Abstract
Pre-sowing seed treatment with nanoparticles has a promising role towards the improvement of seed germination and seedling growth. In the present study, silver nanoparticles (AgNPs) were synthesized from silver nitrate (AgNO3) through green route using aqueous extract of Parthenium hysterophorus L. roots. The synthesized nanoparticles were characterized using various analytical instruments such as UV–Vis spectrophotometer, TEM, SEM, EDX, XRD, and FTIR. Further, the impacts of AgNPs, AgNO3, and plant extract on germination, seedling growth, activity of hydrolytic enzymes, and ROS generation of three pulses (Cicer arietinum L., Pisum sativum L., and Vigna radiata L.) were investigated. Characterization of nanoparticles revealed that the green synthesized AgNPs were mostly spherical with an average size of 11–20 nm and crystallinity was 71.3%. The growth experiment revealed that seed germination and seedling growth were increased under AgNPs (10 and 50 mg/L) and AgNO3 (10 mg/L) treatments as compared to control for three tested pulses. Results also demonstrated the increased hydrolytic enzyme activities during early seedling establishment of three pulses under nanoparticle treatments. Meanwhile, dose dependent increase in ROS production was recorded under both AgNPs and AgNO3 treatments and it was always higher in AgNO3 as compared to AgNPs treatments. However, the growth inhibition at higher concentrations of both AgNPs and AgNO3 treatments suggested that the ROS generation at an optimum level might play an important role towards the enhancement of seed germination. Therefore, AgNPs mediated alteration of the activity of hydrolytic enzymes and generation of ROS might regulate early seedling establishment.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology is an emerging field with wide application in agriculture. The use of nanoparticles in plant growth and productivity is relatively a recent practice and it has the potential to revolutionize agriculture (Guha et al. 2021; Tymoszuk 2021). Since the past decade, a number of nanomaterial-based formulations, such as nanofertilizers, nanopesticides, nanofungicides, and nanosensors have been developed to improve plant growth, protection, and crop productivity (Mahakham et al. 2016). However, several research showed that nanomaterials exhibit a complex interaction and show both positive and negative impact on plants which mainly depends on various factors, such as physicochemical properties of nanoparticles, method of application, concentration of nanoparticles exposure and most important one is the nature of the test organism (Pérez-de-Luque 2017). Nanoparticles of various metals and metal oxides such as silver, gold, iron, zinc, and aluminium have been used for improved germination, seedling growth, and productivity in various crop plants (Guha et al. 2021; Rai-Kalal and Jajoo 2021; Almutairi and Alharbi 2015; Janmohammadi and Sabaghnia 2015). In addition, nanoparticles mediated tolerance against several abiotic stresses such as salinity, chilled and heat stress in plants was also demonstrated by earlier researchers (Khan et al. 2023; Alabdallah and Hasan 2021). However, several adverse effects of nanoparticles including AgNPs such as genotoxicity (Debnath et al. 2020), harmful changes at physiological and biochemical levels on plants have been reported in the literature (Prazak et al. 2020). Among various nanoparticles, AgNPs is the most commonly used one and has tremendous applications in various fields such as biomedical, industry, personal care products, including agriculture and it covers about 25% of total nanoproducts (Tymoszuk 2021). The well-documented antimicrobial property of AgNPs promotes its application in agriculture for crop protection (Prazak et al. 2020). Apart from this, researchers also highlighted the positive impact of AgNPs on germination and growth in a variety of crops such as mung beans (Basheerudeen et al. 2021), onion (Acharya et al. 2019), oat, and radish (Tomacheski et al. 2017) maize, watermelon, zucchini (Almutairi and Alharbi 2015) and so on.
Traditional agricultural practices require rapid and uniform seed germination as well as seedling growth for the successful establishment of crop plants. The germination process starts with the uptake of water by dry seed and subsequent emergence of radicle due to the growth of the embryonic axis, as a consequence the development of root and shoot in later establish successful seedling (Tymoszuk 2021). This involves a variety of physio-biochemical responses including complex enzymatic actions. The hydrolytic enzymes breakdown the reserve food present in the seed and provide energy for seed germination and seedling establishment (Botcha and Prattipati 2020; Laware and Raskar 2014). In agriculture, nanoparticles are used as biological stimulants due to their several advantages over traditional materials: they do not readily break up by light and/or heat, can easily enter through the biological membrane, and can be absorbed fully by plants (Chau et al. 2019). Since past, several strategies have been adopted to improve crop performance and one such method is pre-sowing seed treatment (Rai-Kalal and Jajoo 2021). Pre-sowing seed treatments are well-known agricultural practices to improve seed performance. It helps to initiate early seed metabolism, like DNA, RNA, and protein synthesis which help in early initiation of seed emergence (Bhardwaj et al. 2012). Pre-sowing seed treatment with nanomaterials showed improvement in seed germination and seedling growth in different crops (Guha et al. 2021; Acharya et al. 2019). Improved germination and subsequently seedling growth are chiefly attributed to higher activities of hydrolytic enzymes and increased generation of ROS (Oracz and Karpinski 2016). Generation of oxidative stress and subsequently free radicles mediated polymer breakdown and upregulation of hydrolytic enzyme activity and loosening of cell wall leads to cell expansion and seedling growth (Song and He 2021). Enhanced amylase activity provides more soluble sugar to support rapid germination and seedling growth in nanoprimed seeds (Xu et al. 2021). However, comprehensive studies on the effects of nanoparticles at physiological and biochemical levels during early seedling establishment have not been elucidated and the mechanisms behind the nanoparticles induced enhancement of seed germination remained to be addressed.
