Abstract
Like many vertebrate species, insects rely on a sun compass for spatial orientation and long- range navigation. In addition to the sun, however, insects can also use the polarization pattern of the sky as a reference for estimating navigational directions. Recent analysis of polarization vision pathways in the brain of orthopteroid insects sheds some light onto brain areas that might act as internal navigation centers. Here I review the significance, peripheral mechanisms, and central processing stages for polarization vision in insects with special reference to the locust Schistocerca gregaria. As in other insect species, polarization vision in locusts relies on specialized photoreceptor cells in a small dorsal rim area of the compound eye. Stages in the brain involved in polarized light signaling include specific areas in the lamina, medulla and lobula of the optic lobe and, in the midbrain, the anterior optic tubercle, the lateral accessory lobe, and the central complex. Integration of polarized-light signals with information on solar position appears to start in the optic lobe. In the central complex, polarization-opponent interneurons form a network of interconnected neurons. The organization of the central complex, its connections to thoracic motor centers, and its involvement in the spatial control of locomotion strongly suggest that it serves as a spatial organizer within the insect brain, including the functions of compass orientation and path integration. Time compensation in compass orientation is possibly achieved through a neural pathway from the internal circadian clock in the accessory medulla to the protocerebral bridge of the central complex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many animals show astounding capabilities in spatial orientation and navigation. Sea turtles return to their island of origin from distances of more than 2,000 km in the open ocean; seasonally migrating monarch butterflies migrate to overwintering places in central Mexico some 3,000 km away from summer habitats in the USA (reviewed by Waterman 1989; Papi 1992). On a shorter range of distance, insects such as honeybees or desert ants navigate in straight lines back to their nest after complicated feeding excursions (von Frisch 1967; Wehner 1992; Riley et al. 2003). In many cases the sensory cues used by these animals as guiding signals have been determined and more recent research moves to uncover central nervous mechanisms underlying spatial navigation.
Insects are particularly well suited to the analysis of spatial orientation and animal navigation. In comparison with vertebrates, they have a minute brain, but nevertheless show many navigational mechanisms which are also found in vertebrates, including man (Mouritsen 2001; Wang and Spelke 2002). Research in insects has focused in particular on the honeybee Apis mellifera (Giurfa and Capaldi 1999), and on the desert ant, Cataglyphis bicolor (Wehner 1997, 2003). Both species are central-place foragers, which need to find their way back to their nest or hive after food-searching excursions. Worker honeybees even communicate the directions and distances of a favorable food source to their nestmates. Since the navigational routes in ants and bees depend on the location of a food source relative to the hive/nest, their navigation requires some means of spatial memory (Collett and Collett 2002). At the other extreme, long-range navigators like many butterflies perform regular seasonal migrations, in some cases over several thousand kilometers (Wehner 1984). Here, as in many migratory birds, the distance and direction traveled requires a strong genetic determination.
Behavioral studies in honeybees, ants, and several other species show that insects largely navigate by two mechanisms. In familiar terrain, they use landmarks as guide-posts for navigational routes and nest finding as demonstrated elegantly in the classical study by Tinbergen (1932) on the bee wolf. In addition, and probably more importantly, they rely on a vector-based mechanism of orientation, termed path integration (Wehner 1992, 1994; Collett and Collett 2000). On their foraging trips, bees, ants, and other hymenopteran insects continually monitor their direction of travel using a sun- or a polarized-light compass (Fig. 1A), and estimate the distance to the nest through retinal image motion (in honeybees; Esch and Burns 1996; Srinivasan et al. 2000) or some other mechanism (ants; Wohlgemuth et al. 2002; Labhart and Meyer 2002). Seasonal long-range migratory insects like many butterflies are partly displaced by wind but, likewise, use a celestial compass for maintaining traveling directions at particular angles to the prevailing wind (Wehner 1984). The mechanisms of goal determination in migratory insects may depend on favorable conditions for feeding or egg-laying but have not been studied systematically.
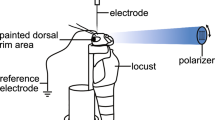
Sensory basis of polarization vision in the locust Schistocerca gregaria. A Pattern of polarized light of the blue sky. Electrical vectors (e-vectors, double arrows) are arranged in concentric circles around the sun (yellow). Direct sunlight is unpolarized. The degree of polarization in the sky increases gradually to a maximum along a circle at an angle of 90° from the sun (large arrowheads). Adapted from Rossel and Wehner (1987). B Polarization-sensitive dorsal rim area (DRA) in the left compound eye of a locust, arrangement of ommatidia, and organization of a DRA ommatidium. In each DRA ommatidium, the microvilli of photoreceptor cell 7 are oriented perpendicularly to the microvilli of photoreceptors 1, 2, 5, 6, and 8. Microvilli of photoreceptors 3 and 4 are irregular. a, anterior. C, D Yaw-torque responses of a tethered flying locust under a slowly rotating dorsal polarizer. Each histogram shows average yaw-torque from six 180° rotations of the polarizer divided into 5° bins. The animal in C shows strong polarotaxis. After painting the DRAs of the eyes black (D), polarotaxis is abolished. B taken from Homberg and Paech (2002), C, D from Mappes and Homberg (2004)
Over recent years, particular advances have been made in identifying a specific region in the compound eye of many insect species that is highly adapted for polarization vision and apparently serves a biological role in the detection of the sky polarization pattern (Labhart and Meyer 1999). We have started to analyze the polarization vision pathway in a suitable insect, the desert locust Schistocerca gregaria, in order to identify brain areas which serve a core function in spatial orientation and compass navigation. This paper reviews the biological significance of polarization vision in the locust, its sensory basis, and discusses polarization vision pathways from the compound eye to the central complex in the median protocerebrum. The specific properties of polarization-sensitive neurons in the central complex, together with other aspects in the neuronal organization of this brain area, suggest that it acts as a spatial organizer in the insect brain, including a role as an internal sky compass.
