Abstract
The pattern of linearly polarized light in the sky can be used for orientation behavior by many insects. Although such behavioral responses have been well described in bees and ants over several decades, until recently it remained largely elusive how polarized-light information is processed in the insect brain. However, over the last decade, substantial advances in understanding polarized-light processing have been made, based on behavioral, electrophysiological, and anatomical data. Particularly, progress was made in the desert locust, but based on comparative work in the field cricket, the monarch butterfly, and the fruit fly broader conclusions about how polarized-light information is encoded in the insect brain in general begin to emerge. After polarized light is detected by photoreceptors of specialized parts of the compound eye, this information passes through the optic lobe, the anterior optic tubercle, and the central complex. In these brain regions, detailed neural responses to polarized light have been characterized in a large set of anatomically defined neurons that together comprise the polarization vision network. This work has begun to unravel how polarized light is integrated with unpolarized light, and how response characteristics of involved neurons are modulated in context-dependent ways. Eventually, all skylight cues appear to be combined to generate a neural representation of azimuthal space around the animal in the central complex of the brain, which could be used as a basis for directed behavior. Polarized-light information is likely contributing to such a representation in many insects and thus this modality could be crucial for illuminating how the insect brain in general encodes the position of the animal in space, a task that all animal brains have to master.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
It has long been known that the sun can be used by insects for orienting in their environment. Surprisingly, early experiments on harvester ants (Messor barbarus) revealed that the animals were still properly orienting on the homebound journey of a foraging trip even though the direct view of the sun had been blocked (Santschi 1923). However, this orientation was abolished when the patch of blue sky visible to the ants was covered with a ground glass plate. Although Felix Santschi could not interpret his results at the time, we now know that the depolarizing effect of the ground glass disturbed the ant’s navigation. Karl von Frisch (1949) eventually showed several decades later through work in the honey bee (Apis mellifera) that the polarization pattern of the blue sky can be used by insects to determine the position of the sun, even when the direct view of the sun is obscured. This pattern is invisible to the human eye and is produced through scattering of sunlight in the atmosphere. Importantly, the plane of polarization—oscillation direction of the electric field vector (E-vector)—is always perpendicular to the scattering plane determined by the sun, the observer, and the observed celestial point. Therefore, the position of the sun can be directly inferred from analyzing the distribution of E-vectors in a patch of blue sky.
Extensive work has been done with honey bees and desert ants over the course of several decades, during which the behavioral responses of these animals to polarized light have been characterized in detail (e.g., Rossel and Wehner 1987; Wehner 1997). But how does the brain of insects process these E-vector signals and transform them into motor commands? Two competing theories were proposed at the time that had very different demands on the nervous system: First, the simultaneous method, in which an animal can immediately determine any E-vector at the sky by combining three parallel analyzer channels and can therefore perceive individual E-vectors (Kirschfeld 1972). Second, the scanning method, in which the animal compares all E-vectors in the sky with a single matched filter (Rossel and Wehner 1986). The output value of this matching can be used to determine the plane of mirror symmetry of the sky (i.e., the solar–antisolar meridian), when the animal rotates around its own body axis by 360° and compares the output over time. While the first method is quite demanding on the nervous system, the second one is much simpler, albeit providing much less information for the animal.
As bees and ants were not easily accessible for electrophysiological recordings, it was the pioneering work of Thomas Labhart on field crickets (Gryllus campestris) that gave the first evidence of how polarized light is processed in the insect brain. He discovered and characterized neurons that responded with changes in their spiking activity in response to different E-vectors (Labhart 1988, 1996; Labhart and Petzold 1993; Labhart and Meyer 2002). These cells (POL1 neurons) were maximally excited at one E-vector orientation, and were maximally inhibited by the orthogonal E-vector angle. This response pattern was called polarization opponency and is found in most polarization-sensitive neurons in the insect nervous system. The fact that the tuning of these cells fell into one of three groups, and therefore POL1 neurons could act as three analyzer channels, was strong support for the hypothesis of instantaneous E-vector detection. A decade later, it was shown by work from the laboratory of Uwe Homberg that the cricket was not the only species in which polarization-sensitive neurons could be analyzed (Homberg and Würden 1997; Vitzthum et al. 2002). In the desert locust (Schistocerca gregaria), several types of neurons were described that also responded to changes in E-vector orientation. Most of these cells were located in the center of the brain in a region called the central complex. It therefore became evident that the complexity of the neural network involved in processing of polarized light was substantial, and much effort was since put into the task of describing additional neural elements that together constitute the polarization vision network of the insect brain.
The main scope of this chapter is to summarize the work over the last decade that immensely widened our knowledge about how polarized light is processed in the brain of insects, particularly in the desert locust, but also in the cricket, in the monarch butterfly (Danaus plexippus), and—very recently—in the fruit fly Drosophila melanogaster. The earlier literature on polarization vision has been reviewed by Horváth and Varjú (2004) and Wehner and Labhart (2006).
2 The Skylight Polarization Pattern
To understand the neural and behavioral responses of insects to polarized-light stimuli, we have to briefly outline the main features of the skylight polarization pattern. In the behavioral and neurophysiological studies considered in this chapter, a simplified version of the natural sky is used, which is based on the single-scattering Rayleigh model (Coulson 1988) (Fig. 4.1). In this approximation to the natural situation, the skylight polarization pattern is described with two variables: the E-vector angle and the degree of linear polarization. The E-vector angles are distributed along concentric circles around the sun, and the degree of polarization is maximal at angles 90° away from the sun. Although the Rayleigh model results in degrees of polarization between 0 and 100 %, the maximal values measured in the clear sky do not exceed 75 % (Brines and Gould 1982; Horváth and Varjú 2004). Hence, in studies dealing specifically with models of the degree of polarization (Heinze and Reppert 2011; Pfeiffer et al. 2011), a correction factor of 0.75 was applied to adapt the Rayleigh equations to the empirical skylight conditions.
Single-scattering Rayleigh model of skylight polarization. (a) Pattern of electric field vectors (E-vectors) in the sky. Orientation of black lines indicate E-vector angles, while their thickness indicates the degree of linear polarization. Numbers represent elevation above the horizon. (b) E-vector angles during different solar elevations; 0° is defined as an E-vector parallel to the solar meridian. (c) Degree of polarization at different solar elevations
Importantly, the E-vector pattern in the sky is not stationary, but moves across the sky according to the apparent movement of the sun (Fig. 4.1b, c). This results in the fact that the overall degree of polarization in the sky is the highest at the lowest solar elevations (i.e., in the morning and evening), when the E-vectors are nearly all oriented in parallel in a wide band passing the zenith perpendicular to the solar–antisolar meridian, while high degrees of polarization can only be found near the horizon at the antisolar half of the sky if the sun is located at high elevations.
The polarization characteristics of real skies (clear, partly cloudy, overcast, foggy, smoky, canopied, moonlit) were investigated both theoretically (Coulson 1988; Schwind and Horváth 1993; Barta and Horváth 2004; Hegedüs et al. 2006) and experimentally (Coulson 1988; Horváth and Wehner 1999; Gál et al. 2001a, b; Pomozi et al. 2001; Horváth et al. 2002a; Hegedüs et al. 2007a, b, c, d) using full-sky imaging polarimetry. These characteristics may considerably differ from the single-scattering Rayleigh model (Suhai and Horváth 2004). The biological implications of these were summarized by Horváth and Varjú (2004) and are discussed in Chaps. 17, 18, 24 and 25.
3 Behavior that Utilizes Linearly Polarized Light
Behavioral experiments with bees and ants were central to discovering that animals can use linearly polarized light and remain the optimal method for describing a species’ ability to use this sensory cue. Additionally, precise behavioral data are extremely valuable as they allow relating electrophysiological data to the biology of a species and thus give relevance to otherwise isolated observations of neuronal responses. In the following sections, a brief overview will be provided over the evidence showing that the species covered in this chapter utilize linearly polarized light.
3.1 Cricket
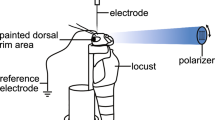
The best studied species with respect to its ability to use linearly polarized light is the field cricket, G. campestris (Fig. 4.2a). In the used experimental paradigm, a tethered cricket is placed on a small sphere, which can rotate freely in all directions and the movement of which can be precisely tracked (Brunner and Labhart 1987). When the animal walks on top of this sphere, the direction as well as the speed of walking can be monitored (Fig. 4.2b).
Behavioral experiments to illuminate polarization vision. (a–c) Crickets. (d–f) Desert locusts (Schistocerca gregaria). (g–i) Monarch butterflies (Danaus plexippus). (j–l) Fruit flies (Drosophila melanogaster). (b, e, h, k) Behavioral assays for testing polarized-light orientation responses in the respective species. In b a linear polarizer is slowly rotated above the tethered cricket, which walks on an air-suspended ball, the movements of which are tracked. In e the tethered locust is placed in front of a wind tunnel and its turning tendencies in response to a rotating linear polarizer are measured by a torque meter. In h the monarch butterfly is tethered inside a flight simulator placed outdoors with clear view of the sky. It can freely rotate and its angular orientation is monitored by an optical encoder. A polarizer can be placed above the simulator to rotate the current E-vector pattern in the sky by 90°. In k a tethered fly is placed inside an outdoor flight arena with clear view of the sky. The fly can rotate freely and its angular orientation is monitored. The current skylight polarization pattern can be switched by 90° by a liquid-crystal-based device, which preserves all skylight features except the polarization angles. (c, f, i, l) Examples of data obtained with the respective assay in each species. All examples show changes in walking or flight direction in response to changing E-vector angles shown from dorsal directions. In i, the arrows indicate 90° rotations of the polarizer. The highlighted circle represents a trial with a UV-interference filter placed above the polarizer. Left column: circular plots of flight orientations accumulated over time. Right column: virtual flight paths calculated from orientation data. Images reproduced with permission from Henze and Labhart (2007) (b, c); Mappes and Homberg (2004) (e, f); Mouritsen and Frost (2002) (Copyright (2002) National Academy of Sciences, USA) (h); Sauman et al. (2005) (i); Weir and Dickinson (2012) (k, l)
When a linear polarizer is slowly rotated around its vertical axis above the walking cricket, the animal shows approximately sinusoidal walking tracks (Fig. 4.2c). This “polarotactic” behavior suggests that the animals possess a preferred E-vector orientation, with which they try to align themselves. Turning tendencies are consequently induced by the mismatch between the preferred orientation and the currently displayed E-vector angle. Consistent with the cricket being a central place forager that uses polarized light for orienting during foraging trips (Beugnon and Campan 1989), the population of all tested animals has no consistently preferred orientation and individual animals even change their preferred E-vector angle between trials. Although on average there is a tendency of either aligning themselves in parallel or perpendicular to the E-vector stimulus (Brunner and Labhart 1987), the preferred directions cover all possible angles with respect to the stimulus, suggesting that the observed behavior is not merely an alignment response. The orientation behavior is completely abolished when the dorsal rim area (DRA) of the compound eye is painted over, revealing that this specialized part of the eye is required for the detection of the E-vector orientation of polarized light.
Over the last two decades, this behavioral response has been studied in more detail and was characterized with respect to absolute sensitivity, spectral tuning, response to low degrees of polarization, and under limited visibility conditions (Herzmann and Labhart 1989; Henze and Labhart 2007). These experiments have shown that the spectral tuning of the behavior matches the spectral tuning of the blue receptors in the DRA of the eyes (Herzmann and Labhart 1989). It has been suggested that the blue sensitivity might benefit species that are active not only during the day but also during crepuscular periods (e.g., crickets), as the absolute radiance of the sky is highest in the blue part of the spectrum at these daytimes (Barta and Horváth 2004). In tune with this argument, the absolute sensitivity of the behavior is remarkably high, with the threshold (2.5 × 107 photons/cm2/s) being below the light levels of a moonless night (Herzmann and Labhart 1989). More recent work determined the abilities of crickets to use polarized light under more natural conditions (Henze and Labhart 2007). This work takes into account that within the natural habitats of these animals, the visibility of the sky is often limited to small patches and that the degree of polarization is much lower than the 100 % used in previous experiments. Remarkably, the behavior is very robust and can be sustained to average degrees of polarization as low as 7 %, both with small polarized-light stimuli embedded in a large unpolarized stimulus (simulating cloud cover with patches of blue sky) as well as homogeneously low degrees of polarization (simulating haze, fog, or overcast sky). Also, the size of the stimulus can be reduced to 1° without abolishing the response of the animals (Henze and Labhart 2007).
3.2 Desert Locust
Very similar to the cricket, polarotactic behavior has been used in desert locusts (S. gregaria) to verify their ability to perceive and use polarized light (Fig. 4.2d–f). Hereby, the animals were tethered in front of a wind tunnel, so that the airflow induced sustained bouts of flight. The lateral torque produced by intended turning responses of the locust was used to monitor the direction of flight (Mappes and Homberg 2004). Similar to crickets, the locusts showed approximately sinusoidal flight tracks when a polarizer was slowly rotated above the animals, indicating that the locusts initiate turning responses when the E-vector orientation of the stimulus does not match an internal preferred orientation (Fig. 4.2f). The preferred orientations of the examined locust population were randomly distributed, showing that locusts can orient in all possible angles relative to the E-vector stimulus, but do not have an overall shared orientation (Mappes and Homberg 2004). Although a common orientation would be expected for a long distant migratory animal such as the desert locust, the lack of finding it in laboratory-raised animals indicates that the common orientation observed in wild locust swarms might be learned, induced by the dynamics of the swarm itself or that polarized light does not play a decisive role in choosing the migratory direction.
As in the cricket, the DRA of the compound eye is also required for polarotaxis in the desert locust (Mappes and Homberg 2004). Additionally, surgically induced lesions in the anterior optic tract completely abolished this behavior as well (Mappes and Homberg 2007), showing that this pathway of the brain is required for a response to polarized light.
3.3 Monarch Butterfly
The migration of the monarch butterfly (Danaus plexippus) is one of the prime examples for long-distance migrations in the animal world, and magnificent swarms of millions of these butterflies fly each year from northeastern North America to their overwintering grounds in central Mexico. Behavioral experiments using a flight simulator have been carried out over the last decade and have shown that monarchs use a time-compensated sun compass to keep a southerly bearing (Mouritsen and Frost 2002; Froy et al. 2003; Reppert et al. 2010). In this simulator, the butterfly is tethered but can rotate freely around its vertical body axis (Fig. 4.2h). The direction of flight can thus be chosen by the animal and is recorded by an optical encoder to calculate virtual flight paths (Fig. 4.2i). While the lateral view of the landscape is obscured by the simulator walls, the natural sky is freely visible to the animal. The question whether monarchs can use polarized skylight for orienting has been addressed in three studies, two of which produced strong evidence that these animals have the capacity for polarized-light guided navigation. Nevertheless, the sun itself must still be regarded as the primary source of information for orientation purposes in these animals, as polarized light is clearly not necessary for time-compensated sun compass orientation (Stalleicken et al. 2005).
The studies showing E-vector-dependent orientation were carried out in situations with low solar elevation, i.e., when the sun was not visible for the butterfly inside the simulator, and the polarization pattern was more or less uniform across the sky. The visible part of the sky was covered with a polarizer, the transmission direction of which was aligned with the dominant E-vector orientation in the sky. While animals flying under this condition did not change their behavior with respect to the control without the polarizer, they changed their flight direction by 90° in either direction, when the polarizer was turned by 90° (Reppert et al. 2004) (Fig. 4.2i). In a subsequent study, a spectral filter that blocked all light below wavelengths of 400 nm (ultraviolet light) was placed above the polarizer. This resulted in complete loss of polarized-light-induced turning responses and reveals that monarch butterflies perceive the polarization pattern of the sky in the UV range (Sauman et al. 2005) (Fig. 4.2i).
