Abstract
Purpose
To evaluate the impact of the fiber–tissue distance on histological parameters in a porcine kidney model.
Methods
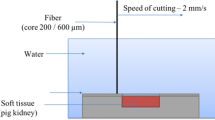
Four lasers were tested at 60 W using a 600-µm bare-ended fiber: a continuous wave (cw) thulium fiber laser (TFL), a super pulsed (SP) TFL, a Ho:YAG laser, and a blue diode laser (BDL). All tissue samples were mounted on a motorized XY-translation stage. The fiber–tissue distance was changed within a range from 0to 6 mm. Ten incisions were made with each laser at each distance. Afterwards, the tissue samples were sliced with a microtome for lactate dehydrogenase staining to determine zones of thermal damage.
Results
In contact mode, the largest incision depth was found for the cw TFL (1.7 ± 0.1 mm) compared to the SP TFL (1.0 ± 0.1 mm), BDL (0.9 ± 0.1 mm) and HoYAG laser (1.1 ± 0.1 mm), respectively. With regard to the coagulative properties, the SP TFL and the Ho:YAG laser showed comparable coagulation depths with 0.7 ± 0.1 and 0.6 ± 0.1 mm, respectively. At 2 mm fiber–tissue distance, the Ho:YAG laser was the only laser that vaporized tissue (incision depth: 0.2 ± 0.1 mm). The BDL was the only laser that caused coagulation at a distance of 3–5 mm.
Conclusion
Our results support the clinical observation that cw TFL must be defocused for best coagulation, while the coagulation depth of the SP TFL remains nearly constant within the range of 0–3 mm. Increasing the distance of the laser fiber to the tissue up to 5 mm did not cause significant differences with regard to coagulation depth using the BDL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laser technologies play a prominent role in modern endoscopic management of benign prostatic hyperplasia (BPH) [1]. Each type of laser is defined by its certain wavelength with a specific absorption in target chromophores [2]. Lasers emit either in a pulsed or in a continuous wave (cw) mode [2]. Therefore, fiber–tissue interaction is not only influenced by the physical properties such as laser power, pulse length and laser emission mode, but also by the soft tissue texture [2, 3]. While pulsed Ho:YAG, cw Tm:YAG and cw Thulium fiber lasers (TFL) are well established for BPH treatment [1], a super pulsed (SP) TFL [3, 4] and a blue diode laser (BDL) have been recently introduced [5, 6].
The SP TFL has recently been approved by the Food and Drug Administration (FDA) and is primarily used for stone lithotripsy with promising results; however, it has not been published outside international conferences yet [7,8,9]. The BDL is already used by dentists to coagulate and cut soft tissue. It demonstrated a fast clot formation with good hemostatic properties [10]. In another study the BDL proved to cause clinically acceptable incision depths without wide tissue denaturation [6]. Until now, no study is available that investigated the laser with regard to BPH treatment.
As mentioned, not only the laser itself might have an impact on histological tissue damage [4]. Various studies have examined the influence of laser energy on tissue effects at different drag speeds [4, 9, 11, 12]; however, the distance between the fiber tip and tissue may also play a crucial role in coagulation of bleeding vessels. This issue has yet not been evaluated sufficiently. Fried et al. previously showed that different lasers necessitate different distances in order to achieve the desired effect [13]. The cw TFL was able to vaporize tissue at 5 mm distance; however, pulsed laser devices needed contact to provoke tissue damage. This topic was also described by Cecchetti et al. who were able to show that the holmium plasma bubble will have the biggest effect on tissue at the distance of 1 mm [14]. The possibility to change the laser effects at different distances and a clear understanding at which distance lasers are safe may improve further clinical work. The aim of our study was, therefore, to investigate the effect of the fiber–tissue distance on fiber–tissue interaction using four different lasers in a non-perfused porcine kidney model: (1) a Ho:YAG, (2) a cw TFL, (3) and the newly introduced SP TFL and (4) BDL.
