanosensory responses are characterized by graded, high frequency firing (> 100 Hz) of a quickly adapting, quickly recovering unit that has a distinct waveform (Gaffin and Brownell 1997a; Peeples and Gaffin (accepted)). Further, the response is not binary; rather, response intensity increases directly with degree of peg deflection (Peeples and Gaffin (accepted)). This response variation has implications for the coding of textural information, a topic we take up at the end of this section.
Another intriguing feature of peg sensilla is that morphological cross-sections of neural elements proximal to the cell body region reveal copious axo-axonic chemical synapses (Foelix and Müller-Vorholt 1983). Several configurations exist, but a common one is a dyad synapse in which one cell is presynaptic to two other neurons. There are also indications of axons being both pre- and post-synaptic to other neurons. The expression of these synapses can be assayed by electrophysiology. For example, in the sand scorpions P. utahensis and S. smeringerus, baseline recordings usually reveal three active neurons (Gaffin and Brownell 1997a, 2001; Gaffin and Walvoord 2004; Knowlton and Gaffin 2011b). The two most active cells (A1 and A2) have similar biphasic waveforms while a third (B) is triphasic and less active (Fig. 9a4). Cross-correlating the activity of the A cells relative to the B cell shows patterns of synaptic interactions where the B cell inhibits the activity of the A cells for tens of milliseconds followed by an inhibitory rebound (Fig. 9a4 lower panel) (Gaffin and Brownell 1997b; Gaffin 2002, 2010). These patterns of synaptic interaction continue even after severing the pectines from the body, verifying that the synaptic effects are confined to the pecten and do not involve CNS feedback (Gaffin and Brownell 1997b). This activity pattern of one cell influencing two others may be the physiological expression of the dyad synapse noted above. Further, especially in higher frequency records, there is evidence that the A cells excite the B cells (directly or indirectly), with the B cells in turn suppressing the A cell activity (Fig. 9a4 sample circuit) (Gaffin and Shakir 2021). This simple feedback mechanism appears to prevent adaptation of the A cells, perhaps keeping them in a dynamic firing range.
Vision
Scorpions have two sets of eyes: the median and lateral eyes. The lateral eyes occur in groups of two to five near the antero-lateral corners of the prosoma, whereas the paired median eyes arise from a small protuberance at the midline of the dorsal prosoma (Fig. 9b). Both median and lateral eyes are ocelli with a single crystalline lens, derived from and continuous with the surrounding cuticle, that focuses light onto a retina (Locket 2001). Because of their position, small size, lack of a vitreous layer, and fused rhabdoms, the lateral eyes do not resolve images and are unlikely to be involved in navigational behaviors. However, they are sensitive to extremely low light levels and are intricately involved (along with the median eyes) in Zeitgeber (timing cue) perception for adjusting the scorpion’s well-described circadian clock system (Fleissner 1974, 1977a, b, c; Schliwa and Fleissner 1980; Fleissner and Fleissner 2001b).
In contrast, the median eyes have many features that make them prime candidates for spatial orientation. These eyes are camera-like, focusing light across a cellular vitreous layer to a dense retina of photoreceptive elements (Fig. 9b1). The eyes are fixed relative to the animal’s orientation and there is no tapetum at the base of the retina (Locket 2001). Together, the median eyes have a 360° panoramic field of view, with 40 degrees of overlap above the animal (Locket 2001). The retina is made up of repeating cylindrical retinular units (Fig. 9b2) that each contain about five receptor cells which have stacked arrays of photo-sensitive microvilli called rhabdomeres (Fleissner 1985; Root 1990; Locket 2001) that abut in the center of the retinular units to form starfish-shaped rhabdoms in cross-section (Belmonte and Stensaas 1975; Root 1990; Fleissner and Fleissner 2001b; Locket 2001). Each retinular unit also has an arhabdomeric cell that extends its dendrites among the receptor cells just proximal to the rhabdomeres (Schliwa and Fleissner 1979). Pigment cells surround and photo-isolate the retinular units from each other (Locket 2001).
The receptor cells contain pigment granules that migrate daily from proximal, peripheral locations to distal, central locations within the cells (Fig. 9b2). The migration is controlled by an intricate efferent neurosecretory fiber (ENSF) system (Fleissner 1974; Fleissner and Fleissner 2001b; Locket 2001) that originates in the protocerebrum and is adjusted by Zeitgeber influences (Fleissner 1977a, b; Fleissner and Fleissner 2001b). Dark adaptation unmasks the median eye rhabdoms, resulting in a significant increase in sensitivity (~ 10–6 W⋅m−2 vs ~ 10–2 W⋅m−2 when light adapted) (Machan 1968; Fleissner and Fleissner 2001a; Locket 2001). The extreme variance in light sensitivity of the median eyes underscores the importance of conducting scorpion navigational studies using dark-adapted animals during their normal nighttime activity periods (Fleissner and Fleissner 2001a) and without extraneous light intrusion.
The discussion of path integration above noted that animals use outbound information to deduce approximate home-bound directions. In some cases, outbound turns can be integrated by comparing orientations to a consistent external reference, such as polarized light direction. Scorpions are known to be behaviorally responsive to polarized light (Brownell 2001; Horváth and Varjú 2004). Polarized light sensitivity may be related to the property of birefringence of the median eye lens, which projects a cross pattern onto the retina that rotates in relation to the direction of polarization (Carricaburu 1968; Locket 2001). While the rhabdomeres of scorpion receptor cells are at large angles relative to each other, and such arrangements have been shown to be important in polarized light detection of other animals (Rossel 1989), it is not known if the divergent angles contribute to polarized light detection in scorpions. Differential stimulus intensity as generated by the birefringent lens would differentially stimulate underlying retinular units and could provide a neural accounting of polarized light from the moon or setting sun (Locket 2001). However, a given set of visual sensors is typically dedicated to object vision or for polarized light vision, but not for both (Wehner 2001; How and Marshall 2014).
What information do scorpions use for navigation?
As suggested in the earlier section on path integration, it is important for navigating animals to use outbound information to calculate the direction and length (distance) of home-directed vectors (Papi 1992a, b). Rotational information can be deduced by integrating outbound turns relative to a steady external referent (such as polarized light direction, geomagnetic fields, or starlight patterns). Rotational and translational (distance walked) information can also be deduced by idiothetic cues such as differential activity of leg joint sensors (proprioceptors), as shown for C. salei (Barth and Seyfarth 1971; Seyfarth and Barth 1972; Seyfarth et al. 1982; Barth 2002) or by monitoring optic flow rates from opposing eyes (Ortega-Escobar 2002, 2006, 2011; Ortega-Escobar and Ruiz 2014). In spiders, cuticular strain and joint displacements are monitored by minute slit sensilla arranged individually or in groups such as lyriform organs (Seyfarth and Barth 1972; Barth 2012).
Mechanoreception-based navigation
Scorpions do not have lyriform organs, but they do have groups of slits in various patterns near the joints along with many isolated slits along the length of the legs (Barth and Wadepuhl 1975; Barth and Stagl 1976; Stemme pers comm). While recordings from sensory elements of the dorsal leg nerve have been correlated electrically with joint movements (Weltzin and Bowerman 1980; Root 1990), and recordings have been made from motor neurons of leg ganglia in semi-intact scorpion preps (Bowerman and Burrows 1980), selective ablation studies are needed to determine the role of slit sensilla in path integration. Nonetheless, the previously discussed study by Prévost and Stemme (2020) provided strong evidence for proprioceptive-based path integration in Mesobuthus eupeus. The most accurate homing bouts were performed without visual information, strongly indicating proprioceptive path integration.
A clear next step in exploring scorpion sensory navigation is to use animals with compromised proprioceptive organs (as discussed in Prévost and Stemme 2020) to disrupt the flow of walking and turning information that can be gleaned from differential leg joint activity (Seyfarth and Barth 1972). Also, the rich mechanosensory input from arrays of peg sensilla and of pectinal hair sensilla on the midventral pectines should be considered. Differential flow of textures across the sweeping right and left pectines could serve to monitor the animal’s movements and turns. Reversibly covering the pectines could shed light on their involvement in path integration.
Vision-based navigation
In addition to mechanoreception-based path integration, idiothetic information could be gained by scorpions by monitoring optic flow past the lateral or medial eyes. Visual information is also likely during learning walks. Although elements of path integration are likely involved in guiding the animals back to their burrows during learning walks, without blinding the animals it is not possible to narrow the sensory information source. Experiments are needed using animals with various structures compromised (eyes, pectines, proprioceptive organs) to deduce the important sensory components in these walks.
How do scorpions process information for navigation?
Vision processing
Visual information gathered in the median eyes flows along two distinct routes, with the receptor and arhabdomeric cells projecting to different neuropils (Fig. 9b3). The receptor cells conduct graded potentials to the first median eye visual neuropil (aka lamina) (Babu 1985; Fleissner and Fleissner 2001b; Locket 2001; Lehmann and Melzer 2013), where they interact with interneurons and terminals from the efferent neurosecretory fiber (ENSF) system involved in circadian rhythmicity. The arhabdomeric cells conduct action potentials to the second median eye visual neuropil (aka medulla) (Fleissner and Fleissner 2001b; Locket 2001; Lehmann and Melzer 2013), which does not receive ENSF input. The terminals of the arhabdomeric neurons synapse with interneurons that may relay information to the arcuate body (Hanström 1923; Babu 1985; Root 1990) and perhaps the mushroom bodies (Fig. 9b4). When thinking about the acquisition and use of visual information for navigation, it is important to consider the spacing and density of the retinular units which send information via the axons of the arhabdomeric cells to higher processing areas. In Androctonus australis, the retina is estimated to contain about 500–600 such units (Fleissner 1985). We will discuss possible implications of these visual projections for navigation following an overview of pectin information processing.