3.4 Houseflies and Fruit Flies
Two species of flies have been examined behaviorally with respect to polarized-light orientation: the fruit fly, Drosophila melanogaster, and the housefly, Musca domestica. Early studies investigated responses to polarized light in tethered walking or flying flies (flying Drosophila: Wolf et al. 1980; walking Musca: Philipsborn and Labhart 1990). The housefly was examined in a setup very similar to the one used for crickets, and results were comparable. The flies showed sinusoidal modulations of their walking direction when a linear polarizer was slowly rotated above the animal (Philipsborn and Labhart 1990). Interestingly, the preferred E-vector orientations were highly significantly clustered around the axis perpendicular to the body length axis of the flies, i.e., flies avoided to align themselves parallel to the E-vector orientation of the stimulus. Furthermore, repeating the experiments with UV and yellow light resulted in the full response amplitude for UV stimuli, while yellow led to no response. This means that UV light was fully sufficient for the observed response.
In Drosophila, orientation responses to rotating E-vectors were examined by recording yaw-torque responses of tethered flies (Wolf et al. 1980). During flight orientation in closed loop conditions (i.e., the flight orientation of the fly generates immediate feedback that controls the stimulus), the flies tended to either align themselves in parallel or perpendicular to the E-vector angle of the stimulus. Surprisingly, significant responses were observed not only in the UV range but also in green light. Moreover, the response was not restricted to the dorsal visual field, but extended into the ventral visual field as well, albeit with reduced amplitude.
Very recently, and after almost three decades of no research, work on polarization vision was revived in Drosophila. Two different behavioral assays were developed that either looked at alignment responses in fly populations (Wernet et al. 2011), or investigated orientation responses of single tethered flies in a flight simulator (Weir and Dickinson 2012) (Fig. 4.2k). In the latter work the flies were stimulated with natural skylight while flying. It revealed that the animals could maintain a straight course over prolonged periods of time and initiated course correction when the flight arena was rotated with respect to the stimulus. This response was abolished by inserting a circular polarizer into the light path, but surprisingly it was maintained under blue light (Weir and Dickinson 2012). Additionally, a second experiment used a liquid-crystal-based polarization-switching device with which the natural skylight E-vector pattern was switched by 90° at 1 min intervals. Importantly, this manipulation only switches the E-vector pattern, but leaves all other skylight cues intact. Also in this setup the flies adjusted their flight direction according to the changing polarization pattern (Fig. 4.2i). This study clearly shows that Drosophila is able to use natural polarization patterns for controlling its flight direction and suggests that, unlike previously thought, the UV receptors of the DRA are not the only means of detecting orientation-relevant E-vectors. These results are strongly supported and expanded by the previously mentioned population assay (Wernet et al. 2011). In this assay, flies generally aligned their body axis parallel to the E-vector. When stimulated with linearly polarized light dorsally, this alignment response was restricted to UV light, while ventral stimulation was also observed for blue and green stimulation. With genetic manipulations the authors showed elegantly that the dorsal response was mediated exclusively by the UV receptors of the DRA. The ventral response, on the other hand, requires a complex interaction of inner (R7/R8) and selected outer photoreceptors (R4–R6) to mediate the observed green, blue, and UV responses (Wernet et al. 2011).
3.5 Polarized Light in the Context of Color Vision
At last, polarized light cannot only be used for orientation behavior. For most purposes, high polarization sensitivity in the main retina of insects would interfere with other visual tasks such as color vision (Horváth and Varjú 2004, pp 362–380, see also Chapter 13 of this book). Polarization sensitivity has therefore been actively reduced in large parts of the eyes in most insects by introducing rhabdomeric twist or bent rhabdomes (Wehner and Bernard 1993). However, some species, particularly Papilio butterflies, have retained moderate degrees of polarization sensitivity in the entire compound eye. In these cases polarized light could be used to enhance the salience of attractive features of the environment for solving specific, species-dependent problems. The ability to distinguish polarized-light-induced false colors and the ability to distinguish isoluminant stimuli of identical color but distinct polarization angles have been revealed through learning paradigms in Papilio butterflies under controlled laboratory conditions (Kelber 1999; Kelber et al. 2001; Kinoshita et al. 2011).
In ovipositing choice experiments in female Papilio butterflies, horizontally polarized green light was strongly preferred over vertically polarized light (Kelber et al. 2001). This choice preference was dependent on the spectrum of the presented stimuli and the authors concluded that the different polarization angles are perceived by the butterfly as having different colors, as they are likely processed by the same neural substrate. In choice experiments involving feeding responses, the animals could also be trained to prefer stimuli of either vertical or horizontal E-vector orientation (Kelber et al. 2001). Whether Papilio perceive different E-vector angles as apparent changes in brightness or changes in color during foraging behavior was examined in a recent study (Kinoshita et al. 2011). During foraging, these animals possess a strong innate preference for vertically polarized light. Choice experiments in this context showed that the dynamics of the learning process resembled that of intensity choices much more closely than that of color-based learning. Hence during foraging, different E-vectors are likely perceived as different intensities rather than different colors (Kinoshita et al. 2011). Whether these laboratory-based findings indeed translate into the natural world and bear behavioral relevance remains hypothetical. Although the described experiments clearly reveal potential interactions between color and polarization processing pathways, the effective crosstalk between both channels is expected to be rather weak, due to the low PS values of the involved photoreceptors (~2). Indeed, modeling combined with imaging polarimetry showed that the color of specularly reflecting leaf surfaces is masked by white glare, which may prevent the perception of polarization-induced hue shifts (Horváth et al. 2002b). Additionally, light reflected from matte flower surfaces can be colorful, but is only weakly polarized or even unpolarized. Modeling degree and angle of polarization of reflections on different surfaces and their dependence on wavelength showed that polarization-induced false colors could help polarization-dependent color vision systems to discriminate between shiny and matte surfaces, but might not be suited to unambiguously encode surface orientation (Hegedüs and Horváth 2004a, b; Chap. 13). However, even if the described experimental results from Papilio butterflies do not fully translate into biologically relevant contexts, they might eventually be highly valuable for disentangling the complex wiring downstream of the multiple spectral types of butterfly photoreceptors.
4 The Detectors of Polarized Light
The sensory periphery for polarization vision has been studied for a long time and consequently has been described in many insect species. As briefly mentioned above, a specialized region of the compound eye called the DRA is generally thought to mediate the detection of linearly polarized light. Fundamental for polarization sensitivity is the alignment and orientation of the microvilli in the rhabdom of DRA ommatidia. In all polarization-sensitive ommatidia, microvilli of the individual photoreceptors are aligned for the entire lengths of the receptor, i.e., the rhabdom is not twisted around its length axis. Second, within each ommatidium the microvilli of one group of photoreceptors are oriented orthogonally to the microvilli of another group of photoreceptors, resulting in two analyzer channels optimized to detect orthogonal E-vector orientations (Fig. 4.3a). Additionally, rhabdoms are often shortened to reduce self-screening, while the area of the cross section is widened to maintain high absolute sensitivity. Also, receptors within the DRA are generally homochromatic, a feature that renders the polarized-light responses indifferent to the spectral composition of the stimulus. Additional anatomical specializations like enlarged receptive fields, lack of screening pigments, or degraded optics are often present as well, but depend on the species studied (summarized by Labhart and Meyer 1999).
The dorsal rim areas (DRAs) of the compound eyes across insect species. (a) Anatomical layout of the DRA in the monarch butterfly, the cricket, and the desert locust. Shown for each species are the outlines of a single ommatidium with the microvilli orientation of the individual retinula cells. The dominant microvilli orientations are shown for all ommatidia of the DRA in the overview images. Note the fan-shaped organization of the ommatidial arrays in all species. Images reproduced with permission from Labhart et al. (2009) (monarch butterfly); Blum and Labhart (2000) (cricket); Homberg and Paech (2002) (locust). (b) Estimated region of sky viewed by the DRA in Drosophila, the monarch butterfly, and the locust/cricket. The area of sky viewed by the left eye is indicated in blue (dark), while the area viewed by the right eye is shown in yellow (light). Estimates are based on data from Henze (2009) (Drosophila); Stalleicken et al. (2006), Labhart et al. (2009) (monarch butterfly); Blum and Labhart (2000) (cricket); Homberg and Paech (2002) (locust). Cartoons of cricket and Drosophila adapted from Henze (2009)
The four species considered in this chapter show several differences in the organization of their DRAs (Fig. 4.3a). These differences are particularly strong in the overall layout and orientation of the DRA. As the direction of view and size of the DRA, combined with the receptive field size of the photoreceptors, determine which part of the sky can be viewed and analyzed by an animal (Fig. 4.3b), these features are exceptionally important for interpreting the characteristics of later-stage neural responses to polarized light. Only when the information available to the animal is known, it can be combined with behavioral observations to reveal the algorithms operating on a neuronal level that transform sensory information into motor commands.
4.1 Which Part of the Sky Is Viewed by the DRAs of the Different Species?
In principle, two types of DRA can be distinguished in the four species covered in this chapter: First, crickets and locusts possess short but wide DRAs directed towards a large, elliptical region of the contralateral sky, centered at an elevation of around 60° (Blum and Labhart 2000; Homberg and Paech 2002). Second, elongated and narrow DRAs are found in monarch butterflies and Drosophila. These long DRAs are directed towards a narrow strip of sky approximately parallel to the body length axis of the animal (Stalleicken et al. 2006; Henze 2009; Labhart et al. 2009) (Fig. 4.3b). In all species the microvilli orientations of the ommatidia are arranged in a fan-like manner across the DRA (Fig. 4.3a). While the acceptance angle of individual ommatidia is large in crickets (ca. 20°, Labhart et al. 1984) and locusts (ca. 30°, Eggers et al. 1993), it is small in monarch butterflies and Drosophila (ca. 4°, Stalleicken et al. 2006; Henze 2009). Consequently, the receptive fields of individual ommatidia overlap substantially in locusts and crickets, so that each part of the sky within the acceptance range of the DRA is viewed by many ommatidia at the same time. Due to the fan-shaped nature of microvilli orientations, all possible E-vector angles can be simultaneously detected at each point of sky within the receptive field of the DRA. In contrast, in monarchs and Drosophila the stretched out, narrow fan of microvilli orientations, combined with the small acceptance angles of individual ommatidia, implicates that each part of the DRA is optimized to perceive a different E-vector angle. Thus, the information transmitted from the overall DRA is expected to differ substantially between locusts and crickets on the one hand, and monarchs and flies on the other hand, even in identical skylight situations.
4.2 Which DRA Photoreceptors Are Involved in Polarized-Light Perception?
The answer to this question depends strongly on which of the four species covered in this chapter is considered. Most similar is the situation in the cricket and the locust. In both species, the majority of DRA photoreceptors is blue sensitive, while a small proportion is UV sensitive (Labhart et al. 1984; Eggers et al. 1993). The major blocks of orthogonal microvilli are produced by the R7 and the R1, R2, R5, R6 receptors, all of which are blue sensitive and thus together provide two homochromatic polarization analyzers. Interestingly, one microvilli orientation (R1, R2, R5, R6) contributes considerably more area to the cross section of the rhabdom than the orthogonal one (R7) (Blum and Labhart 2000; Homberg and Paech 2002) (Fig. 4.3a). While the R8 receptor is a fully developed receptor cell in the locust, it is much shorter in the cricket and is restricted to the proximal part of the rhabdom. In both species it contributes microvilli to the R1, R2, R5, R6 group of photoreceptors. Recent data from the cricket surprisingly show that the R8 cell expresses UV opsin (Henze et al. 2012), suggesting that this might also be the case in locusts, for which similar data do not yet exist. At last, R3, R4 receptors lack microvilli in the cricket and are substantially reduced in the locust as well (Blum and Labhart 2000; Homberg and Paech 2002). Whereas the majority of receptors target the lamina in both species, a few receptors target the medulla. While no further detail is known in the locust, dye fills in the cricket suggest that the long projections to the medulla originate from the R7, R8 cells (Blum and Labhart 2000). Overall, both species possess a photoreceptor organization in the DRA that indicates a high degree of specialization for the detection of polarized light. With the partial reduction of the R8 receptor and the complete lack of microvilli in the R3, R4 receptors, the cricket DRA ommatidia appear slightly more specialized than their locust counterparts.
In the monarch butterfly, all eight DRA receptor cells in each ommatidium ubiquitously express UV opsins and thus comprise a completely homochromatic system for polarization vision (Sauman et al. 2005). Receptors R3 and R7 comprise one analyzer channel, while R1, R2, R4, R5, R6, R8 cells constitute the orthogonal channel (Labhart et al. 2009). Similar to locusts and crickets, the area of cross section allocated to the R3, R7 channel is considerably smaller than the area taken up by the orthogonal analyzers, indicating that this finding might bear functional significance. Dye fills into the DRA of the monarch suggest that all projections terminate in the medulla, as known for UV-opsin-expressing photoreceptors in other species (Sauman et al. 2005).
In Drosophila the existence of neural superposition eyes with their unfused rhabdomes precludes orthogonal analyzer channels to which all photoreceptors of one ommatidium contribute, as the optical axes of the individual receptor cells are not aligned. Here, the two only receptors with identical optical axes are the inner receptors, i.e., R7 and R8. In the DRA, these cells express exclusively UV opsins and show orthogonal microvilli orientations with respect to one another (Wernet and Desplan 2004; Wernet et al. 2011). As in the monarch butterfly, the UV-sensitive receptors possess long projections that target the medulla (Fischbach and Dittrich 1989).
4.3 A Second Specialized Area for Detecting Polarized Light?
Recently, data has accumulated suggesting that the DRA is likely not the only region in the eye specialized for detecting polarized light. The most direct evidence results from data in Drosophila. Here, the existence of a ventral alignment response to polarized light has led to a thorough investigation of ventral photoreceptors (Wernet et al. 2011). Although no clearly defined ventral region could be identified, in which ommatidia were as highly specialized as in the DRA, the rhabdomeres of individual receptors showed only low to moderate twist compared to surrounding receptors, a crucial prerequisite for detecting polarized light. These untwisted receptor cells were either UV-opsin-expressing R7 or blue-opsin-expressing R4, R5, consistent with the above-described behavioral data. Additionally, the microvilli orientation of R7 cells was highly aligned across neighboring ommatidia, allowing extraction of E-vector information even from only moderately polarization-sensitive receptors by means of spatial integration (Wernet et al. 2011). Green-opsin-expressing R8 receptors might be needed in conjunction with outer receptors to mediate a behavioral response to green stimuli, although the mechanism remains unclear. As the R8 receptor is highly twisted (and expresses a different opsin than R7), no crossed analyzers exist in the ventral eye.
In crickets, in situ hybridization data revealed that blue opsins are not only expressed in the DRA as previously thought but also occur in a band-like region of the ventral eye (Henze et al. 2012). Other than in the DRA, the involved receptors are likely only R1, R3, R5, R7, while the remaining R2, R4, R6, R8 cells express green opsins. Although the ultrastructure of this eye region is unknown and no behavioral data exist in crickets that suggest the use of ventral sources of polarized light, such data exist for the closely related desert locusts. These animals have been reported to avoid extended bodies of water (Shashar et al. 2005). As such behavior is likely mediated via detection of horizontally polarized light (Schwind 1985; Horváth and Varjú 2004; Chaps. 5 and 16), a ventral band of specialized photoreceptors for polarized-light detection might be a shared feature among orthopteran insects. Indirect evidence for this speculation was found through anatomical and physiological data in the locust brain. First, in the medulla, two neuron types that arborize in the dorsal rim medulla possess a second arborization tree in a ventral part of the medulla, either ipsilaterally or contralaterally (el Jundi et al. 2011). Second, the lateral extent of receptive fields of polarization-sensitive neurons in the optic lobe, as well as in the central brain, extends to sky regions close to the horizon, and thus cannot solely result from the activation of DRA photoreceptors. This includes optic lobe neurons (el Jundi et al. 2011), several types of central-complex neurons (Heinze et al. 2009), and descending neurons of the ventral nerve cord (Träger and Homberg 2011).
