Abstract
Purpose
To report on the first short-term oncologic outcomes of percutaneous irreversible electroporation for small renal masses.
Methods
Patients with cT1a renal masses treated with irreversible electroporation from April 2013 through December 2016 were reviewed. Small, low complexity tumors were generally selected for irreversible electroporation using the NanoKnife® System (Angiodynamics, Latham, NY, USA). Surveillance imaging was performed post-operatively, and survival analysis was completed using the Kaplan–Meier method.
Results
A total of 42 tumors in 41 patients underwent irreversible electroporation. Mean tumor size was 2.0 cm with a median R.E.N.A.L nephrometry score of 5. Twenty-nine patients (71%) were discharged the same day of the procedure and no major (Clavien grade II or higher) intraoperative or post-operative complications occurred. Initial treatment success rate was 93%; our three failures (7%) underwent salvage radiofrequency ablation. With a mean follow-up of 22 months, 2-year local recurrence-free survival was 83% for patients with biopsy confirmed renal cell carcinoma, 87% with biopsy confirmed or a history of renal cell carcinoma, and 92% for the intent-to-treat cohort.
Conclusions
Although with low morbidity, in comparison to extirpation and conventional thermal ablation technologies, irreversible electroporation has suboptimal short-term local disease control results in this series of small, low complexity tumors. Larger series and longer follow-up will determine the durability of this modality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Long-term data now supports the oncologic efficacy of thermal ablative therapies such as radiofrequency ablation (RFA) and cryoablation (CA) in treating small (<4 cm) renal cell carcinoma (RCC) [1–5]. However, these techniques have limitations for larger tumors, those adjacent to the collecting system, or if the tumor is in close proximity to other structures [6–8]. In addition, efficacy of thermal ablative techniques can be limited by the vascular “heat sink,” phenomena [9]. An alternative ablative approach to classic thermal techniques may overcome some of these limitations.
Irreversible electroporation (IRE) is an ablative technology that employs short pulses of high voltage electrical current across the target tissue. This creates irreversible nanopores in the phospholipid bilayer of the cell membrane causing membrane destabilization and thereby lethal changes in cell permeability [10, 11].
IRE does not rely on extreme temperatures to ablate the target tissue and theoretically is not affected by the “heat sink,” of intratumoral or adjacent vasculature. Pre-clinical data has demonstrated homogenous areas of non-viability within the ablation zone with preservation of the extracellular matrix [12]. The majority of the published literature regarding IRE has been performed on other organs such as the liver, pancreas, and prostate [13–15]. Most renal evaluations of IRE have been in porcine models [16–18], with few human feasibility trials [19–21].
We recently published our clinical experience in which we describe our technique, radiographic appearance, and very early results of percutaneous IRE ablation [22]. This report details the first clinical and oncologic outcomes of percutaneous IRE ablation of small renal masses (SRM).
Materials and methods
Patients
All patients with cT1a renal masses, and cN0M0, treated with IRE from April 2013 through December 2016 were reviewed under Institutional Review Board approval. Patients were counseled by the treating urologist regarding all therapeutic options for RCC including active surveillance, ablation and surgical extirpation. Management was determined based on tumor size and location, patient comorbidities, and the patient’s preference after lengthy discussion of the risks and benefits. Small, peripheral, posterior or laterally located tumors with low R.E.N.A.L. nephrometry scores [23] were generally selected for IRE to minimize complexity of treatment in this initial experience. Informed consent was obtained from all individual participants included in this study.