Pectines processing
Neurons from the bimodal peg sensilla travel in the pectinal nerves to neuropils located ventrally and caudally in the subesophageal ganglion (Fig. 9a5). The neuropils parse to posterior pectinal neuropils (PPN1-3) and an anterior pectinal neuropil (APN). Notably, peg afferents remain topographically arranged in all subregions of PPN, with proximal pegs projecting medially and distal pegs laterally (Brownell 1998, 2001; Wolf 2008, 2017; Wolf and Harzsch 2012; Hughes and Gaffin 2019; Drozd et al. 2020). It will be interesting to trace second order neurons from the PPN and APN to see if a relationship exists with the mushroom bodies and/or arcuate body. Neurons from mechanosensory sensilla on the pectinal spine (labeled “hs” in Fig. 9a1) also converge and travel the pectinal nerve to the posterior CNS (Melville 2000), but do not enter the PPN (Drozd et al. 2022). Instead, they diverge and send collaterals to the lateral mechanosensory pecten neuropil (LMPN) as well as fibers to the neuromere region of the 3rd and 4th walking legs and branches to contralateral arborizations dorsal to the PPN and ipsilaterally to the 3rd and 4th walking leg neuromeres (Drozd et al. 2022). This parallel mechanosensory system appears to be a circuit for regulating pecten height and thus position of peg fields relative to the surface. The spine of each pecten precedes the pegs as the organ travels across the ground, and the protruding mechanosensory hairs are the first points of obstacle contact (Schneider et al. 2003). The stimulation of the sensilla (Kladt et al. 2007) appears to reflexively summon leg 3 and 4 motor neurons to alter body posture (Drozd et al. 2022). The pectines are also supplied with muscle and it will be interesting to see if there is a direct relay back to the organs for further micro adjustments.
Chemo-textural familiarity hypothesis
Clearly, scorpions have rich sensory capabilities. Of particular interest are the enormous matrices of sensory units present in the pectines and median eyes. The number of chemo-tactile peg sensilla exceeds 100,000 in some species (Swoveland 1978), and there are at least 1000 visual arhabdomeric projections from the two median eyes (Fleissner 1985). But how could such matrices be deployed to guide navigation back to a home burrow? One possibility is the navigation by chemo-textural familiarity hypothesis (Gaffin and Brayfield 2017; Gaffin and Curry 2020), which was inspired by the navigation by scene familiarity hypothesis for ants (Baddeley et al. 2011, 2012). The premise is that on early homebound journeys, scorpions use their pectines to gather textural and chemical information that they later use to guide their movements towards familiar tastes and touches to return home after longer forays. Of course, for this hypothesis to be valid, there must be innate behaviours for acquiring home-directed “glimpses” of the environment, since everything experienced while moving away from their burrow is 180-degrees rotated from glimpses directed towards their burrow (Gaffin et al. 2022). In this regard, the recent discovery of path integration (Prévost and Stemme 2020) and learning walks (Gaffin et al. 2022) in scorpions is exciting and could be crucial for acquiring such home-directed information.
Additionally, for navigation by familiarity to be valid, the sensor and environmental complexity must be sufficient to avoid aliasing (confusing similar appearing scenes from different locations). From a sensor complexity point of view, the pectines and median eyes are exceptional. For example, the pectines of a female P. utahensis have about 20 teeth per pecten and about 100 pegs per tooth, adding up to about 4000 pegs (2*20*100) between the two pectines. Even if the pegs generated only two states of neural activity (“on” or “off”), there would be 2^4000 detectable patterns, more than an Excel spreadsheet can express. In reality, the pegs are not binary; they respond to many different chemicals and concentrations, as well as to mechanical deflections that also are not binary (Peeples and Gaffin (accepted)). The number of detectable stimuli increases the number of states (the base number) of our equation, which increases the number of detectable patterns precipitously. If we add the 1000 visual units to the mix (i.e., trimodal sensory input) … well, you get the point.
Some computer simulations have been developed based on known parameters of the scorpion pectinal system (Gaffin and Brayfield 2017; Musaelian and Gaffin 2020). In some of these models, substrate textural differences are represented by shades of black and white and chemicals by colors. Simulations are useful in that thousands of simultaneous navigational experiments can be performed using high throughput supercomputers, and sensory and environmental variables can be systematically altered to determine optimal performance conditions (Musaelian and Gaffin 2020). These simulations are generating proof-of-concept models of familiarity navigation while also shedding light on what could be the unit of information in terms of pectinal input to the CNS. Along these lines, since the neural projections from pectinal teeth to the CNS are topographically maintained (Brownell 1998, 2001; Wolf 2008; Drozd et al. 2020) and since the size of sand grains from the animals’ natural habitat span multiple teeth (Brownell 2001; Gaffin and Walvoord 2004), it is tempting to suggest that the tooth may be the unit of information with the aggregate activity of the pegs on each tooth contributing to a greater number of states. Going back to our female P. utahensis example with 40 teeth across two pectines and assuming (very conservatively) that the pegs have only two states, then there would be 100 possible states per tooth when aggregating the peg population. This leads to 100^40 = 1 × 1080 detectable pattern (a number Excel can handle, but enormous nonetheless). Further, computer models of scene familiarity suggest that such blurring of the environment actually enhances route following fidelity (Gaffin et al. 2015; Gaffin and Brayfield 2016, 2017; Musaelian and Gaffin 2020).
A lot more research is needed to describe the kinetics of pecten movement relative to the substrate. Accounts have been mostly anecdotal, yet descriptions vary from the pectines intermittently tapping the ground to continuously dragging across the substrate as the animal walks (Alexander 1958; Williams 1966; Gaffin and Brownell 1992; Gaffin and Walvoord 2004). Navigation via purely chemo-textural familiarity would argue for continuous substrate sampling via dragging. Even blurring through parallel summation of peg responses would not seem adequate in highly discontinuous sampling. However, we must also realize that scorpions have outstanding median eyes that cover all 360 around them and are sensitive to star light level. As such, it is possible that the chemo-tactile information from the pectines and visual panoramic information from the eyes blend together at higher levels to further lock the animal onto familiar paths. In addition, home-bound vectors gleaned by path integration must always be considered. It could also be that pecten draggers become pecten tappers with multiple experiences with a given substrate. Simply put, much more behavioral research is needed to resolve all these questions.
Discussion
Amblypygids, spiders, and scorpions each bring something unique to the table when it comes to understanding the mechanisms underlying spatial orientation and navigation. Importantly, they expand our knowledge of sensory systems involved in arthropod navigation well beyond vision and contribute to our understanding of neural processes underlying such complex behavior. While synthesizing the research advances associated with amblypygid, spider and scorpion navigational prowess and associated mechanisms, we identified both gaps in our understanding of each taxonomic group and missed research opportunities. These gaps and missed opportunities appear to be the result primarily of (i) biases in sensory focus, (ii) distinct conceptual areas of focus, and (iii) different levels of baseline neuroanatomical information. Likely due to distinct natural histories and potentially their phylogenetic distance (though many hypothesize that all three orders belong to Arachnopulmonata, see Sharma et al. 2014; Giribet and Edgecombe 2019; Ballesteros et al. 2022), each system appears to have developed its own research culture and approach, with minimal overlap across systems. We urge future research to integrate across these systems to advance a broader understanding of arachnid and arthropod navigation.
The primary sensory modalities tested for influencing navigation in amblypygids, spiders and scorpions are not the same, making it challenging to identify generalized patterns among the three groups. Building off the olfactory capabilities of the antenniform legs, for example, amblypygid studies have focused primarily on testing the role of olfaction in navigation, while olfaction has not been studied at all in spiders. There are a few accounts of olfactory-guided orientation in scorpions, but the dense contact chemoreceptive peg sensilla on the pectines and their topographically ordered CNS projections have commanded most of the research attention. In contrast, spider studies have focused predominantly on visual information, with particular attention paid to polarized light and substrate-based cues. Indeed, the role of polarized light and associated eye structure in spiders has greatly expanded our appreciation for this sensory modality in arachnid navigation. In amblypygids, however, visual masking studies suggest that vision is unimportant. But recent findings in amblypygids demonstrate that individuals can learn overhead visual patterns. This visual learning, combined with the knowledge that there are visual inputs into the mushroom bodies of amblypygids, suggests the potential for visual canopy orientation; a suggestion that remains to be tested. The jury is still out on visual navigation in scorpions—few studies have addressed this question. Yet anatomical and physiological accounts suggest that vision could play a very important role, especially under very low light conditions (Fleissner and Fleissner 2001a). Finally, mechanoreception-based navigation has been studied in spiders and scorpions, with a strong focus on proprioception and path integration. In contrast, despite antenniform leg ablation studies likely impacting mechanoreception (Santer 2019), this sensory modality has not been the focus of amblypygid studies. Perhaps surprisingly, manipulations of mechanoreception have not been carried out in amblypygids or scorpions, yet such experiments could provide critical information on the importance and use of this sensory input.