Pulses are the major source of dietary proteins, including other nutrients, and play a major role in the diets of many developing countries (Stefano et al. 2019). Among different pulses, such as chickpeas, lentils, beans, peas, and vetch are the major cultivated crops. These crops are relevant to promote sustainable agricultural practices due to their ability to fix the atmospheric nitrogen through nodule formation and thus low requirement of fertilizers (Avezum et al. 2022). So, in the present study, three pulses (Cicer arietinum L., Pisum sativum L., and Vigna radiata L.) were selected to evaluate the effect of pre-sowing seed treatment with phytosynthesized AgNPs on their germination and seedling growth. Further, the status of hydrolytic enzymes and generation of ROS were also studied to analyse the probable role of AgNPs towards enhanced seed germination and early seedling establishment.
2 Materials and methods
2.1 Synthesis and characterization of silver nanoparticles
To synthesize of AgNPs, 90 mL of 0.1 M AgNO3 salt solution was mixed with 10 mL of aqueous extract (1:10, w/v) of roots of Parthenium hysterophorus L. and kept in the dark at room temperature for 24 h. Further, the bioreduction of silver ions into silver nanoparticles in the mixture was determined by UV–Vis spectrophotometer (Optizen Pop) by using a wavelength range of 320–500 nm. The size and morphology of nanoparticles were determined by transmission electron microscopy (TEM) (JEOL JEM 1400 plus). The surface morphology and elemental analysis of synthesized nanoparticles were determined by field emission scanning electron microscopy (FESEM) and energy dispersive X-ray (EDX) (Zeiss Sigma 300) analysis, respectively. The X-ray diffraction (XRD) (Bruker D8) determined the crystalline nature, average crystallite size, and crystallinity percentage of the particles according to Mondal et al. (2022). The surface functional groups of synthesized nanoparticles were determined by Fourier transform infrared spectroscopy (FTIR) (Agilent 650).
2.2 Plant growth condition and seed treatment
Seeds of three different pulses such as chickpea (Cicer arietinum L.), pea (Pisum sativum L.), and mung bean (Vigna radiata L.) were collected from Crop Research and Seed Multiplication Farm, The University of Burdwan, West Bengal, India. Healthy seeds were surface sterilized and imbibed in different concentrations (0, 10, 50, and 100 mg/L) of AgNPs, AgNO3, and Parthenium root extract for 12 h. Then seeds were washed with distilled water and placed on separate petri plates containing moist filter paper (Whatman no.1) with distilled water in triplicates. All petri plates were kept in the dark for germination at 25 ± 2 °C. After germination, seedlings were grown under an average 14 h photoperiod with 200 μM/m2/s light intensity at 25 ± 2 °C in a growth chamber.
2.3 Germination and growth measurement
The seeds were allowed to germinate and grown for 10 days with distilled water. The germination percentage (GP), mean germination time (MGT), and seedling vigour indices (VI) were calculated following Feizi et al. (2013). The root and shoot length, fresh and dry biomass of seedlings were measured after ten days following Rai-Kalal and Jajoo (2021).
2.4 Measurement of photosynthetic pigments, soluble sugar, and protein content
The photosynthetic pigments content was measured from ten days old seedlings. The photosynthetic pigments, such as chlorophyll (chlorophyll a and chlorophyll b) and carotenoid contents were calculated by the method of MaClachlan and Zalik (1963). The soluble sugar of seedlings was determined by the Anthrone method (McCready et al. 1950) and protein content was determined by the method described by Lowry et al. (1951).
2.5 Estimation of hydrolytic enzymes activity
The activity of amylase and protease were measured after imbibition and then periodically after 24 h, for three days from germinating seeds, and after seven and ten days from seedlings. The amylase activity was measured by the method described by Laware and Raskar (2014) with minor modifications. Briefly, 0.2 g of sample was homogenized in 1 mL of 0.1 M sodium acetate buffer, pH 4.8 in a prechilled mortar and pestle. The homogenate was then centrifuged at 10,000 rpm at 4 °C for 10 min and the supernatant was used as the source of enzyme. Then the reaction mixture contained 2 mL of acetate buffer, 0.5 mL of 1% starch, and 0.5 mL of enzyme extract was set for 10 min at 37 °C. After 10 min of incubation, 2 mL of DNS reagent was added and boiled for 10 min, and red colour was measured at 510 nm. The enzyme activity was estimated by a standard curve using maltose and enzyme activity was expressed as mg of maltose released per min per gram fresh tissue.