Methods
All experiments were carried out using four lasers: a pulsed 100 W 2.1 µm Ho:YAG laser (peak power ~ 2–10 kW, Lumenis, Inc, Palo Alto, CA, USA), a 120-W cw 1.9 µm TFL (peak power 120 W, NTO IRE-Polus, Fryazino, Russia), a 120-W SP 1.9 µm TFL (peak power 500 W, NTO IRE-Polus, Fryazino, Russia) and a 60-W cw 445 nm BDL (NTO IRE-Polus, Fryazino, Russia). The mean power output of all lasers was set at 60 W, respectively. A 600-µm bare-ended laser fiber was used for all incisions. The physical properties of the lasers and the laser settings are summarized in Table 1.
An adjusted ex-vivo model of an isolated non-frozen porcine kidney was used to investigate fiber–tissue interaction as previously described [4, 15]. The fresh non-frozen porcine kidney was cut with an electric slicer into 8 × 4 cm pieces, put into a metal box and fixed on a motorized translation XY stage. A fixed fiber holder was used which allowed to change the distance to the tissue surface in steps of 1 mm. The gap between the fiber and tissue was filled with normal saline which was changed within a range from 0 to 6 mm. The cutting speed was set at 2 mm/s. All experiments were performed by the same investigator examining ten incisions with each laser at each distance for better comparison. The tissue samples were sliced with a microtome for subsequent lactate dehydrogenase staining to determine zones of thermal damage [16]. A microtome (3550 TECHNICAL, Genelabotech, China) was used to produce 300 μm-thick sagittal cryosections. Ethical approval was not necessary, because the kidneys were obtained from animals that were killed for other purposes.
All samples were analyzed using a LEICA DM4000 B LED microscope equipped with a LEICA DFC7000 T digital camera and a LAS V4.8 software (Leica Microsystems, Wetzlar, Germany). The incision depths (mm) and superficial coagulation depths (mm) were measured. Carbonization grad (CG) was qualitatively estimated from 0 to 3 (zero no carbonization, three extensive carbonization). Needles with thermocouples (type K thermocouples (Chromel/Alumel)) were inserted into the tissue at different depths to estimate temperature related effects in the tissue. WinDaq™ (DATAQ Instruments, Akron, Ohio, USA) version 4.00 was used for thermal data acquisition and analysis. Achieved data on temperature changes and incision depths were expressed as mean ± SD. After measurement, the mean was calculated for better comparison of the lasers.
Results
The physical properties of the lasers, all applied laser settings and histological findings are presented in Table 1. Thermal analysis of the tissue with thermocouples during laser treatment revealed the following: coagulation was found at temperatures > 60 °C, characterized by protein denaturation and beginning of pyrolysis. Carbonization took place between 150 and 350 °C considered as protein denaturation and complete pyrolysis of the tissue.
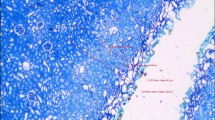
In contact mode (0 mm fiber-tissue distance), the deepest incision depth was found for the cw TFL (1.7 ± 0.1 mm) compared to the SP TFL (1.0 ± 0.1 mm), BDL (0.9 ± 0.1 mm) and HoYAG laser (1.1 ± 0.1 mm) (Table 1). It further showed the deepest coagulation depth (1.1 ± 0.1 mm) among all other devices (Fig. 1). The graph presents the estimated area of vaporization (width × depth). Extensive carbonization of CG 2–3 was found using the cw TFL and BDL in contact mode. In contrast, the SP TFL and the Ho:YAG laser showed minimal carbonization of CG 0–1 and CG 0, respectively.