Conceptually, path integration and learning walks, both mechanisms frequently studied in insects, have helped guide spider and scorpion navigation studies. In contrast, amblypygid studies have focused more on allothetic, sensory-based learning and its relationship to navigation. As such, it is difficult to discern at this point whether amblypygids use path integration or learning walks. Simultaneously, we know little about whether spiders or scorpions display modality-specific learning capacities. Furthermore, the hypothesized integration center of the enlarged mushroom bodies in amblypygids has led to multiple studies exploring multimodal navigation and learning. Multimodality per se, in contrast, is only now beginning to be discussed and integrated into spider and scorpion research. Given that many spider or scorpion experiments were designed to test path integration while amblypygid studies were designed to test modality-specific learning, synthesizing results across systems is currently challenging. Nonetheless, the advances made in each group can be adapted for other groups. Moving forward, amblypygid studies focused explicitly on path integration and mechanoreception-based navigation borrowing techniques and designs from spider and scorpion are needed, as are modality-specific and multimodal studies of spider and scorpion learning.
Elegant electrophysiological studies have provided a wealth of information on the sensory capacities of scorpion pectines, paving the path for future behavioural studies. The giant fiber system of amblypygids has also been explored with electrophysiology, as has their olfactory capacity, but its structure and potential function has not been directly related to a potential role in navigation in the same manner that the pectines have. The computer simulations using scorpion pectinal systems, for example, might be useful for exploring the antenniform leg system of amblypygids.
Finally, our knowledge of basic neuroanatomy and sensory processing across all three groups is non-uniform and there are several notable gaps. In amblypygids, we now know that visual and olfactory information send inputs into the mushroom bodies, for example, but we know little about mechanoreceptive inputs into higher order processing centers. The behavioural data suggesting that amblypygids are capable of configural multimodal, but not multicomponent unimodal, stimuli open new questions regarding sensory processing and functional integration. In spiders with different lifestyles, a polarized-light pattern analyzer analogous to that in insect compound eyes has been described, but its connection with the central nervous system is unknown. Similarly, little is known about the inputs to the mushroom body or arcuate body in scorpions. Many exciting research questions remain.
Conclusions
No matter the reason—that they are nocturnal, cryptic, secretive, venomous, and/or subjects of scary fables and movies—arachnids are not well represented in the scientific literature compared to their arthropod relatives. A cursory Google Scholar search with the terms “bee” and “ant” yields a combined 6,510,000 entries, while “spider”, “scorpion”, and “whip spider” produces only 365,700. This ~ 18:1 literature ratio is far out of line with the number of described species for these groups (~ 34 K to ~ 50 K respectively). Arachnids are also overlooked compared to their insect relatives in spatial orientation studies. For example, of the 18 papers in this special edition, 15 have titles where the animal group can be deduced. All 15 papers concern arthropods and 14 cover insects in subphylum Mandibulata, 10 of which reference hymenopterans. The only non-mandibulate paper is this one that covers arachnids in subphylum Chelicerata. This bee/ant prevalence may also lead to oversampling of vision compared to other modalities. Judging from the titles, 10 appear related to vision, 2 indicate multisensory, and 1 olfaction. Arachnids may thus be useful animals for closing this sensory-bias gap owing to their significant mechanosensory, chemosensory (olfaction and contact chemoreception), and multisensory abilities. Finally, the diversity of taxa studied within the arachnids is also quite small. In this review, we discuss only 4 amblypygid species, 7 spider species, and 4 scorpion species. If we are to ever obtain a broad understanding of navigational capacities and their underlying neural mechanisms, we need to broaden the representation of arachnids in navigation studies.
Availability of data and materials
Not applicable.
Change history
10 March 2023
Article note related to special issue deleted
References
Adams AM, Marais E, Turner JS, Prendini L, Pinshow B (2016) Similar burrow architecture of three arid-zone scorpion species implies similar ecological function. Sci Nat 103:56. https://doi.org/10.1007/s00114-016-1374-z
Alexander AJ (1958) On the stridulation of scorpions. Behaviour 12:339–352. https://doi.org/10.1163/156853958X00028
Ali MO, Saber S, Elmenshawy O, El Bakary Z, Sarhan M (2001) A comparative morphological study of the pectines of three scorpion species (Scorpionida, Buthidae) from Assiut, Egypt. Serket 7:94–105
Ashford K, Blankenship R, Carpenter W, Wheeler I, Gaffin D (2018) Response of the eastern sand scorpion, Paruroctonus utahensis, to air movement from a moth analog. J Arachnol 46:226–230. https://doi.org/10.1636/JoA-S-17-097.1
Avarguès-Weber A, Giurfa G (2013) Conceptual learning by miniature brains. Proc R Soc Lond B Biol Sci 280:20131907. https://doi.org/10.1098/rspb.2013.1907
Babu KS (1985) Patterns of arrangement and connectivity in the central nervous system of arachnids. In: Barth FG (ed) Neurobiology of arachnids. Springer-Verlag, Berlin, pp 3–19
Baddeley B, Graham P, Philippides A, Husbands P (2011) Holistic visual encoding of ant-like routes: navigation without waypoints. Adapt Behav 19:3–15. https://doi.org/10.1177/1059712310395410
Baddeley B, Graham P, Husbands P, Philippides A (2012) A model of ant route navigation driven by scene familiarity. PLoS Comput Biol 8:e1002336. https://doi.org/10.1371/journal.pcbi.1002336
Ballesteros JA, Santibáñez-López CE, Baker CM, Benavides LR, Cunha TJ, Gainett G, Sharma PP (2022) Comprehensive species sampling and sophisticated algorithmic approaches refute the monophyly of Arachnida. Mol Biol Evol 39:msac021. https://doi.org/10.1093/molbev/msac021
Ban XC, Shao ZK, Wu LJ, Sun JT, Xue XF (2022) Highly diversified mitochondrial genomes provide new evidence for interordinal relationships in the Arachnida. Cladistics 38:462–464. https://doi.org/10.1111/cla.12504
Barth FG (2000) How to catch the wind: spider hairs specialized for sensing the movement of air. Naturwissenschaften 87:51–58
Barth FG (2002) A spider’s world: Senses and behavior. Springer, Berlin
Barth FG (2012) Arthropod strain sensors. In: Bhushan B (ed) Encyclopedia of nanotechnology. Springer, Netherlands, Dordrecht, pp 127–136
Barth FG (2020) A spider in motion: facets of sensory guidance. J Comp Physiol A 207:239–255. https://doi.org/10.1007/s00359-020-01449-z
Barth FG, Seyfarth E-A (1971) Slit sense organs and kinesthetic orientation. Z Vergl Physiol 74:326–328. https://doi.org/10.1007/BF00297732
Barth FG, Stagl J (1976) The slit sense organs of arachnids: a comparative study of their topography on the walking legs (Chelicerata, Arachnida). Zoomorphologie 86:1–23. https://doi.org/10.1007/BF01006710
Barth FG, Wadepuhl M (1975) Slit sense organs on the scorpion leg (Androctonus australis L., Buthidae). J Morphol 145:209–227. https://doi.org/10.1002/jmor.1051450207
Beck L, Gorke K (1974) Tagesperiodik, revierverhalten und beutefang der geisselspinne Admetus pumilio CL Koch im freiland. Z Tierpsychol 35:173–186
Beck L, Foelix R, Godeke E, Kaiser R (1977) Morphology, larval development, and hair sensilla of antenniform legs of whip spider Heterophrynus longicornis Butler (Arachnida Amblypygi). Zoomorphologie 88:259–276
Becker JE, Brown CA (2016) Reliable refuge: two sky island scorpion species select larger, thermally stable retreat sites. PLOS ONE 11:e0168105. https://doi.org/10.1371/journal.pone.0168105
Belmonte C, Stensaas LJ (1975) Repetitive spikes in photoreceptor axons of the scorpion eye. Invertebrate eye structure and tetrodotoxin. J Gen Physiol 66:649–655. https://doi.org/10.1085/jgp.66.5.649
Bingman VP, Graving JM, Hebets EA, Wiegmann DD (2017) Importance of the antenniform legs, but not vision, for homing by the neotropical whip spider Paraphrynus laevifrons. J Exp Biol 220:885–890. https://doi.org/10.1242/jeb.149823
Blest AD (1985) The fine structure of spider photoreceptors in relation to function. In: Barth FG (ed) Neurobiology of arachnids. Springer-Verlag, Berlin, pp 79–102