Similarly, the protease activity was measured by the method described by Laware and Raskar (2014) with minor modifications. In brief, 0.2 g of sample was homogenized in 2 mL of 10 mM sodium acetate buffer, pH 7.5 in a prechilled mortar and pestle. The homogenate was then centrifuged at 10,000 rpm at 4 °C for 10 min and the supernatant was used as the source of enzyme. The supernatant (1 mL) was added to 5 mL of 0.65% casein and incubated for 10 min at 37 °C. Then 5 mL of 5% H2SO4 was added to the reaction mixture and set for 30 min at 37 °C. Centrifuge the reaction mixture at 10,000 rpm for 10 min. The supernatant (2 mL) was mixed with 5 mL of 5% Na2CO3 and 1 mL of 0.5 N Folin-phenol and kept for 30 min in the dark. The blue colour developed was recorded at 660 nm. The enzyme activity was measured by a standard curve using L-tyrosine and enzyme activity was expressed as mg of tyrosine released per min per gram fresh tissue.
2.6 Analysis and histochemical localization of ROS
Superoxide radical (O2·−) was estimated from germinating seeds after 48 h following the method described by Wu and Tiedemann (2002) with minor modifications. In brief, radicles (0.5 g) were homogenized with 1.8 mL of 50 mM phosphate buffer (pH 7.5) and 0.2 mL of 10 mM hydroxylammonium chloride and set for 30 min at room temperature. The homogenate was then centrifuged at 10,000 rpm for 10 min and keep the supernatant. Then add 0.5 mL of 17 mM sulphanilamide (in 30% acetic acid) and 0.5 mL of 7 mM naphthalene diamine dihydrochloride to 0.5 mL of supernatant in order and set for 20 min at room temperature. The absorbance was recorded at 540 nm and a calibration curve was made using NaNO2. The concentration of O2·− was calculated according to 2[NO2−] = [O2·−] (mM) from the calibration curve. The histochemical localization of ROS in radicles of 12 h imbibed seeds was made following the method of Mahakham et al. (2017) and photographed through a stereomicroscope (Olympus SZ51).
2.7 Data analysis
The data of three replicates were analysed statistically by one-way analysis of variance (ANOVA) and Tukey-HSD multiple comparisons tests with IBM SPSS Statistics (Version 26). All the data shown in this study were the mean of three replicates along with standard deviation (SD). The statistical significance was made at 5% probability level.
3 Results and discussion
3.1 Synthesis and characterization of nanoparticles
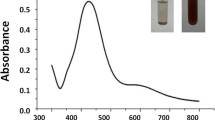
The colourless mixture of salt solution and root extract turned yellowish brown upon 24 h incubation, indicating the synthesis of silver nanoparticles (Mondal et al. 2022). In the reaction mixture, the silver ions (Ag+) were reduced to zero valent silver (Ag0) upon acceptance of an electron from the phytochemicals present in the root extract and subsequently capping turned the silver ions into silver nanoparticles (Acharya et al. 2019). The UV–Vis spectra of synthesized AgNPs from Parthenium root extract with AgNO3 is shown in Fig. 1a. Results clearly demonstrated that a sharp peak at 420 nm of AgNPs solution when evaluated with a wavelength range of 320–500 nm, indicating the formation of silver nanoparticles (Mondal et al. 2022). However, both AgNO3 and plant extract did not show any sharp peaks when they were evaluated alone (Fig. 1a). The TEM analysis of synthesized AgNPs showed that the particles are mostly spherical in nature and all particles are freely distributed (Fig. 1b). Figure 1b also represents the average particle diameter was in a range of 11–20 nm. In another microscopic study, the FESEM of synthesized AgNPs showed the particles are almost spherical in shape (Fig. 1c) and the EDX analysis ensured the presence of silver in the synthesized nanoparticles (Fig. 1d). However, a few peaks indicated the presence of gold and other elements in EDX analysis is due to the gold coating for sample preparation and presence of biomolecules as capping agents, respectively (Mondal et al. 2022). The prominent X-ray diffraction peaks (Fig. 1e) at 2θ values of 28.73°, 40.97°, 50.71°, 67.19°, and 74.23° represent the crystalline nature of synthesized AgNPs. The XRD spectra also revealed the average crystallite size of synthesized nanoparticles was 17.2 nm with crystallinity of 71.3%. Analysis of surface chemistry and possible functional groups of phytochemicals present in root extract responsible for the reduction and stabilization of synthesized AgNPs was conducted by FTIR spectra. The FTIR spectra (Fig. 1f) showed a number of prominent peaks of which a broad peak at 3122 cm−1 indicated the stretching of the alcoholic -OH group (Mondal et al. 2022). Other sharp peaks at 1516, 1398, 1201 and 880 cm−1 correspond to functional groups of NO2 of nitro compounds, CH3 and CH2 of alkanes or alkenes, C–O–C of ethers, and P-O of phosphorous compounds, respectively (Yuen et al. 2005). The functional groups were derived from the phytochemicals present in the root extract, which reduced and stabilized the AgNPs (Acharya et al. 2019). The presence of a variety of phytochemicals including alkaloids, steroids, glycosides, phenolic compounds, saponins, flavonoids, proteins, amino acids, and sugar in the aqueous extract of the root of Parthenium hysterophorus was also reported in the literature (Mondal et al. 2014; Rauf et al. 2022).