At a fiber-tissue distance of 1 mm, the incision depth decreased in all four lasers. At 1 mm, the Ho:YAG and cw TFL produced the deepest incisions of 0.9 ± 0.1 mm and 1.0 ± 0.1 mm with a similar coagulation depth of 0.8 ± 0.1 mm, respectively. The BDL showed an incision depth of 0.7 ± 0.1 mm with a coagulation depth of 0.3 ± 0.1 mm, which was similar to the coagulation depth in contact mode. A minimal incision depth was found using the SP TFL (0.3 ± 0.1 mm) with a coagulation zone of 0.5 ± 0.1 mm (Fig. 2). At a fiber–tissue distance of 2 mm, the Ho:YAG was the only laser that showed vaporized tissue (incision depth 0.2 ± 0.1 mm). The coagulation depth decreased in all four lasers at a fiber–tissue distance of 2 mm. At a distance of 2 mm, the coagulation depth of the cw TFL (0.7 ± 0.1 mm) and the Ho:YAG (0.7 ± 0.1 mm) was still comparable. The coagulation depth at 2 mm distance was similar between the SP TFL (0.4 ± 0.1 mm) and the BDL (0.5 ± 0.1 mm). At 3–5 mm fiber–tissue distance, tissue was not vaporized with any of the four lasers. At these distances, the BDL was the only laser that caused coagulation of the porcine kidney (Table 1).
Histological evaluation of the effects of laser treatment: incision is caused by explosive vaporization of tissue water (a); thermo-mechanical damage is created by generation and collapse of gas bubbles at the fiber tip (b); carbonization occurs due to complete pyrolysis of tissue (c); protein denaturation (coagulation), water vaporization and beginning of pyrolysis (d); protein denaturation (coagulation), no water vaporization (e). Arrows indicate the widths of the respective zones
Discussion
Various studies have shown that the theoretical incision depth of the lasers does not match the clinically tested penetration depths in different study models [4, 11]. The key findings of our experiments are the well-comparable incision depths of all four lasers at short distances. The cw TFL showed the deepest incision depth of 1.7 mm and as well the deepest coagulation depth of 1.1 mm in contact mode. After defocusing the laser beam to a distance of 2 mm, the cw TFL showed significantly reduced ablative properties with a shallow coagulation zone comparable to that of the Ho:YAG laser. These findings support the clinical observation that the cw TFL must be defocused for superficial coagulation during BPH treatment.
To date, no study has yet dealt with different distances with regard to laser–tissue interaction. It is important to understand the influence of laser energy at different distances to perform further investigations in a more clinical setting. Several studies have proven the safety and efficiency in soft tissue ablation with a TFL [9]. Enikeev et al. demonstrated a comparable outcome after thulium fiber enucleation of the prostate (ThuFLEP) to TURP with less time of catheterization and hospitalization [17]. In another study, the same group presented data of the erectile function which is not affected after ThuFLEP with even an increase in the IIEF-5 score after 6 months’ follow-up [18].
A novel super pulsed TFL has recently been approved by the FDA and is primarily used for stone lithotripsy, but is also applicable for soft tissue vaporization. Clinical studies that compare the SP TFL with the Ho:YAG lithotripsy are not published yet; however, preclinical experiments seem promising [9]. Some studies have shown that the TFL ablates up to four times more stone material, in both fragmentation and dusting mode, than the holmium laser [7, 8]. It is to note that the TFL technology totally differs from the better known Tm:YAG laser. In short, the TFL uses laser diodes as the energy light source which are powered by electric current. The generated laser emission is then transferred within a 10–30 m-long thulium-ion containing active fiber and transferred to a very thin fiber core [4, 8, 9].
Comparing the cw with the SP TFL, the latter showed a slightly deeper incision. However, the difference of 0.7 mm seems insignificant in clinical practice. These results are not consistent with a previously published study by Becker et al. in which they demonstrated a mean incision depth of 2.9 and 3.7 mm (drag speed 2 mm/s, 60 W, 0.5 mm fiber-tissue distance) using a cw TFL and a SP TFL, respectively [4]. In their study, three incisions were used at each power setting; however, in the actual study, ten incisions were performed with each laser. Therefore, a higher reliability of the results could be accepted. Also, the data can be compared much better with the company’s values, although the theoretical penetration depth of 0.2 mm is not reached.
