Abstract
Purpose
To compare task performances of novices and experts using advanced high-definition 3D versus 2D optical systems in a surgical simulator model.
Methods
Fifty medical students (novices in laparoscopy) were randomly assigned to perform five standardized tasks adopted from the Fundamentals of Laparoscopic Surgery (FLS) curriculum in either a 2D or 3D laparoscopy simulator system. In addition, eight experts performed the same tasks. Task performances were evaluated using a validated scoring system of the SAGES/FLS program. Participants were asked to rate 16 items in a questionnaire.
Results
Overall task performance of novices was significantly better using stereoscopic visualization. Superiority of performances in 3D reached a level of significance for tasks peg transfer and precision cutting. No significant differences were noted in performances of experts when using either 2D or 3D. Overall performances of experts compared to novices were better in both 2D and 3D. Scorings in the questionnaires showed a tendency toward lower scores in the group of novices using 3D.
Conclusions
Stereoscopic imaging significantly improves performance of laparoscopic phantom tasks of novices. The current study confirms earlier data based on a large number of participants and a standardized task and scoring system. Participants felt more confident and comfortable when using a 3D laparoscopic system. However, the question remains open whether these findings translate into faster and safer operations in a clinical setting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With James Cameron’s Avatar in 2009, three-dimensional imaging began its triumphant march from cinemas around the world into our living rooms. Although 3D imaging had been introduced to laparoscopy as early as 1996, it took years of technological development to overcome limitations in ergonomics and quality of these systems [1]. In parallel, the introduction of stereoscopic viewing with the da Vinci system for robotically assisted laparoscopy presumably triggered the demand for 3D systems in conventional laparoscopy. The da Vinci system not only offered a higher degree of freedom in instrument movements, but also added depth perception to laparoscopy, thus leading to improved surgical performance and a higher comfort for the operating surgeon [2, 3]. However, differences between the da Vinci’s parallel rod lenses system with direct vision through separate lenses for each eye and 3D-systems using a monitor system for visualization as in conventional laparoscopy, are considerable, and potential benefits of one system may not apply to the other.
Today, a number of systems are on the market ready to enter our operating theaters to replace 2D laparoscopy and/or challenge more expensive robotic systems comprising potential disadvantages such as the lack of tactile feedback. Possible benefits of adding depth perception to laparoscopy may be able to alleviate shortcomings of laparoscopy, such as a loss of dexterity, reduced tactile sensation, and the fulcrum effect (instruments moving in the opposite direction to the surgeon’s hands due to the pivot point) [4]. Procedures may potentially become faster and safer with surgeons in training going through shorter learning curves. Several in vitro studies including limited numbers of participants suggested a superiority of modern 3D laparoscopic systems as compared to conventional 2D systems in laboratory settings [5–13]. In addition, a limited number of clinical studies using stereoscopic systems demonstrated favorable results [14–16]. The current study is to verify these findings in a standardized surgical simulator model using validated tasks adopted from the Fundamentals of Laparoscopic Surgery (FLS) curriculum with a high number of participants.
Materials and methods
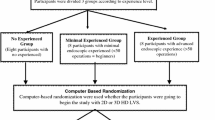
Fifty medical students without previous experience in laparoscopic surgery (novices) were randomly assigned to groups N2D (n = 25) and N3D (n = 25). Participating subjects were asked to perform five standardized tasks adopted from the Fundamentals of Laparoscopic Surgery (FLS) and European Training in Basic Laparoscopic Urological Skills (E-BLUS) curricula [17, 18]. A standardized instruction was given using a pre-recorded video manual that showed the five tasks. The students then were given a short time (5 min) to become familiar with the instruments and in case of the 3D group to become adopted to 3D vision.
In a second part of the study, eight experts with an experience of at least 50 laparoscopic procedures were asked to perform the same tasks in 2D and 3D in random order (groups E2D and E3D).
A standard laparoscopic box trainer was used, holding two 5-mm working ports and a 10-mm camera port in a typical triangle position. Both the 2D and 3D optical systems were mounted to a holding arm and held in a fixed position showing the complete area of interest within the box trainer (Fig. 1).