Technique
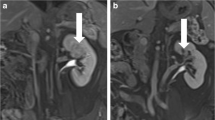
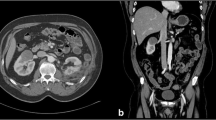
Our IRE technique has been previously published [22]. Briefly, all IRE procedures are performed with the NanoKnife® System (Angiodynamics, Latham, NY, USA). IRE probes are placed percutaneously under computed tomography (CT) guidance by an experience interventional radiologist, with the patient under general anesthesia and neuromuscular blockade. The goal is to bracket the tumor with the number and configuration of probes determined by tumor size and location. For masses less than 1.5 cm in diameter and for those 1.5–2.5 cm, three and four electrodes are typically placed, respectively. Masses 2.5–4 cm are treated with multiple probes, including probes within the center of the tumor, thus requiring probe repositioning during the procedure to ensure adequate treatment coverage. As discussed by Trimmer et al., IRE probe exposure length of 2.0 cm was elected for the first three patients. After consultation with the manufacturer and others familiar with IRE, the exposure length was decreased to 1.5 cm for all subsequent patients to maximize current density [22].
Renal mass biopsy was performed at a separate earlier clinical setting or after IRE probe placement at the time of ablation. Performance of biopsy in patients with a prior RCC diagnosis was up to clinician discretion. In some patients, clinician concern for disrupting or displacing the IRE probes at the time of ablation precluded biopsy.
Following verification of electrode placement with CT reformatted images, a 10-pulse “trial poration,” series for each probe pairing was performed. Voltage adjustments were made as needed to set the initial current delivered between 30 and 40 A. All renal tumors were then treated with the determined voltage pulsed for 100 ms at 1 Hz with cardiac synchronization, delivered for 70 pulses between each electrode pair. The polarity on the electrodes was subsequently reversed and 70 pulses between each electrode pair were again discharged to complete the treatment. Finally, a repeat CT with intravenous contrast was performed to assess adequate treatment by loss of tumor enhancement.
Imaging and follow-up
Post-IRE efficacy was initially assessed by radiologists experienced in ablation imaging at 6-weeks, by verifying no contrast enhancement on cross-sectional imaging. Patients without enhancement continued imaging evaluation at 3 and/or 6 months, 12 months and annually thereafter. Patients underwent standard chest X-ray surveillance based on AUA guidelines post-ablation [24]. Serum creatinine levels were monitored preoperatively and 3–6 months post-operatively to trend glomerular filtration rate (GFR) changes.
Outcome variables
Patient demographics and tumor characteristics were collected. We monitored procedural time, defined from skin incision to dressing complete, and recorded the number of IRE probes used per case. Procedural and post-operative complications were reviewed and adjudicated using the Clavien–Dindo classification [25]. Length of hospitalization was also recorded.
Survival analysis
Residual enhancement at initial 6-week imaging was classified as treatment failure, and these patients were censored from further survival analysis after salvage treatment. Subsequent contrast enhancement or lesion growth in the zone of ablation was considered evidence of local recurrence. Cancer-specific (CSS) and overall survival (OS) was determined based on death from RCC or other causes, respectively. Survival Analysis was performed on three levels: patients with biopsy confirmed RCC, patients with either biopsy confirmed or a history of RCC, and an intent-to-treat (ITT) group.
Statistics
Statistical analysis was performed with SPSS version 22 (Armonk, NY, USA). Means or medians were calculated for each variable determined by the normality of the data. Survival analysis was performed using the Kaplan–Meier method.
Results
Clinical outcomes
A total of 42 tumors in 41 patients were treated with curative intent using IRE. Patient demographics, tumor characteristics, intraoperative outcomes, and final pathology are listed in Table 1. Mean tumor size was 2.0 cm (range 1.0–3.6 cm) with a median R.E.N.A.L. nephrometry score of 5.
Median operative time was 94 min. Twenty-five (60%) tumors were biopsied either pre-procedure or intraoperatively. Of the tumors (40%) that were not biopsied, six patients (14%) had prior RCC diagnoses. The reason for not performing a biopsy was often due to patient preference pre-procedure or difficulty positioning the biopsy needle with IRE probes in place intraoperatively. Pathology confirmed RCC in 80% (20/25) of biopsies, with 8% (2/25) oncocytic neoplasms favoring oncocytoma, and 12% (3/25) non-diagnostic.