Spatial navigation in locusts
In contrast to ants and honeybees, locusts have not often been the subject of research on insect spatial orientation, although some information on orientation mechanisms exists, particularly from observations of migrating locust swarms or marching hopper bands. Locusts are polymorphic grasshoppers which aggregate in bands (larvae) or swarms (adults) in response to high density of individuals. In the desert locust, as in some other species, two extreme phases, a gregarious and a solitary phase, can be distinguished which differ in behavior, coloration, and morphological characters (Uvarov 1966). Both phases perform long-range migratory flights, but while solitary individuals generally fly at night, gregarious locusts migrate in large swarms during the day (Baker 1978; Farrow 1990). Radar observations have shown that migratory directions of night-flying grasshoppers and locusts are remarkably oriented in a common compass direction that is not affected by the presence of the moon, but implicates other mechanisms of active, celestial or magnetic, compass orientation (Schaefer 1976; Riley and Reynolds 1986). Long-range migrations of gregarious locusts during the day occur in late larvae (marching hopper bands) and in adults, and have been studied in most detail for the desert locust Schistocerca gregaria. The desert locust is most common around the desert belt of Africa but also occurs on the Arabian Peninsula, and as far east as northern India. Annual migrations are adapted to the seasonal shifts in zones of rainfall and occur in more or less regular circuits or to-and fro-migrations performed by different generations (Baker 1978). Swarms usually maintain constant compass directions over several hours despite changing wind conditions (Kennedy 1945, 1951; Baker et al. 1984). Kennedy (1945, 1951), moreover, demonstrated in field studies that migratory directions in marching hopper bands and in flying adults can be changed predictably by shifting the sun position through a mirror, indicating that a sky compass is essential for maintaining migratory directions.
Laboratory experiments on walking larvae and tethered flying adults suggest that the sky polarization pattern, in addition to direct sun light, is a prominent compass cue for navigation (Fig. 1C, D). Locust larvae walking on a Kramer sphere oriented themselves menotactically to polarized light (Eggers and Weber 1993), and in tethered flying animals, a slowly rotating dorsally presented polarizer induced periodic and robust yaw-torque responses corresponding to the 180° periodicity of the stimulus (Mappes and Homberg 2004). These experiments strongly suggest that locusts, like many other insect species such as flies, butterflies, honeybees, ants, and crickets (reviewed by Horváth and Varjú 2003), can extract compass information for spatial navigation not only from the solar azimuth – the horizontal component of the sun’s direction – but also from the sky polarization pattern.
Sensory basis of polarization vision
The photoreceptor cells of insects are inherently sensitive to polarized light owing to the parallel orientation of the dichroic photoreceptor molecule, rhodopsin, in microvillar membranes. Photon absorption is maximal for light with an e-vector parallel to the microvillus axis (Israelachvili and Wilson 1976; Goldsmith and Wehner 1977). However, because polarization sensitivity interferes with the perception of color and brightness, it is reduced or actively suppressed in most parts of the eye and visual system by misalignment of microvillar orientation along the rhabdomere (Nilsson et al. 1987; Wehner and Bernhard 1993; Wehner 2001) and, in addition, by convergence of outputs from photoreceptor cells with different microvillar orientations (Strausfeld and Nässel 1981; Meinertzhagen and Sorra 2001). In many insect species, including locusts, ommatidia in a small dorsal margin of the compound eye, termed the dorsal rim area (DRA), are particularly well adapted for high polarization sensitivity (Labhart and Meyer 1999; Homberg and Paech 2002). The DRA faces the sky, with optic axes directed upwards and slightly contralaterally, suggesting its role in the analysis of sky polarization. In DRA photoreceptor cells, microvilli are precisely aligned in parallel within the rhabdomeres. Self-screening is reduced by the short length of the rhabdoms. Furthermore, two blocks of photoreceptor cells with orthogonal microvillar orientations and, therefore, with sensitivity to perpendicular e-vectors, occur in each ommatidium. The visual fields of DRA photoreceptors are often increased by degraded optics, reduction or lack of screening pigment between adjacent ommatidia, and enlarged cross-sectional area of the rhabdoms. Finally, the two sets of photoreceptor cells within each ommatidium are homochromatic, consistent with their exclusive specialization for polarized light detection (reviewed by Labhart and Meyer 1999).
The compound eye of the desert locust has a particularly prominent DRA which shows all specializations described for other insects (Eggers and Gewecke 1993; Homberg and Paech 2002). Owing to its dark pigmentation in S. gregaria, the DRA can even be identified with the unaided eye (Fig. 1B). As in many other insects, the locust DRA faces an area in the contralateral hemisphere of the sky with optical axes of ommatidia pointing 15–30° contralaterally. The locust DRA consists of about 400 ommatidia, and microvillar orientations are arranged in a fan-like pattern similar to that of the field cricket, the honeybee, and the desert ant (Fig. 1B). Polarotaxis in tethered flying locusts is lost after painting the DRAs black (Fig. 1C, D), which indicates that polarization sensitivity is mediated exclusively by the DRA (Mappes and Homberg 2004). Although the degree of sky polarization, especially under partly cloudy conditions and under canopy, is highest in the UV (Horváth and Varjú 2003), DRA photoreceptors in the locust – as in the cricket – are most sensitive in the blue (Eggers and Gewecke 1993). The reason for this is not completely clear but it may be an adaptation to the use of the system at very low light intensities during nighttime flights (Zufall et al. 1989). Interestingly, each DRA ommatidium in the locust, as in many other insects, contains, in addition to the two blocks of photoreceptor cells with perpendicular microvilli, two photoreceptor cells, numbered R3 and R4, which have small rhabdomeres with irregularly oriented microvilli and, thus, most likely low polarization sensitivity. Therefore, each DRA ommatidium contains two orthogonal polarization analyzers and a possible intensity detector, whose contribution to spatial orientation is at present not clear.