Booncham U, Sitthicharoenchai D, Pradatsundarasar A, Prasarnpun S, Thirakhupt K (2007) Sexual Dimorphism in the Asian Giant Forest Scorpion, Heterometrus laoticus Couzijn, 1981. NU Int J Sci 4:42–52
Bost K, Gaffin DD (2004) Sand scorpion home burrow navigation in the laboratory. Euscorpius 17:1–5
Bowerman RF, Burrows M (1980) The morphology and physiology of some walking leg motor neurones in a scorpion. J Comp Physiol 140:31–42. https://doi.org/10.1007/BF00613745
Bradley R (1982) Digestion time and reemergence in the desert grassland scorpion Paruroctonus utahensis (Williams) (Scorpionida, Vaejovidae). Oecologia 55:316–318. https://doi.org/10.1007/BF00376918
Bradley RA (1988) The influence of weather and biotic factors on the behaviour of the scorpion (Paruroctonus utahensis). J Anim Ecol 57:533–551. https://doi.org/10.2307/4923
Brownell PH (1977) Compressional and surface waves in sand: used by desert scorpions to locate prey. Science 197:479–482. https://doi.org/10.1126/science.197.4302.479
Brownell PH (1998) Glomerular cytoarchitectures in chemosensory systems of arachnids. Ann N Y Acad Sci 855:502–507. https://doi.org/10.1111/j.1749-6632.1998.tb10614.x
Brownell P (2001) Sensory ecology and orientational behaviors. In: Brownell P, Polis G (eds) Scorpion biology and research. Oxford University Press, Oxford, pp 159–183
Brownell P, Farley RD (1979a) Detection of vibrations in sand by tarsal sense organs of the nocturnal scorpion, Paruroctonus mesaensis. J Comp Physiol 131:23–30. https://doi.org/10.1007/BF00613080
Brownell P, Farley RD (1979b) Orientation to vibrations in sand by the nocturnal scorpion Paruroctonus mesaensis: mechanism of target localization. J Comp Physiol 131:31–38. https://doi.org/10.1007/BF00613081
Brownell P, Farley RD (1979c) Prey-localizing behaviour of the nocturnal desert scorpion, Paruroctonus mesaensis: Orientation to substrate vibrations. Anim Behav 27:185–193. https://doi.org/10.1016/0003-3472(79)90138-6
Camp EA, Gaffin DD (1999) Escape behavior mediated by negative phototaxis in the scorpion Paruroctonus utahensis (Scorpiones, Vaejovidae). J Arachnol 27:679–684
Carricaburu P (1968) Dioptrique oculaire du scorpion Androctonus australis. Vis Res 8:1067–1072. https://doi.org/10.1016/0042-6989(68)90078-3
Casto P, Gosser J, Wiegmann DD, Hebets EA, Bingman VP (2019) Self-derived chemical cues support home refuge recognition in the whip spider Phrynus marginemaculatus (Amblypygi: Phrynidae). J Arachnol 47:290–292. https://doi.org/10.1636/JoA-S-18-067
Casto P, Wiegmann DD, Coppola VJ, Nardi D, Hebets EA, Bingman VP (2020) Vertical-surface navigation in the Neotropical whip spider Paraphrynus laevifrons (Arachnida: Amblypygi). Anim Cogn 23:1205–1213. https://doi.org/10.1007/s10071-020-01420-0
Chapin KJ, Hebets EA (2016) The behavioral ecology of amblypygids. J Arachnol 44:1–14. https://doi.org/10.1636/V15-62.1
Collett TS (2019) Path integration: how details of the honeybee waggle dance and the foraging strategies of desert ants might help in understanding its mechanisms. J Exp Biol 222:jeb205187. https://doi.org/10.1242/jeb.205187
Collett TS, Zeil J (2018) Insect learning flights and walks. Curr Biol 28:R984–R988. https://doi.org/10.1016/j.cub.2018.04.050
Cruse H, Wehner R (2011) No need for a cognitive map: decentralized memory for insect navigation. PLoS Comput Biol 7:e1002009. https://doi.org/10.1371/journal.pcbi.1002009
Dacke M, Nilsson DE, Warrant EJ, Blest AD, Land MF, O’Carroll DC (1999) Built-in polarizers form part of a compass organ in spiders. Nature 401:470–473. https://doi.org/10.1038/46773
Dacke M, Doan TA, O’Carroll DC (2001) Polarized light detection in spiders. J Exp Biol 204:2481–2490. https://doi.org/10.1242/jeb.204.14.2481
Deeti S, Cheng K (2021) Learning walks in an Australian desert ant. Melophorus bagoti J Exp Biol 224:jeb242177. https://doi.org/10.1242/jeb.242177
Devaud J-M, Papouin T, Carcaud J, Sandoz J-C, Grünewald B, Giurfa M (2015) Neural substrate for higher-order learning in an insect: mushroom bodies are necessary for configural discriminations. Proc Natl Acad Sci USA 112:E5854–E5862. https://doi.org/10.1073/pnas.1508422112
Drozd D, Wolf H, Stemme T (2020) Structure of the pecten neuropil pathway and its innervation by bimodal peg afferents in two scorpion species. PLoS One 15:e0243753. https://doi.org/10.1371/journal.pone.0243753
Drozd D, Wolf H, Stemme T (2022) Mechanosensory pathways of scorpion pecten hair sensillae—adjustment of body height and pecten position. J Comp Neurol 530:2918–2937. https://doi.org/10.1002/cne.25384
Dunlop JA (2010) Geological history and phylogeny of Chelicerata. Arthropod Struct Dev 39:124–142. https://doi.org/10.1016/j.asd.2010.01.003
Eakin RM, Brandenburger JL (1971) Fine structure of the eyes of jumping spiders. J Ultrastruct Res 37:618–663. https://doi.org/10.1016/S0022-5320(71)80029-1
Eastwood EB (1978) Notes on the scorpion fauna of the Cape. IV. The burrowing activities of some scorpionids and buthids (Arachnida, Scorpionida). Ann S Afr Mus 74:249–255
Farris SM (2005) Evolution of insect mushroom bodies: old clues, new insights. Arthropod Struct Dev 34:211–234. https://doi.org/10.1016/j.asd.2005.01.008
Fet V, Brewer MS, Soleglad ME, Neff DPA (2006) Constellation array: a new sensory structure in scorpions (Arachnida: Scorpiones). Boletín de la Sociedad Eentomológica Aragonesa /S.E.A.) 38:269–278.
Flanigan KAS, Wiegmann DD, Casto P, Coppola VJ, Flesher NR, Hebets EA, Bingman VP (2021a) Visual control of refuge recognition in the whip spider Phrynus marginemaculatus. J Comp Physiol A 207:729–737. https://doi.org/10.1007/s00359-021-01509-y
Flanigan KAS, Wiegmann DD, Hebets EA, Bingman VP (2021) Multisensory integration supports configural learning of a home refuge in the whip spider Phrynus marginemaculatus. J Exp Biol 224:jeb238444. https://doi.org/10.1242/jeb.238444
Fleischmann PN, Christian M, Müller VL, Rössler W, Wehner R (2016) Ontogeny of learning walks and the acquisition of landmark information in desert ants, Cataglyphis fortis. J Exp Biol 219:3137–3145. https://doi.org/10.1242/jeb.140459
Fleissner G (1974) Circadiane Adaptation und Schirmpigmentverlagerung in den Sehzellen der Medianaugen yon Androctonus australis L. (Buthidae, Scorpiones). J Comp Physiol 91:399–416
Fleissner G (1977a) Scorpion lateral eyes: Extremely sensitive receptors of Zeitgeber stimuli. J Comp Physiol 118:101–108. https://doi.org/10.1007/BF00612340
Fleissner G (1977b) Entrainment of the scorpion’s circadian rhythm via the median eyes. J Comp Physiol 118:93–99. https://doi.org/10.1007/BF00612339
Fleissner G (1977c) The absolute sensitivity of the median and lateral eyes of the scorpion, Androctonus australis L. (Buthidae, Scorpiones). J Comp Physiol 118:109–120. https://doi.org/10.1007/BF00612341
Fleissner G (1985) Intracellular recordings of light responses from spiking and nonspiking cells in the median and lateral eyes of the scorpion. Naturwissenschaften 72:46–48. https://doi.org/10.1007/BF00405333
Fleissner G, Fleissner G (2001a) Night vision in desert scorpions. In: Fet V, Selden PA (eds) Scorpions 2001. British Arachnological Society, In Memoriam Gary A. Polis, pp 317–324
Fleissner G, Fleissner G (2001b) Neuronal organization of circadian systems. In: Brownell P, Polis G (eds) Scorpion biology and research. Oxford University Press, pp 107–137
Foelix RF (1975) Occurrence of synapses in peripheral sensory nerves of arachnids. Nature 254:146–148. https://doi.org/10.1038/254146a0
Foelix RF (2011) Biology of spiders, 3rd edn. Oxford University Press, Oxford
Foelix R, Hebets EA (2001) Sensory biology of whip spiders (Arachnida, Amblypygi). Andrias 15:129–140
Foelix RF, Müller-Vorholt G (1983) The fine structure of scorpion sensory organs. II. Pecten sensilla. Bull Br Arachnol Soc 6:68–74
Foelix RF, Schabronath J (1983) Fine structure of scorpion sensory organs. I. Tarsal sensilla. Bull Br Arachnol Soc 6:53–67
Fowler-Finn KD, Hebets EA (2006) An examination of agonistic interactions in the whip spider Phrynus marginemaculatus (Arachnida, Amblypygi). J Arachnology 34:62–76. https://doi.org/10.1636/S04-104.1
French AS, Torkkeli PH, Seyfarth E-A (2002) From stress and strain to spikes: mechanotransduction in spider slit sensilla. J Comp Physiol A 188:739–752. https://doi.org/10.1007/s00359-002-0363-1
Frost L, Butler D, O’Dell B, Fet V (2001) A coumarin as a fluorescent compound in scorpion cuticle. In: Fet V, Felden PA (eds) Scorpions 2001. British Arachnological Society, Burham Beeches, Bucks, UK, In Memoriam Gary A. Polis, pp 363–368