Technical specifications of applied imaging systems
3D: dual-channel parallel rod lenses optical system with integrated 2× CMOS stereo native full-HD camera (Einstein Vision®, Aesculap AG & Co. KG, Tuttlingen/B. Braun Melsungen AG, Melsungen, Germany), transmitting 2× HD-SDI signals to a standard 3D full-HD medical monitor, passive polarization glasses.
2D: identical system using a single channel.
Performance tasks and calculations of task scores
Task 1: Peg transfer
Participants were asked to pick up six objects from the left side of a peg board with their non-dominant hand (i.e., left hand), transfer them to their right hand, and place the objects over pegs on the right side and vice versa (Fig. 2a). An overall task score was calculated according to the FLS scheme (based on the recorded video sequences). This includes an error score being subtracted from a time score with the time score being calculated as cutoff time (300 s) − time needed and the error score as the added numbers of dropped or lost pegs or forgotten hand changes (as weighted by a multiplier of 10.25 and 10, respectively).
Task 2: Threading eyelets
A 2/0 polyglactin suture with an SH needle had to be threaded through nine eyelets (ring screws with an opening of 6 mm fixed to a board, space between screws 45–100 mm, Fig. 2b). The task score was calculated as cutoff time (600 s) − actual time.
Task 3: Ligating loop
A pre-tied ligating loop had to be placed and fixed around a peg in accordance with a drawn line (Fig. 2c). Task score was calculated as time score (180 s − actual time) − error score (failed knot = 50, missing drawn line in mm 10×).
Task 4: Intracorporeal knot
A suture had to be fed into two 4 × 500-mm rubber bands at designated entry points and tied using a needle holder and a grasper (Fig. 2d). Since students may have had varying degrees of experience in knot tying, all the participants were allowed to practice knot tying with a conventional needle driver before starting the task. Task score was calculated as time score (600 s − actual time) − error score (distance from missed entry points in mm 10×).
Task 5: Precision cutting
Precision cutting involves cutting a marked circle with a diameter of 50 mm on a mounted piece of cellular rubber (Fig. 2e). Task score was calculated as time score (600 s − actual time) − error score (length of deviations >1 mm in mm).
After completing the tasks, participants were asked to rate 16 items in a questionnaire. This included assessments of the difficulty of each task, self-evaluations of performance (implementation of intended actions, satisfaction with results, successful learning), ratings of different qualities (haptics, vision), and personal experience (dexterity, visual-motor coordination, fun, challenge, ambition/impetus to give up, learning experience, and self-perception as a surgeon). Ratings used a numeric Likert scale (1–5, 1 representing the best experience and 5 the worst).
Scorings in the questionnaires showed a tendency toward lower scores (fewer difficulties, better personal experience).
Continuous variables were compared by using a Student’s T test for normally distributed but independent populations. All continuous data were reported as mean ± SD. Statistical analyses were conducted by using Excel (Microsoft Deutschland, Unterschleissheim, Germany) and IBM SPSS Statistics, version 21 (IBM Deutschland, Ehningen, Germany).
Results
Overall task performance (added total scores of tasks 1–5) of novices was significantly better using stereoscopic compared to monoscopic visualization (groups N2D = 579.4 and N3D = 823.1, p = 0.006). No significant difference was noted between expert groups E2D and E3D (1679.0 and 1733.0, respectively, p = 0.346). The overall performance of experts compared to novices was better in both 2D and 3D (p = 7.9 × 10−11 and p = 9.2 × 10−11) (Table 1; Fig. 3a–e).
Task performance scores; a peg transfer, b threading eyelets, c ligating loop, d intracorporeal knot, e precision cutting, f overall score (mean total scores ± SD), (p values see Table 1), g mean scores questions 1–16 (novices), scores 1–5 (1 = best, 5 = worst). p > 0.05 in all except *p = 0.01; **p = 0.03
Novices showed variable differences in performances of individual tasks using 3D and 2D visualization. Superiority of performances in 3D reached a level of significance for tasks peg transfer (p = 0.016) and precision cutting (p = 0.001). 3D performances of tasks threading eyelets, ligating loop, and intracorporeal knot (p = 0.297, p = 0.083 and p = 0.066, respectively) were superior but did not reach statistical significance.
In the expert group, no significant differences in the task scores or total score between 2D and 3D performances was observed.