3.2 Germination performance
The overall impact of AgNPs, AgNO3, and root extract on the germination of chickpea, pea, and mung bean are depicted in Fig. 2. From Fig. 2, it is clear that the germination percentage was increased in nanotreated seeds of three tested pulses. Germination percentage was significantly increased (p < 0.05) after 24 and 48 h for both pea and mung bean at 10 and 50 mg/L AgNPs treatments over control, respectively. Moreover, the highest germination percentage was recorded at 50 and 10 mg/L AgNPs treatment for pea and mung bean, respectively. A significant (p < 0.05) increase in seed germination at lower concentration (10 mg/L) of AgNO3 treatment over control was also recorded in peas. However, a reduction in seed germination at higher concentration (100 mg/L) of both AgNPs and AgNO3 was recorded over control for all three plant species. However, no significant change in germination was recorded at different treatments over control for chickpeas. In addition, mean germination time (MGT) was also calculated and it was reduced in nanotreated seeds as compared to control for all tested plant species (Table 1). A significant (p < 0.05) reduction (15%) of MGT was recorded at 50 mg/L of AgNPs treatment in peas as compared to control. Similarly, lower concentration of AgNO3 (10 mg/L) also showed significant (p < 0.05) reduction in MGT for peas. Nanoparticles mediated higher water uptake and upregulation of hydrolytic enzymes could be attributed to higher seed germination under AgNPs treatments. Chandrasekaran et al. (2020) also highlighted the upregulation of α-amylase activity and higher starch metabolism support the rapid seed germination in nanotreated seeds. It was also suggested that upregulation of the aquaporin gene and thus faster water uptake also helps in the early initiation of seed metabolism. In the present study, higher seed germination at lower concentrations of AgNPs treatments and subsequent inhibition in a dose dependent manner suggests that the slow release of Ag+ ions maintains the ROS generation below the oxidative window and facilitates ROS signalling for a prolonged period, which upregulated the process of seed germination. The oxidative dissolution of Ag+ ions at a slower rate from AgNPs in aqueous medium was also reported by Dobias and Bernier-Latmani (2013). Increased germination under lower concentration (10 mg/L) of silver salt could be due to the Ag+ ions mediated ROS generation, which helps in seed germination (Basheerudeen et al. 2021). Overall improvement of seed germination at lower concentration of AgNO3 treatment over control and subsequent decrease with higher concentrations also support the oxidative window hypothesis (Guha et al. 2021; Xu et al. 2021). Higher seed germination in rice upon primed with zero valent iron nanoparticles suggested that nanoparticles mediated intracellular ROS generation and complex ROS signalling leads to gene expression, cell wall loosening, cellular growth, and the subsequent emergence of radicle (Song and He 2021; Basheerudeen et al. 2021). However, disruption of cellular metabolism under higher concentration of AgNO3 (100 mg/L) could be a probable reason for increased MGT for all tested plant species. Disruption of cellular metabolism could be attributed to increased accumulation of ROS in presence of AgNO3 (Vishwakarma et al. 2017). A reduction in GMT in two cultivars of beans upon AgNPs treatment was also demonstrated by Prazak et al. (2020). On the other hand, the allelopathic effect of plant extract might be a reason for the reduction in germination for both pea and mung bean under plant extract treatments (Rashid et al. 2008).
3.3 Seedling growth parameters
Root and shoot length of ten days old seedlings showed variation in different treatments for all three plant species (Table 1). The highest increase (p < 0.05) in root length of about 20% and 24% was recorded for chickpea and mung bean seedlings at 10 mg/L AgNPs treatment, respectively, whereas 9.5% (p < 0.05) for pea at 50 mg/L AgNPs treatment as compared to control. In addition, a significant increase (p < 0.05) in root length at 10 mg/L AgNO3 treatment was also recorded for chickpea over control. However, significant (p < 0.05) reduction in root length at higher concentration (100 mg/L) of both AgNPs and AgNO3 treatments were recorded for all tested plant species. Similarly, significant (p < 0.05) increase in shoot length of chickpea (13%) and mung bean (17%) were recorded at 10 mg/L AgNPs treatment, while 13% increase in shoot length was recorded at 50 mg/L AgNPs for peas as compared to control. An increase (p < 0.05) in shoot length at 10 mg/L AgNO3 treatment was also recorded in pea (Table 1). However, shoot length was reduced at higher concentrations (100 mg/L) of both AgNPs and AgNO3 treatments. A decrease in shoot length of about 20 and 16% (chickpea), 19 and 11% (pea), and 16 and 27% (mung bean) were recorded at 100 mg/L AgNPs and AgNO3 treatments, respectively, as compared to control. Moreover, shoot length was also significantly (p < 0.05) reduced only for peas under plant extract at 50 and 100 mg/L treatments. AgNPs mediated upregulation of growth hormones and activity of hydrolytic enzymes, resulting in higher availability of simple sugar support growth promotion in nanotreated seedlings (Mahakham et al. 2017). However, higher concentration of both AgNPs and AgNO3 treatments generate more oxidative stress and subsequent cell damage leading to the growth impairment of seedlings (Basheerudeen et al. 2021). Moreover, seedling growth in terms of root and shoot length was improved in plant extract treatments for chickpeas might be nutritional supplementation from biomolecules present in the plant extract. However, the decline of seedling growth of pea and mung beans under Parthenium root extract is probably due to its allelopathic effect on seedlings (Tefera 2002). Increased seedling growth upon seed priming with low concentrations (10 and 20 mg/L) of AgNPs was also reported by Mahakham et al. (2017). Janmohammadi and Sabaghnia (2015) demonstrated the improvement of shoot length in sunflowers upon pre-sowing seed treatment with silicon nanoparticles. Almost similar growth retardation of oat, lettuce, and radish seedlings upon AgNO3 treatment was also demonstrated by Tomacheski et al. (2017).