With regard to the hemostatic properties, the coagulation depth of the SP TFL remains nearly constant within the fiber–tissue distance of 0–3 mm. The lower coagulation ability of the SP TFL in comparison to the cw TFL was already demonstrated in a previous study [4]. Our experiments demonstrated comparable hemostatic properties of the novel SP TFL to the already sufficiently examined Ho:YAG laser that showed clinically less bleeding rates compared to TURP and OP in BPH treatment [19]. Emiliani and colleagues investigated the histological ablation effect of a Ho:YAG laser at different power output settings in an in vitro model [11]. The mean coagulation depth was 0.48 mm. These findings corroborate our results with a mean coagulation depth of 0.6 mm in contact mode using the Ho:YAG laser. The overall mean incision depth was measured at 2 mm. This incision depth is significantly higher compared to a study by Johnson et al. in 1992 [20] and higher to our findings with 0.4 and 1.1 mm, respectively. However, Johnson et al. used a study design in which they performed hand-held incisions in air. It is to be noted that the Ho:YAG laser has its absorption maximum in water; hence, Johnsons´ study design is not well comparable to present studies and clinically not transferable. The difference between the study by Emiliani et al. and ours is the frequency of incisions per setting. They used two incisions per setting, whereas we performed ten. At the same frequency, it would be possible for the results to come closer together.
The main difference between the BDL and the other lasers that were tested is that it targets hemoglobin instead of water due to its certain wavelength of 445 nm. Targeting hemoglobin as the main chromophore might be advantageous for surgery on highly vascularized organs, e.g., prostate tissue [2]. In our experiments, the BDL has shown the smallest coagulation depth of all tested lasers and it was the only laser that still coagulated tissue at a distance of > 2 mm. So far, the BDL has not been used clinically in urological practice [5, 21]. However, its properties (i.e., deep coagulation and fast tissue ablation) could become rather useful for prostate vaporization. As mentioned before, the BDL might challenge the current gold standard for prostate vaporization, that are KTP/LBO:YAG lasers. These lasers share a deep penetration depth, extensive carbonization and effective coagulation [22]. Nevertheless, further comparative in-vitro and in-vivo trials are necessary. A surprising finding was that by increasing the distance of the laser fiber to the tissue did not cause significant differences with regard to coagulation depth using the BDL. These findings support previously published data by Arkhipova et al. in which they showed narrow coagulation zones of the BDL comparable to a KTP laser in an animal model for partial laparoscopy [21]. They stated that the BDL has drawbacks in terms of coagulation, but in the hands of experienced surgeons it might still be a good tool to manage these operations [21]. Jiang et al. demonstrated a significant increase in the ablation rate of the BDL compared to the KTP laser with 5.14 and 1.20 mm3/s, respectively, with a pronounced carbonization layer and comparable coagulation depths [5]. The higher carbonization grade after the use of the cw TFL and the BDL in contact mode could lead to higher rates of urge incontinence in the immediate postoperative course as stated by Chen et al. [23]. The results of Jiang et al. are well comparable to ours with an incision depth of 1.0 mm without any ablation at distances > 2 mm. The work by Braun et al. also supports our findings. The authors provided evidence of a better cutting ability of the BDL comparing to a 970*nm diode laser (incision depth 0.61 mm vs. 0.36 mm) and acceptable denaturation depths [6]. In another study, Hess et al. suggested that the BDL is at least as good as the available KTP laser for tissue cutting [24].