Scorings in the questionnaires showed a tendency toward lower scores in group N3D (less difficulties, better personal experience). Questions 12 (“How would you rate depth perception during task performances?”) and 13 (“How would you rate visual-motor coordination?”) were rated significantly better by participants of the N3D group (Fig. 3g).
Discussion
Since its introduction in the early 1980s, laparoscopy has developed from an experimental magnifying to standard of care for many surgical diseases. Today, even complex operations such as Whipple procedures or radical cystectomies can be performed laparoscopically with low complications rates and good functional and oncological outcomes [19]. However, inherent restrictions of laparoscopy, such as limitations in range of motion, an indirect working mode and decreased spatial orientation, demand a great deal of skill and experience and therefore a long learning curve [15]. In particular, it is the lack of sufficient depth perception that may affect surgical performance of complex tasks, such as intracorporeal knot tying and precise dissection [9]. In 2D laparoscopy, depth perception can only be achieved by mental processing of indirect references, such as motion parallax, relative position and size of anatomic structures, shading, and tissue grading [1, 9].
3D technology
Stereoscopic imaging in laparoscopy significantly differs from other modes of stereoscopic visualization applied in surgery. Compared to direct stereoscopic visualization like using a stereo microscope or magnifying glasses or the double-lens system of the da Vinci robot, 3D imaging in laparoscopy necessitates an additional camera and monitor system. It was only after the development of smaller lenses systems and enhanced modes of transmission (polarization or wavelength multiplexing instead of shutter systems) that 3D systems today offer a comfortable visual impression avoiding detrimental effects such as ghosting (double images), keystone effect (distortion), or vertical errors (caused by imprecise adjustment of double-lens systems) [1, 20].
3D systems for laparoscopy
Currently available 3D systems for laparoscopy either use bi-channel optical systems incorporating two parallel rod lenses (Einstein Vision®, Aesculap AG & Co. KG, Tuttlingen/B. Braun Melsungen AG, Melsungen, Germany) or two “chip-on-the-tip” digital image sensors (Olympus Medical Systems Corp., Tokyo, Japan; Karl Storz GmbH & Co., Tuttlingen, Germany). Both technologies offer full-HD resolution (1920 × 1080 pixels) transmitted to polarization monitors, thereby halfing the vertical resolution to 1920 × 540 due to a line-by-line assignment of the right- and left-eye image.
Influence of 3D imaging on surgical performance
Available studies comparing 2D and 3D laparoscopic imaging show conflicting data concerning a potential benefit of stereoscopic visualization on surgical performance. This may be attributed to the technology at hand in earlier studies that were not able to show significant difference in surgical performance in both experimental and clinical settings [21, 22]. Current experimental studies suggest that 3D imaging improves phantom task performance as measured by number of errors and time needed [5, 7, 9–13]. Experts seem to gain similarly in precision and time needed as compared to novices [9]. In addition, stereoscopic vision seems to improve learning curves in novices [6, 8]. However, most studies included relatively small numbers of participants (5–10 per subgroup) and/or a small number of phantom tasks with some including non-validated tasks.
Our study by and large confirms the findings of the aforementioned studies. All tasks were performed better by novices using the stereoscopic system as measured by the Fundamentals of Laparoscopic Surgery evaluation system integrating performance time and errors [23]. Experts performed better than novices in all tasks in both 2D and 3D with differences being markedly higher in the more difficult tasks 4 and 5. However, stereoscopic visualization did not translate into a markedly better performance in the expert group. Similar findings have been described by others [13]. Although this may be attributed to the rather low number of expert participants, it may as well point to a professionally acquired ability of mentally processing a virtual 3D reconstructed space from a 2D picture.
To our knowledge, the current study is the first to use the validated scoring system of the SAGES/FLS program. This system allowed us to calculate overall performances of both groups (novices and experts). In addition, the high number of participants guaranteed the participation of an unselected group of students, possibly including some with impaired stereo vision. It has been suggested by others to test and potentially exclude subjects with impaired stereoscopic vision [9]. However, we believe that a high number of unselected participants corresponds well a real-life situation.
The study is limited by a low number of expert participants and its in vitro nature. After stereoscopic laparoscopy has now been proven superior in a sufficient number of simulator studies, future research should aim at its clinical application.