These findings in orthopteran insects and flies are in tune with long-standing observations in aquatic insects (e.g., water beetles, water bugs, dragonflies, tabanid flies, mayflies, stoneflies, caddisflies), which are attracted to bodies of water and use horizontally polarized light for this task (Horváth and Varjú 2004; Chaps. 5, 16, and 22). The backswimmer, Notonecta glauca, indeed possesses a ventral eye region specialized for detecting polarized light and might be an extreme example of a more general principle applicable to many insects (Schwind 1983, 1985).
4.4 Polarization Sensitivity in the Main Retina
For the main retina outside the DRA, moderate polarization sensitivity has been described in some insects, particularly in butterflies (Kelber et al. 2001; Pirih et al. 2010) but also in the cricket (Labhart et al. 1984). The values of polarization sensitivity (PS) range from 2 to 5, as opposed to much higher values in the DRA (up to 40). In crickets the strongest polarization sensitivity for non-DRA photoreceptors was found in UV receptors, while in Papilio butterflies all spectral classes of photoreceptors possess PS values of around 2 (Kelber et al. 2001; Kinoshita et al. 2011). In the eastern pale clouded yellow butterfly (Colias erate), the highest polarization sensitivity was found for blue and red receptors (Pirih et al. 2010). Interestingly, in both butterfly species fixed sets of photoreceptors possess specific microvilli orientations, which are 0°, 90°, 35°, and 145° (Kelber et al. 2001; Pirih et al. 2010; Kinoshita et al. 2011), possibly providing a substrate for the described innately preferred E-vector orientations.
Additionally in Drosophila, polarization sensitivity in the dorsal eye outside the DRA is suggested by behavioral experiments (Weir and Dickinson 2012). Hereby, the ability of the fly to respond to the skylight polarization pattern with changes in flight direction was not affected, when only blue light was available and thus the UV part of the spectrum was excluded from the stimulus. As the polarization-sensitive inner receptors of the DRA are exclusively UV sensitive, the behavior is either mediated by the outer receptor cells of the DRA (expressing rh1 blue opsins) or by ommatidia of the dorsal eye outside the DRA, both of which would be surprising findings.
5 The Optic Lobe
Like all visual information detected by the compound eyes, polarized-light information is first processed in the optic lobes. The layout of these large structures on either side of the central brain can be described as a series of stacked, retinotopically organized neuropils adjacent to the retina (Fig. 4.4). In all species, the outermost neuropil region is the lamina, which is proximally followed by the medulla. The third region, the lobula complex, varies considerably between locusts and crickets on the one hand and butterflies and flies on the other hand. In flies and monarchs it consists of the actual lobula and the posteriorly located lobula plate, whereas in locusts and crickets, the lobula complex comprises four subunits, none of which can be easily homologized to the lobula and lobula plate of the other group. The last neuropil of the optic lobe is the accessory medulla, a small, spherical brain area that lies just anterior of the medulla and has been shown to house the circadian clock in cockroaches and Drosophila (Helfrich-Forster et al. 1998). Although its role is not known in the locust, anatomical similarity suggests a circadian function as well (Homberg et al. 1991). In monarchs, fibers stained for the clock protein CRYPTOCHROME-1 connect this region to the pacemaker cells in the pars lateralis and therefore also implicate a circadian function for the accessory medulla in that species (Sauman et al. 2005).
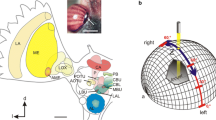
Polarization vision pathway in the brain of the desert locust (a) and the monarch butterfly (b). Brain regions involved in polarization vision are highlighted. Known neural elements are illustrated by lines. Proposed output regions are symbolized by filled circles, while proposed input areas are represented by open half circles. Note that input elements of the polarization vision pathway are shown on the left brain hemisphere (as viewed by the animal), while output elements are shown on the right hemisphere. The mushroom body pedunculus and lobes have been eliminated on the right hemisphere for clarity. DRA dorsal rim area, DRLa dorsal rim lamina, DRMe dorsal rim medulla, La lamina, Me medulla, aMe accessory medulla, LoX lobula complex, Lo lobula, MB mushroom body, AOTu anterior optic tubercle, LAL lateral accessory lobe, BU bulbs, AL antennal lobe, CBL lower unit of the central body, CBU upper unit of the central body, PB protocerebral bridge, POTu posterior optic tubercle, pPC posterior protocerebrum. Figure reproduced with permission from Merlin et al. (2012)
The photoreceptors of the DRA terminate either in the lamina (majority of receptors in locusts and crickets) or in the medulla (all other receptors). In the locust, these projections form specialized regions at the dorsal rims of both neuropils that are clearly distinct from the main lamina and medulla (Homberg and Paech 2002) (Fig. 4.4a). Unfortunately, to date no neurons directly postsynaptic to DRA photoreceptors have been physiologically described.
Nearly all neurons of the brain that respond to polarized light exhibit a feature called polarization opponency, i.e., they are maximally excited when the animal is stimulated at one particular E-vector orientation, while they are maximally inhibited at the perpendicular E-vector orientation. This behavior, first described by Labhart (1988) for neurons of the cricket optic lobe, has led to a model that describes how photoreceptors could transmit signals to their postsynaptic partner neurons (Labhart 1988). Hereby, the two blocks of photoreceptors with orthogonally oriented microvilli converge on a common neuron. While one would inhibit the neuron, the other one would excite it. As all insect photoreceptors contain histamine as transmitter (leading to postsynaptic inhibition; Nässel 1999), the excitatory pathway must comprise an indirect connection. This model additionally suggests that polarized-light perception is independent of the stimulus intensity, a feature confirmed by several studies in the cricket as well as the locust (Labhart 1988; Herzmann and Labhart 1989; Kinoshita et al. 2007; el Jundi and Homberg 2012).
An alternative model of how polarization opponency could be produced has been proposed recently by Pfeiffer et al. (2011). In this model, the release of histamine inhibits the postsynaptic neuron, but additionally leads to rebound excitation. Therefore, at moderately spaced bouts of transmitter release (at nonoptimal E-vectors), the postsynaptic cell would be excited due to rebound excitation in between transmitter release events. At higher activation levels of the photoreceptors, the transmitter release would become contiguous and thus the postsynaptic neuron would only be inhibited. This model predicts otherwise difficult to explain behavior of certain locust neurons in response to different degrees of polarization and to unpolarized light (see below). Additionally, the same neurons also show higher activation at higher light intensities and therefore are not intensity insensitive, a feature easier to explain with the second model.
The neurons that likely receive input from the dorsal rim medulla have only been described anatomically in the locust. These neurons, termed transmedulla neurons (formerly line-tangential neurons), possess input fibers in the dorsal rim medulla, as well as a single input neurite that runs vertically through the medulla (Homberg et al. 2003; el Jundi et al. 2011) (Fig. 4.5a). Thus, each of these cells should be responsive to polarized light and to unpolarized light from one vertical row of ommatidia, i.e., to illumination from a specific azimuth angle. The axonal projections of these cells are located within a small region of the anterior lobula and in the lower division of the anterior optic tubercle (AOTu). Overall, the population of these cells is suited to transmit polarized-light information from the dorsal rim medulla to the central brain and combine it with information about unpolarized light from all possible azimuth angles. Whether similar neurons exist in the other species covered in this chapter is still unclear, as is the proof that these neurons are indeed polarization sensitive in locusts.
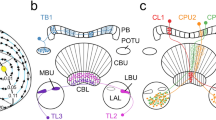
Polarization-sensitive neurons of the optic lobe. (a) Photoreceptor projections and transmedulla neurons revealed from dye injections in the desert locust (data from el Jundi et al. 2011). (b) Frontal reconstruction of a cricket POL1 neuron. (c) Frontal reconstruction of a locust MeMe1 neuron (data from el Jundi et al. 2011). (d) Neural activity in response to a rotating linear polarizer in a cricket POL1 neuron. Shown are two successive 360° rotations of the polarizer (black lines below spike trace). (e, f) Distribution of E-vector tunings in POL1 neurons of the cricket (e) and in MeMe1 neurons (blue/light) as well as TIM1 neurons (green/dark) of the locust (f). Mean orientations of the locust neurons are illustrated by arrows. Note that in the cricket angles are plotted clockwise, while in locusts they are plotted counterclockwise (see Supplementary Fig. 4.1). (g, h) Receptive fields of cricket POL1 neurons (g; schematic illustration) and locust MeMe1 neurons (h). Data in h show the lateral extent of the receptive field orthogonal to the body length axis. Plotted is the normalized response amplitude against the elevation of the stimulus (data from el Jundi et al. 2011). (i) Schematic illustration of neural elements involved in polarization vision in the optic lobe of the locust. All neurons converge in an individual layer of the medulla. Neuron types are color-coded and named in italics. Target neuropils (not included in the image) are named in normal font. Note that TIM2 and MeMe2 neurons have been omitted for clarity. DRLa dorsal rim lamina, DRMe dorsal rim medulla, Me medulla, aMe accessory medulla, LoX lobula complex, AOTu anterior optic tubercle, La lamina, DRA dorsal rim area, LU lower unit; c contralateral. Images reproduced/adapted with kind permission from: Springer Science + Business Media (Labhart and Petzold 1993) (b); Cambridge University Press (Wehner and Labhart 2006) (d); Labhart and Meyer (2002) (e); Journal of Experimental Biology (Labhart et al. 2001) (g); el Jundi et al. (2011) (h)
The single vertical neurites of the transmedulla neurons are confined to one layer of this neuropil. Interestingly, all polarization-sensitive neurons of the locust medulla are equally confined to the identical layer, implying that this layer provides the neural substrate for processing of polarized-light information in the optic lobe (el Jundi et al. 2011) (Fig. 4.5i). These neurons all possess large tangential arborization trees that cover a large number of medulla cartridges (Homberg and Würden 1997; el Jundi and Homberg 2010; el Jundi et al. 2011). Additionally, they fall into one of two groups, as they either possess input and output fibers within the ipsilateral optic lobe, or they possess a midline crossing neurite that connects ipsilateral dendrites with contralateral axonal endings. Three types of locust neurons fall into the first group (TIM1, TIM2, and TML; names from el Jundi et al. 2011), all of which appear to receive input from the dorsal rim medulla. While the TIM1 neuron possesses input and output fibers throughout the innervated medulla layer, the TIM2 neuron receives input only in the dorsal medulla (including the dorsal rim medulla) and projects to ventral parts of the same layer. The TML neuron receives input throughout the medulla, but possesses its output fibers in the lamina. Whereas the TIM1 neuron appears to receive additional input from the accessory medulla, the TML neuron possesses potential output fibers in this neuropil (Fig. 4.5i). The second group of cells comprises two types of intermedulla neurons (MeMe1 and MeMe2). MeMe1 cells receive input from large parts of the ipsilateral medulla and project to equally big areas of the same layer in the contralateral medulla, while giving rise to additional output fibers in the contralateral accessory medulla (Fig. 4.5c). Surprisingly, this cell type does not arborize in the dorsal rim medulla. MeMe2 cells receive input from dorsal parts of the medulla (including the dorsal rim medulla), while projecting to ventral parts of the contralateral medulla. Additionally, these cells project to extensive areas within the median, posterior protocerebrum (el Jundi et al. 2011).
Although the set of polarization-sensitive neurons of the optic lobe has been most extensively described in the locust, a cell type similar to locust MeMe1 cells has been long known in the cricket and in fact was the first polarization-sensitive neuron discovered in any insect (Labhart and Petzold 1993; Labhart 1996; Labhart et al. 2001) (Fig. 4.5b). These POL1 neurons have long been viewed as the prototype of all polarization-sensitive insect neurons, and indeed share many features with all other polarization-sensitive cells in different brain areas and in other insect species. The defining feature of POL1 neurons is a tonic change of action potential frequency in response to changing E-vector orientations presented to the animal from the zenith. When a linear polarization filter is slowly rotated above the animal, this leads to a sinusoidal modulation of firing frequency (Fig. 4.5d). Thus, characteristics of this sine function can be used to describe the neurons in detail. First, the activity peak is defined as the preferred E-vector orientation (Φmax-value), while the activity trough is called the Φmin-value. Additionally, the summed amplitude of frequency modulation is defined as the response strength (R). This term is calculated by summing up all absolute deviations from the mean activity during a rotation of the polarization filter in bins of 20° (Labhart 1996, modified by Heinze et al. 2009). As the background frequency, i.e., the activity of the cell without any stimulation, lies in between maximal and minimal activity during stimulation, the neuron is excited at Φmax and inhibited at Φmin, a behavior termed polarization opponency. At last, the receptive field of the neuron is defined as the spatial region in which stimulation leads to at least 25 % of maximal excitation.
The recorded population of POL1 neurons can be divided into three groups, which are distinguished by their Φmax-values. These distinct tuning directions are roughly 10°, 60°, and 130° for neurons with the soma in the left optic lobe (Labhart et al. 2001) and suggest that POL1 neurons occur as three individual neurons per brain hemisphere (Fig. 4.5e). The receptive fields of these cells are large and centered at around 60° elevation in the contralateral sky hemisphere, suggesting that around one-third of all DRA ommatidia converge on each POL1 neuron (Labhart et al. 2001) (Fig. 4.5g). The E-vector tuning does neither depend on the position within the receptive field nor on the degree of polarization or the stimulus intensity (Labhart and Petzold 1993; Labhart 1996; Labhart et al. 2001). Importantly, POL1 neurons do not respond to unpolarized light stimuli, but are extremely sensitive to polarized light, even at very low degrees of polarization (threshold 5 %) (Labhart 1996).
How do the polarization-sensitive neurons of the locust optic lobe compare to the described cricket POL1 neurons? First, only one of the five neuron types of the locust medulla shows polarization opponency (el Jundi et al. 2011). While all cell types respond with sinusoidal modulation of spiking frequency, responses are generally exclusively excitatory. The receptive fields for polarized-light stimulation were tested for TIM1, MeMe1, and TML neurons. Whereas TIM1 and MeMe1 cells possess receptive fields comparable to cricket POL1 neurons (Fig. 4.5h), the TML neurons (innervating the lamina) possess an ipsilaterally centered receptive field. Interestingly, all three neuron types respond exclusively to stimulation from the ipsilateral eye for zenithal stimulation (el Jundi et al. 2011). The combination of input from the ipsilateral eye and an ipsilateral receptive field in TML neurons suggests that information from the main retina outside the DRA is responsible for the polarization sensitivity of this neuron, as the DRA is directed towards the contralateral sky hemisphere. Another interesting difference between locusts and crickets is found in the distribution of E-vector tunings. In no single cell type of the locust, one finds the characteristic three tuning groups of the cricket POL1 neurons. Nevertheless, individual cell types possess distinct E-vector tunings. Sufficient numbers of recordings only exist for TIM1 and MeMe1 neurons, of which the first is tuned to 113°, while the second is tuned to 160° (el Jundi et al. 2011) (Fig. 4.5f). In this context it has to be noted that in crickets Φmax-angles have been plotted clockwise, while in locusts they have been plotted counterclockwise (Supplementary Fig. 4.1). Considering this, the tuning groups found in the locust cells coincide remarkably well with two of three tuning groups of the cricket POL1 neurons (in cricket coordinates: TIM1 = 67° and MeMe1 = 20°). As for these cells the receptive fields are also similar between the species, one could speculate that the function of cricket POL1 neurons is distributed over several cell types in the locust. On the other hand, a detailed anatomical analysis of the cricket optic lobe has not yet been performed, so that the existence of locust-like cell types in the cricket cannot be ruled out.