AgNPs treatments also exhibited pronounced effects on both fresh and dry biomass of seedlings for all tested pulses as compared to other treatments (Table 1). The highest increase (p < 0.05) in fresh biomass of about 15 and 14% for both pea and mung bean were recorded at 50 and 10 mg/L AgNPs treatments over control, respectively. However, a significant (p < 0.05) reduction in fresh biomass was also observed at 50 and 100 mg/L AgNO3 treatment for both chickpea and mung bean as compared to control. Moreover, no significant effect of plant extract on fresh biomass was recorded for all tested plant species. In addition, a stimulatory effect of nanotreatment on the dry biomass of seedlings was also recorded for all tested plant species (Table 1). A significant (p < 0.05) increase in dry biomass of about 17% (pea) and 24% (mung bean) was recorded at 50 and 10 mg/L AgNPs treatments as compared to control, respectively. Reduced (p < 0.05) dry biomass at higher concentration (100 mg/L) of both AgNPs and AgNO3 for pea and mung bean was also observed over control. However, no impact of plant extract on dry biomass was found in three tested plant species. Faster germination, increased root and shoot length, and improved photosynthetic pigments in nanotreated seedlings are responsible for higher accumulation of biomass (Rai-Kalal and Jajoo 2021). Improved photosynthetic activity in the presence of metal nanoparticles is chiefly attributed to a greater accumulation of photosynthates and finally the accumulation of biomass. Acharya et al. (2019) also highlighted the role of metal NPs towards the improved light-harvesting system and enhanced efficiency of chemical energy production in the chloroplast. Very recently, a stimulatory effect of metal nanoparticles on photosynthetic apparatus and photosynthetic efficiency was also described by Kumar et al. (2023). Almost similar impact of AgNPs on fresh biomass of watermelon, zucchini, and corn was reported by Almutairi and Alharbi (2015). Pre-sowing seed treatment with various metal nanoparticles (Zn, Cu, Mn, and Fe) also showed increased biomass accumulation in Cyperus esculentus (Honchar et al. 2021). However, decreased dry weight at higher concentrations of metal nanoparticles (ZnO nanoparticles) exposure in mung bean was also reported by Lakshmi et al. (2017).
On the other hand, seedling vigour indices (vigour index I and vigour index II) which correlated with seedling growth and biomass are shown in Table 1. The highest increase in vigour index I of about 19% (at 10 mg/L), 35% (at 50 mg/L), and 28% (at 10 mg/L) was recorded under AgNPs treatment for chickpea, pea, and mung bean, respectively, over control. Higher vigour index I was also recorded at lower concentration of AgNO3 treatment and it was significantly (p < 0.05) higher at 10 mg/L AgNO3 treatment over control for pea. However, the vigour index I was significantly decreased at higher concentration (100 mg/L) of both AgNPs and AgNO3 treatments for all tested plant species. Similarly, vigour index II was also significantly (p < 0.05) increased by 15% (at 10 mg/L), 25% (at 50 mg/L), and 30% (at 10 mg/L) under AgNPs treatment for chickpea, pea, and mung bean, respectively, over control. However, dose dependent decrease in vigour index II with higher concentrations of both AgNPs and AgNO3 treatments was recorded for all tested plant species. Moreover, no significant difference in vigour indices were recorded under plant extract treatment. Moreover, AgNPs mediated faster seed germination supports the early seedling establishment and higher biomass accumulation could be a reason for the improvement of seedling vigour as we found in our study. Lakshmi et al. (2017) demonstrated the improvement of seedling vigour indices in Vigna radiata upon ZnO NPs treatment and suggested that the ZnO NPs mediated improvement of seed quality during germination and subsequently results in the quenching of the free radicals in germinating seeds. In turn, the oxygen produced through this process could also be used for seed respiration, which would further promote germination and result in improving seedling vigour. Improved vigour indices were also reported in sunflowers upon pre-sowing seed treatment with silicon nanoparticles (Janmohammadi and Sabaghnia 2015).