Conclusions
Stereoscopic imaging improves performance of laparoscopic phantom tasks. Participants felt more confident and comfortable when using a 3D laparoscopic system. However, the question remains open whether these findings translate into faster and safer operations in a clinical setting.
Abbreviations
- 2D:
-
Two dimensional
- 3D:
-
Three dimensional
- CMOS:
-
Complementary metal-oxide semiconductor
- E2D:
-
Experts in 2D laparoscopy group
- E3D:
-
Experts in 3D laparoscopy group
- FLS:
-
Fundamentals of laparoscopic surgery
- HD:
-
High definition
- N2D:
-
Novices in 2D laparoscopy group
- N3D:
-
Novices in 3D laparoscopy group
- SAGES:
-
Society of American Gastrointestinal and Endoscopic Surgeons
- SDI:
-
Serial digital interface
References
Kunert W, Storz P, Müller S, Axt S, Kirschniak A (2013) 3D in laparoscopy: state of the art. Chirurg 84(3):202–207
Santos-Carreras L, Hagen M, Gassert R, Bleuler H (2012) Survey on surgical instrument handle design: ergonomics and acceptance. Surg Innov 19(1):50–59
Byrn JC, Schluender S, Divino CM, Conrad J, Gurland B, Shlasko E, Szold A (2007) Three-dimensional imaging improves surgical performance for both novice and experienced operators using the da Vinci Robot System. Am J Surg 193(4):519–522
Gallagher A, McClure N, McGuigan J, Ritchie K, Sheehy N (1998) An ergonomic analysis of the fulcrum effect in the acquisition of endoscopic skills. Endoscopy 30(7):617–620
Alaraimi B, El Bakbak W, Sarker S, Makkiyah S, Al-Marzouq A, Goriparthi R, Bouhelal A, Quan V, Patel B (2014) A randomized prospective study comparing acquisition of laparoscopic skills in three-dimensional (3D) vs. two-dimensional (2D) laparoscopy. World J Surg 38(11):2746–2752
Ashraf A, Collins D, Whelan M, O’Sullivan R, Balfe P (2015) Three-dimensional (3D) simulation versus two-dimensional (2D) enhances surgical skills acquisition in standardised laparoscopic tasks: a before and after study. Int J Surg 14:12–16
Lusch A, Bucur PL, Menhadji AD, Okhunov Z, Liss MA, Perez-Lanzac A, McDougall EM, Landman J (2014) Evaluation of the impact of three-dimensional vision on laparoscopic performance. J Endourol 28(2):261–266
Özsoy M, Kallidonis P, Kyriazis I, Panagopoulos V, Vasilas M, Sakellaropoulos GC, Liatsikos E (2015) Novice surgeons: Do they benefit from 3D laparoscopy? Lasers Med Sci 30(4):1325–1333
Storz P, Buess GF, Kunert W, Kirschniak A (2012) 3D HD versus 2D HD: surgical task efficiency in standardised phantom tasks. Surg Endosc 26(5):1454–1460
Tanagho YS, Andriole GL, Paradis AG, Madison KM, Sandhu GS, Varela JE, Benway BM (2012) 2D versus 3D visualization: impact on laparoscopic proficiency using the fundamentals of laparoscopic surgery skill set. J Laparoendosc Adv Surg Tech A 22(9):865–870
Usta TA, Ozkaynak A, Kovalak E, Ergul E, Naki MM, Kaya E (2015) An assessment of the new generation three-dimensional high definition laparoscopic vision system on surgical skills: a randomized prospective study. Surg Endosc 29(8):2305–2313
Wagner OJ, Hagen M, Kurmann A, Horgan S, Candinas D, Vorburger SA (2012) Three-dimensional vision enhances task performance independently of the surgical method. Surg Endosc 26(10):2961–2968
Cicione A, Autorino R, Breda A, De Sio M, Damiano R, Fusco F, Greco F, Carvalho-Dias E, Mota P, Nogueira C, Pinho P, Mirone V, Correia-Pinto J, Rassweiler J, Lima E (2013) Three-dimensional vs standard laparoscopy: comparative assessment using a validated program for laparoscopic urologic skills. Urology 82(6):1444–1450
Bilgen K, Ustün M, Karakahya M, Işik S, Sengül S, Cetinkünar S, Küçükpinar TH (2013) Comparison of 3D imaging and 2D imaging for performance time of laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech 23(2):180–183