Maybe the most fundamental difference between locust and cricket neurons is that in the locust unpolarized light also results in neuronal responses, whereas cricket POL1 neurons are insensitive to unpolarized light. When an unpolarized light spot is rotated around the animal at constant elevation, all described locust neurons show excitation when the stimulus is present at a specific azimuth (el Jundi et al. 2011). This so-called “azimuth tuning” occurs independent of the used spectral range of the stimulus, and tuning is largely identical for UV and green light. Interestingly, the distribution of these tunings within the population of medulla neurons depends on whether laboratory-raised animals are used or animals that have been raised with a clear view of the sky. In laboratory-raised locusts, the distribution of preferred azimuth angles is random, implying that the observed excitation can be mediated by both eyes. On the other hand, in the second locust group, all neurons showed an identical tuning at an azimuth of ca. 100°, i.e., on the left side of the animal (ipsilateral to the soma of the recorded cell) (el Jundi et al. 2011). This means that the sensory experience of the locust during development shapes the response properties of the studied neurons in the optic lobe. Thus, data obtained from animals raised under laboratory conditions allow drawing conclusions about the genetically determined default state of these cells. The detailed comparison of these polarization-sensitive neurons in animals with and without sensory experience of skylight cues could therefore provide an excellent model for studying experience-dependent modulation of neural networks.
6 The Anterior Optic Tubercle
The first processing stage of polarized-light information in the central brain of insects is the anterior optic tubercle (AOTu) (Fig. 4.4). This relatively small neuropil is composed of several subunits and receives input from the optic lobe through fibers of the anterior optic tract, while fibers originating in the AOTu project to the lateral accessory lobes (LALs) and to the contralateral AOTu. Generally, one can distinguish a large subunit and either a single (locust) or several (monarch) small subunits (Homberg et al. 2003; Heinze and Reppert 2012). Polarization-sensitive neurons have only been described in the small subunits. These neurons belong to one of two groups: First, neurons projecting from the AOTu to specialized regions of the LAL (TuLAL neurons), and second, neurons interconnecting the AOTu’s of both brain hemispheres (intertubercle neurons; Fig. 4.6a). The latter cell types from the locust (LoTu1 and TuTu1 cells) are probably the best studied polarization-sensitive neurons in the central brain of any insect (Pfeiffer et al. 2005, 2011; Kinoshita et al. 2007; Pfeiffer and Homberg 2007; el Jundi and Homberg 2012). As these cells occur only once (LoTu1) or as a single pair (TuTu1) per brain hemisphere, they can be uniquely identified and the same individual neurons were studied in hundreds of recordings. They have been examined with respect to their response to different E-vector angles (Pfeiffer et al. 2005), degrees of polarization (Pfeiffer et al. 2011), and stimulus intensities (Kinoshita et al. 2007; el Jundi and Homberg 2012). Additionally, their spectral response properties have been described (Kinoshita et al. 2007), as well as the lateral extent of their receptive fields for polarized-light stimuli (el Jundi and Homberg 2012). Some of these characteristics have been compared between laboratory-raised animals and animals reared with clear view of the sky (Pfeiffer and Homberg 2007), as well as between the solitary and gregarious forms of the desert locust (el Jundi and Homberg 2012). Furthermore, these cells respond to unpolarized light stimuli in a complex way as well, both in locusts and in monarch butterflies (Kinoshita et al. 2007; Pfeiffer and Homberg 2007; Heinze and Reppert 2011).
Polarization-sensitive neurons in the anterior optic tubercle. (a) Reconstructions of the intertubercle neurons of desert locusts (top: LoTu1; bottom: TuTu1; data from el Jundi and Homberg 2012). (b) Circular plots of mean activity during rotations of a linear polarizer above the locust for LoTu1 (top) and TuTu1 (bottom) neurons (modified from el Jundi and Homberg 2012). (c) Circular plots of mean spiking activity of a locust LoTu1 neuron during stimulation with an unpolarized light spot moving around the locust at constant elevation. (d) As c, but data from a monarch butterfly TuLAL neuron. Lines in b–d indicate background firing frequency of the respective neurons. (e) Schematic illustration of polarization-sensitive neural connections of the anterior optic tubercle of the locust. Input is drawn on the left side of the image. Highlighted (blue/dashed) neurons have been shown to receive input from both eyes. While the number of LoTu1 and TuTu1 cells is shown accurately, all other cell types occur in large, but unknown numbers. Somata of TL2 and TL3 cells have been omitted for clarity. ant. Lo anterior lobula, AOTu anterior optic tubercle, UU upper unit, LU lower unit, LBU lateral bulb, MBU medial bulb, CX central complex. Images reproduced with permission from: Pfeiffer and Homberg (2007) (c); Heinze and Reppert (2011) (d)
6.1 Polarized-Light Responses of AOTu Neurons
In response to polarized light, all types of locust AOTu neurons (LoTu, TuTu, and TuLAL) show strong modulations of their firing frequency when the animal is stimulated with a rotating linear polarizer, resulting in a preferred E-vector orientation at the point of maximal excitation (Φmax) typical for each cell type (Fig. 4.6b). Except for LoTu1 neurons, all cell types show polarization opponency, thus suggesting converging inhibitory and excitatory channels with opposite E-vector preference. Contrary, E-vector responses of LoTu1 neurons lack an inhibitory component and are excited by all E-vector orientations, i.e., maximally excited at Φmax and minimally excited at Φmin (Pfeiffer et al. 2005). As expected from the spectral sensitivity of the locust DRA photoreceptors, the polarization response is most pronounced in the blue range (tested for intertubercle neurons) (Kinoshita et al. 2007). LoTu and TuTu cells receive signals almost exclusively from the ipsilateral eye, and, in tune with the geometry of the DRA, the receptive fields of these cells are located in the contralateral sky hemisphere. They are centered at around 60° elevation and have a diameter of ca. 120° (el Jundi and Homberg 2012). Although TuLAL neurons are much more numerous, they have been studied much less due to their smaller fiber diameters. The available data suggest that their receptive fields are variable both in lateral extension as well as in the location of the receptive field center. Based on a single recording, TuLAL1a neurons receive input from both eyes, while the ocular dominance of TuLAL1b cells remains unknown (el Jundi and Homberg 2012) (Fig. 4.6e).
6.2 Stimulus Intensity
The polarized-light responses of TuTu neurons as well as TuLAL projection neurons are remarkably indifferent with respect to the presented light intensity. The full amplitude of the frequency modulation is maintained over several orders of magnitude of decreasing light levels and breaks down completely within one to two orders of magnitude below levels of 1010 photons/cm2/s (Kinoshita et al. 2007; el Jundi and Homberg 2012). This means that these neurons are not suited to encode stimulus intensity, but possess an all-or-nothing response pattern above their sensitivity threshold. Differently, LoTu1 neurons possess an intensity-response curve with a shallower slope and therefore respond with stronger frequency modulations at more intense light levels over at least four orders of magnitude (Kinoshita et al. 2007; el Jundi and Homberg 2012). Additionally, very bright light levels comparable to midday conditions lead to a reduction in the response amplitude of LoTu cells, resulting in a uniquely bell-shaped intensity-response curve (el Jundi and Homberg 2012). Together with a described increase in response amplitude towards the end of the day (el Jundi and Homberg 2012), these characteristics suggest that LoTu1 cells may constitute a polarization channel specialized for dim light conditions around sunset.
6.3 E-Vector Tuning
In laboratory-raised locusts, the single LoTu1 cell in the right hemisphere is tuned to ~45° and the one in the left hemisphere to ~135°, i.e., their tuning is mirror symmetrical with respect to the midline of the brain. Similarly, TuTu cells (two per hemisphere) are tuned to ~45° and ~0° in the right hemisphere and ~135° and ~0° in the left hemisphere (Pfeiffer et al. 2005). While the tunings of LoTu neurons were confirmed in a second study, the same study showed no significant tuning groups for TuTu cells (el Jundi and Homberg 2012), indicating that there might be differences in the characteristics of these neurons between different locust populations. Interestingly, in animals that have been raised with clear view of the sky, the described distinct tunings change for both cell types, and the distributions become much broader (TuTu1) or even completely random (LoTu1) (Pfeiffer and Homberg 2007). Similarly, when solitary locusts are compared to gregarious locusts, the same broadening to the point of randomness was also observed in the solitary animals (el Jundi and Homberg 2012). This shows that the E-vector tunings of intertubercle neurons can be influenced by a variety of factors, including behavioral state and sensory experience. Together with the pronounced change of E-vector tuning in response to the time of day (see section “Time Compensation”), this raises the question of how the different E-vector tunings of these neurons are generated from the presumably constant photoreceptor input in a fixed receptive field.
For TuLAL neurons, E-vector tunings were randomly distributed in all studies, in line with the large number of these cells (ca. 60 per hemisphere) and their variable receptive fields. The latter suggests that different sets of ommatidia from the locust DRA are integrated by different TuLAL neurons, with the resulting E-vector tuning reflecting the average orientation of the input ommatidia.
6.4 Degree of Polarization
Interestingly, LoTu neurons are exclusively excited when presented with polarized light from dorsal directions, but are strongly inhibited by unpolarized light from the same direction (Pfeiffer et al. 2005; Kinoshita et al. 2007). As unpolarized light is merely a blend of polarized light with all possible E-vector orientations, this seems a paradox. When these neurons were presented with successively reduced degrees of polarization d, the purely excitatory response became polarization opponent at intermediate d-values and purely inhibitory at low d-values (Pfeiffer et al. 2011). This means that the mean spiking activity is correlated with the degree of polarization. Additionally, the response amplitude linearly decreased with smaller values of d and became indistinguishable from background variability at d-values of ca. 30 %. Importantly, this limits the area of the sky containing useful information for these cells (and for the locust) to the region further away than 50° from the sun (and the anti-sun). For TuTu neurons, lower d-values also lead to decreased response amplitudes as well as to lower mean spiking rates, albeit not reaching near total inhibition as in LoTu neurons. Although lower response amplitudes at lower d-values are intuitive (more E-vector noise), the reduction in overall activity found for both neurons when adding unpolarized light to a polarized-light stimulus is more difficult to explain. Pfeiffer et al. (2011) addressed this neuronal behavior in an elegant model and proposed that the release of well-spaced bouts of inhibitory transmitter (histamine) from photoreceptors in response to polarized light allows for rebound excitation in the postsynaptic lamina cells, and therefore leads to the observed excitatory responses. In contrast, when stimulated with unpolarized light or very high intensities of polarized light (el Jundi and Homberg 2012), transmitter release from photoreceptors would become contiguous and is thus increased to a level that does no longer allow for rebound excitation, which consequently leads to an inhibitory response. The E-vector tuning of LoTu neurons would result from combining all ommatidia of the DRA while considering the asymmetry of rhabdom area devoted to one of the two dominant microvilli orientations within each ommatidium. Estimating from the microvilli orientation of R7 photoreceptors across the DRA, the resulting tuning of 35° is reasonably close to the observed 45° in laboratory-raised animals (Pfeiffer et al. 2011).
6.5 Integration of Unpolarized Skylight Cues
Neurons of the AOTu also respond to unpolarized light (Pfeiffer et al. 2005; Kinoshita et al. 2007). Experiments in which unpolarized light spots were moved around the animal at constant elevation showed that these cells are strongly excited when the stimulus passes through a specific azimuth (Pfeiffer and Homberg 2007; Heinze and Reppert 2011) (Fig. 4.6c, d). This azimuth tuning has been shown for all recorded AOTu neuron types in locusts and monarch butterflies (locusts: TuTu1, LoTu1, TuLAL1a; monarch: TuLAL1a, TuLAL1b). In monarchs, the color of the unpolarized light does not affect the responses, and a strong response is found with UV, blue, and green light. The tuning for all three colors is indistinguishable (Heinze and Reppert 2011) (Fig. 4.6d). In locusts on the other hand, the neurons show color opponency with green stimuli being excitatory and UV stimuli being inhibitory (Fig. 4.6c). Consequently, the monarch cells appear to encode the azimuth of the brightest spot in the sky across all wavelengths, and thus likely provide direct information about the position of the sun, whereas the locust neurons are suited to encode the spectral gradient of the sky, another sun-derived skylight cue. As longer wavelengths dominate the solar hemisphere of the sky, while short wavelengths are more evenly distributed, the ratio of green to UV light in each point in the sky indicates the angular distance of that point from the sun. Thus, for example, if a locust neuron has a preferred azimuth lying directly ahead of the animal, the cell would be activated when the locust faces towards the solar sky hemisphere, but would be inhibited when facing in the opposite direction, whereas zenithal E-vector information is identical in both situations. Thus, Pfeiffer and Homberg (2007) suggest that these response characteristics are well suited to provide an unambiguous direction signal in polarization-sensitive neurons. This disambiguation of the E-vector information is necessary due to the axial symmetry of zenithal E-vectors, which allow finding the solar meridian, but cannot be used to distinguish the solar from the antisolar hemisphere of the sky.
An interesting question arises when one asks how the locust neurons would respond to direct illumination by the sun. As the sun is the brightest source of UV light as well as of green light, the described opponent response to both wavelengths would block an activation of the neuron. Remarkably, a reversal in the response from inhibition to excitation has been observed for some LoTu1 neurons between low and high intensities of UV light (Kinoshita et al. 2007). This suggests that when the sun is not visible, low intensity UV light received from the sky would inhibit the neuron and thus facilitate encoding of the spectral gradient, while during times of visibility of the sun, high intensity UV light from the sun itself would activate the neuron and lead to a response like the one described for the monarch butterfly. As responses to unpolarized light were mostly stronger than the responses to polarized light in both species (Pfeiffer and Homberg 2007; Heinze and Reppert 2011), the sun as the most prominent skylight cue would dominate the neuron’s response when it is present in the sky. Only when the sun is not available as orientation cue, the spectral gradient of the sky and the polarization pattern would dominate the neuronal response and thus guarantee a robust encoding of the solar azimuth under more difficult sky conditions. Behavioral data from the monarch butterfly strongly support such an hierarchy of skylight cues by showing that although these animals have the capacity for using polarized light for navigation, the sun is used as the primary orientation cue (Mouritsen and Frost 2002; Froy et al. 2003; Reppert et al. 2004; Sauman et al. 2005; Stalleicken et al. 2005).
Another interesting difference between locusts and monarchs becomes apparent when one compares the distribution of azimuth tunings in these neurons. In locusts, this distribution depends on the cell type. LoTu1 neurons recorded from the left brain hemisphere share a common excitatory azimuth tuning of 90°, i.e., on the left side of the animal, while TuTu neurons show a double peaked tuning distribution centered contralaterally with a strong inhibitory component ipsilaterally (Pfeiffer and Homberg 2007). In the UV range, this reverses and TuTu neurons share one ipsilateral excitatory peak, while LoTu neurons show a broad distribution with a shared ipsilateral, inhibitory azimuth tuning. At last, TuLAL neurons show no shared common azimuth tuning at all (Pfeiffer and Homberg 2007). On the contrary, all recorded neuron types in the monarch butterfly possess a tuning distribution for all tested colors with a highly significant maximum near 270°, i.e., on the right side of the animal (for neurons recorded in the left brain hemisphere) (Heinze and Reppert 2011). Although the functional significance of this finding is not yet known, it clearly suggests species-dependent differences in polarized-light processing at the level of the AOTu.