3.4 Photosynthetic pigments
The photosynthetic pigments content (chlorophyll and carotenoids) under different treatments are presented in Table 2. Results revealed that about 11 and 10% (p < 0.05) increase in chlorophyll a for chickpea and mung bean was recorded at 10 mg/L AgNPs treatment respectively, while it increased for about 7% in pea at 50 mg/L AgNPs treatment with respect to control. Moreover, no significant effect of plant extract on chlorophyll a content was observed in this study. However, chlorophyll b content was decreased under most of the treatment conditions. An increase in chlorophyll b content of about 5% was observed only in pea at 50 mg/L AgNPs treatment with respect to control. However, a reduction in all the pigments content at higher concentration (100 mg/L) of both AgNPs and AgNO3 treatments were also observed for all three tested plant species. On the other hand, carotenoids content was also improved in nanotreated seedlings of both pea and mung bean as compared to untreated control. However, it was decreased for chickpeas at higher concentrations (100 mg/L) of AgNPs and AgNO3 treatments. However, no significant impact of plant extract on carotenoids was observed in this study. Therefore, this dose dependent variation in pigment level clearly revealed that the AgNPs might have some role in chlorophyll biosynthesis (Anusuya and Banu 2016). The decrease in chlorophyll content at higher concentrations of both AgNPs and AgNO3 is probably due to oxidative damage caused by the release of Ag+ ions which disrupt chlorophyll synthesis (Ma et al. 2015). The ROS generation within the oxidative window influences several electron acceptors of photosystem II and the efficient electron acceptors are the indicators of higher chlorophyll content in leaves (Pereira et al. 2019). Slow release of Ag+ ions from AgNPs for a longer period helps in the maintenance of oxidative stress below the oxidative window and provides better chlorophyll synthesis up to the later stage of seedlings. Very recently, Basheerudeen et al. (2021) demonstrated an almost similar effect of AgNPs on photosynthetic pigments of Vigna radiata. Nair and Chung (2015) also showed the improvement of total chlorophyll in Vigna radiata upon AgNPs treatments up to 20 mg/L and subsequently decreased the chlorophyll content at higher concentrations.
3.5 Soluble sugar and protein content
Along with the improvement of photosynthetic pigments, higher amounts of soluble sugar and protein content in nanotreated seedlings were recorded as compared to control for three tested plant species, and the entire results are depicted in Table 2. Results revealed that significant increase (p < 0.05) in sugar levels of about 1.31- and 1.16-folds over control were recorded in pea and mung bean at 50 and 10 mg/L AgNPs treatments, respectively. About 1.54-folds increase in sugar content was also recorded for chickpeas at 10 mg/L AgNPs with respect to control. However, decreased sugar content at higher concentration (100 mg/L) of both AgNPs and AgNO3 for chickpeas (p < 0.05), peas, and mung beans indicated their toxic effect on plants beyond a threshold level. On the other hand, the protein content was increased at lower concentration (10 mg/L) of AgNPs and decreased in higher concentrations (50 and 100 mg/L) as well as in all concentrations of AgNO3 treatment in chickpea. However, a significant increase (p < 0.05) in protein content was recorded at 50 mg/L in peas, but subsequently, at higher concentration of both AgNPs and plant extract showed a sharp reduction in protein level over control. About 12.3% increase in protein content in mung bean at 10 mg/L AgNPs treatment was also recorded as compared to control. Moreover, the highest reduction in protein content was found at 100 mg/L AgNO3 treatment for mung bean, suggesting the toxic effect of silver ions on plant metabolism. Higher accumulation of both sugar and protein content in nanotreated seedlings might be due to the improved rate of photosynthesis. It was suggested that soluble sugar content is directly linked with the photosynthetic efficiency and total chlorophyll content of seedlings (Mahakham et al. 2016). Increased sugar content in onion upon seeds primed with AgNPs was also reported by Acharya et al. (2019). They also suggested that the higher chlorophyll content is directly linked with increased total photosynthates in plants. Tymoszuk (2021) also highlighted the increased accumulation of photosynthetic pigments and higher protein content in radish seedlings at lower concentration (50 mg/L) of AgNPs treatment and subsequently declined at higher concentration (100 mg/L).
3.6 Activity of hydrolytic enzymes
During seed germination, the reserved starch is hydrolyzed into soluble sugar by amylase, whereas storage protein breaks down into amino acids by protease to provide nutrients and energy for germination (Botcha and Prattipati 2020; Laware and Raskar 2014). The activity of both amylase and protease was measured starting from seed imbibition to the later stage of seedling growth under different treatments and presented in Figs. 3, 4, 5, 6, 7 and 8. Results revealed that a significant increase (p < 0.05) of amylase activity during the early stage of seed emergence (up to 24 h) of AgNPs (10 mg/L) treated seeds of chickpea, while subsequently reduced in the later stage of seedling growth with respect to control (Fig. 3). In peas, up to 72 h of germination, the amylase activity was higher and subsequently decreased after the seventh and tenth day at 50 mg/L AgNPs treatment over control and other treatments (Fig. 4). Similarly, in mung bean, at 10 mg/L AgNPs treatment the amylase activity was significantly (p < 0.05) higher up to 72 h and decreased at seventh day as compared to control (Fig. 5). Enhancement of enzyme activity in early stage of seedling growth could be a positive crosstalk between phytohormones (gibberellin and auxin) and ROS and which subsequently triggered the gene expression for amylase (Chandrasekaran et al. 2020). Moreover, the highest reduction (p < 0.05) in amylase activity at 100 mg/L AgNO3 treatment as well as decreased at higher concentrations of AgNPs (50 and 100 mg/L) suggested the toxic effect of silver might cause oxidative stress generation above the threshold level, which in turn adversely affected cellular metabolism and resulted in decreased expression of amylase. It was also suggested that the Ag+ ions can easily interact with enzymes and inhibit their activity (Xu et al. 2021). However, other treatments (AgNO3 and plant extract) showed lower amylase activity after the tenth day over control. This might be due to the early seedling establishment and subsequent growth along with improved photosynthetic pigment helps in efficient photosynthesis and therefore higher availability of sugar responsible for lowering the amylase activity (Botcha and Prattipati 2020). An almost similar observation of increased amylase activity during germination in AgNPs treated chickpea seeds was described by Anusuya and Banu (2016). Higher amylase activity and starch metabolism in AgNPs primed rice seeds were also demonstrated earlier by Mahakham et al. (2017).