Kyriazis I, Özsoy M, Kallidonis P, Vasilas M, Panagopoulos V, Liatsikos E (2015) Integrating three-dimensional vision in laparoscopy: the learning curve of an expert. J Endourol 29(6):657–660
Bove P, Iacovelli V, Celestino F, De Carlo F, Vespasiani G, Finazzi Agrò E (2015) 3D vs 2D laparoscopic radical prostatectomy in organ-confined prostate cancer: comparison of operative data and pentafecta rates: a single cohort study. BMC Urol 15:12
Peters JH, Fried GM, Swanstrom LL, Soper NJ, Sillin LF, Schirmer B, Hoffman K, Committee SF (2004) Development and validation of a comprehensive program of education and assessment of the basic fundamentals of laparoscopic surgery. Surgery 135(1):21–27
Brinkman WM, Tjiam IM, Schout BMA, Muijtjens AMM, Van Cleynenbreugel B, Koldewijn EL, Witjes JA (2014) Results of the European basic laparoscopic urological skills examination. Eur Urol 65(2):490–496
Novara G, Catto JWF, Wilson T, Annerstedt M, Chan K, Murphy DG, Motttrie A, Peabody JO, Skinner EC, Wiklund PN, Guru KA, Yuh B (2015) Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur Urol 67(3):376–401
Zhang L, Zhang Y-Q, Zhang J-S, Xu L, Jonas JB (2013) Visual fatigue and discomfort after stereoscopic display viewing. Acta Ophthalmol 91(2):e149–e153
Chan AC, Chung SC, Yim AP, Lau JY, Ng EK, Li AK (1997) Comparison of two-dimensional vs three-dimensional camera systems in laparoscopic surgery. Surg Endosc 11(5):438–440
Hanna GB, Shimi SM, Cuschieri A (1998) Randomised study of influence of two-dimensional versus three-dimensional imaging on performance of laparoscopic cholecystectomy. Lancet 351(9098):248–251
Fraser SA, Klassen DR, Feldman LS, Ghitulescu GA, Stanbridge D, Fried GM (2003) Evaluating laparoscopic skills: setting the pass/fail score for the MISTELS system. Surg Endosc 17(6):964–967
Acknowledgments
We would like to thank Schoelly Fiberoptic GmbH, Denzlingen, Germany, for providing the instruments and technical support for this study.
Authors’ contribution
Martin Schoenthaler: Protocol/project development, data collection and management, data analysis, manuscript writing/editing, supervision. Daniel Schnell: Protocol development, data collection and management, data analysis. Konrad Wilhelm: Data collection and management, data analysis. Daniel Schlager: Manuscript writing/editing. Fabian Adams: Manuscript writing/editing. Simon Hein: Manuscript editing, figure editing. Ulrich Wetterauer: Data interpretation, supervision. Arkadiusz Miernik: Protocol/project development, data analysis, manuscript writing/editing, supervision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclosure
Martin Schoenthaler: consultant contract with Schoelly Fiberoptic GmbH, Denzlingen, Germany, and NeoTract Inc., Pleasanton, USA. Daniel Schnell: no conflicts of interest or financial ties to disclose. Konrad Wilhelm: no conflicts of interest or financial ties to disclose. Daniel Schlager: no conflicts of interest or financial ties to disclose. Fabian Adams: no conflicts of interest or financial ties to disclose. Simon Hein: no conflicts of interest or financial ties to disclose. Ulrich Wetterauer: advisory board, DR. KADE Pharmazeutische Fabrik GmbH, Berlin. Arkadiusz Miernik: consultant contract with Schoelly Fiberoptic GmbH, Denzlingen, Germany.
Ethical standard
The study has been approved by the local ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All experiments were performed according the guidelines of GLP (Good Laboratory Practice).
Rights and permissions
About this article
Cite this article
Schoenthaler, M., Schnell, D., Wilhelm, K. et al. Stereoscopic (3D) versus monoscopic (2D) laparoscopy: comparative study of performance using advanced HD optical systems in a surgical simulator model. World J Urol 34, 471–477 (2016). https://doi.org/10.1007/s00345-015-1660-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-015-1660-y