Gaffin DD (2001) Electrophysiological evidence of synaptic interactions between sensory neurons in peg sensilla of Centruroides vittatus (Say, 1821) (Scorpiones: Buthidae). In: Fet V, Felden PA (eds) Scorpions 2001. In Memoriam Gary A. Polis. British Arachnological Society, Burham Beeches, Bucks, UK, pp 325–330
Gaffin DD (2002) Electrophysiological analysis of synaptic interactions within peg sensilla of scorpion pectines. Microsc Res Tech 58:325–334. https://doi.org/10.1002/jemt.10140
Gaffin DD (2010) Analysis of sensory processing in scorpion peg sensilla. J Arachnol 38:1–8. https://doi.org/10.1636/SH08-60.1
Gaffin D (2011) In situ infrared videography of sand scorpion nighttime surface activity. Euscorpius 2011:1–13. https://doi.org/10.18590/euscorpius.2011.vol2011.iss122.1
Gaffin DD, Brayfield BP (2016) Autonomous visual navigation of an indoor environment using a parsimonious, insect inspired familiarity algorithm. PLoS One 11:e0153706. https://doi.org/10.1371/journal.pone.0153706
Gaffin DD, Brayfield BP (2017) Exploring the chemo-textural familiarity hypothesis for scorpion navigation. J Arachnol 45:265–270. https://doi.org/10.1636/JoA-S-16-070.1
Gaffin DD, Brownell PH (1992) Evidence of chemical signaling in the sand scorpion Paruroctonus mesaensis (Scorpionida: Vaejovidae). Ethology 91:59–69. https://doi.org/10.1111/j.1439-0310.1992.tb00850.x
Gaffin DD, Brownell PH (1997a) Response properties of chemosensory peg sensilla on the pectines of scorpions. J Comp Physiol A 181:291–300. https://doi.org/10.1007/s003590050115
Gaffin DD, Brownell PH (1997) Electrophysiological evidence of synaptic interaction within chemosensory peg sensilla of scorpion pectines. J Comp Physiol A 181:301–307. https://doi.org/10.1007/s003590050116
Gaffin D, Brownell P (2001) Chemosensory behavior and physiology. In: Brownell P, Polis G (eds) Scorpion biology and research. Oxford University Press, Oxford, pp 184–203
Gaffin DD, Curry CM (2020) Arachnid navigation-a review of classic and emerging models. J Arachnol 48:1–25. https://doi.org/10.1636/0161-8202-48.1.1
Gaffin DD, Shakir SF (2021) Synaptic interactions in scorpion peg sensilla appear to maintain chemosensory neurons within dynamic firing range. Insects 12:904. https://doi.org/10.3390/insects12100904
Gaffin DD, Walvoord ME (2004) Scorpion peg sensilla: are they the same or are they different? Euscorpius 17:7–15
Gaffin DD, Wennstrom KL, Brownell PH (1992) Water detection in the desert sand scorpion, Paruroctonus mesaensis (Scorpionida, Vaejovidae). J Comp Physiol A 170:623–629. https://doi.org/10.1007/BF00199338
Gaffin DD, Dewar A, Graham P, Philippides A (2015) Insect-inspired navigation algorithm for an aerial agent using satellite imagery. PLoS One 10:e0122077. https://doi.org/10.1371/journal.pone.0122077
Gaffin DD, Muñoz MG, Hoefnagels MH (2022) Evidence of learning walks related to scorpion home burrow navigation. J Exp Biol 225:jeb243947. https://doi.org/10.1242/jeb.243947
Geethabali RKP (1973) A metasomatic neural photoreceptor in the scorpion. J Exp Biol 58:189–196. https://doi.org/10.1242/jeb.58.1.189
Giribet G, Edgecombe GD (2019) The phylogeny and evolutionary history of arthropods. Curr Biol 29:R592–R602. https://doi.org/10.1016/j.cub.2019.04.057
Giurfa M (2013) Cognition with few neurons: higher-order learning in insects. Trends Neurosci 36:285–294. https://doi.org/10.1016/j.tins.2012.12.011
Görner P (1958) Die optische und kinästhetische orientierung der trichterspinne Agelena labyrinthica (Cl.). Z Vgl Physiol 41:111–153. https://doi.org/10.1007/BF00345583
Görner P, Claas B (1985) Homing behavior and orientation in the funnel-web spider, Agelena labyrinthica Clerck. In: Barth FG (ed) Neurobiology of arachnids. Springer-Verlag, Berlin, pp 275–297
Graving JM, Bingman VP, Hebets EA, Wiegmann DD (2017) Development of site fidelity in the nocturnal amblypygid, Phrynus marginemaculatus. J Comp Physiol A 203:313–328. https://doi.org/10.1007/s00359-017-1169-5
Hanström B (1923) Further notes on the central nervous system of arachnids: Scorpions, phalangids, and trap-door spiders. J Comp Neurol 35:249–274. https://doi.org/10.1002/cne.900350402
Harland DP, Li D, Jackson RR (2012) How jumping spiders see the world. In: Lazareva OF, Shimizu T, Wasserman EA (eds) How animals see the world. Oxford University Press, Oxford, pp 133–163
Haug C, Haug JT (2021) The fossil record of whip spiders: the past of Amblypygi. PalZ 95:387–412. https://doi.org/10.1007/s12542-021-00552-z
Hebets EA (2002) Relating the unique sensory system of amblypygids to the ecology and behavior of Phrynus parvulus from Costa Rica (Arachnida, Amblypygi). Can J Zool 80:286–295. https://doi.org/10.1139/Z02-006
Hebets EA, Chapman RF (2000) Electrophysiological studies of olfaction in the whip spider Phrynus parvulus (Arachnida, Amblypygi). J Insect Physiol 46:1441–1448. https://doi.org/10.1016/S0022-1910(00)00068-8
Hebets EA, Aceves-Aparicio A, Aguilar-Argüello S, Bingman VP, Escalante I, Gering EJ, Nelsen DR, Rivera J, Sanchez-Ruiz JA, Segura-Hernandez L, Settepani V, Wiegmann DD, Stafstrom JA (2014a) Multimodal sensory reliance in the nocturnal homing of the amblypygid Phrynus pseudoparvulus (Class Arachnida, Order Amblypygi)? Behav Processes 108:123–130. https://doi.org/10.1016/j.beproc.2014.09.014
Hebets EA, Gering EJ, Bingman VP, Wiegmann DD (2014b) Nocturnal homing in the tropical amblypygid Phrynus pseudoparvulus (Class Arachnida, Order Amblypygi). Anim Cogn 17:1013–1018. https://doi.org/10.1007/s10071-013-0718-8
Heinze S (2014) Polarized-light processing in insect brains: Recent insights from the desert locust, the monarch butterfly, the cricket, and the fruit fly. In: Horváth G (ed) Polarized light and polarization vision in animal sciences. Springer-Verlag, Berlin, pp 61–111
Heinze S, Gotthardt S, Homberg U (2009) Transformation of polarized light information in the central complex of the locust. J Neurosci 29:11783–11793. https://doi.org/10.1523/JNEUROSCI.1870-09.2009
Heinze S, Narendra A, Cheung A (2018) Principles of insect path integration. Curr Biol 28:R1043–R1058. https://doi.org/10.1016/j.cub.2018.04.058
Homann H (1971) Die Augen der Araneae. Z Morph Tiere 69:201–272. https://doi.org/10.1007/BF00277623
Homberg U (2004) In search of the sky compass in the insect brain. Naturwissenschaften 91:199–208. https://doi.org/10.1007/s00114-004-0525-9
Horváth G, Varjú D (2004) Polarization sensitivity in spiders and scorpions. In: Horváth G, Varjú D (eds) Polarized light in animal vision: Polarization patterns in nature. Springer, Berlin Heidelberg, pp 243–246
How MJ, Marshall NJ (2014) Polarization distance: a framework for modelling object detection by polarization vision systems. Proc R Soc Lond B Biol Sci 281:20131632. https://doi.org/10.1098/rspb.2013.1632
Hughes KL, Gaffin DD (2019) Investigating sensory processing in the pectines of the striped bark scorpion. Centruroides Vittatus Invert Neurosci 19:9. https://doi.org/10.1007/s10158-019-0228-8
Igelmund P (1987) Morphology, sense-organs, and regeneration of the forelegs (whips) of the whip spider Heterophrynus elaphus (Arachnida, Amblypygi). J Morphol 193:75–89. https://doi.org/10.1002/jmor.1051930108
Igelmund P, Wendler G (1991a) The giant fiber system in the forelegs (whips) of the whip spider Heterophrynus elaphus Pocock (Arachnida: Amblypygi). J Comp Physiol A 168:63–73. https://doi.org/10.1007/BF00217104
Igelmund P, Wendler G (1991b) Morphology and physiology of peripheral giant interneurons in the forelegs (whips) of the whip spider Heterophrynus elaphus Pocock (Arachnida, Amblypygi). J Comp Physiol A 168:75–83. https://doi.org/10.1007/BF00217105
Ivanov V, Balashov Y (1979) The structural and functional organization of the pectine in a scorpion Buthus eupeus Koch (Scorpiones, Buthidae) studied by electron microscopy. The fauna and ecology of Arachnida. Trudy Zoological Institute, Leningrad, pp 73–87
Jayatilaka P, Murray T, Narendra A, Zeil J (2018) The choreography of learning walks in the Australian jack jumper ant Myrmecia croslandi. J Exp Biol 221:jeb185306. https://doi.org/10.1242/jeb.185306
Kaltsas D, Mylonas M (2010) Locomotory activity and orientation of Mesobuthus gibbosus (Scorpiones: Buthidae) in central Aegean Archipelago. J Nat Hist 44:1445–1459. https://doi.org/10.1080/00222931003632732
Kaltsas D, Stathi I, Mylonas M (2009) Intraspecific differentiation of social behavior and shelter selection in Mesobuthus gibbosus (Brullé, 1832) (Scorpiones: Buthidae). J Ethol 27:467–473. https://doi.org/10.1007/s10164-008-0144-6
Kladt N, Wolf H, Heinzel H-G (2007) Mechanoreception by cuticular sensilla on the pectines of the scorpion Pandinus cavimanus. J Comp Physiol A 193:1033–1043. https://doi.org/10.1007/s00359-007-0254-6
Knowlton ED, Gaffin DD (2009) A new approach to examining scorpion peg sensilla: the mineral oil flood technique. J Arachnol 37:379–382. https://doi.org/10.1636/SH08-79SC.1