On the other hand, protease activity was also increased in AgNPs treated seeds during early stage of germination and decreased at later stage growth for all tested plant species. The maximum protease activity was recorded after 48 h in chickpea seeds at 10 mg/L AgNPs treatment and it was about 1.42-folds higher (p < 0.05) than control (Fig. 6). However, protease activity in early stage (up to 72 h) was decreased at higher concentrations (50 and 100 mg/L) of both AgNPs and AgNO3 treatments. Both pea (Fig. 7) and mung bean (Fig. 8) showed similar patterns of protease activity under AgNPs treatments at 50 and 10 mg/L, respectively. A significant increase (p < 0.05) of about 26% in protease activity was recorded after 24 h at 50 mg/L AgNPs treatment for pea and of about 33 and 55% at 10 mg/L AgNPs treatment after 48 and 72 h for mung bean as compared to control, respectively. However, more interestingly, protease activity decreased after the seventh and tenth day at 10 mg/L (for chickpea and mung bean) and 50 mg/L (for pea) AgNPs treatments as compared to control and other treatments. Almost similar observation was made during rice seed germination, where protease activity was higher at 60 h and subsequently decreased upon exposure of zero valent iron nanoparticles (Guha et al. 2021). However, no significant impact of plant extract on protease activity was recorded for all tested plant species. Rapid utilization of storage protein and subsequent growth of radicle enables faster seed germination and later growth of seedlings. Lower protease activity after 48 h also suggested the early utilization of storage protein in seeds (Botcha and Prattipati 2020). AgNPs and AgNO3 mediated oxidative stress might regulate the dose dependent protease activity during seed germination. Previously, TiO2 NPs mediated enhancement of protease activity during seed germination in Allium cepa was also reported by Laware and Raskar (2014).
3.7 Analysis and histochemical localization of ROS
Accumulation of superoxide anion (O2−) in germinating seeds (after 48 h) at different treatments was measured and is shown in Table 2. Dose dependant significant increase (p < 0.05) of O2− content was recorded under both AgNPs and AgNO3 treatments in all tested plant species. However, the highest increase in O2− content of about 1.67, 2.23- and 2.42-folds over control was recorded in chickpea, pea, and mung bean at 100 mg/L of AgNO3 treatment, respectively. Moreover, a higher accumulation of O2− content at 100 mg/L of AgNPs treatment was also recorded over both control and other concentrations of AgNPs treatments. Ag+ ions mediated generation of ROS leads to the highest accumulation of O2− under AgNO3 treatment and slow release of Ag+ ions from AgNPs might restrict over production of O2− as compared to AgNO3. The slow release of Ag+ ions from AgNPs was also previously described by earlier researchers (Tomacheski et al. 2017; Dobias and Bernier-Latmani 2013; Kittler et al. 2010). Significant increase (p < 0.05) of O2− at lower dose of AgNPs treatments suggested the ROS generation at a certain level might help in seed metabolism and subsequent seedling establishment. Therefore, the generation of oxidative stress below the ‘oxidative window’ and subsequent ROS signalling promote seed germination. Almost a similar effect of zero valent iron nanoparticles on ROS homeostasis and ROS signalling mediated regulation of hydrolytic enzymes during seed germination in rice was also explained by Guha et al. (2021). Moreover, greater accumulation of O2− at higher concentrations of both AgNPs and AgNO3, and subsequent growth inhibition might be due to ROS mediated damages in the cell membrane, nucleic acid, and disruption of cellular metabolisms (Vishwakarma et al. 2017). Nair and Chung (2014) also highlighted the similar dose dependent increase in ROS generation under AgNPs treatment in Arabidopsis thaliana. Metal nanoparticles mediated ROS generation and their toxicity in plants were also reported by several researchers (Guha et al. 2021; Ma et al. 2015). However, no significant increase in O2− content was found in root extract treatments and this suggested the O2− generation in germinating seeds due to the action of Ag+ ions released from either AgNPs or AgNO3.