Polarization vision pathways in the brain
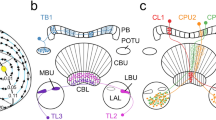
Tracer injections and single-cell dye fills in the cricket and locust revealed parts of the polarization vision pathways in the insect brain. Photoreceptors of the DRA project to distinct dorsal areas in the lamina and medulla which, as shown for the cricket, maintain a retinotopic organization (Blum and Labhart 2000; Homberg and Paech 2002). In the cricket, one type of heterolateral interneuron, termed POL1, has been studied extensively. These neurons have dendritic arborizations in the dorsal medulla and send axonal processes to the contralateral medulla (Labhart and Petzold 1993; Petzold 2001). Similar polarization-sensitive interneurons were subsequently found in the locust (Homberg and Würden 1997) and in the cockroach Leucophaea maderae (Loesel and Homberg 2001). POL1 neurons have no processes in the median protocerebrum, and it is therefore unclear how they are involved in navigational control of the animal. Fortunately, an anatomical study on a small brain area in the desert locust, the anterior optic tubercle, revealed what might be a pathway to an internal navigation compass (Fig. 2; Homberg et al. 2003a). The anterior optic tubercle is a small neuropil in the anterior lateral brain of the locust and consists of two substructures, an upper and a lower unit. Both units receive massive input from visual neurons of the medulla and lobula of the optic lobe. One of these cell types has a peculiar dendritic tree. It consists of a single process with sparse ramifications that extend dorso-ventrally through the medulla (Fig. 2B). In butterflies, neurons of this type have been most appropriately termed “line tangentials” of the medulla (Strausfeld and Blest 1970). Interestingly, line tangentials with axonal fibers to the lower unit of the anterior optic tubercle have projections extending to the dorsal rim of the medulla, and thus provide a connection between the polarization-sensitive dorsal rim areas of the visual system and the median protocerebrum (Fig. 2A, B). The anterior optic tubercles of both brain hemispheres are interconnected by heterolateral interneurons with processes extending into the lobula (Fig. 2A, C). All other outputs from the lower unit of the tubercle specifically invade two small structures in the lateral accessory lobe of the brain, termed the median olive and the lateral triangle (Fig. 2A; Homberg et al. 2003a). These subfields of the lateral accessory lobe are, finally, connected by third-order interneurons of the polarization vision pathway to the lower division of the central body (Fig. 2A, D; Homberg, 1994a, 1994b; Müller et al. 1997). Interestingly, a parallel visual pathway originating from medulla line tangentials provides visual input via the upper unit of the anterior optic tubercle and the lateral accessory lobe to the upper division of the central body, but the physiological properties of this pathway are poorly known. We thus discovered two parallel visual pathways from the medulla to the upper and lower divisions of the locust central body. One of these pathways, leading to the lower division of the central body, originates in the dorsal rim of the medulla (Fig. 2), and is therefore most likely to carry polarized light signals. What are the functional properties of neurons in the different processing stages of this polarization vision system?
Polarization vision pathways (A) and constituent neuronal cell types (B–D) in the brain of the locust Schistocerca gregaria. A Polarization-sensitive photoreceptors of the dorsal rim area of the compound eye (asterisk) project to dorsal rim areas in the lamina and medulla (DRLa, DRMe) in the optic lobe. First-order interneurons with dendritic ramifications in the dorsal rim of the medulla and tangential arborizations in the medulla send axonal processes to the ventralmost layer 1 of the anterior lobula (ALo1) and to the lower unit of the anterior optic tubercle (LU). Second-order interneurons connect the anterior optic tubercle to the median olive (MO) and lateral triangle (LT) of the lateral accessory lobe (LAL), and third-order interneurons with ramifications in the lateral triangle or median olive provide input to the central body (CB). Heterolateral interneurons connect the anterior lobes of the lobula and the lower units of the anterior optic tubercles of both brain hemispheres. ALo2 layer 2 of the anterior lobe of the lobula, Ca calyces of the mushroom body, La lamina, Me medulla, OLo outer lobe of the lobula, P pedunculus of the mushroom body, PB protocerebral bridge, UU upper unit of the anterior optic tubercle. B Mass staining of medulla line tangentials with processes in the dorsal rim of the medulla (DRMe) and projections to layer 1 of the anterior lobe of the lobula (ALo1) and to the lower unit of the anterior optic tubercle (LU). C Heterolateral interneuron with processes in ALo1 and LU of both brain hemispheres. D Tangential neuron of the lower division of the central body (CBL) and dendritic processes in the lateral triangle of the lateral accessory lobe (LT). Scale bars: 200 µm (in A–C), 100 µm (D). Modified from Vitzthum et al. (2002) and Homberg et al. (2003a)
Physiological properties of POL neurons
Physiological studies of polarization-sensitive (POL) neurons have focused on POL1 neurons in the cricket medulla (Labhart 1988; Labhart et al. 2001; Petzold 2001), while in the locust, neurons have been studied at the levels of the anterior optic tubercle and the central complex (Vitzthum et al. 2002; Pfeiffer and Homberg 2003). Some additional data on medulla neurons exist for the cockroach, the locust, and the desert ant (Homberg and Würden 1997; Labhart 2000; Loesel and Homberg 2001). All the POL neurons studied so far respond tonically to illumination with polarized light, and spiking activity depends in a sinusoidal fashion on e-vector orientation. Neurons encountered in the optic lobe of the locust and cockroach receive maximum excitatory input at a particular e-vector (Φmax) and apparently no input at an e-vector perpendicular to Φmax (Homberg and Würden 1997; Loesel and Homberg 2001). POL1 neurons of the cricket and central-complex neurons in the locust, on the other hand, show polarization opponency, i.e., spiking activity changes in a sinusoidal manner with e-vector-dependent inhibitory and excitatory parts (Fig. 3A, B). Polarization-opponent responses correspond to the perpendicular orientation of the two e-vector analyzers in each DRA ommatidium and suggest that early in sensory processing, signals from photoreceptors with orthogonal microvilli provide antagonistic excitatory and inhibitory input to follower interneurons. Together with the homochromacy of the DRA photoreceptors, opponent inputs enhance e-vector contrast and simultaneously render the neurons insensitive to unpolarized light and chromatic contrast, as demonstrated particularly well for the cricket POL1 neurons (Labhart 1988, 1996; Labhart and Petzold 1993). POL1 neurons of the cricket medulla have been characterized most extensively. The neurons have wide receptive fields of 60° or more. They receive polarization input from the ipsilateral eye and, corresponding to the contralateral optic axis of the dorsal rim area, have receptive fields in the contralateral visual field of the animal. Three physiological types of POL1 neurons can be distinguished by their different tuning to e-vector orientations of 10°, 60°, and 130° relative to the longitudinal axis of the animal (Labhart and Meyer 2002). Morphologically, POL1 neurons therefore exist at least as three bilateral pairs of neurons with identical morphology but different e-vector tuning. The functional role of the POL1 neurons in the spatial navigation of the cricket is not known. It has been suggested that they serve as the input stage to a central navigation compass (Labhart and Petzold 1993; Wehner 2003), but since the neurons interconnect the right and left medulla and lack significant ramifications in the median protocerebrum, a role in bilateral signal exchange at the medulla level seems more likely.