Knowlton ED, Gaffin DD (2010) A new tip-recording method to test scorpion pecten chemoresponses to water-soluble stimulants. J Neurosci Methods 193:264–270. https://doi.org/10.1016/j.jneumeth.2010.09.002
Knowlton ED, Gaffin DD (2011) Electrophysiology of scorpion peg sensilla. JoVE 50:e2642. https://doi.org/10.3791/2642
Knowlton ED, Gaffin DD (2011b) Functionally redundant peg sensilla on the scorpion pecten. J Comp Physiol A 197:895. https://doi.org/10.1007/s00359-011-0650-9
Kovoor J, Muñoz Cuevas A, Ortega Escobar J (1993) Microanatomy of the anterior median eyes and its possible relation to polarized-light reception in Lycosa tarentula (Araneae, Lycosidae). Boll Zool 60:367–375. https://doi.org/10.1080/11250009309355841
Kovoor J, Muñoz-Cuevas A, Ortega-Escobar J (2005) The visual system of Lycosa tarentula (Araneae, Lycosidae): Microscopic anatomy of the protocerebral optic centres. Ital J Zool 72:205–216. https://doi.org/10.1080/11250000509356673
Labhart T (1980) Specialized photoreceptors at the dorsal rim of the honeybee’s compound eye: polarizational and angular sensitivity. J Comp Physiol 141:19–30. https://doi.org/10.1007/BF00611874
Labhart R (1986) The electrophysiology of photoreceptors in different eye regions of the desert ant, Cataglyphis bicolor. J Comp Physiol A 158:1–7. https://doi.org/10.1007/BF00614514
Labhart T (1988) Polarization-opponent interneurons in the insect visual system. Nature 331:435–437. https://doi.org/10.1038/331435a0
Labhart T, Meyer EP (1999) Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc Res Tech 47:368–379. https://doi.org/10.1080/11250000509356673
Land MF (1985) The morphology and optic of spider eyes. In: Barth FG (ed) Neurobiology of arachnids. Springer-Verlag, Berlin, pp 53–78
Lehmann T, Melzer RR (2013) Looking like Limulus?- Retinula axons and visual neuropils of the median and lateral eyes of scorpions. Front Zool 10:40. https://doi.org/10.1186/1742-9994-10-40
Lehmann T, Melzer RR (2018) Also looking like Limulus?- retinula axons and visual neuropils of Amplypygi (whip spiders). Front Zool 15:52. https://doi.org/10.1186/s12983-018-0293-6
Lehmann T, Melzer RR (2019) The visual system of Thelyphonida (whip scorpions): support for Arachnopulmonata. Arthropod Struct Dev 51:23–31. https://doi.org/10.1016/j.asd.2019.06.002
Lehmann KDS, Shogren FG, Fallick M, Colton Watts J, Schoenberg D, Wiegmann DD, Bingman VP, Hebets EA (2022) Exploring higher-order conceptual learning in an arthropod with a large multisensory processing center. Insects 13:81. https://doi.org/10.3390/insects13010081
Locket A (2001) Eyes and vision. In: Brownell PH, Polis GA (eds) Scorpion biology and research. Oxford University Press, Oxford, pp 79–106
Loesel R, Heuer CM (2010) The mushroom bodies–prominent brain centres of arthropods and annelids with enigmatic evolutionary origin. Acta Zool 91:29–34. https://doi.org/10.1111/j.1463-6395.2009.00422.x
Machan L (1968) Spectral sensitivity of scorpion eyes and the possible role of shielding pigment effect. J Exp Biol 49:95–105. https://doi.org/10.1242/jeb.49.1.95
Magni F, Papi F, Savely HE, Tongiorgi P (1965) Research on the structure and physiology of the eyes of a lycosid spider. III. Electroretinographic responses to polarized light. Arch Ital Biol 103:146–158
Mathejczyk TF, Wernet MF (2017) Sensing polarized light in insects. In: Sherman SM (ed) Oxford research encyclopedia neuroscience. Oxford University Press, Oxford, pp 1–33
McCormick SJ, Polis GA (1990) Prey, predators, and parasites. In: Polis G (ed) The biology of scorpions. Stanford University Press, Stanford, pp 294–320
Melville JM (2000) The pectines of scorpions: analysis of structure and function. Oregon State University, Ph.D.
Menzel R (2001) Searching for the memory trace in a mini-brain, the honeybee. Learn Mem 8:53–62. https://doi.org/10.1101/lm.38801
Meßlinger K (1987) Fine structure of scorpion trichobothria (Arachnida, Scorpiones). Zoomorphology 107:49–57. https://doi.org/10.1007/BF00312129
Mittelstaedt H (1983) The role of multimodal convergence in homing by path integration. Fortsch Zool 28:197–212
Mizunami M, Weibrecht JM, Strausfeld NJ (1998) Mushroom bodies of the cockroach: their participation in place memory. J Comp Neurol 402:520–537. https://doi.org/10.1002/(SICI)1096-9861(19981228)402:4%3c520::AID-CNE6%3e3.0.CO;2-K
Morehouse N (2020) Spider vision. Curr Biol 30:R975–R980. https://doi.org/10.1016/j.cub.2020.07.042
Mueller KP, Labhart T (2010) Polarizing optics in a spider eye. J Comp Physiol A 196:335–348. https://doi.org/10.1007/s00359-010-0516-6
Murayama GP, Willemart RH (2019) Are trichobothria used in terrestrial prey capture by the yellow scorpion Tityus serrulatus Lutz & Mello, 1922 (Buthidae)? Arachnology 18:287–290. https://doi.org/10.13156/arac.2019.18.3.287
Musaelian A, Gaffin DD (2020) High-throughput simulations indicate feasibility of navigation by familiarity with a local sensor such as scorpion pectines. bioRxiv. https://doi.org/10.1101/2020.06.17.156612
Narendra A, Gourmaud S, Zeil J (2013) Mapping the navigational knowledge of individually foraging ants, Myrmecia croslandi. Proc R Soc Lond B Biol Sci 280:20130683. https://doi.org/10.1098/rspb.2013.0683
Nentwig W (1987) The prey of spiders. In: Nentwig W (ed) Ecophysiology of spiders. Springer-Verlag, Heidelberg, pp 249–273
Nisani Z, Honaker A, Jenne V, Loya F, Moon H (2018) Evidence of airborne chemoreception in the scorpion Paruroctonus marksi (Scorpiones: Vaejovidae). J Arachnol 46:40–44. https://doi.org/10.1636/JoA-16-092.1
NØrgaard T (2005) Nocturnal navigation in Leucorchertris arenicola (Araneae, Sparassidae). J Arachnol 33:533–540. https://doi.org/10.1636/04-113.1
NØrgaard T, Henschel JR, Wehner R (2003) Long-distance navigation in the wandering desert spider Leucorchestris arenicola: can the slope of the dune surface provide a compass cue? J Comp Physiol A 189:801–809. https://doi.org/10.1007/s00359-003-0455-6
NØrgaard T, Henschel JR, Wehner R (2006) The night-time temporal window of locomotor activity in the Namib desert long-distance wandering spider, Leucorchestris arenicola. J Comp Physiol A 192:365–372. https://doi.org/10.1007/s00359-005-0072-7
NØrgaard T, Nilsson D-E, Henschel JR, Garm A, Wehner R (2008) Vision in the nocturnal wandering spider Leucorchestris arenicola (Araneae: Sparassidae). J Exp Biol 211:816–823. https://doi.org/10.1242/jeb.010546
NØrgaard T, Gagnon YL, Warrant EJ (2012) Nocturnal homing: Learning walks in a wandering spider? PLoS ONE 7:e49267. https://doi.org/10.1371/journal.pone.0049263
Oliveira PS, Hölldobler B (1989) Orientation and communication in the Neotropical ant Odontomachus bauri Emery (Hymenoptera, Formicidae, Ponerinae). Ethology 83:154–166. https://doi.org/10.1111/j.1439-0310.1989.tb00525.x
Ortega-Escobar J (2002) Evidence that the wolf-spider Lycosa tarentula (Araneae, Lycosidae) needs visual input for path integration. J Arachnol 30:481–486. https://doi.org/10.1636/0161-8202(2002)030[0481:ETTWSL]2.0.CO;2
Ortega-Escobar J (2006) Role of the anterior lateral eyes of the wolf spider Lycosa tarentula (Araneae, Lycosidae) during path integration. J Aracnol 34:51–61. https://doi.org/10.1636/S04-103.1
Ortega-Escobar J (2011) Anterior lateral eyes of Lycosa tarantula (Araneae: Lycosidae) are used during orientation to detect changes in the visual structure of the substratum. J Exp Biol 214:2375–2380. https://doi.org/10.1242/jeb.055988
Ortega-Escobar J (2017) Polarized-light vision in spiders. Trends Entomol 13:25–34
Ortega-Escobar J (2020) Homing in the arachnid taxa Araneae and Amblypygi. Anim Cogn 23:1189–1204. https://doi.org/10.1007/s10071-020-01424-w
Ortega-Escobar J, Munoz-Cuevas A (1999) The anterior median eyes of Lycosa tarentula (Araneae, Lycosidae) detect the polarized light: Behavioral experiments and electroretinographic analysis. J Arachnol 27:663–671
Ortega-Escobar J, Ruiz MA (2014) Visual odometry in the wolf spider Lycosa tarantula (Araneae: Lycosidae). J Exp Biol 217:395–401. https://doi.org/10.1242/jeb.091868
Ortega-Escobar J, Ruíz MA (2017) Role of the different eyes in the visual odometry in the wolf spider Lycosa tarantula (Araneae, Lycosidae). J Exp Biol 220:259–265. https://doi.org/10.1242/jeb.145763
Papi F (1955) Sull’orientamento astronomico in specie del gen. Arctosa (Araneae Lycosidae). Z Vergl Physiol 41:481–489. https://doi.org/10.1007/BF00547387
Papi F (1992a) Animal homing. Chapman & Hall, London
Papi F (1992b) General aspects. In: Papi F (ed) Animal homing. Chapman & Hall, London, pp 1–18
Pearce JM (2002) Evaluation and development of a connectionist theory of configural learning. Anim Learn Behav 30:73–95. https://doi.org/10.3758/BF03192911