7 The Central Complex
The final processing stage for polarized-light information in the brains of all examined animals to date is the central complex (CX) (Homberg et al. 2011; Merlin et al. 2012) (Fig. 4.4). This midline-spanning group of neuropils is located at the center of the brain. It consists of four major compartments: the upper and lower divisions of the central body (CBU, CBL; called fan-shaped body and ellipsoid body in flies), the protocerebral bridge (PB), and the paired noduli. Although the overall orientation of the CX within the brain differs substantially between locusts and crickets on the one hand, and butterflies and flies on the other hand, its components are highly conserved (Williams 1975; el Jundi et al. 2010; Heinze and Reppert 2012; Ito et al. 2014). Moreover, their internal neuroarchitecture appears to be remarkably well conserved down to the level of single cell types (Hanesch et al. 1989; Heinze and Homberg 2008; Heinze et al. 2013; Lin et al. 2013). In all analyzed species these cell types can be divided into three major classes of neurons: tangential neurons, columnar neurons, and pontine neurons (in most detail described in the desert locust, the monarch butterfly, and Drosophila).
Tangential neurons connect a variety of brain regions outside the CX with complete layers of either one of the CX-compartments, generating a characteristic stratified layout in the central body and in the noduli. These cells, of which many different types have been found, are generally thought to constitute the principal input to the CX (Hanesch et al. 1989; Li et al. 2009; el Jundi et al. 2010; Heinze et al. 2013).
In contrast, columnar neurons connect small, well-defined regions between several CX-compartments. As these cell types generally occur in isomorphic sets of 16 individual neurons, the arborization trees of which are evenly distributed across the width of the innervated CX-compartment, they generate a repetitive neuroarchitecture consisting of 16 “columns” or “slices” (Williams 1975; Hanesch et al. 1989; Heinze and Homberg 2008; Heinze et al. 2013; Ito et al. 2014). While existing in a linear array in the unlayered PB, in the central body, the columns orthogonally intersect with the layers generated by tangential neurons. Ultimately, many columnar cell types converge with their proposed output arborizations in the LALs, paired neuropils on either side of the central body. These neurons are thought to constitute the major output pathway from the CX (Heinze and Homberg 2008; el Jundi et al. 2010; Heinze et al. 2013).
Cells of the third group, the pontine neurons, connect individual columns on either side of the midline with one another. They only occur in the CBU and also exist as isomorphic sets of cells (Heinze and Homberg 2008; Siegl et al. 2009; Heinze et al. 2013). Thus, these cell types provide the basis for complex, interhemispheric information flow within the CBU. Similarly, sets of tangentially oriented, multicolumnar neurons of the PB provide another, even more complex way of information exchange between CX-regions on either side of the midline (Heinze and Homberg 2007; Heinze et al. 2013). In some species, these neurons additionally arborize in the posterior optic tubercle, a small neuropil near the posterior brain boundary, and are therefore considered tangential neurons of the PB. As these fibers are lacking in other species (Homberg 1985; Hanesch et al. 1989; Young and Armstrong 2010; Lin et al. 2013), only the intrinsic arborizations restricted to columns of the PB appear to be a commonly shared feature of the CX in many insects.
The vast majority of the mentioned types of neuron have been reported to respond to linearly polarized light and together form the CX-polarization vision network. However, most knowledge about how this information propagates through this intricate network is indirect, i.e., either derived from anatomical data or from comparisons of response characteristics between individual cell types. Nevertheless, a substantial amount of evidence has accumulated over the recent years that indicates that, in principle, there are three processing stages present in the CX neuronal network: First, the input stage, represented by tangential neurons of the CBL (Fig. 4.7); second, an intermediate stage, represented by special types of columnar neurons, as well as multicolumnar neurons of the PB (Fig. 4.8); and third, the output stage, represented by a group of large columnar neurons projecting to the LALs (Fig. 4.9). In the following subsections, all three stages will be examined in detail.
The input stage of polarized-light processing in the central complex. (a) TuLAL neurons and their likely postsynaptic tangential neurons of the lower division of the central body (CBL) registered into the standardized compass neuropils of the monarch butterfly (data from Heinze et al. 2013). The pathway originating in the lower unit of the anterior optic tubercle (AOTu-LU) is shown in red (dark), while the pathway originating in the strap region (SP) of the AOTu is shown in green (light). Pathway 1 passes through the dorsal sector of the bulbs (BU) and ends in a central layer of the CBL. Pathway 2 passes through the ventral sector of the BU and ends in peripheral layers of the CBL. (b) Frontal reconstructions of TuLAL neurons and tangential neurons of the CBL in the desert locust. Likely parallel pathways are illustrated in red (dark) and green (light). Pathway 1 passes predominantly through the medial bulb (MBU) and ends in a central layer of the CBL, while pathway 2 passes through the lateral bulb (LBU) and ends in a peripheral layer of the CBL (reconstructions modified after Pfeiffer et al. 2005; Träger et al. 2008). (c, d) Schematic illustrations of the input pathways to the CBL from the AOTu in the locust (c) and the monarch butterfly (d). Names of cell types are shown next to the lines illustrating the neurons. Colors as in A/B. LAL lateral accessory lobe, Lo lobula, UU upper unit
The intermediate stage of polarized-light processing in the central complex of the desert locust. (a) Proposed information flow to the intermediate stage neurons of the protocerebral bridge (PB). Based on receptive field organization, information from input neurons of the right side of the midline (black TL2 neurons) appears to be passed on to CL1a neurons of the contralateral brain side (black), and vice versa for information from the left side (red/gray neurons). Within the PB (top), TB1 neurons are suited to integrate information from both hemispheres with their bilateral input fibers and generate zenith-centered receptive fields. TB1 and TL2 neurons are shown as frontal reconstructions (neurite to posterior optic tubercle POTu in TB1 cells has been omitted for clarity), while CL1a neurons are drawn schematically. Nomenclature for PB-columns is shown on top. (b) Arborization scheme of TB1 neurons in the locust brain. Each row represents one individual neuron, with black squares indicating varicose (output) fibers and gray squares indicating smooth (input) fibers. The example shown in a is highlighted. (c) Preferred E-vector tuning of TB1 neurons plotted against the position of the output fibers along the PB. As each TB1 neuron possesses two columns filled with output fibers at a distance of eight columns, the analysis was restricted to one hemisphere and the dataset was shown twice for illustration purposes. Note that E-vector tunings of the neurons change linearly with the position of the arborization tree along the PB. (d) Schematic illustration of the linear regression data shown in c. Each column of the PB is associated with a particular E-vector tuning, overall covering all possible E-vectors (180°) once per PB hemisphere. CBL lower division of the central body; CBU upper division of the central body, BU bulb. Images were reproduced with permission from: Heinze et al. (2009) (a); Heinze and Homberg (2007) (b, c)
The output stage of the central-complex and polarization-sensitive downstream connections. (a) Schematic illustration of the systematic E-vector tunings in the protocerebral bridge (PB) in columnar neurons as opposed to TB1 neurons. The 90° shift in tunings between TB1 and columnar neurons indicates an inhibitory connection. (b, c) Anatomy of major output neurons of the central complex (CPU1 neurons) in the monarch butterfly (b) and the desert locust (c). The left panels show two individual neurons (registered into the standardized central complex in the monarch; frontal reconstructions projected onto 3D reconstruction of neuropils in the locust), while the right panel shows the heterolateral connectivity scheme for these neurons. The asterisks indicate actually identified neurons. Note the similarity between the species, despite 360 million years of separated evolutionary history. (d) Reconstruction of a polarization-sensitive neuron with input fibers in the lateral accessory lobes (LAL), providing a potential link to descending pathways. (e) Reconstruction of a polarization-sensitive descending neuron with input fibers in the posterior protocerebrum and output arborizations in the subesophageal ganglion (SEG) and in all three thoracic ganglia (TG). CBU upper division of the central body, CBL lower division of the central body, vLAL ventral LAL, dLAL dorsal LAL, dS dorsal shell, vS ventral shell, MBU medial bulb, LBU lateral bulb. Images have been reproduced with permission from: Heinze et al. (2013) (b); Heinze and Homberg (2008) (c); Heinze and Homberg (2009) (d); Träger and Homberg (2011) (e)
7.1 The Input Stage of the Central-Complex Network
How does polarized-light information reach the CX? The output neurons of the AOTu (TuLAL cells) carry polarized-light information to a specialized part of the LAL, which is characterized by a microglomerular structure and strong GABA (γ-aminobutyric acid) immunoreactivity. This region serves as a relay between the AOTu and the CX and is now referred to as the bulb (in previous literature: lateral triangle; Hanesch et al. 1989; Träger et al. 2008; Heinze and Reppert 2012) (Fig. 4.7a, b). It is divided into two distinct parts in the locust (called the lateral bulb [lateral triangle] and the medial bulb [median olive]), whereas it consists of two to three fused compartments in Drosophila, the monarch butterfly, and the cricket. Its microglomerular structure originates from very large synapses formed between the AOTu-projection neurons and tangential neurons of the CBL (TL neurons). Here, large, cup-shaped presynaptic terminals (TuLAL neurons) engulf tangles of very fine postsynaptic endings (TL neurons) (Träger et al. 2008; Heinze et al. 2013). These synaptic complexes have been particularly well described in the locust and are reminiscent of the specialized giant synapses (Calyx of Held) in the vertebrate auditory pathway. Their function within the polarization vision pathway remains elusive, but both pre- and postsynaptic neurons are tightly tuned to particular E-vectors when the animal is stimulated with a rotating linear polarizer in locusts and monarchs (Vitzthum et al. 2002; Pfeiffer et al. 2005; Heinze and Reppert 2011). Träger et al. (2008) speculate that the anatomical resemblance with the Calyx of Held might reflect a functional similarity, suggesting a role of these synapses in ensuring the precise timing of E-vector signals provided by the right and the left eye, before these signals reach the CX as central integration center.
Tangential neurons of the CBL (TL neurons) are the principal input for polarized-light information into the CX. They receive signals from projection neurons of the AOTu and project to individual layers of the CBL (Träger et al. 2008; Heinze et al. 2009; Heinze and Reppert 2011). Depending on which CBL layer is innervated, several types of these cells can be distinguished (Fig. 4.7). First defined for the locust (Müller et al. 1997), these neuronal subtypes have also been identified in the monarch butterfly (Heinze et al. 2013), and similar polarization-sensitive cells exist also in the cricket (Sakura et al. 2008). In Drosophila, the ring neurons of the ellipsoid body are the homologous counterparts to these neurons (Hanesch et al. 1989), but have so far not been shown to respond to polarized light. Interestingly, anatomical data suggest that the different subtypes of TL neurons receive input from different types of AOTu-projection neurons in monarchs and locusts, and hence constitute at least two parallel pathways which carry information about polarized light from early processing stages to the CX (Träger et al. 2008; Heinze et al. 2013) (Fig. 4.7c, d). In locusts, these pathways have been examined in most detail and can be distinguished by the response characteristics of the involved neurons: TL2 neurons respond to stimuli from both eyes, while TL3 neurons only receive ipsilateral input (Vitzthum et al. 2002; Heinze et al. 2009). Although the ocular dominance of these cells has not been examined in other species to date, the striking anatomical similarity, particularly between the monarch butterfly and the locust, suggests that two parallel input pathways carrying nonredundant E-vector information are a fundamental computational element found in the polarization vision network of the central brain of insects.
7.2 The Intermediate Stage of the Central-Complex Network
How is the information arriving in the CX via TL neurons processed through further stages? The most likely target neurons are a group of columnar cells, called CL1 neurons (Heinze and Homberg 2009). These cells connect individual columns of the CBL with columns of the PB, while also projecting to a small region of the LAL. Interestingly, the detailed structure of the neuronal terminals suggests that there are two subtypes of these cells with opposite polarity: the first with input regions in the CBL and output regions in the PB and the second with input regions in the PB and output regions in the CBL (Hanesch et al. 1989; Heinze and Homberg 2008, 2009; Heinze et al. 2013; Lin et al. 2013). The LAL projections appear to be output areas in all types. This complex anatomy has been described in all examined species (locusts, monarchs, and Drosophila) and thus the possibility for bidirectional information flow between the PB and the CBL appears to be a shared feature of the insect CX. The input arborizations of these cells in the CBL coincide with the location of the output fibers of major TL neurons in locusts and monarchs, and indeed CL1 cells exhibit exquisite polarization sensitivity (Heinze and Homberg 2009; Heinze et al. 2009; Heinze and Reppert 2011). In fact, in locusts these neurons are most narrowly tuned to their preferred E-vector compared to all other cell types. As for TL-neurons, the tuning of each individual CL1 neuron is not correlated with its anatomical characteristics (Vitzthum et al. 2002; Heinze and Homberg 2009). This is remarkable and difficult to explain for the columnar neurons, as each individual cell can be uniquely identified based on the location of its columnar arborization tree along the width of the CBL/PB. Either different sets of these neurons possess different tuning directions in identical columns or the tuning of each cell is dynamically adjusted over time (or between individual animals).
Intriguingly, the potentially postsynaptic partners of CL1 cells show a precise correlation between their E-vector tuning and the position of their arborization trees along the width of the PB (Heinze and Homberg 2007). These cells, called TB1 cells, possess two columnar output arborizations, filling two of the 16 PB-columns at a distance of eight columns apart. The space not covered by these arborization trees is largely filled with smooth input fibers, while only the columns directly adjacent to the output fibers remain devoid of terminals (Fig. 4.8a). As at least one TB neuron exists for each PB-column (Fig. 4.8b), the location of the output fiber trees can be correlated to the zenithal E-vector tuning of the cell. When this was performed in the locust, a significant relation between the physiology and the morphology of the neurons emerged (Fig. 4.8c). Moreover, the E-vector tunings of these neurons are distributed in such a way along the PB that all possible E-vectors (range from 0° to 180°) are mapped precisely on each PB hemisphere (Heinze and Homberg 2007) (Fig. 4.8d). This means that when a given E-vector is shown to the locust from the zenith, two activity maxima are produced along the length of the PB, and the location of these maxima changes systematically with the orientation of the animal. That is, the readout of these activity maxima can serve as a predictor of body orientation relative to the zenithal E-vector. Given the fact that the zenithal E-vector orientation depends strictly on the azimuth of the sun, this population of neurons can act as an ordered array of head direction cells within the global frame of reference provided by the sun, i.e., they can serve as an internal sun compass.
The question of how this E-vector map is computed remains one of the major questions related to polarization vision. The complex interplay of CL1 and TB1 neurons may be suited to perform the necessary computations, especially as these neuron types are highly conserved in many insect species. An additional piece in the puzzle, however, is provided by neurons described in the locust that interconnect the posterior optic tubercles on either side of the midline (el Jundi and Homberg 2010). As these small neuropils are also innervated by TB1 neurons (Heinze and Homberg 2007), a potentially closed loop between TB1 cells on either hemisphere of the PB is created that might play a role in shaping and stabilizing activity peaks within the PB through mutual inhibition (U. Homberg, personal communications).