Polarization-sensitivity in the central complex of the locust brain. A, B, D Physiology and morphology of a polarization-opponent interneuron of the central complex. A Intracellular recording. Rotation of the polarizer through 360° results in two alternating periods of excitation and inhibition at characteristic e-vectors, independent of turning direction. Calibration 30 mV, 2 s. B E-vector response plot (means ± SD; n=2). Straight line indicates background activity. Fitting a sin2-function to the data reveals a Φmax of 117.7°. D The neuron has minute dendritic arborizations in the median olive (arrows) of the lateral accessory lobe (LAL) and innervates a single layer of the lower division of the central body (CBL). CBU upper division of the central body. Scale bar: 100 µm. C Φmax-orientations from 55 neurons of the central complex are broadly distributed from 0° to 180°. E, F Visual field and Φmax-orientations of a POL neuron from the central complex. E Relative response amplitude (relative amplitude of sin2-fit) to a 2°-polarization stimulus at different positions around the animal (center bar: zenithal stimulation, r right, l left, a anterior, p posterior; stimulus elevation indicated by concentric circles). F Φmax-orientations at different stimulus positions. Orientations are given with respect to the tangents to the parallels of latitude of stimulus elevation. Φmax values are similar throughout the visual field except for the most eccentric positions (at 20° right and left). A, B, D from Vitzthum et al. (2002); E, F from Sascha Gotthardt (Diploma Thesis, University of Marburg, 2003)
Intracellular recordings in the locust have focused on interneurons of the anterior optic tubercle and neurons of the central complex (Vitzthum et al. 2002; Pfeiffer and Homberg 2003). Recordings, particularly from heterolateral interneurons connecting the anterior optic tubercles of both brain hemispheres, consistently showed polarization sensitivity in neurons of the lower unit of the tubercle, but – except for a single centrifugal cell type (Vitzthum et al. 2002) – a conspicuous lack of polarization sensitivity in neurons of the upper unit of the tubercle. In the central complex, various types of columnar and tangential neuron were polarization sensitive, comprising a network of at least 300 neurons but, except for one type of columnar neuron of the upper division, all neurons were associated with the lower division of the central body and had ramifications either in the median olive or in the lateral triangle of the lateral accessory lobe (Figs. 2D, 3D; Vitzthum et al. 2002). The physiological data, therefore, fully support the anatomical studies on the locust polarization vision pathway and demonstrate the functional differences between the parallel visual pathways through the upper and lower units of the tubercle.
POL neurons of the locust midbrain differ in several physiological properties from the POL1 neurons in the cricket optic lobe. In contrast to POL1 neurons in the cricket, many POL neurons of the locust optic tubercle and central complex showed responses to unpolarized light, in addition to polarization opponency (Vitzthum et al. 2002). Thus, POL neurons in the locust midbrain appeared to be less specific than those in the cricket optic lobe. A hint of the possible biological significance of the responses to unpolarized light came from our anatomical studies, particularly from the peculiar shape of the dendritic trees of the medulla line tangentials, the input elements to the anterior optic tubercle. Judged from the dendritic trees of these neurons, they receive polarization input via the dorsal rim area of the eye from a patch of sky in the contralateral visual hemisphere. In addition, however, they should also receive input from ipsilateral unpolarized light, in particular from stimuli at all elevations of a particular azimuthal angle, reflecting the position of their line-tangential dendrite along the fronto-caudal axis of the medulla. It is tempting to speculate that already at the level of these medulla line tangentials, two visual inputs, one signaling the azimuthal position of the sun irrespective of its elevation and the second, signaling corresponding input from the sky polarization pattern, are combined to further enhance the directional tuning of these neurons. Unfortunately, the small fiber diameters of these neurons have so far prevented any attempts at intracellular recordings, but current experiments on postsynaptic elements in the optic tubercle (Fig. 2C) are underway to test this hypothesis (K. Pfeiffer and U. Homberg, unpublished).