Post-operatively, 71% (29/41) of patients were discharged home while the remaining 29% (12/41) were admitted for one night. All complications were Clavien grade I with an overall rate of 22% (9/41). Four patients had small asymptomatic perinephric hematomas on immediate post-IRE CT scan (10%). Two of these patients were discharged from recovery, while the other two were admitted for overnight observation. They both had stable vital signs and complete blood counts, and were discharged the following morning. Two patients had post-operative urinary retention (5%) and were observed overnight. One patient (2%) had significant pain that led to overnight observation. Lastly, two patients (5%) developed respiratory difficulty in the recovery unit and required non-invasive positive pressure ventilation. Both subsequently had uneventful hospitalizations and were discharged the following day. Mean post-operative change in GFR was −6 ml/min for the entire cohort (Table 1).
Oncologic outcomes
Initial treatment success rate was 93% (39/42) on 6-week follow up CT scan. The three failures were patients #3, #11, and #22 in our experience. Mean tumor size of the failures was 2.3 cm (range 1.5–3.6 cm). Each failure underwent salvage RFA, without confirmatory biopsy, and has remained without evidence of disease recurrence for a mean of 21 months (range 3–35 months).
Tumors with less than 3 months of follow-up (n = 4) were excluded from further survival analysis. Mean follow-up for the remaining ITT cohort (n = 35) was 22 months (SD 12.4) with 21 (60%) having at least 18 months of surveillance. Two patients developed local recurrences at 11 and 23 months, respectively. The 2-year actuarial local recurrence-free survival (LRFS) rate in patients with biopsy confirmed RCC was 83% (Fig. 1). In patients with biopsy confirmed or a history of RCC, and in our ITT cohort, 2-year LRFS was 87 and 92%, respectively.
The patient who developed a local recurrence at 11 months (initial biopsy 3.0 cm clear cell RCC) underwent salvage treatment with a robot-assisted partial nephrectomy. Pathology demonstrated 3.5 cm clear cell RCC, Fuhrman grade 3, with invasion into perinephric fat (pT3a) and negative margins. He remained free of disease until 32 months when he developed a 1.7 cm adrenal metastasis and underwent robotic adrenalectomy with confirmed pathology. At 35 months surveillance from his initial IRE treatment he is currently without evidence of disease. The other patient developed a 1.2 cm mass in the ablation track at 23 months (initial biopsy 1.4 cm clear cell RCC). Unfortunately, she had colonic adenocarcinoma at the time of initial IRE and subsequently developed peritoneal carcinomatosis prior to her local recurrence. Although the mass has not been biopsied, based on the location it is highly suspicious for RCC recurrence. She is currently receiving chemotherapy, and will not be pursuing additional treatment for RCC.
One patient with a 2.2 cm right renal mass, who did not undergo biopsy prior to or during his ablation procedure, passed away from an unrelated cause 16 months after IRE. The 2-year actuarial OS rates for the biopsy confirmed, biopsy confirmed or history of RCC, and for the ITT cohorts was 100, 100, and 95%, respectively. No patient has passed away from RCC.
Discussion
Both of the current American Urological Association’s [26] and European Association of Urology’s [27] guidelines recognize tumor extirpation as standard management for a clinical T1 renal mass, with thermal ablation as an option. IRE, on the other hand, is an athermal minimally invasive technology that has FDA 510k clearance for ablation of soft tissue. In this first reported series of 42 cT1a masses undergoing IRE, we demonstrate an initial treatment success rate of 93% (39/42) with a 2-year LRFS rate of 83% in patients with at least 3 months of follow-up and biopsy confirmed RCC. Although our clinical outcomes have been appropriate with no major complications and the majority of our patients (71%) discharged the day of surgery, the short-term LRFS is suboptimal compared to thermal ablation or partial nephrectomy outcomes.