Although this is the first study that investigated the histological impact of the laser fiber–tissue distance in a standardized experimental setup, some limitations have to be depicted. First, it is an in vitro study with fresh non-frozen pig kidneys. Therefore, this non-vascularized model can give only a partial evaluation of the coagulation effect of the lasers. Further studies should include in vivo protocols to filter out the lack of blood flow and to better illustrate the situation in clinical use. Second, the distance was chosen in 1 mm steps. In clinical application, the laser fiber might be adjusted in approximately 0.1 mm steps. However, it is of note that the histological differences were rather small, which is why smaller distances would not provide any further information about the histological damage. Due to the novelty of the SP TFL, further studies are of utmost importance to corroborate its pre-clinical results in tissue ablation and stone lithotripsy. That also counts for the BDL; however, other in vitro studies are first necessary if this laser can compete with the already existing ones. Despite these limitations, some conclusions can be drawn for clinical practice. First, we were able to show that the term “defocusing” must be considered carefully, since the laser effects vary depending on the distance from tissue. Second, our results can have an impact on the learning curve during laser lithotripsy and in the area of enucleation, since it can be seen at which distance the best coagulation effect occurs.
Conclusions
These in-vitro tests showed that the SP TFL had a better safety profile than the Ho:YAG laser with no tissue ablation at 2 mm which suggests that it can be safely adopted for general use. Conversely, the Ho:YAG laser produced thermo-mechanical damage to tissue at a distance of 2 mm. The cwTFL was the best device for tissue cutting and cauterizing in contact mode. The BDL had the strongest coagulation effect in blood-rich tissue among the four lasers, without significant changes in coagulation depth at different distances.
References
Herrmann TRW, Liatsikos EN, Nagele U et al (2012) EAU guidelines on laser technologies. Eur Urol 61:783–795. https://doi.org/10.1016/j.eururo.2012.01.010
Bach T, Muschter R, Sroka R et al (2012) Laser treatment of benign prostatic obstruction: basics and physical differences. Eur Urol 61:317–325. https://doi.org/10.1016/j.eururo.2011.10.009
Enikeev D, Okhunov Z, Rapoport L et al (2018) Novel thulium fiber laser for enucleation of prostate: a retrospective comparison with open simple prostatectomy. J Endourol. https://doi.org/10.1089/end.2018.0791
Becker B, Enikeev D, Glybochko P et al (2019) Effect of optical fiber diameter and laser emission mode (cw vs pulse) on tissue damage profile using 1.94 µm Tm:fiber lasers in a porcine kidney model. World J Urol. https://doi.org/10.1007/s00345-019-02944-y
Jiang D-L, Yang Z, Liu G-X et al (2019) A novel 450-nm blue laser system for surgical applications: efficacy of specific laser-tissue interactions in bladder soft tissue. Lasers Med Sci 34:807–813. https://doi.org/10.1007/s10103-018-2668-5
Braun A, Kettner M, Berthold M et al (2018) Efficiency of soft tissue incision with a novel 445-nm semiconductor laser. Lasers Med Sci 33:27–33. https://doi.org/10.1007/s10103-017-2320-9
Traxer O, Rapoport L, Tsarichenko D et al (2018) V03–02 first clinical study on superpulse thulium fiber laser for lithotripsy. J Urol 199:e321–e322. https://doi.org/10.1016/j.juro.2018.02.827
Traxer O, Keller EX (2019) Thulium fiber laser: the new player for kidney stone treatment? A comparison with Holmium:YAG laser. World J Urol. https://doi.org/10.1007/s00345-019-02654-5
Kronenberg P, Traxer O (2019) The laser of the future: reality and expectations about the new thulium fiber laser-a systematic review. Transl Androl Urol 8:S398–S417. https://doi.org/10.21037/tau.2019.08.01
Ishikawa I, Okamoto T, Morita S et al (2011) Blue-violet light emitting diode (LED) irradiation immediately controls socket bleeding following tooth extraction: clinical and electron microscopic observations. Photomed Laser Surg 29:333–338. https://doi.org/10.1089/pho.2010.2856