Peeples HM, Gaffin DD (accepted) An assessment of the mechanosensory responses of peg sensilla on scorpion pectines. J Arachnol
Polis GA (1979) Prey and feeding phenology of the desert sand scorpion Paruroctonus mesaensis (Scorpionidae: Vaejovidae). J Zool 188:333–346. https://doi.org/10.1111/j.1469-7998.1979.tb03419.x
Polis GA (1980) Seasonal patterns and age-specific variation in the surface activity of a population of desert scorpions in relation to environmental factors. J Anim Ecol 49:1–18. https://doi.org/10.2307/4275
Polis GA (1990) Ecology. In: Polis G (ed) The biology of scorpions. Stanford University Press, Stanford, pp 247–293
Polis GA, Farley RD (1979) Behavior and ecology of mating in the cannibalistic scorpion, Paruroctonus mesaensis Stahnke (Scorpionida: Vaejovidae). J Arachnol 7:33–46
Polis GA, Farley RD (1980) Population biology of a desert scorpion: survivorship, microhabitat, and the evolution of life history strategy. Ecology 61:620–629. https://doi.org/10.2307/1937428
Polis GA, Sissom WD (1990) Life history. In: Polis G (ed) The biology of scorpions. Stanford University Press, Stanford, pp 161–223
Polis GA, McReynolds CN, Ford RG (1985) Home range geometry of the desert scorpion Paruroctonus mesaensis. Oecologia 67:273–277. https://doi.org/10.1007/BF00384298
Polis GA, Myers C, Quinlan M (1986) Burrowing biology and spatial distribution of desert scorpions. J Arid Environ 10:137–146. https://doi.org/10.1016/S0140-1963(18)31254-0
Prendini L, Volschenk ES, Maaliki S, Gromov AV (2006) A ‘living fossil’ from Central Asia: The morphology of Pseudochactas ovchinnikovi Gromov, 1998 (Scorpiones: Pseudochactidae), with comments on its phylogenetic position. Zool Anz 245:211–248. https://doi.org/10.1016/j.jcz.2006.07.001
Prévost ED, Stemme T (2020) Non-visual homing and the current status of navigation in scorpions. Anim Cogn 23:1215–1234. https://doi.org/10.1007/s10071-020-01386-z
Reissland A, Görner P (1985) Trichobothria. In: Barth FG (ed) Neurobiology of arachnids. Springer, Berlin, Heidelberg, pp 138–161
Root TM (1990) Neurobiology. In: Polis G (ed) The biology of scorpions. Stanford University Press, Stanford, pp 341–413
Rossel S (1989) Polarization sensitivity in compound eyes. In: Stavenga DG, Hardie RC (eds) Facets of vision. Springer, Berlin, Heidelberg, pp 298–316
Sancer G, Kind E, Plazaola-Sasieta H, Balke J, Pham T, Hasan A, Münch LO, Courgeon M, Mathejczyk TF, Wernet MF (2019) Modality-specific circuits for skylight orientation in the fly visual system. Curr Biol 29:2812–2825. https://doi.org/10.1016/j.cub.2019.07.020
Santer RD (2019) Olfactory and tactile cues can guide near-distance location of a refuge by whip spiders (Arachnida, Amblypyi). Anim Behav 158:e1–e2. https://doi.org/10.1016/j.anbehav.2019.10.020
Santer RD, Hebets EA (2008) Agonistic signals received by an arthropod filiform hair allude to the prevalence of near-field sound communication. Proc R Soc Lond B Biol Sci 275:363–368. https://doi.org/10.1098/rspb.2007.1466
Santer RD, Hebets EA (2009) Tactile learning by a whip spider, Phrynus marginemaculatus CL Koch (Arachnida, Amblypygi). J Comp Physiol A 195:393–399. https://doi.org/10.1007/s00359-009-0417-8
Santer RD, Hebets EA (2011) Evidence for air movement signals in the agonistic behaviour of a nocturnal arachnid (Order Amblypygi). PLoS One 6:e22473. https://doi.org/10.1371/journal.pone.0022473
Santer RD, Hebets EA (2011b) The sensory and behavioural biology of whip spiders (Arachnida, Amblypygi). Adv Insect Physiol 41:1–64. https://doi.org/10.1016/B978-0-12-415919-8.00001-X
Santschi F (1911) Observations et remarques critiques sur le mécanisme de l’orientation chez les fourmis. Rev Suisse Zool 19:305–338
Schliwa M, Fleissner G (1979) Arhabdomeric cells of the median eye retina of scorpions: I. Fine Structural Analysis J Comp Physiol 130:265–270. https://doi.org/10.1007/BF00614613
Schliwa M, Fleissner G (1980) The lateral eyes of the scorpion, Androctonus australis. Cell Tissue Res 206:95–114. https://doi.org/10.1007/BF00233611
Schneider W, Böhm H, Heinzel HG (2003) The role of the pecten organs in the regulation of the body height in walking scorpions. Verh Dtsch Zool Ges 96:152
Schröer W-D (1974) Zum Mechanismus der Analyse polarisierten Lichtes bei Agelena gracilens C. L. Koch (Araneae, Agelenidae). I. Die Morphologie der Retina der vorderen Mittelaugen (Hauptaugen). Z Morph Tiere 79:215–231
Schröer W-D (1976) Polarisationsempfindlichkeit rhabdomerialer Systeme in den Hauptaugen der Trichterspinne Agelena gracilens (Arachnida: Araneae: Agelenidae). Ent Germ 3:88–92. https://doi.org/10.1127/entom.germ/3/1976/88
Schröer W-D (2017) Fine structure of the anterior median eyes of the funnel-web spider Agelena labyrinthica (Araneae: Agelenidae). Arthropod Struct Dev 46:196–214. https://doi.org/10.1016/j.asd.2017.01.001
Seiter M, Strobl L, Schwaha T, Prendini L, Schramm FD (2022) Morphometry of the pedipalp patella provides new characters for species-level taxonomy in whip spiders (Arachnida, Amblypygi): a test case with description of a new species of Phrynus. Zool Anz 298:10–28. https://doi.org/10.1016/j.jcz.2022.02.004
Sergi CM, Antonopoulos T, Rodríguez RL (2021) Black widow spiders use path integration on their webs. Behav Ecol Sociobiol 75:73. https://doi.org/10.1007/s00265-021-03009-0
Seyfarth E-A, Barth FG (1972) Compound slit sense organs on the spider leg: mechanoreceptors involved in kinesthetic orientation. J Comp Physiol 78:176–191. https://doi.org/10.1007/BF00693611
Seyfarth E-A, Hergenröder R, Ebbes H, Barth FG (1982) Idiothetic orientation of a wandering spider: compensation of detours and estimates of goal distance. Behav Ecol Sociobiol 11:139–148. https://doi.org/10.1007/BF00300103
Sharma PP, Kaluziak ST, Pérez-Porro AR, González VL, Hormiga G, Wheeler WC, Giribet G (2014) Phylogenomic interrogation of Arachnida reveals systemic conflicts in phylogenetic signal. Mol Biol Evol 3:2963–2984. https://doi.org/10.1093/molbev/msu235
Sinakevitch I, Long SM, Gronenberg W (2021) The central nervous system of whip spiders (Amblypygi): Large mushroom bodies receive olfactory and visual input. J Comp Neurol 529:1642–1658. https://doi.org/10.1002/cne.25045
Sombke A, Klann AE, Lipke E, Wolf H (2019) Primary processing neuropils associated with the malleoli of camel spiders (Arachnida, Solifugae): a re-evaluation of axonal pathways. Zoological Lett 5:1–13. https://doi.org/10.1186/s40851-019-0137-z
Spence AJ, Hebets EA (2006) Anatomy and physiology of giant neurons in the antenniform leg of the amblypygid Prynus marginemaculatus. J Arachnol 34:566–577. https://doi.org/10.1636/S05-53.1
Stachel SJ, Stockwell SA, Van Vranken DL (1999) The fluorescence of scorpions and cataractogenesis. Chem Biol 6:531–539. https://doi.org/10.1016/S1074-5521(99)80085-4
Stahnke HL (1972) UV light, a useful field tool. Bioscience 22:604–607. https://doi.org/10.2307/1296207
Steinhoff PO, Uhl G, Harzsch S, Sombke A (2020) Visual pathways in the brain of the jumping spider Marpissa muscosa. J Comp Neurol 528:1883–1902. https://doi.org/10.1002/cne.24861
Stemme T, Pfeffer SE (2022) Anatomy of the nervous system in Chelifer cancroides (Arachnida: Pseudoscorpiones) with a distinct sensory pathway associated with the pedipalps. Insects 13:25. https://doi.org/10.3390/insects13010025
Strausfeld NJ (1998) Crustacean - Insect relationships: the use of brain characters to derive phylogeny amongst segmented invertebrates. Brain Behav Evol 52:186–206. https://doi.org/10.1159/000006563
Strausfeld NJ, Barth FG (1993) Two visual systems in one brain: Neuropils serving the secondary eyes of the spider Cupiennius salei. J Comp Neurol 328:43–62. https://doi.org/10.1002/cne.903280104
Strausfeld NJ, Reisenman CE (2009) Dimorphic olfactory lobes in the Arthropoda. Ann N Y Acad Sci 1170:487–496. https://doi.org/10.1111/j.1749-6632.2009.04020.x
Strausfeld NJ, Weltzien P, Barth FG (1993) Two visual systems in one brain: neuropils serving the principal eyes of the spider Cupiennius salei. J Comp Neurol 328:63–75. https://doi.org/10.1002/cne.903280105
Strausfeld NJ, Hansen L, Li YS, Gomez RS, Ito K (1998) Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn Mem 5:11–37. https://doi.org/10.1101/lm.5.1.11
Strausfeld NJ, Sinakevitch I, Brown SM, Farris SM (2009) Ground plan of the insect mushroom body: functional and evolutionary implications. J Comp Neurol 513:265–91. https://doi.org/10.1002/cne.21948
Strausfeld NJ, Wolff GH, Sayre ME (2020) Mushroom body evolution demonstrates homology and divergence across Pancrustacea. Elife. https://doi.org/10.7554/eLife.52411
Swoveland ML (1978) External morphology of scorpion pectines. PhD Thesis, San Francisco State University.