The emergence of IRE as a treatment modality for malignancies has grown over the past decade. It has long been recognized that applying electrical impulses across cellular membranes induces poration, and the reversible nature of this has been utilized for drug delivery and gene therapy for decades [11]. More recently, Rubinsky and colleagues modulated these impulses with increased voltage and number of pulses, and noted the irreversible results with subsequent cell death [10]. This novel ablative technique offers potential advantages over traditional ablation in the treatment of SRM due to its predominant manner of non-thermal cell death. It has been reported to be safe with good clinical success in the treatment of hepatic, pancreatic, and prostate lesions [13–15].
Experience with renal tumor IRE is limited. Pech et al. demonstrated feasibility and safety of IRE in the clinical phase I study of patients undergoing curative resection of RCC [19]. Of six patients with 2.0–3.5 cm renal masses who underwent IRE immediately prior to surgical resection, all tolerated the IRE procedure well with no complications. Wendler and colleagues performed partial nephrectomy 4 weeks after IRE in three patients to assess histologic changes [20]. Although histology demonstrated high degrees of damage to the renal tumors, they noted small tumor residues without proliferative activity within each ablation zone. More recently, in a series of five patients Diehl et al. demonstrated the safety of IRE in treating SRM in solitary kidneys [21]. However, no reports regarding early oncologic outcomes have been published to date.
Our initial treatment success rate of 93% is consistent with prior reports of both CA (97%) [5] and RFA (87%) [2], although it should be emphasized that two of the three failures in our series occurred relatively early in our experience. Additionally, while treatment failure was not confirmed with a biopsy, based on our experience with other ablation techniques these were in fact failures as opposed to reactive hyperemia that could be monitored. We suspect the learning curve with IRE application was a factor. It is our opinion that IRE is technically more challenging than RFA or CA due to the need for precise placement of multiple probes. Probes must bracket the tumor, and be positioned parallel and to the same depth to ensure uniform voltage fields. After review of our three failures, we hypothesize that non-parallel probes and exposure of the active probe zone to peri-tumoral adipose tissue may have resulted in non-uniform current flow and incomplete poration/ablation [22]. Further monitoring of treatment failures going forward will help elucidate our hypothesis.
Long-term favorable oncologic outcomes for ablative treatment of SRM have been established. Contemporary 5-year LRFS for T1a lesions is 93–96% [1–3] and 86–87% [4, 5] for RFA and CA, respectively. In addition, prior partial nephrectomy literature has demonstrate LRFS rates of greater than 97% for tumors <3.0 cm [28]. We recognize our 2-year LRFS rate of 83% in this small series with short follow-up does not compare favorably to this mature literature, especially in the context of small and minimally complex tumors. We also acknowledge that our low biopsy rate (60%) hinders interpretation of potentially more favorable early outcomes of the entire series (92% ITT LRFS). A concerted effort to improve our biopsy rate is underway to better define the potential role of athermal ablation in treating SRM.
One of our recurrences appeared in the ablation track, potentially from seeding. A rare (0.3%) occurrence in the contemporary literature [6], this needs further monitoring in larger studies to determine the true risk from IRE. Our CSS and OS are not surprising given the short follow up. We anticipate a greater understanding of this technology’s long-term oncologic applications with further surveillance.
As for complications, the four (10%) small perinephric hematomas identified were similar to prior experience with RFA and CA [29, 30] and were not clinically significant. The two patients who had respiratory difficulty in recovery (5%) were morbidly obese (BMI 37 and 38.5, respectively), but with no respiratory comorbidities (e.g. sleep apnea, chronic obstructive pulmonary disease). Due to the need for complete paralysis during the IRE procedure, it is possible this was due to residual paralytic medications, as these patients gradually recovered after receiving non-invasive positive pressure ventilation for a few hours. Within the context of the small tumor size and low complexity, we did not experience a major (Clavien grade II or higher) complication.