7.3 The Output Stage of the Central-Complex Network
How is the information stored in the azimuth representation of the PB transmitted to the motor system? Judged by anatomical data as well as their physiological response characteristics, the most likely candidates for this task are columnar neurons that receive their input in the PB and project to different areas of the LALs. Among these, found in all insects studied so far, is a group of cell types called CPU1 neurons, which likely constitute the major output pathway from the CX in general (Homberg 1985; Hanesch et al. 1989; Vitzthum et al. 2002; Heinze and Homberg 2008; Heinze et al. 2009, 2013; el Jundi et al. 2010; Heinze and Reppert 2011; Phillips-Portillo 2012; Lin et al. 2013) (Fig. 4.9b, c). Aside from input fibers in the PB, these cells have additional input-type branches propagating through the layers of the CBU, whereas their output terminals cover large regions of the contralateral LAL. Detailed analyses in the locust and the monarch revealed several subtypes of these neurons, all of which were shown to respond to polarized light in both species (Heinze and Homberg 2008; Heinze et al. 2013). In locusts, the E-vector tunings of these cells are arranged in a systematic way and depend on the PB-column in which each neuron receives its input (Heinze and Homberg 2007). As for TB1 neurons, this map-like E-vector representation covers a range of 180° in each PB hemisphere, but is shifted by 90° with respect to TB1 cells (Fig. 4.9a). This means that in PB-columns in which TB1 neurons exhibit maximal activity at a given E-vector, the corresponding CPU1 cell shows minimal activity (i.e., maximal inhibition). If CPU1 neurons are indeed postsynaptic to TB1 cells, this implies an inhibitory connection between those two types of neuron. Although TB1 cells are not GABAergic, the presence of serotonin and several neuropeptides (shown through immunocytochemistry) in these cells in the locust does at least permit such inhibitory effects (Heinze and Homberg 2007). Preliminary data in the monarch butterfly reveal a very similar correlation between E-vector tuning and location of arborization trees along the PB in that species and suggest that systematic representations of E-vector angles are not a specialization of the desert locust (S. Heinze, unpublished observation; J. Phillips-Portillo, personal communications).
In the locust, two more columnar cell types with input-type fibers in the PB have been consistently shown to respond to polarized light: CP1 and CP2 neurons (Vitzthum et al. 2002; Heinze et al. 2009). These cells project to small regions of the LAL, closely associated (but likely not overlapping) with the lateral and medial bulbs (lateral triangle and median olive). The tuning angles of these neurons follow the same pattern as CPU1 neurons with respect to the position of the input arborization in the PB, i.e., they might also receive inhibitory input from TB1 neurons (Heinze and Homberg 2007). Unlike CPU1 cells though, these cell types have not been reported in any other insect to date.
A separate possible output pathway is present in the LAL projections of CL1 neurons. This projection is very small (and even missing in one subtype of these cells) in the locust (Heinze and Homberg 2008, 2009), but is much more prominent in the monarch butterfly (Heinze et al. 2013). In the monarch these cells show an increased fiber diameter and higher numbers of axonal terminals in the LAL. Additionally, the CL1 projection area in the monarch LAL stands out as a distinct region (called anterior loblet) not found in the locust. Importantly, this pathway does not necessarily receive input from the PB, and could thus mediate a direct route between tangential input neurons of the CBL and LAL cells serving as input to the motor system.
At last, a group of neurons found in many insects, but physiologically examined only in the locust, shows a behavior termed “conditional polarization sensitivity” (Heinze and Homberg 2009). These cells, which are all columnar neurons of the PB that project either to the noduli (CL2, CPU4) or to bilateral regions of the LAL (CPU2), do not consistently respond to polarized-light stimulations, but appear to be recruited to the polarized-light processing network of the CX in a context-dependent manner. What might trigger the switch in their responsiveness is unknown. Also the question of whether homologous neurons show similar behavior in other insects remains to be solved. However, the presence of these cells underlines the potential complexity and dynamic nature of the polarization vision network of the CX at least in the desert locust. The striking anatomical resemblance of the locust neurons to those in other insects (particularly in the monarch butterfly; Heinze et al. 2013) across extremely wide evolutionary distances makes it likely that the described network is not only a specialization of the locust brain but also exists in other insect species, possibly exhibiting similar functions.
7.4 Physiological Evidence for Proposed Information Flow
Aside from anatomical data and the fact that all described neurons respond to polarized light, what is the evidence supporting the laid out polarization vision network of the CX? Despite the fact that E-vector response curves appear to be similar between different cell types at first sight, detailed analysis in the locust has revealed characteristics that systematically vary from “early stage” to “late stage” neurons of the POL network in the CX (Heinze et al. 2009). This includes the signal-to-noise ratio of the neurons, their background activity, and the size and orientation of their receptive fields. In detail, tangential neurons of the CX (input stage) possess comparably small receptive fields centered in the contralateral hemisphere (in tune with the viewing direction of the ipsilateral DRA), while exhibiting low background activity and high signal-to-noise ratios (Fig. 4.10b). On the other hand, all neurons participating in the map-like E-vector representation of the PB show zenith-centered, very large receptive fields, high background activity, and lower signal-to-noise ratios (Fig. 4.10d, e). Furthermore, columnar neurons of the CBL, which are proposed to serve as the link between the input stage and the later stages, possess intermediate physiological characteristics. Their receptive fields are larger compared to tangential input stage cells, but are not zenith-centered as in later processing stages (Fig. 4.10c). In fact, the observed slight shift in receptive field center towards the ipsilateral hemisphere in these neurons suggests that information present in TL neurons from one side of the brain is transmitted to only those CL neurons projecting to the contralateral hemisphere of the PB (CL1 cell bodies are located close to the PB, i.e., contralaterally to TL neurons). Therefore, information from each sky hemisphere would be projected to the ipsilateral half of the PB (Fig. 4.8a). The previously described multiglomerular neurons of the PB (TB neurons) would then be ideally suited to integrate this information with their bilateral input fibers to generate the observed very large, zenith-centered receptive fields (Heinze et al. 2009).
Physiology of polarization-sensitive neurons across processing stages in the desert locust. Left column: Activity of neurons during one rotation of a linear polarizer above the animal. Top trace shows mean activity (gliding average); bottom trace shows voltage trace of intracellular recording. Counterclockwise rotations: 0°–360°; clockwise rotations: 360°–0°. Right column: Lateral extent of receptive fields for stimulation with polarized light. Plotted is the normalized response amplitude against the elevation of the stimulus along the meridian orthogonal to the body length axis of the animal. The line indicates background variability of the neuron type without stimulation. Neuron types are arranged from early to late processing stages: (a) TuLAL1a neurons (modified after el Jundi and Homberg 2012). (b) TL2 neurons. (c) CL1 neurons. (d) TB1 neurons. (e) CPU1 neurons. (f) Ipsilaterally descending neuron. No receptive field data exist for this cell type. Receptive field data show data from individual neurons in a and b, while showing mean ± standard error for c–e. Note that receptive fields change from small, variable, often contralaterally centered fields (TuLAL1a, TL2) to broad, ipsilaterally centered fields in CL1 neurons, to zenith-centered, very wide fields (TB1, CPU1). Within spike trains, comparison between cell types reveals that signal-to-noise ratio decreases the further the cell type is removed from the sensory periphery, while background activity and variability increase. (g) Illustration of stimulus delivery for measuring lateral extent of receptive fields. a anterior, p posterior, Z zenith. Images are reproduced with permission from: Heinze et al. (2009) (b–e, g); Träger and Homberg (2011) (f)
The most direct evidence for the directionality of signal flow in neurons of the POL network are intracellular recordings from several types of columnar neurons from either the PB or the LAL. When recorded in the PB (their proposed input region), postsynaptic potentials were frequently observed in CPU1 and CP2 neurons (locusts; CPU1 also in monarchs), while recordings from the same cell types in the LAL (proposed output region) revealed no such potentials (Heinze et al. 2009). These observations are strongly supporting the direction of signal flow within the output stage of the CX-POL network. Interestingly, and despite large numbers of recorded cells, no such clear distinction could be made for CL1 neurons, supporting the idea of bidirectional information flow between the PB and the CBL.
8 Beyond the Central Complex
In order to guide an insect’s behavior, the information about polarized light represented in the central brain has to reach the motor centers of the thorax. Indeed, intracellular recordings performed in the locust have demonstrated the presence of such a polarization-sensitive descending pathway (Träger and Homberg 2011) (Fig. 4.9e). Two pairs of neurons, one descending through the ipsilateral neck connective and the other one descending through the contralateral connective, possess proposed input fibers in the posterior protocerebrum of the brain and have their proposed output fibers in the subesophageal and thoracic ganglia. These cells respond with modulations of their spiking frequency when the animal is presented with a rotating linear polarizer (Fig. 4.10f). The responses are relatively weak and are imposed on a high background variability and variable background firing frequency (Träger and Homberg 2011). However, such behavior is expected for neurons many synapses away from the sensory input and is in line with the increasing variability and decreasing signal-to-noise ratio in late processing stages of the CX (Heinze et al. 2009) (Fig. 4.10). Both neurons also show strong, direction-selective responses to moving gratings, indicating that information from more than one sensory system has converged onto these cells. As responses to these motion stimuli were much stronger than responses to polarized light, the latter might carry less behavioral relevance. Indeed, so far it has not been shown that locusts use polarized-light information for flight orientation in a natural setting, and potentially only use it as a backup system or in particular behavioral contexts.
Where do these descending neurons receive their information from, and which cells might be their postsynaptic targets? A conclusive answer for these questions remains yet to be found, but candidate neurons for both tasks have been identified in the locust. As the described descending neurons do not have input fibers in the LAL, they cannot receive information directly from the CX output cells. So far, two types of polarization-sensitive neurons have been found that could be postsynaptic to CX output cells (Heinze and Homberg 2009). The first one projects from the ipsilateral LAL to large bilateral regions of the posterior protocerebrum (Fig. 4.9d) and has also been anatomically identified in the monarch butterfly (Heinze and Reppert 2011). It is therefore the most promising candidate for integrating information from CX-output cells and relaying it to descending neurons. The second cell connects the ipsilateral LAL to its contralateral counterpart. Although it cannot contact the described descending neurons directly, it might provide an intermediate step in integrating CX-output signals.
Within the ventral nerve cord, one type of neuron has been described that shows remarkably robust polarization sensitivity. It projects from the ipsilateral hemisphere of the subesophageal ganglion to the contralateral hemisphere of the first thoracic ganglion and might be suited to link polarization-sensitive descending neurons to motor neurons, if the descending brain neurons do not directly target motor neurons themselves (Träger and Homberg 2011). Interestingly, the polarization sensitivity of these neurons is much more pronounced than in the potentially presynaptic descending cells. Also, the found motion sensitivity in these cells is not direction selective and weaker than the polarized-light response. This either suggests that there is another unidentified polarization-sensitive descending pathway converging onto these neurons, or that there is substantial local processing of information within the networks of the ventral cord ganglia, which amplifies the descending polarized-light signals. Altogether, this underlines that there are still significant gaps in our understanding of the pathways that carry polarized-light information from the neural circuits of the central brain to the thoracic motor circuits.
9 Time Compensation and Circadian Input
Two of the three species that have been most extensively studied in the context of polarization vision, the desert locust and the monarch butterfly, are both insects that perform spectacular long-distance migrations. This implies that these animals are capable of maintaining a straight course over prolonged periods of time. If they indeed use the sun and the sun-derived polarization pattern of the sky as major orientation cues, these insects must compensate for the changing solar position over the course of the day. That means they have to adjust their desired heading with respect to the sun by using information about the time of day derived from the circadian clock. To perform this task, a neural connection between the circadian clock and the polarization vision network of the central brain is necessary.
Two parameters about solar position change differentially over the course of the day: solar elevation and solar azimuth. As compass information can be exclusively derived from the solar azimuth, only changes in this parameter have to be compensated to maintain a steady course over the duration of the day (“azimuth compensation”). Additionally, however, when polarized-light cues are used to derive the solar azimuth, another problem occurs: Changes in solar elevation strongly influence the interrelation between skylight polarization within the receptive fields of the DRA photoreceptors and solar azimuth. As the information content of detected polarized light consequently depends on how high the sun is up in the sky, reliable information about solar azimuth can only be derived from polarized-light information, when the animal accounts for the current solar elevation (Fig. 4.11a). This process (“elevation compensation”) is needed independent of whether the compass information will be used for long-distance migration or local foraging, as it is required to generate an intrinsically consistent representation of the current solar azimuth from more than one skylight compass cue. The fact that elevation compensation is needed by all insects that use polarized light to detect the solar azimuth, while azimuth compensation is only required by long-distance migrants to maintain a steady course, suggests that these processes are distinct from each other. In the following, both processes and their potential neural substrates will be discussed in more detail.
Time compensation of skylight compass cues. (a) Relation between solar elevation and skylight polarization pattern. At low solar elevations (top), all E-vectors (short gray lines) are arranged in parallel, so that the correct solar azimuth is always indicated when a 90° relation between E-vector and solar azimuth is assumed. When the solar elevation is higher, the 90° relation between the solar azimuth and the E-vector angle is only valid in the zenith. Outside the zenith, assuming a 90° relation results in a large error (black double arrow) in the solar azimuth estimate. Therefore, when E-vectors outside the zenith are used for detecting the solar azimuth, the current solar elevation has to be accounted for. (b) Tuning of locust neurons from the anterior optic tubercle to polarized light from the zenith (black) and unpolarized light spots moving around the animal at constant elevation (green light, ultraviolet light). The difference between the azimuth tuning in response to green light and the E-vector tuning is termed ΔΦmax. This ΔΦmax value is plotted in the bottom panel against the time of recording of the neurons (bins of 72 min). The data are fitted with a function that describes the angular difference between solar azimuth and E-vector orientation for individual points of the sky over the course of the day. The fit is calculated for August 1 (within the rearing period of the used animals) and 60° elevation above the horizon according to the visual axis of the DRA. The fit line is calculated for the coordinates of Tropic of Cancer (23.4°N), which is in the natural habitat of the locusts. (c) As b, but data for neurons recorded from the bulbs of the monarch butterfly (AOTu neurons and TL neurons). ΔΦmax is defined as the difference of E-vector tuning to the mean of the azimuth tunings of all tested colors. The line in the bottom panel represents the mean perceived E-vector in the region of sky viewed by the dorsal rim of the monarch over the course of the day, calculated for the location and date of capture of the used animals. Data are binned in 1 h bins and plotted against the Zeitgeber time (0 = light on). Note that in both species, the angular difference between azimuth and E-vector tuning is large in the evening and morning (at low solar elevations) and small around noon (at high solar elevations). Thus, the E-vector tuning of the neurons changes over the course of the day to match the skylight situation in the region of sky viewed by each species’ dorsal rim area, a process called elevation compensation. (d) The principle of azimuth compensation. When an animal has to maintain a constant flight bearing over the course of the day by using a sun compass, it has to adjust its orientation with respect to the sun, i.e., keep the sun on the left in the morning, while keeping it on its right in the evening. (e) Behavioral data revealing that the antennae are required for a time-compensated sun compass in the monarch butterfly. Left: Circular plot of flight directions of a population of butterflies in a flight simulator. All animals maintain a steady southwesterly bearing. Right: When antennae are clipped, the butterflies still show directed flight; however, they become disoriented as a group. This implies that the timing information needed to adjust flight direction according to the daytime is housed within the antennae (modified after Merlin et al. 2009)
9.1 Elevation Compensation
The concept of elevation compensation was first introduced by Pfeiffer and Homberg (2007) through work in the desert locust. The authors recorded from neurons of the AOTu and determined the cell’s E-vector tuning as well as their azimuth tuning to unpolarized light points. As the polarized-light stimuli were presented from the zenith, the expectation was that, within an individual neuron, the difference between azimuth tuning and E-vector tuning would be 90° (E-vectors in the zenith are perpendicular to the solar azimuth). However, it was found that this difference varied greatly and, surprisingly, its absolute value depended on the daytime of the recording (Fig. 4.11b). Large values (close to 90°) occurred in the morning and evening, while during midday small values dominated. This behavior could be explained by taking into account that the locust DRA has a receptive field centered at around 60° elevation on either side of the animal. In this region of the sky, the E-vector orientation of skylight strongly depends on the solar elevation: For any given solar azimuth, the E-vector orientation in the DRA receptive field changes when the sun is located at different heights above the horizon. In other words, the identical solar azimuth is indicated by different E-vectors depending on the solar elevation, which itself depends on the time of day. If the correct solar azimuth should be derived from the detected E-vectors, they have to be interpreted differently according to the current time of day. Pfeiffer and Homberg (2007) related the rate of change of the E-vector tuning in the recorded neurons to the rate of change of celestial E-vectors in the center of the receptive field of the DRA and found them to be virtually identical. The match was particularly strong when the skylight conditions of the locust’s native habitat (latitude of northern Africa) were taken into account (Fig. 4.11b). Several conclusions can be drawn from these results: First, the zenithal E-vectors presented in the experiments are likely interpreted as if they came from the complete DRA, i.e., that information from all parts of the DRA is pooled before reaching the recorded cells. Second, the neurons anticipate the changing interrelation of celestial E-vectors and solar azimuth by adjusting their E-vector tuning in a way that ensures that it encodes the correct solar azimuth throughout the day (consistent with the azimuth obtained from the presented unpolarized stimuli). Third, this correction function is optimized to match the sensory periphery of the locust (i.e., shape and orientation of the DRA), as well as the skylight conditions of the native habitat of the animals.