A second difference from POL1 neurons of crickets concerns the visual fields of POL neurons in the locust midbrain. Certain neurons at the level of the central complex receive binocular input in contrast to purely ipsilateral input of cricket POL1 neurons of the medulla. Visual fields of central-complex neurons can be extremely large and can cover a visual angle of more than 90° (Fig. 3E). As in POL1 neurons, the e-vector tuning of these neurons is largely independent of the position of the stimulus within the visual field (Fig. 3F). In contrast to the eccentric position of the visual fields of POL1 neurons, however, the visual fields of certain central-complex neurons are roughly bilaterally symmetric, with the strongest responses near the zenith (Fig. 3E). The neurons, therefore, could signal head direction of the locust relative to solar azimuth but independent of solar elevation. Finally, in contrast to the three e-vector tuning types of POL1 neurons, no distinct physiological classes of preferred e-vector orientations were found in the central complex, but instead a continuum of Φmax-orientations ranging from 0° to 180° (Fig. 3C; Vitzthum et al. 2002; Labhart and Meyer 2002). Therefore, during rotation of the animal under the open sky, different populations of neurons become active as their respective Φmax-orientations match the e-vector orientations in the sky. With zenith-centered visual fields, these responses are robust against different solar elevations. Under the open sky, these neurons, therefore, may act as “head-direction cells”; they signal the directional heading of the locust and might serve a similar biological function for spatial orientation as head direction cells in the mammalian brain (Taube 1998; Sharp et al. 2001). The representation of compass orientation by the activity profiles of a set of “compass neurons” in the insect brain has, in fact, been proposed before (Hartmann and Wehner 1995; Wehner 2001; Labhart and Meyer 2002).
The central complex: an internal orientation compass?
The central complex has a midline-spanning position in the insect brain and is of extraordinary regularity in neuroarchitecture. It consists of four interconnected neuropils, the protocerebral bridge, the upper division of the central body (also termed ‘fan-shaped body’), the lower division of the central body (also termed ‘ellipsoid body’), and a pair of ventral noduli (Fig. 4; reviewed by Homberg 1987; Hanesch et al. 1989). The upper and lower divisions of the central body are substructured into several layers. The protocerebral bridge and each of these layers can be regarded as linear arrays of 16 columns, eight columns in the right and eight columns in the left brain hemisphere (Fig. 4). Tangential neurons provide input from several areas in the right or left brain hemisphere to all columns of a particular array, while numerous sets of columnar neurons, each consisting of 16 neural elements, interconnect the columns of different layers in a precise order, and finally send converging axonal projections to the lateral triangles or other foci in the lateral accessory lobes (Fig. 4; Williams 1975; Hanesch et al. 1989). The lateral accessory lobes are invaded by the dendritic trees of descending neurons which provide the most direct pathway from the central complex to thoracic motor centers.
Organization of columnar neurons of the locust central complex. A Ensemble tracing of 16 locustatachykinin-immunostained CL1 columnar neurons with ramifications in the protocerebral bridge (PB), the lower division of the central body (CBL), and the lateral triangles (LT) of the lateral accessory lobes. Scale bar: 100 µm. B Schematic diagram showing the pattern of connections between columns of the protocerebral bridge and columns of the central body. Axonal fibers project to the LT in the contralateral brain hemisphere. CBU upper division of the central body. A modified from Vitzthum and Homberg (1998)
Polarization-sensitive neurons comprise perhaps 20% or less of all neurons of the locust central complex and are associated particularly with the lower division of the central body. The upper division of the central body and the protocerebral bridge also receive prominent visual input, as judged from anatomical data, but the nature of these signals is still unclear. Is the central complex, therefore, a higher-order visual brain area? The lower division of the central body, indeed, appears to be dominated by visual functions. In contrast to the upper division and the protocerebral bridge, the lower division is not present in early larval brains of various Lepidoptera which lack compound eyes (Panov 1959; Homberg and Hildebrand 1994). In blind cave beetles, the lower division of the central body is poorly developed, while the upper division and the protocerebral bridge appear to be normal (Ghaffar et al. 1984). In addition to visual functions, several studies on the central complex have emphasized a role in motor control (reviewed by Homberg 1987; Strauss and Heisenberg 1993; Strausfeld 1999; Strauss 2002). The lateral accessory lobes, the major targets of central-complex outputs, communicate through ascending and descending neurons with the ventral nerve cord (Homberg 1994a), and research, particularly in the silk moth Bombyx mori, has demonstrated a role of descending neurons from the lateral accessory lobes in directional walking of male moths toward calling females (Kanzaki 1998; Mishima and Kanzaki 1998, 1999). Structural mutants of the fruit fly Drosophila melanogaster with disruptions of certain parts of the central complex provided further insights into the role of the central complex in walking and flight (reviewed by Strauss 2002). Mutant flies have defects in goal-oriented straight walking, object-induced turning responses (in flight), and show a generally reduced walking motivation. Several mutant lines are unable to continue steering courses toward a target landmark after the landmark becomes invisible (Strauss 2002). While these mutants have not been studied with respect to possible defects in compass orientation, the data clearly point toward a role of the central complex in spatial orientation, particularly in processes of decisions and maintenance about particular directions in locomotion. The presence of a well-developed protocerebral bridge and upper division of the central body in cave beetles without compound eyes and optic lobes suggests that the central complex is, indeed, not a visual brain center per se, but instead an internal compass and navigation center which, in many insect species, relies heavily on visual inputs.
An important aspect of spatial orientation is idiothetic feedback from various proprioreceptors to navigational control centers to allow for internal monitoring of walking paths and, together with external compass cues, to provide the means for path integration, which is the ultimate mechanism for many insects to navigate back to their nests after food searching excursions (Wehner et al. 1996; Collett and Collett 2000). Brain areas involved in path integration have not been identified in insects, but the central complex is certainly a promising candidate. Single-cell recordings in the locust demonstrated self-movement generated mechanosensory input from the wing base to the central complex (Homberg 1994a), but how this reafferent feedback is integrated with sky compass information is still unclear.