There exist several limitations to our study in addition to aforementioned concerns about our biopsy rate. First, though this is the only and largest series to date, our patient population is small. Second, there is selection bias in our study. Our mean tumor size of 2.0 cm and median R.E.N.A.L. nephrometry score of 5 demonstrate the small size and low complexity in our series, a notable intention in our early investigations with this new technology. We believe that treatment of larger cT1a tumors should be avoided unless appropriate oncologic efficacy is first demonstrated in low complexity tumors. In addition, there are presently potential limitations with IRE tumor size as probes must be placed a maximum of 2.0 cm apart. Therefore, complex and expensive probe formations may limit the ability to treat larger tumors. Lastly, our mean follow-up of 22 months is expectedly short compared to that in the mature thermal ablation literature. Larger prospective studies with longer follow-up are needed to demonstrate our ultimate ability with IRE.
Conclusions
The short-term experience with IRE demonstrates suboptimal oncologic, although appropriate clinical, outcomes in this small series. However, salvage treatment for local recurrences is feasible. Increasing our biopsy rate and longer follow-up will determine the durability of this modality to warrant further use in treating RCC.
References
Tracy CR, Raman JD, Donnally C, Trimmer CK, Cadeddu JA (2010) Durable oncologic outcomes after radiofrequency ablation: experience from treating 243 small renal masses over 7.5 years. Cancer 116(13):3135–3142
Psutka SP, Feldman AS, McDougal WS, McGovern FJ, Mueller P, Gervais DA (2013) Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol 63(3):486–492
Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA (2012) Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol 61(6):1156–1161
Kim EH, Tanagho YS, Saad NE, Bhayani SB, Figenshau RS (2014) Comparison of laparoscopic and percutaneous cryoablation for treatment of renal masses. Urology 83(5):1081–1087
Caputo PA, Ramirez D, Zargar H, Akca O, Andrade HS, O’Malley C, Remer EM, Kaouk JH (2015) Laparoscopic cryoablation for renal cell carcinoma: 100-month oncologic outcomes. J Urol 194(4):892–896
Atwell TD, Carter RE, Schmit GD, Carr CM, Boorjian SA, Curry TB, Thompson RH, Kurup AN, Weisbrod AJ, Chow GK, Leibovich BC, Callstrom MR, Patterson DE (2012) Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol 23(1):48–54
Best SL, Park SK, Youssef RF, Yaacoub RF, Olweny EO, Tan YK, Trimmer C, Cadeddu JA (2012) Long-term outcomes of renal tumor radio frequency ablation stratified by tumor diameter: size matters. J Urol 187(4):1183–1189
Park BK, Kim CK (2009 Sep) Complications of image-guided radiofrequency ablation of renal cell carcinoma: causes, imaging features and prevention methods. Eur Radiol 19(9):2180–2190
Lay AH, Faddegon S, Olweny EO, Morgan M, Lorber G, Trimmer C, Leveillee R, Cadeddu JA, Gahan JC (2015) Oncologic efficacy of radio frequency ablation for small renal masses: clear cell vs papillary subtype. J Urol 194(3):653–657
Davalos RV, Mir ILM, Rubinsky B (2005 Feb) Tissue ablation with irreversible electroporation. Ann Biomed Eng 33(2):223–231
Gehl J (2003 Apr) Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand 177(4):437–447
Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B (2006) In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE T Bio-Med Eng 53(7):1409–1415
Kingham TP, Karkar AM, D’Angelica MI, Allen PJ, Dematteo RP, Getrajdman GI, Sofocleous CT, Solomon SB, Jarnagin WR, Fong Y (2012) Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg 215(3):379–387
Martin RCG, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, McMasters KM, Watkins K (2015 Sep) Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg 262(3):486–494
Ting F, Tran M, Böhm M, Siriwardana A, Van Leeuwen PJ, Haynes A-M, Delprado W, Shnier R, Stricker PD (2016) Focal irreversible electroporation for prostate cancer: functional outcomes and short-term oncological control. Prostate Canc P D 19(1):46–52
Deodhar A, Monette S, Single GW, Hamilton WC, Thornton R, Maybody M, Coleman JA, Solomon SB (2011) Renal tissue ablation with irreversible electroporation: preliminary results in a porcine model. Urology 77(3):754–760
Tracy CR, Kabbani W, Cadeddu JA (2011) Irreversible electroporation (IRE): a novel method for renal tissue ablation. BJU Int 107(12):1982–1987
Olweny EO, Kapur P, Tan YK, Park SK, Adibi M, Cadeddu JA (2013) Irreversible electroporation: evaluation of nonthermal and thermal ablative capabilities in the porcine kidney. Urology 81(3):679–684
Pech M, Janitzky A, Wendler JJ, Strang C, Blaschke S, Dudeck O, Ricke J, Liehr U-B (2011) Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Inter Rad 34(1):132–138
Wendler JJ, Ricke J, Pech M, Fischbach F, Jürgens J, Siedentopf S, Roessner A, Porsch M, Baumunk D, Schostak M, Köllermann J, Liehr U-B (2015) First delayed resection findings after irreversible electroporation (IRE) of human localised renal cell carcinoma (RCC) in the IRENE Pilot Phase 2a trial. Cardiovasc Inter Rad 4(39):239–250.