Emiliani E, Talso M, Haddad M et al (2018) The true ablation effect of holmium YAG laser on soft tissue. J Endourol 32:230–235. https://doi.org/10.1089/end.2017.0835
Kang HW, Choi BB (2019) Dependence of laser-induced tissue ablation on optical fiber movements for laser prostatectomy. World J Urol. https://doi.org/10.1007/s00345-019-03019-8
Fried NM, Murray KE (2005) High-power thulium fiber laser ablation of urinary tissues at 1.94 microm. J Endourol 19:25–31. https://doi.org/10.1089/end.2005.19.25
Cecchetti W, Zattoni F, Nigro F, Tasca A (2004) Plasma bubble formation induced by holmium laser: an in vitro study. Urology 63:586–590. https://doi.org/10.1016/j.urology.2003.09.010
Yaroslavsky I, Kovalenko A, Arkhipova V et al (2018) Comparison of a novel 450-nm laser with Ho:YAG (2100 nm), Tm fiber (1940 nm), and KTP (532 nm) lasers for soft-tissue ablation. In: Kang HW, Chan KF (eds) Therapeutics and diagnostics in urology 2018. SPIE, San Francisco, p 15
Sherwood ME, Flotte TJ (2007) Improved staining method for determining the extent of thermal damage to cells. Lasers Surg Med 39:128–131. https://doi.org/10.1002/lsm.20450
Enikeev D, Netsch C, Rapoport L et al (2019) Novel thulium fiber laser for endoscopic enucleation of the prostate: a prospective comparison with conventional transurethral resection of the prostate. Int J Urol 26:1138–1143. https://doi.org/10.1111/iju.14115
Enikeev D, Glybochko P, Rapoport L et al (2018) Impact of endoscopic enucleation of the prostate with thulium fiber laser on the erectile function. BMC Urol 18:87. https://doi.org/10.1186/s12894-018-0400-1
Cornu J-N, Ahyai S, Bachmann A et al (2015) A systematic review and meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic obstruction: an update. Eur Urol 67:1066–1096. https://doi.org/10.1016/j.eururo.2014.06.017
Johnson DE, Cromeens DM, Price RE (1992) Use of the holmium:YAG laser in urology. Lasers Surg Med 12:353–363
Arkhipova V, Enikeev M, Laukhtina E et al (2019) Ex vivo and animal study of the blue diode laser, Tm fiber laser, and their combination for laparoscopic partial nephrectomy. Lasers Surg Med. https://doi.org/10.1002/lsm.23158
Meskawi M, Hueber P-A, Valdivieso R et al (2019) Complications and functional outcomes of high-risk patient with cardiovascular disease on antithrombotic medication treated with the 532-nm-laser photo-vaporization Greenlight XPS-180 W for benign prostate hyperplasia. World J Urol 37:1671–1678. https://doi.org/10.1007/s00345-018-2560-8
Chen C-H, Chiang P-H, Lee W-C et al (2012) High-intensity diode laser in combination with bipolar transurethral resection of the prostate: a new strategy for the treatment of large prostates (>80 ml). Lasers Surg Med 44:699–704. https://doi.org/10.1002/lsm.22081
Hess MM, Fleischer S, Ernstberger M (2018) New 445 nm blue laser for laryngeal surgery combines photoangiolytic and cutting properties. Eur Arch Otorhinolaryngol 275:1557–1567. https://doi.org/10.1007/s00405-018-4974-8
Funding
No funding was obtained.
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore, share collective responsibility and accountability for the results. T: project development, data collection, data analysis, manuscript editing. N: project development, data collection, data analysis, manuscript editing. E: project development, data collection, data analysis, manuscript editing. G: project development, manuscript editing. H: project development, manuscript editing. K: project development, manuscript editing. L: project development, data collection, data analysis. G: project development, manuscript editing. B: project development, data collection, data analysis, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was not necessary in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taratkin, M., Netsch, C., Enikeev, D. et al. The impact of the laser fiber-tissue distance on histological parameters in a porcine kidney model. World J Urol 39, 1607–1612 (2021). https://doi.org/10.1007/s00345-020-03326-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03326-5