A final problem: time compensation
Animals using a sky compass for spatial navigation face a serious problem. As the solar azimuth changes over the course of the day, a constant migratory direction can only be maintained with permanent adjustment of the angle between the navigational vector and the solar azimuth. Behavioral experiments in bees, ants and, more recently, in monarch butterflies, clearly showed that these insects are, indeed, capable of time-compensated sky compass orientation, i.e., they adjust their compass bearings in relation to solar azimuth with time of day (reviewed by Lindauer 1960; Wehner 1992; Mouritsen and Frost 2002). How is this achieved? One has to assume that the internal navigation compass is under the permanent control of an internal circadian clock, since these adjustments are also made without external zeitgeber signals, e.g., within the dark hive of a honeybee colony. Under certain circumstances returning foraging honeybees perform their waggle dances for extended time periods within the hive. These dances communicate the distance and direction of a favorable food source relative to solar azimuth to nestmates. Lindauer (1954) observed that the direction of these dances gradually changes over several hours, thus compensating for the gradually changing solar azimuth. The location of the internal circadian clock has not been determined in species such as honeybees, ants, and locusts, but in the cockroach Leucophaea maderae and in the fruit fly Drosophila melanogaster, a small area in the optic lobe, the accessory medulla and its associated neurons, has been identified as the site of the circadian pacemaker controlling circadian rhythms in behavior (reviewed by Helfrich-Förster et al. 1998; Homberg et al. 2003b). An accessory medulla is also present in the locust brain and this closely resembles the accessory medulla of the cockroach in anatomical organization, concentration of neuropeptides, and electrophysiological properties of neurons (Homberg et al. 1991; Würden and Homberg 1995; Homberg and Würden 1997). Immunocytochemistry and single-cell dye fills revealed a prominent connection from the locust accessory medulla via the posterior optic tubercle to the protocerebral bridge (Homberg et al. 1991; Vitzthum et al. 1996; Homberg and Würden 1997). These “visual” inputs to the central complex at the level of the protocerebral bridge may actually be timing information from an internal circadian clock, which would constantly adjust directional output from the central complex with respect to time of day (Fig. 5). If so, another input essential for an internal sky compass would be present in the central complex, but exactly how time compensation is achieved within the central complex network, is still open to speculation.
Fiber pathways to and from the central complex in the locust brain. Red visual pathways via the anterior optic tubercle (AOTu) carrying solar azimuth/sky polarization signals; blue connections with ascending and descending pathways possibly carrying reafferent signals (ascending) and motor commands (descending); green pathways from the accessory medulla (AMe) via the posterior optic tubercle (POTu) to the protocerebral bridge (PB), possibly providing circadian signals essential for time compensation. CB central body, LAL lateral accessory lobe, Lo lobula, Me medulla. Scale bar: 200 µm
Outlook and perspectives
The neural substrate for vector orientation has not been identified in any animal species, although there is considerable evidence for a strong involvement of the hippocampal formation in vertebrates (e.g., Taube 1998; Bingman and Able 2002). Analysis of the polarization vision system in the brain of the locust has allowed us to gain preliminary insights into the neuronal mechanisms and brain areas involved in sky compass orientation in insects. However, many questions at the neuronal level still need to be resolved before we have even a basic understanding of the neuronal outline of the navigation network in the insect brain. It is still highly hypothetical, how and where information about the sky polarization pattern, solar azimuth, and daytime are integrated into a compass signal. In a step further, a combination with proprioceptive input for path integration and spatial memory, and an interaction with landmark (olfactory and/or visual) information is required. Can all of these functions be attributed to the central complex/lateral accessory lobes, or are certain aspects of spatial orientation (e.g., spatial memory) shared functions between the mushroom body and the central complex? What kinds of descending pathways are involved in spatial navigation? Some of these questions can be analyzed best in a large well-studied insect such as the locust, while the honeybee or the fruit fly are more advantageous for aspects concerning spatial memory. As we learn more about the neuroarchitectures involved in spatial navigation, network models may be helpful to understand how computational interactions, e.g., in the central complex network, may lead to motor commands for vector orientation and path integration. Some of the existing models (Wittmann and Schwegler 1995; Hartmann and Wehner 1995; Lambrinos 2003) already contain elements and neuronal arrays reminiscent of central-complex neuroarchitecture.
References
Baker RR (1978) The evolutionary ecology of animal migration. Hodder and Stoughton, London
Baker PS, Gewecke M, Cooter RJ (1984) Flight orientation of swarming Locusta migratoria. Physiol Entomol 9:247–252
Bingman VP, Able KP (2002) Maps in birds: representational mechanisms and neural bases. Curr Opin Neurobiol 12:745–750
Blum M, Labhart T (2000) Photoreceptor visual fields, ommatidial array, and receptor axon projections in the polarisation-sensitive dorsal rim area of the cricket compound eye. J Comp Physiol A 186:119–128
Collett M, Collett TS (2000) How do insects use path integration for their navigation? Biol Cybern 83:245–259
Collett TS, Collett M (2002) Memory use in insect visual navigation. Nat Rev Neurosci 3:542–552
Eggers A, Gewecke M (1993) The dorsal rim area of the compound eye and polarization vision in the desert locust (Schistocerca gregaria). In: Wiese K, Gribakin FG, Popov AV, Renninger G (eds) Sensory systems of arthropods. Birkhäuser, Basel, pp 101–109
Eggers A, Weber T (1993) Behavioural evidence for polarization vision in locusts. In: Elsner N, Heisenberg M (eds) Gene–brain–behaviour. Thieme, Stuttgart, p 336
Esch HE, Burns JE (1996) Distance estimation by foraging honeybees. J Exp Biol 199:155–162
Farrow RA (1990) Flight and migration in acridoids. In Chapman RF, Joern A (eds) Biology of grasshoppers. Wiley, New York, pp 227–314
Frisch K von (1967) The dance language and orientation of bees. Harvard University Press, Cambridge, Mass.