Diehl SJ, Rathmann N, Kostrzewa M, Ritter M, Smakic A, Schoenberg SO, Kriegmair MC (2016) Irreversible electroporation for surgical renal masses in solitary kidneys: short-term interventional and functional outcome. J Vasc Interv Radiol 27(9):1407–1413
Trimmer CK, Khosla A, Morgan M, Stephenson SL, Ozayar A, Cadeddu JA (2015 Oct) Minimally invasive percutaneous treatment of small renal tumors with irreversible electroporation: a single-center experience. J Vasc Interv Radiol 26(10):1465–1471
Kutikov A, Uzzo RG (2009) The R.E.N.A.L nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 182(3):844–853
Donat SM, Diaz M, Bishoff JT, Coleman JA, Dahm P, Derweesh IH, Herrell SD, Hilton S, Jonasch E, Lin DW, Reuter VE, Chang SS (2013) Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol 190:407–416
Dindo D, Demartines N, Clavien P-A (2004 Aug) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, Faraday MM, Kaouk JH, Leveillee RJ, Matin SF, Russo P, Uzzo RG, Practice Guidelines Committee of the American Urological Association (2009) Guideline for management of the clinical T1 renal mass. J Urol 182:1271–1279
Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, Volpe A, Bex A (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67(5):913–924
Crispen PL, Boorjian SA, Lohse CM, Sebo TS, Cheville JC, Blute ML, Leibovich BC (2008) Outcomes following partial nephrectomy by tumor size. J Urol 180(5):1912–1917
Balageas P, Cornelis F, Le Bras Y, Hubrecht R, Bernhard JC, Ferrière JM, Ravaud A, Grenier N (2013) Ten-year experience of percutaneous image-guided radiofrequency ablation of malignant renal tumours in high-risk patients. Eur Radiol 23(7):1925–1932
Zargar H, Samarasekera D, Khalifeh A, Remer EM, O’Malley C, Akca O, Autorino R, Kaouk JH (2015 Apr) Laparoscopic vs percutaneous cryoablation for the small renal mass: 15-year experience at a single center. Urology 85(4):850–855
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
NEC: Data collection, Data analysis, Manuscript writing and editing. IS: Data collection, Data analysis, Manuscript writing and editing. AHL: Data collection. MSCM: Data collection, Manuscript writing. AO: Data collection. CT: Protocol/project development. JAC: Protocol/project development, Data analysis, Manuscript editing.
Conflict of interest
The authors declare that they have no conflict of interest.
Extra-institutional funding
None.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the Institutional Review Board and with the 1964 Helsinki declaration and its later amendments.
Rights and permissions
About this article
Cite this article
Canvasser, N.E., Sorokin, I., Lay, A.H. et al. Irreversible electroporation of small renal masses: suboptimal oncologic efficacy in an early series. World J Urol 35, 1549–1555 (2017). https://doi.org/10.1007/s00345-017-2025-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-017-2025-5