Recent work in the AOTu of migratory monarch butterflies has led to very similar results in this species (Heinze and Reppert 2011). Here, the E-vector tuning is also changing according to the time of day, so that the difference between E-vector tuning and tuning to the azimuth position of unpolarized light spots follows a function similar to the locust neurons (Fig. 4.11c). However, the function derived from the locust neurons did not match the monarch data precisely, but needed to be modified in a way that accounted for the different receptive fields of the monarch DRA and the date and location that the animals had been caught from the wild. This underlines the fundamental importance of the elevation-compensation process: Both species modify the E-vector tuning of their AOTu neurons according to the time of day to consistently combine otherwise ambiguous skylight compass cues into a consistent representation of the solar azimuth. To reach this identical outcome, both species have optimized the correction function in a way that accounts for their different eye shapes and habitats.
Where in the brain is timing information combined with E-vector information? A conclusive answer to this question has not been found to date. However, the location must occur upstream of the AOTu neurons that exhibit the described response features. Therefore, polarization-sensitive neurons of the optic lobe are promising candidates for integrating timing information. The source of this information would likely be the circadian clock of the animals. Whether the daytime-dependent change of neural tuning is truly circadian or is merely a response to rhythmic lighting conditions remains to be shown by repeating recordings in animals kept in constant darkness.
The pacemaker of the circadian clock in the monarch brain is located within few neurons in the pars lateralis (Reppert 2006; Zhu et al. 2008). Through tracing neurons expressing the clock protein CRYPTOCHROME-1, fibers have been identified that might colocalize with projections from DRA photoreceptors in the medulla and this way provide a possible interaction site between the circadian system and polarization-sensitive neurons (Sauman et al. 2005; Reppert et al. 2010; Merlin et al. 2012). In locusts, the seat of the clock is unknown, but through anatomical comparison with data from cockroaches is postulated to be located in the accessory medulla, a small neuropil of the optic lobes. Indeed, several polarization-sensitive neurons have been found in the optic lobe of locusts and crickets that possess side branches in this neuropil. As some of these likely serve as input fibers, they would be suited to transfer timing information from the clock to the compass system (Labhart and Petzold 1993; Homberg and Würden 1997; el Jundi and Homberg 2010; el Jundi et al. 2010).
9.2 Azimuth Compensation
Much less is known about the neural mechanisms of azimuth compensation (Fig. 4.11d, e). Through behavioral experiments with clock-shifted animals, this process has only been conclusively shown to exist in the monarch butterfly to date (Mouritsen and Frost 2002; Froy et al. 2003). Interestingly, this behavior depends on the presence of the antennae, particularly of functional antennal clocks (Merlin et al. 2009) (Fig. 4.11e). When the antennal clocks become interrupted or desynchronized by either cutting them or blocking their exposure to light, the animals lose their ability for time-compensating their flight direction (Merlin et al. 2009; Guerra et al. 2012). How timing information from the antenna feeds into the polarization-sensitive network of the brain remains one of the major questions of the field. A promising candidate region of the brain is the LAL, where the output of the CX-polarization vision network converges and which, in moths, receives indirect input from the antennal lobes, while it also appears to play a crucial role in guiding steering movements (Iwano et al. 2010). Whether this mechanism is a specialization of lepidopteran insects, or whether it is applicable for insects in general, remains to be shown.
In the desert locust, a connection between the accessory medulla, the presumed seat of the clock, and the posterior optic tubercle has been anatomically identified (Homberg and Würden 1997; el Jundi and Homberg 2010). As the posterior optic tubercle is an intrinsic part of the locust polarization vision network (Heinze and Homberg 2007; el Jundi and Homberg 2010), the authors hypothesize that timing information needed to compensate for the changing solar azimuth over the course of the day might be transmitted through this connection. However, no physiological evidence supporting this hypothesis exists to date. Interestingly, the E-vector tunings of descending brain neurons of the locust are linearly correlated with the time of day of the recording (Träger and Homberg 2011). As alternate explanations for this correlation still cannot be ruled out and no evidence exists for time-dependent changes of flight direction in locust behavioral experiments, it remains to be confirmed whether this is the first direct observation of daytime-dependent alterations of E-vector tunings downstream of the CX. If these data indeed show azimuth-compensated E-vector tunings in premotor command neurons, it is puzzling that this does not manifest itself in the behavior of the animals.
10 Putting Polarized Light Into Context
Polarized light cannot be viewed as an isolated sensory cue, as it is used by the animal in conjunction with other available skylight cues. Together they provide the animal with a global frame of reference, in which it can embed sensory information that is of immediate behavioral relevance. Moreover, detecting and perceiving directional skylight cues does not provide the insect with any useful information, unless they are combined with features of the environment that have to be either avoided (e.g., predators) or approached (e.g., food, mating partners, nest). Additionally, whether features are attractive or repulsive depends on the behavioral context. For example, the nest of a central place forager is only attractive on the inbound journey after a foraging trip. Hence, modulating factors, like the motivational state of the animal or the current time of day, have to be integrated when transforming sensory signals into motor commands. An animal constantly has to evaluate whether its current direction of movement does match its desired heading. Any discrepancy has to be translated into compensatory movements. If the behavior of the animal involves prolonged straight segments or a central point to which it has to return, having a global frame of reference is of great importance for ensuring accuracy. It is in this context, in which the sun and other celestial features like polarized skylight can provide most valuable orientation cues (Lambert et al. 2011).
Through the described data from desert locusts and monarch butterflies, it became evident that, early in the system, information about the skylight polarization pattern is combined with information obtained from observing the sun itself or the skylight spectral gradient. This way, a more robust signal is produced that reliably encodes head direction even if one of the cues is noisy or missing. This implies, however, that the activity of the involved neurons does not serve the perception of polarized light as such, as action potentials in these cells are generated by all skylight cues and cannot be separated according to which sensory cue caused them. Rather, these cells are suited to encode body orientation with respect to the solar azimuth. Later in the system, this information is used to generate an ordered representation of azimuthal space in the CX, for which polarized skylight is likely only one of many contributing factors (Fig. 4.12). One could speculate that this azimuth representation provides the frame of reference in which behaviorally relevant features of the environment are associated with directional information. This process of combining a “Where-pathway” (azimuth representation) with a “What-pathway” (behaviorally relevant aspects of the sensory world) could provide the basis for generating motor commands. Promising candidate neurons have been identified that occur in all insects studied and likely receive input from the CBU as well as from the azimuth representation of the PB (CPU1, CPU2 cells; Figs. 4.9b, c and 4.12). As the CBU receives neural connections from many brain areas (Heinze et al. 2013) and has been implicated in memory formation of acutely relevant visual stimuli (Liu et al. 2006), it could indeed be the endpoint of a “What-pathway.” Exceptionally rich supply of neuromodulatory substances (Homberg 2002; Nässel and Homberg 2006; Kahsai et al. 2010; Kahsai and Winther 2011) together with the observation of context-dependent switches in sensory responses (conditionally polarization-sensitive cells; Heinze and Homberg 2009) additionally suggest that modulating factors required to direct behavior are also integrated within the CX.
Polarized-light perception in the context of central-complex function. In summary, the data accumulated over the last decade suggest that polarized light is one of several skylight cues that helps to establish a global frame of reference in the central complex. The neural basis of this is an ordered array of neurons in the protocerebral bridge (PB), which encodes the body orientation of the animal with respect to the sun, i.e., they provide an internal representation of the azimuthal space around the animal. This information is reaching the PB through specialized regions of the lateral accessory lobes (blue/dark: lateral bulb and medial bulb) and the lower division of the central body (CBL) (the “Where-pathway”). Behaviorally relevant features of the environment, e.g., conspecifics, food, obstacles, etc., are thought to be represented in the upper division of the central body (CBU), a neuropil that receives input from many areas of the brain (the “What-pathway”). Additionally, the CBU contains a large variety of neuromodulatory substances, which potentially confer information about the motivational state of the animal or its arousal level. At last, output neurons of the central complex (CPU1 cells) that receive input from the azimuth representation of the PB as well as from the CBU are ideally suited to combine information about body orientation with interesting features of the environment and thereby produce signals that may be used to induce motor commands guiding the behavior of the animal
11 Conclusions
In summary, despite remaining gaps in understanding, a more and more complete picture of the neural pathways involved in the processing of polarized-light information has emerged recently, especially through work in the desert locust. This extensive neuronal network spans nearly all processing stages from photoreceptors of the retina to neurons in the thorax that are potentially directly involved in controlling steering movements of the animal. The significant challenge that lies ahead is to verify the proposed links between these neural elements to combine them to a comprehensive network that performs the computations needed to explain an animal’s behavior in response to polarized-light stimuli.
To extract general computational principles and to find the key shared elements that define the polarization vision network in the insect brain, work is needed in species other than the desert locust and the monarch butterfly. Particularly, species that do not share the migratory behavior of these animals will give important insights into how widely applicable the findings from locusts and monarchs are. The rich behavior described in bees, as well as the already extensive knowledge from crickets, makes these species ideal for expanding electrophysiological studies. Additionally, the powerful genetic methods available in Drosophila, combined with the recent description of well-defined behavioral responses to linearly polarized light, open up a new line of research, which will enable us to examine polarization vision on completely new levels.
Importantly, all this will not only be relevant for the understanding of polarized-light orientation of insects, but will shed light on more general principles of how the brain transforms sensory signals into motor commands that guide an animal’s behavior.