Ghaffar H, Larsen JR, Booth GM, Perkes R (1984) General morphology of the brain of the blind cave beetle, Neaphaenops tellkampfii Erichson (Coleoptera: Carabidae). Int J Insect Morphol Embryol 13:357–371
Giurfa M, Capaldi E (1999) Vectors, routes and maps: new discoveries about navigation in insects. Trends Neurosci 22:237–242
Goldsmith TH, Wehner R (1977) Restrictions on rotational and translational diffusion of pigment in the membranes of a rhabdomeric photoreceptor. J Gen Physiol 70:453–490
Hanesch U, Fischbach KF, Heisenberg M (1989) Neuronal architecture of the central complex in Drosophila melanogaster. Cell Tissue Res 257:343–366
Hartmann G, Wehner R (1995) The ant’s path integration system: a neural architecture. Biol Cybern 73:483–497
Helfrich-Förster C, Stengl M, Homberg U (1998) Organization of the circadian system in insects. Chronobiol Int 15:567–594
Homberg U (1987) Structure and functions of the central complex in insects. In Gupta AP (ed) Arthropod brain: its evolution, development, structure, and functions. Wiley, New York, pp 347–367
Homberg U (1994a) Flight-correlated activity changes in neurons of the lateral accessory lobes in the brain of the locust Schistocerca gregaria. J Comp Physiol A 175:597–610
Homberg U (1994b) Distribution of neurotransmitters in the insect brain. (Progress in Zoology 40) Fischer, Stuttgart
Homberg U, Hildebrand JG (1994) Postembryonic development of γ-aminobutyric acid-like immunoreactivity in the brain of the sphinx moth Manduca sexta. J Comp Neurol 339:132–149
Homberg U, Paech A (2002) Ultrastructure and orientation of ommatidia in the dorsal rim area of the locust compound eye. Arthropod Struct Dev 30:271–280
Homberg U, Würden S (1997) Movement-sensitive, polarization-sensitive, and light-sensitive neurons of the medulla and accessory medulla of the locust, Schistocerca gregaria. J Comp Neurol 386:329–346
Homberg U, Würden S, Dircksen H, Rao KR (1991) Comparative anatomy of pigment-dispersing hormone-immunoreactive neurons in the brain of orthopteroid insects. Cell Tissue Res 266:343–357
Homberg U, Hofer S, Pfeiffer K, Gebhardt S (2003a) Organization and neural connections of the anterior optic tubercle in the brain of the locust, Schistocerca gregaria. J Comp Neurol 462:415–430
Homberg U, Reischig T, Stengl M (2003b) Neural organization of the circadian system of the cockroach Leucophaea maderae. Chronobiol Int 20:577–591
Horváth G, Varjú D (2003) Polarization patterns in nature and polarized light in animal vision. Springer, Berlin Heidelberg New York
Israelachvili JN, Wilson M (1976) Absorption characteristics of oriented photopigments in microvilli. Biol Cybern 21:9–15
Kanzaki R (1998) Coordination of wing motion and walking suggests common control of zigzag motor program in a male silkworm moth. J Comp Physiol A 182:267–276
Kennedy JS (1945) Observations on the mass migration of desert locust hoppers. Trans R Entomol Soc Lond 95:247–262
Kennedy JS (1951) The migration of the desert locust (Schistocerca gregaria FORSK.). I. The behaviour of swarms. II. A theory of long-range migrations. Philos Trans R Soc Ser B 235:163–290
Labhart T (1988) Polarization-opponent interneurones in the insect visual system. Nature 331:435–437
Labhart T (1996) How polarization-sensitive interneurones of crickets perform at low degrees of polarization. J Exp Biol 199:1467–1475
Labhart T (2000) Polarization-sensitive interneurons in the optic lobe of the desert ant Cataglyphis bicolor. Naturwissenschaften 87:133–136
Labhart T, Meyer EP (1999) Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc Res Tech 47:368–379
Labhart T, Meyer EP (2002) Neural mechanisms in insect navigation: polarization compass and odometer. Curr Opin Neurobiol 12:707–714
Labhart T, Petzold J (1993) Processing of polarized light information in the visual system of crickets. In Wiese K, Gribakin F, Popov AV, Renninger G (eds) Sensory system of arthropods. Birkhäuser, Basel, pp 158–168
Labhart T, Petzold J, Helbling H (2001) Spatial integration in polarization-sensitive interneurones of crickets: a survey of evidence, mechanisms and benefits. J Exp Biol 204:2423–2430
Lambrinos D (2003) Navigation in desert ants: the robotic solution. Robotica 21:407–426
Lindauer M (1954) Dauertänze im Bienenstock und ihre Beziehung zur Sonnenbahn. Naturwissenschaften 41:506–507
Lindauer M (1960) Time-compensated sun orientation in bees. Cold Spring Harbor Symp Quant Biol 25:371–377
Loesel R, Homberg U (2001) Anatomy and physiology of neurons with processes in the accessory medulla of the cockroach Leucophaea maderae. J Comp Neurol 439:193–207
Mappes M, Homberg U (2004) Behavioral analysis of polarization vision in tethered flying locusts. J Comp Physiol A 190:61–68
Meinertzhagen IA, Sorra KE (2001) Synaptic organization in the fly’s optic lamina: few cells, many synapses and divergent microcircuits. In: Kolb H, Robbs H, Wu S (eds) Progress in brain research, vol 131. Elsevier, New York, pp 53–69
Mishima T, Kanzaki R (1998) Coordination of flipflopping neural signals and head turning during pheromone-mediated walking in a male silkworm moth Bombyx mori. J Comp Physiol A 183:273–282
Mishima T, Kanzaki R (1999) Physiological and morphological characterization of olfactory descending interneurons of the male silkworm moth, Bombyx mori. J Comp Physiol A 184:143–160
Mouritsen H (2001) Navigation in birds and other animals. Image Vis Comput 19:713–731
Mouritsen H, Frost, BJ (2002) Virtual migration in tethered flying monarch butterflies reveals their orientation mechanisms. Proc Natl Acad Sci USA 99:10162–10166
Müller M, Homberg U, Kühn A (1997) Neuroarchitecture of the lower division of the central body in the brain of the locust (Schistocerca gregaria). Cell Tissue Res 288:159–176