Vinnedge JE, Gaffin DD (2015) Determination of in-lab site fidelity and movement patterns of Paruroctonus utahensis (Scorpiones: Vaejovidae). J Arachnol 43:54–58. https://doi.org/10.1636/J14-36.1
Warburg MR (2013) The locomotory rhythmic activity in scorpions: with a review. Arthropods 2:95–104
Warburg MB, Polis GA (1990) Behavioral responses, rhythms, and activity patterns. In: Polis G (ed) The biology of scorpions. Stanford University Press, Stanford, pp 224–246
Webb B, Wystrach A (2016) Neural mechanisms of insect navigation. Curr Opin Insect Sci 15:27–39. https://doi.org/10.1016/j.cois.2016.02.011
Wehner R (2001) Polarization vision—a uniform sensory capacity? J Exp Biol 204:2589–2596. https://doi.org/10.1242/jeb.204.14.2589
Wehner R (2016) Early ant trajectories: spatial behaviour before behaviourism. J Comp Physiol A 202:247–266. https://doi.org/10.1007/s00359-015-1060-1
Wehner R, Labhart T (2006) Polarization vision. In: Warrant E, Nilsson D-E (eds) Invertebrate vision. Cambridge University Press, Cambridge, pp 291–348
Wehner R, Strasser S (1985) The POL area of the honeybee’s eye: behavioural evidence. Physiol Entomol 10:337–349. https://doi.org/10.1111/j.1365-3032.1985.tb00055.x
Wehner R, Meier C, Zollikofer C (2004) The ontogeny of foraging behaviour in desert ants, Cataglyphis bicolor. Ecol Entomol 29:240–250. https://doi.org/10.1111/j.0307-6946.2004.00591.x
Weltzin R, Bowerman RF (1980) Scorpion Walking Leg Proprioceptors. Am Zool 20:940
Wessnitzer J, Webb B (2006) Multimodal sensory integration in insects—towards insect brain control architectures. Bioinspir Biomim 1:63. https://doi.org/10.1088/1748-3182/1/3/001
Weygoldt P (1977) Coexistence of two species of whip spiders (Genus Heterophrynus) in neotropical rain forest (Arachnida, Amblypygi). Oecologia 27:363–370. https://doi.org/10.1007/BF00345569
Weygoldt P (2000) Whip spiders (Chelicerata: Amblypygi). Their biology, morphology and systematics. Apollo Books, Stenstrup.
Wiegmann DD, Hebets EA, Gronenberg W, Graving JM, Bingman VP (2016) Amblypygids: Model organisms for the study of arthropod navigation mechanisms in complex environments? Front Behav Neurosci 10:47. https://doi.org/10.3389/fnbeh.2016.00047
Wiegmann DD, Moore CH, Flesher NR, Harper ED, Keto KR, Hebets EA, Verner P Bingman VP (2019) Nocturnal navigation by whip spiders: antenniform legs mediate near-distance olfactory localization of a shelter. Anim Behav 149:45–54. https://doi.org/10.1016/j.anbehav.2019.01.005
Wiegmann DD, Casto P, Hebets EA, Bingman VP (2020) Distortion of the local magnetic field appears to neither disrupt nocturnal navigation nor cue shelter recognition in the amblypygid Paraphrynus laevifrons. Ethology 126:403–412. https://doi.org/10.1111/eth.12985
Williams SC (1966) Burrowing activities of the scorpion Anuroctonus phaeodactylus (Wood) (Scorpionida: Vejovidae). Proc Calif Acad Sci 34:419–428
Wolf H (2008) The pectine organs of the scorpion, Vaejovis spinigerus: structure and (glomerular) central projections. Arthropod Struct Dev 37:67–80. https://doi.org/10.1016/j.asd.2007.05.003
Wolf H (2011) Odometry and insect navigation. J Exp Biol 214:1629–1641. https://doi.org/10.1242/jeb.038570
Wolf H (2017) Scorpions pectines – Idiosyncratic chemo- and mechanosensory organs. Arthropod Struct Dev 46:753–764. https://doi.org/10.1016/j.asd.2017.10.002
Wolf H, Harzsch S (2012) Serotonin-immunoreactive neurons in scorpion pectine neuropils: similarities to insect and crustacean primary olfactory centres? Zoology 115:151–159. https://doi.org/10.1016/j.zool.2011.10.002
Wolff GH, Strausfeld NJ (2015) Genealogical correspondence of mushroom bodies across invertebrate phyla. Curr Biol 25:38–44. https://doi.org/10.1016/j.zool.2011.10.002
Wolff GH, Strausfeld NJ (2016) Genealogical correspondence of a forebrain centre implies an executive brain in the protostome–deuterostome bilaterian ancestor. Philos Trans R Soc Lond B Biol Sci 371:20150055. https://doi.org/10.1098/rstb.2015.0055
Wolff GH, Thoen HH, Marshall J, Sayre ME, Strausfeld NJ (2017) An insect-like mushroom body in a crustacean brain. elife 6:e29889. https://doi.org/10.7554/eLife.29889.001
World Spider Catalog (2022). World Spider Catalog. Version 23.5. Natural History Museum Bern, online at http://wsc.nmbe.ch, accessed on 8 Sep 2022
Zeil J, Fleischmann PN (2019) The learning walks of ants (Hymenoptera: Formicidae). Myrmecol News 29:93–110. https://doi.org/10.25849/MYRMECOL.NEWS_029:093
Zeil J, Ribi WA, Narendra A (2014) Polarisation vision in ants, bees and wasps. In: Horváth G (ed) Polarized light and polarization vision in animal sciences. Springer-Verlag, Berlin, pp 41–60
Zwicky KT (1968) A light response in the tail of Urodacus, a scorpion. Life Sci 7:257–262. https://doi.org/10.1016/0024-3205(68)90020-9
Zwicky KT (1970a) The spectral sensitivity of the tail of Urodacus, a scorpion. Experientia 26:317–317. https://doi.org/10.1007/BF01900120
Zwicky KT (1970b) Behavioural aspects of the extraocular light sense of Urodacus, a scorpion. Experientia 26:747–748. https://doi.org/10.1007/BF02232523
Acknowledgements
We would like to thank Uwe Homberg for his kind invitation to contribute a manuscript to this Special Issue. We would like to thank Harald Wolf, Torben Stemme, Denise Drozd and Mariëlle Hoefnagels for reviewing early drafts of part of this review and providing outstanding suggestions. We would also like to thank Jake Graving for the SEM images used in Fig. 1 and Wulfila Gronenberg for creating the beautiful Fig. 3. We are grateful to the arachnology community and the researchers whose curiosity about amblypygid, spider, and scorpion navigation has contributed to our growing understanding of this field. Finally, we thank the reviewers of the manuscript for their useful edits and suggestions.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All the authors were involved in drafting and revising the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Uwe Homberg.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ortega-Escobar, J., Hebets, E.A., Bingman, V.P. et al. Comparative biology of spatial navigation in three arachnid orders (Amblypygi, Araneae, and Scorpiones). J Comp Physiol A 209, 747–779 (2023). https://doi.org/10.1007/s00359-023-01612-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-023-01612-2