References
Barta A, Horváth G (2004) Why is it advantageous for animals to detect celestial polarization in the ultraviolet? Skylight polarization under clouds and canopies is strongest in the UV. J Theor Biol 226:429–437
Beugnon G, Campan R (1989) Homing in the field cricket, Gryllus campestris. J Insect Behav 2:187–198
Blum M, Labhart T (2000) Photoreceptor visual fields, ommatidial array, and receptor axon projections in the polarization-sensitive dorsal rim area of the cricket compound eye. J Comp Physiol A 186:119–128
Brines ML, Gould JL (1982) Skylight polarization patterns and animal orientation. J Exp Biol 96:69–91
Brunner D, Labhart T (1987) Behavioural evidence for polarization vision in crickets. Physiol Entomol 12:1–10
Coulson KL (1988) Polarization and intensity of light in the atmosphere. A. Deepak Publishing, Hampton, VA, USA
Eggers A, Gewecke M, Wiese K, Popov AV, Renninger G (1993) The dorsal rim area of the compound eye and polarization vision in the desert locust (Schistocerca gregaria). In: Wiese K (ed) Sensory systems in arthropods. Birkhäuser, Basel, pp 101–109
el Jundi B, Homberg U (2010) Evidence for the possible existence of a second polarization-vision pathway in the locust brain. J Insect Physiol 56:971–979
el Jundi B, Homberg U (2012) Receptive field properties and intensity-response functions of polarization-sensitive neurons of the optic tubercle in gregarious and solitarious locusts. J Neurophys 108:1695–1710
el Jundi B, Heinze S, Lenschow C, Kurylas AE, Rohlfing T, Homberg U (2010) The locust standard brain: a 3D standard of the central complex as a platform for neural network analysis. Front Syst Neurosci 3:21
el Jundi B, Pfeiffer K, Homberg U (2011) A distinct layer of the medulla integrates sky compass signals in the brain of an insect. PLoS One 6:e27855
Fischbach KF, Dittrich APM (1989) The optic lobe of Drosophila melanogaster. I.: a Golgi analysis of wild-type structure. Cell Tissue Res 258:441–475
Froy O, Gotter AL, Casselman AL, Reppert SM (2003) Illuminating the circadian clock in monarch butterfly migration. Science 300:1303–1305
Gál J, Horváth G, Barta A, Wehner R (2001a) Polarization of the moonlit clear night sky measured by full-sky imaging polarimetry at full moon: comparison of the polarization of moonlit and sunlit skies. J Geophys Res D 106:22647–22653
Gál J, Horváth G, Meyer-Rochow VB, Wehner R (2001b) Polarization patterns of the summer sky and its neutral points measured by full-sky imaging polarimetry in Finnish Lapland north of the Arctic Circle. Proc R Soc A 457:1385–1399
Guerra PA, Merlin C, Gegear RJ, Reppert SM (2012) Discordant timing between antennae disrupts sun compass orientation in migratory monarch butterflies. Nat Commun 3, article no 958
Hanesch U, Fischbach KF, Heisenberg M (1989) Neuronal architecture of the central complex in Drosophila melanogaster. Cell Tissue Res 257:343–366
Hegedüs R, Horváth G (2004a) How and why are uniformly polarization-sensitive retinae subject to polarization-related artefacts? Correction of some errors in the theory of polarization-induced false colours. J Theor Biol 230:77–87
Hegedüs R, Horváth G (2004b) Polarizational colours could help polarization-dependent colour vision systems to discriminate between shiny and matt surfaces, but cannot unambiguously code surface orientation. Vis Res 44:2337–2348
Hegedüs R, Horváth Á, Horváth G (2006) Why do dusk-active cockchafers detect polarization in the green? The polarization vision in Melolontha melolontha is tuned to the high polarized intensity of downwelling light under canopies during sunset. J Theor Biol 238:230–244
Hegedüs R, Åkesson S, Horváth G (2007a) Polarization patterns of thick clouds: overcast skies have distribution of the angle of polarization similar to that of clear skies. J Opt Soc Am A 24:2347–2356
Hegedüs R, Åkesson S, Horváth G (2007b) Anomalous celestial polarization caused by forest fire smoke: why do some insects become visually disoriented under smoky skies? Appl Opt 46:2717–2726
Hegedüs R, Åkesson S, Wehner R, Horváth G (2007c) Could Vikings have navigated under foggy and cloudy conditions by skylight polarization? On the atmospheric optical prerequisites of polarimetric Viking navigation under foggy and cloudy skies. Proc R Soc A 463:1081–1095
Hegedüs R, Barta A, Bernáth B, Meyer-Rochow VB, Horváth G (2007d) Imaging polarimetry of forest canopies: how the azimuth direction of the sun, occluded by vegetation, can be assessed from the polarization pattern of the sunlit foliage. Appl Opt 46:6019–6032
Heinze S, Homberg U (2007) Maplike representation of celestial E-vector orientations in the brain of an insect. Science 315:995–997
Heinze S, Homberg U (2008) Neuroarchitecture of the central complex of the desert locust: intrinsic and columnar neurons. J Comp Neurol 511:454–478
Heinze S, Homberg U (2009) Linking the input to the output: new sets of neurons complement the polarization vision network in the locust central complex. J Neurosci 29:4911–4921
Heinze S, Reppert SM (2011) Sun compass integration of skylight cues in migratory monarch butterflies. Neuron 69:345–358
Heinze S, Reppert SM (2012) Anatomical basis of sun compass navigation I: the general layout of the monarch butterfly brain. J Comp Neurol 520:1599–1628
Heinze S, Gotthardt S, Homberg U (2009) Transformation of polarized light information in the central complex of the locust. J Neurosci 29:11783–11793
Heinze S, Florman J, Asokaraj S, el Jundi B, Reppert SM (2013) Anatomical basis of sun compass navigation II: the neuronal composition of the central complex of the monarch butterfly. J Comp Neurol 521:267–298
Helfrich-Forster C, Stengl M, Homberg U (1998) Organization of the circadian system in insects. Chronobiol Int 15:567–594
Henze MJ (2009) Two facets of insect vision: polarization sensitivity and visual pigments. PhD thesis, Universität Zürich, Zürich, Switzerland
Henze MJ, Labhart T (2007) Haze, clouds and limited sky visibility: polarotactic orientation of crickets under difficult stimulus conditions. J Exp Biol 210:3266–3276
Henze MJ, Dannenhauer K, Kohler M, Labhart T, Gesemann M (2012) Opsin evolution and expression in arthropod compound eyes and ocelli: insights from the cricket Gryllus bimaculatus. BMC Evol Biol 12:163
Herzmann D, Labhart T (1989) Spectral sensitivity and absolute threshold of polarization vision in crickets: a behavioral study. J Comp Physiol A 165:315–319
Homberg U (1985) Interneurones of the central complex in the bee brain (Apis mellifera, L.). J Insect Physiol 31:251–264
Homberg U (2002) Neurotransmitters and neuropeptides in the brain of the locust. Microsc Res Tech 56:189–209
Homberg U, Paech A (2002) Ultrastructure and orientation of ommatidia in the dorsal rim area of the locust compound eye. Arthropod Struct Dev 30:271–280
Homberg U, Würden S (1997) Movement-sensitive, polarization-sensitive, and light-sensitive neurons of the medulla and accessory medulla of the locust, Schistocerca gregaria. J Comp Neurol 386:329–346
Homberg U, Würden S, Dircksen H, Rao K (1991) Comparative anatomy of pigment-dispersing hormone-immunoreactive neurons in the brain of orthopteroid insects. Cell Tissue Res 266:343–357
Homberg U, Hofer S, Pfeiffer K, Gebhardt S (2003) Organization and neural connections of the anterior optic tubercle in the brain of the locust, Schistocerca gregaria. J Comp Neurol 462:415–430
Homberg U, Heinze S, Pfeiffer K, Kinoshita M, el Jundi B (2011) Central neural coding of sky polarization in insects. Philos Trans R Soc Lond B 366:680–687
Horváth G, Varjú D (2004) Polarized light in animal vision—polarization patterns in nature. Springer, Heidelberg
Horváth G, Wehner R (1999) Skylight polarization as perceived by desert ants and measured by video polarimetry. J Comp Physiol A 184:1–7 [Erratum 184: 347-349 (1999)]
Horváth G, Barta A, Gál J, Suhai B, Haiman O (2002a) Ground-based full-sky imaging polarimetry of rapidly changing skies and its use for polarimetric cloud detection. Appl Opt 41:543–559
Horváth G, Gál J, Labhart T, Wehner R (2002b) Does reflection polarization by plants influence colour perception in insects? Polarimetric measurements applied to a polarization-sensitive model retina of Papilio butterflies. J Exp Biol 205:3281–3298
Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, Hartenstein V et al (2014) A systematic nomenclature for the insect brain. Neuron 81(4):755–765
Iwano M, Hill ES, Mori A, Mishima T, Mishima T, Kei Ito K, Kanzaki R (2010) Neurons associated with the flip-flop activity in the lateral accessory lobe and ventral protocerebrum of the silkworm moth brain. J Comp Neurol 518:366–388
Kahsai L, Winther AME (2011) Chemical neuroanatomy of the Drosophila central complex: distribution of multiple neuropeptides in relation to neurotransmitters. J Comp Neurol 519:290–315
Kahsai L, Martin J-R, Winther AME (2010) Neuropeptides in the Drosophila central complex in modulation of locomotor behavior. J Exp Biol 213:2256–2265
Kelber A (1999) Why “false” colours are seen by butterflies. Nature 402:251
Kelber A, Thunell C, Arikawa K (2001) Polarisation-dependent colour vision in Papilio butterflies. J Exp Biol 204:2469–2480
Kinoshita M, Pfeiffer K, Homberg U (2007) Spectral properties of identified polarized-light sensitive interneurons in the brain of the desert locust Schistocerca gregaria. J Exp Biol 210:1350–1361
Kinoshita M, Yamazato K, Arikawa K (2011) Polarization-based brightness discrimination in the foraging butterfly, Papilio xuthus. Philos Trans R Soc Lond B 366:688–696
Kirschfeld K (1972) The number of receptors necessary for determining the position of the E-vector of linearly polarized light. Z Naturforsch B 27:578–579
Labhart T (1988) Polarization-opponent interneurons in the insect visual system. Nature 331:435–437
Labhart T (1996) How polarization-sensitive interneurons of crickets perform at low degrees of polarization. J Exp Biol 199:1467–1475
Labhart T, Meyer EP (1999) Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc Res Tech 47:368–379
Labhart T, Meyer EP (2002) Neural mechanisms in insect navigation: polarization compass and odometer. Curr Opin Neurobiol 12:707–714
Labhart T, Petzold J (1993) Processing of polarized light information in the visual system of crickets. In: Wiese K (ed) Sensory systems in arthropods. Birkhäuser, Basel, pp 158–169
Labhart T, Hodel B, Valenzuela I (1984) The physiology of the cricket’s compound eye with particular reference to the anatomically specialized dorsal rim area. J Comp Physiol A 155:289–296
Labhart T, Petzold J, Helbling H (2001) Spatial integration in polarization-sensitive interneurones of crickets: a survey of evidence, mechanisms and benefits. J Exp Biol 204:2423–2430
Labhart T, Baumann F, Bernard GD (2009) Specialized ommatidia of the polarization-sensitive dorsal rim area in the eye of monarch butterflies have non-functional reflecting tapeta. Cell Tissue Res 338:391–400
Lambert A, Furgale P, Barfoot TD, Enright J (2011) Visual odometry aided by a sun sensor and inclinometer. In: 2011 I.E. aerospace conference, 5–11 March 2011, Big Sky, MT, pp 1–14
Li W, Pan Y, Wang Z, Gong H, Gong Z, Liu L (2009) Morphological characterization of single fan-shaped body neurons in Drosophila melanogaster. Cell Tissue Res 336:509–519
Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, Heisenberg M, Liu L (2006) Distinct memory traces for two visual features in the Drosophila brain. Nature 439:551–556
Mappes M, Homberg U (2004) Behavioral analysis of polarization vision in tethered flying locusts. J Comp Physiol A 190:61–68
Mappes M, Homberg U (2007) Surgical lesion of the anterior optic tract abolishes polarotaxis in tethered flying locusts, Schistocerca gregaria. J Comp Physiol A 193:43–50
Merlin C, Gegear RJ, Reppert SM (2009) Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies. Science 325:1700–1704
Merlin C, Heinze S, Reppert SM (2012) Unraveling navigational strategies in migratory insects. Curr Opin Neurobiol 22:353–361
Mouritsen H, Frost BJ (2002) Virtual migration in tethered flying monarch butterflies reveals their orientation mechanisms. Proc Natl Acad Sci USA 99:10162–10166
Müller M, Homberg U, Kühn A (1997) Neuroarchitecture of the lower division of the central body in the brain of the locust (Schistocerca gregaria). Cell Tissue Res 288:159–176
Nässel DR (1999) Histamine in the brain of insects: a review. Microsc Res Tech 44:121–136
Nässel DR, Homberg U (2006) Neuropeptides in interneurons of the insect brain. Cell Tissue Res 326:1–24
Pfeiffer K, Homberg U (2007) Coding of azimuthal directions via time-compensated combination of celestial compass cues. Curr Biol 17:960–965
Pfeiffer K, Kinoshita M, Homberg U (2005) Polarization-sensitive and light-sensitive neurons in two parallel pathways passing through the anterior optic tubercle in the locust brain. J Neurophys 94:3903–3915
Pfeiffer K, Negrello M, Homberg U (2011) Conditional perception under stimulus ambiguity: polarization- and azimuth-sensitive neurons in the locust brain are inhibited by low degrees of polarization. J Neurophys 105:28–35
Philipsborn A, Labhart T (1990) A behavioural study of polarization vision in the fly, Musca domestica. J Comp Physiol A 167:737–743
Phillips-Portillo J (2012) The central complex of the flesh fly, Neobellieria bullata: recordings and morphologies of protocerebral inputs and small-field neurons. J Comp Neurol 520:3088–3104
Pirih P, Arikawa K, Stavenga DG (2010) An expanded set of photoreceptors in the Eastern pale clouded yellow butterfly, Colias erate. J Comp Physiol A 196:501–517
Pomozi I, Horváth G, Wehner R (2001) How the clear-sky angle of polarization pattern continues underneath clouds: full-sky measurements and implications for animal orientation. J Exp Biol 204:2933–2942
Reppert SM (2006) A colorful model of the circadian clock. Cell 124:233–236
Reppert SM, Zhu H, White RH (2004) Polarized light helps monarch butterflies navigate. Curr Biol 14:155–158
Reppert SM, Gegear RJ, Merlin C (2010) Navigational mechanisms of migrating monarch butterflies. Trends Neurosci 33:399–406
Rossel S, Wehner R (1986) Polarization vision in bees. Nature 323:128–131
Rossel S, Wehner R (1987) The bee’s E-vector compass. In: Menzel R, Mercer A (eds) Neurobiology and behaviour of honeybees. Springer, Heidelberg, pp 76–93
Sakura M, Lambrinos D, Labhart T (2008) Polarized skylight navigation in insects: model and electrophysiology of E-vector coding by neurons in the central complex. J Neurophys 99:667–682
Santschi F (1923) L’orientation siderale des fourmis, et quelques consideration sur leurs differentes possibilites d’orientation. Mem Soc Vaudoise Sci Nat 4:137–175
Sauman I, Briscoe AD, Zhu H, Shi D, Froy O, Stalleicken J, Yuan Q, Casselman A, Reppert SM (2005) Connecting the navigational clock to sun compass input in monarch butterfly brain. Neuron 46:457–467
Schwind R (1983) Zonation of the optical environment and zonation in the rhabdom structure within the eye of the backswimmer, Notonecta glauca. Cell Tissue Res 232:53–63
Schwind R (1985) Sehen unter und über Wasser, Sehen von Wasser. Naturwissenschaften 72:343–352
Schwind R, Horváth G (1993) Reflection-polarization pattern at water surfaces and correction of a common representation of the polarization pattern of the sky. Naturwissenschaften 80:82–83
Shashar N, Sabbah S, Aharoni N (2005) Migrating locusts can detect polarized reflections to avoid flying over the sea. Biol Lett 1:472–475
Siegl T, Schachtner J, Holstein GR, Homberg U (2009) NO/cGMP signalling: L-citrulline and cGMP immunostaining in the central complex of the desert locust Schistocerca gregaria. Cell Tissue Res 337:327–340
Stalleicken J, Mukhida M, Labhart T, Wehner R, Frost B, Mouritsen H (2005) Do monarch butterflies use polarized skylight for migratory orientation? J Exp Biol 208:2399–2408
Stalleicken J, Labhart T, Mouritsen H (2006) Physiological characterization of the compound eye in monarch butterflies with focus on the dorsal rim area. J Comp Physiol A 192:321–331
Suhai B, Horváth G (2004) How well does the Rayleigh model describe the E-vector distribution of skylight in clear and cloudy conditions? A full-sky polarimetric study. J Opt Soc Am A 21:1669–1676
Träger U, Homberg U (2011) Polarization-sensitive descending neurons in the locust: connecting the brain to thoracic ganglia. J Neurosci 31:2238–2247
Träger U, Wagner R, Bausenwein B, Homberg U (2008) A novel type of microglomerular synaptic complex in the polarization vision pathway of the locust brain. J Comp Neurol 506:288–300
Vitzthum H, Müller M, Homberg U (2002) Neurons of the central complex of the locust Schistocerca gregaria are sensitive to polarized light. J Neurosci 22:1114–1125
von Frisch K (1949) Die Polarisation des Himmelslichtes als orientierender Faktor bei den Tänzen der Bienen. Experientia 5:142–148
Wehner R (1997) The ant’s celestial compass system: spectral and polarization channels. In: Lehrer M (ed) Orientation and communication in arthropods. Birkhäuser, Basel, pp 145–186
Wehner R, Bernard GD (1993) Photoreceptor twist: a solution to the false-color problem. Proc Natl Acad Sci USA 90:4132–4135
Wehner R, Labhart T (2006) Polarization vision. In: Warrant E, Nilsson DE (eds) Invertebrate vision. Cambridge University Press, Cambridge, UK, pp 291–348
Weir PT, Dickinson MH (2012) Flying Drosophila orient to sky polarization. Curr Biol 22:21–27
Wernet MF, Desplan C (2004) Building a retinal mosaic: cell-fate decision in the fly eye. Trends Cell Biol 14:576–584
Wernet MF, Velez MM, Clark DA, Baumann-Klausener F, Brown JR, Klovstad M, Labhart T, Clandinin TR (2011) Genetic dissection reveals two separate retinal substrates for polarization vision in Drosophila. Curr Biol 21:1–9
Williams JLD (1975) Anatomical studies of the insect central nervous system: a ground-plan of the midbrain and an introduction to the central complex in the locust, Schistocerca gregaria (Orthoptera). J Zool 176:67–86
Wolf R, Gebhardt B, Gademann R, Heisenberg M (1980) Polarization sensitivity of course control in Drosophila melanogaster. J Comp Physiol A 139:177–191
Young JM, Armstrong J (2010) Structure of the adult central complex in Drosophila: organization of distinct neuronal subsets. J Comp Neurol 518:1500–1524
Zhu H, Sauman I, Yuan Q, Casselman A, Emery-Le M, Emery P, Reppert SM (2008) Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol 6:e4
Acknowledgments
I am grateful to Basil el Jundi, Almut Kelber, and Gábor Horváth for valuable comments on earlier drafts of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Fig. 4.1
Different ways in which E-vector angles have been plotted for desert locusts and crickets. For crickets angles increase clockwise, while they increase counter-clockwise for locusts. To transform cricket angles into the locust system, one has to subtract them from 180°. Note that the locust system has also been adopted for the monarch butterfly (PNG 273 kb)
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Heinze, S. (2014). Polarized-Light Processing in Insect Brains: Recent Insights from the Desert Locust, the Monarch Butterfly, the Cricket, and the Fruit Fly. In: Horváth, G. (eds) Polarized Light and Polarization Vision in Animal Sciences. Springer Series in Vision Research, vol 2. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54718-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-54718-8_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54717-1
Online ISBN: 978-3-642-54718-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)