Nilsson D, Labhart T, Meyer EP (1987) Photoreceptor design and optical properties affecting polarization sensitivity in ants and crickets. J Comp Physiol A 161:645–658
Panov AA (1959) Structure of the insect brain at successive stages of postembryonic development. II. The central body. Entomol Rev URSS 38:276–284
Papi F (1992) Animal homing. Chapman and Hall, London
Petzold J (2001) Polarisationsempfindliche Neuronen im Sehsystem der Feldgrille, Gryllus campestris: Elektrophysiologie, Anatomie und Modellrechnungen. PhD thesis, University of Zurich
Pfeiffer K, Homberg U (2003) Neurons of the anterior optic tubercle of the locust Schistocerca gregaria are sensitive to the plane of polarized light. In Elsner N, Zimmermann N (eds) The neurosciences from basic research to therapy. Thieme, Stuttgart, pp 567–568
Riley JR, Reynolds DR (1986) Orientation at night by high-flying insects. In Danthanarayana W (ed) Insect flight: dispersal and migration. Springer, Berlin Heidelberg New York, pp 71–87
Riley JR, Greggers U, Smith AD, Stach S, Reynolds DR, Stollhoff N, Brandt R, Schaupp F, Menzel R (2003) The automatic pilot of honeybees. Proc R Soc Lond B 270:2421–2424
Rossel S, Wehner R (1987) The bee’s e-vector compass. In Menzel R, Mercer A (eds) Neurobiology and behaviour of honeybees. Springer, Berlin Heidelberg New York, pp 76–93
Schaefer GW (1976) Radar observations of insect flight. In Rainey RC (ed) Insect flight. (Symposium of the Royal Entomological Society vol 7) Blackwell, Oxford, pp 157–197
Sharp PE, Blair HT, Cho J (2001) The anatomical and computational basis of the rat head-direction cell signal. Trends Neurosci 24:289–294
Srinivasan VM, Zhang SW, Altwen M, Tautz J (2000) Honeybee navigation: nature and calibration of the odometer. Science 287:851–853
Strausfeld NJ (1999) A brain region in insects that supervises walking. In Binder MD (ed) Progress in brain research, vol 123. Elsevier, Amsterdam, pp. 273–284
Strausfeld NJ, Blest AD (1970) Golgi studies on insects. Part I. The optic lobes of Lepidoptera. Philos Trans R Soc Lond Ser B 258:81–134
Strausfeld NJ, Nässel DR (1981) Neuroarchitectures serving compound eyes of Crustacea and insects. In: Autrum H (ed) Handbook of sensory physiology, vol VII/6B. Springer, Berlin Heidelberg New York, pp 1–132
Strauss R (2002) The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol 12:633–638
Strauss R, Heisenberg M (1993) A higher control center of locomotor behavior in the Drosophila brain. J Neurosci 13:1852–1861
Taube JS (1998) Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol 55:225–256
Tinbergen N (1932) Über die Orientierung des Bienenwolfes (Philanthus triangulum Fabr.). Z Vgl Physiol 16:305–334
Uvarov BP (1966) Grasshoppers and locusts, vol 1. Cambridge University Press, New York
Vitzthum H, Homberg U (1998) Locustatachykinin I/II-immunoreactive neurons in the central complex of the locust brain. J Comp Neurol 390:455–469
Vitzthum H, Homberg U, Agricola H (1996) Distribution of Dip-allatostatin I-like immunoreactivity in the brain of the locust Schistocerca gregaria with detailed analysis of immunostaining in the central complex. J Comp Neurol 369:419–437
Vitzthum H, Müller M, Homberg U (2002) Neurons of the central complex of the locust Schistocerca gregaria are sensitive to polarized light. J Neurosci 22:1114–1125
Wang RF, Spelke ES (2002) Human spatial representation: insights from animals. Trends Cogn Sci 6:1114–1125
Waterman TH (1989) Animal navigation. Freeman, New York
Wehner R (1984) Astronavigation in insects. Annu Rev Entomol 29:277–298
Wehner R (1992) Arthropods. In: Papi F (ed) Animal homing. Chapman and Hall, London, pp 45–144
Wehner R (1994) The polarization-vision project: championing organismic biology. In: Schildberger K, Elsner N (eds) Neural basis of behavioural adaptations. Fischer, Stuttgart, pp 103–143
Wehner R (1997) The ant’s celestial compass system: spectral and polarization channels. In: Lehrer M (ed) Orientation and communication in arthropods. Birkhäuser, Basel, pp 145–185
Wehner R (2001) Polarization vision: a uniform sensory capacity? J Exp Biol 204:2589–2596
Wehner R (2003) Desert ant navigation: how miniature brains solve complex tasks. J Comp Physiol A 189:579–588
Wehner R, Bernhard GD (1993) Photoreceptor twist: a solution to the false color problem. Proc Natl Acad Sci USA 90:4132–4135
Wehner R, Michel B, Antonsen P (1996) Visual navigation in insects: coupling of egocentric and geocentric information. J Exp Biol 199:129–140
Williams JLD (1975) Anatomical studies of the insect central nervous system: a ground-plan of the midbrain and an introduction to the central complex in the locust, Schistocerca gregaria (Orthoptera). J Zool 76:67–86
Wittmann T, Schwegler H (1995) Path integration: a network model. Biol Cybern 73:569–575
Wohlgemuth S, Ronacher B, Wehner R (2002) Distance estimation in the third dimension in desert ants. J Comp Physiol A 188:273–281
Würden S, Homberg U (1995) Immunocytochemical mapping of serotonin and neuropeptides in the accessory medulla of the locust, Schistocerca gregaria. J Comp Neurol 362:305–319
Zufall F, Schmitt M, Menzel R (1989) Spectral and polarized light sensitivity of photoreceptors in the compound eye of the cricket (Gryllus bimaculatus). J Comp Physiol A 164:597–608
Acknowledgements
I am grateful to Dr. Monika Stengl for her helpful comments on the manuscript and to Sascha Gotthardt for providing Fig. 3E, F. The research described in this paper is supported by the Deutsche Forschungsgemeinschaft, currently HO 950/13-2 and HO 950/14-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Homberg, U. In search of the sky compass in the insect brain. Naturwissenschaften 91, 199–208 (2004). https://doi.org/10.1007/s00114-004-0525-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-004-0525-9