On the other hand, histochemical localization of ROS (O2− and H2O2) after 12 h imbibition under different treatments is shown in Fig. 9. Maximum intensity of NBT staining at higher concentration of both AgNPs and AgNO3 treated seeds for all tested plant species as compared to control, suggested the higher accumulation of both O2− and H2O2 (Mahakham et al. 2017). Almost similar effects of both AgNPs and AgNO3 on the generation of oxidative stress in the roots of mustard using NBT staining were shown by Vishwakarma et al. (2017).
Histochemical localization of ROS (O2− and H2O2) after 12 h imbibition of seeds with NBT staining. Representative images of imbibed seeds of a control, b 10 mg/L AgNPs, c 50 mg/L AgNPs, d 100 mg/L AgNPs, e 10 mg/L AgNO3, f 50 mg/L AgNO3, g 100 mg/L AgNO3, h 10 mg/L extract, i 50 mg/L extract, and j 100 mg/L extract treatments are presented
3.8 Mechanism of nanoparticles induced early seedling establishment
Figure 10 demonstrated a tentative mechanism of AgNPs induced seed germination and seedling growth. From Fig. 10, it is clear that AgNPs easily penetrated the seed coat and generated Ag+ ions inside the seed, which is supposed to accelerate the generation of ROS. Almost similar line of thought was reported in earlier literature (Xu et al. 2021; Tomacheski et al. 2017; Dobias and Bernier-Latmani 2013). Therefore, AgNPs might maintain the Ag+ ions at the threshold level and generate oxidative stress below the ‘oxidative window’ for a prolonged time as compared to AgNO3. This nanoinduced mechanism also revealed that the balanced ROS acting as a messenger for cell signalling (MAPK cascade) towards the upregulation of hydrolytic enzymes (i.e. amylase, protease, etc.) which is essential for the mobilization of stored food (Guha et al. 2021). The generation of oxidative stress below the ‘oxidative window’ triggers the essential metabolic processes during seed germination (Xu et al. 2021). Therefore, this ROS could be the main driving force towards the dislocation of cell walls, weakening of seed coat and endosperm, and subsequently early emergence of the radicle (Song and He 2021; Basheerudeen et al. 2021).
4 Conclusion
The present study showed the application of AgNPs enhanced the seed germination and seedling growth in three pulses. Nanoparticles mediated alteration of cellular metabolism related to enhanced enzyme activity and ROS generation at threshold level during germination might alter the seed physiology and metabolism that favoured rapid seed germination and subsequent seedling growth. The increased activity of both amylase and protease in nanotreated seeds indicates that the AgNPs has some role in the modulation of activity of hydrolytic enzymes during early seedling establishment. However, further studies are needed to investigate the exact mechanism of action of nanoparticles on enzyme activity, whether silver nanoparticle enhances gene expression for hydrolytic enzymes through phytohormones signalling or directly binds with enzymes as an activator and alter their activity.
References
Acharya P, Jayaprakasha GK, Crosby KM, Jifon JL, Patil BS (2019) Green-synthesized nanoparticles enhanced seedling growth, yield, and quality of onion (Allium cepa L.). ACS Sustain Chem Eng 7(17):14580–14590. https://doi.org/10.1021/acssuschemeng.9b02180
Alabdallah NM, Hasan MM (2021) Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J Biol Sci 28(10):5631–5639. https://doi.org/10.1016/j.sjbs.2021.05.081
Almutairi ZM, Alharbi A (2015) Effect of silver nanoparticles on seed germination of crop plants. Int J Sci Res 9(6):594–598
Anusuya S, Banu KN (2016) Silver-chitosan nanoparticles induced biochemicalvariations of chickpea (Cicer arietinum L.). Biocatal Agric Biotechnol 8:39–44. https://doi.org/10.1016/j.bcab.2016.08.005
Avezum L, Rondet E, Mestres C, Achir N, Madode Y, Gibert O, Lefevre C, Hemery Y, Verdeil JL, Rajjou L (2022) Improving the nutritional quality of pulses via germination. Food Rev Int. https://doi.org/10.1080/87559129.2022.2063329
Basheerudeen MAH, Mushtaq SA, Soundhararajan R, Nachimuthu SK (2021) Marine endophytic fungi mediated Silver nanopa application in plant growth promotion in rticles and their Vigna radiata L. Int J Nano Dimens 12(1):1–10
Bhardwaj J, Anand A, Nagarajan S (2012) Biochemical and biophysical changes associated with magnetopriming in germinating cucumber seeds. Plant Physiol Biochem 57:67–73. https://doi.org/10.1016/j.plaphy.2012.05.008
Botcha S, Prattipati SD (2020) Role of amylase and protease in germinating Sterculia urens Roxb. Bangladesh J Sci Ind Res 55(2):107–112. https://doi.org/10.3329/bjsir.v55i2.47631
Chandrasekaran U, Luo X, Wang Q, Shu K (2020) Are there unidentified factors involved in the germination of nanoprimed seeds? Front Plant Sci 11:832. https://doi.org/10.3389/fpls.2020.00832
Chau NH, Doan QH, Chu TH, Nguyen TT, Dao Trong H, Ngo QB (2019) Effects of different nanoscale microelement-containing formulations for presowing seed treatment on growth of soybean seedlings. J Chem 20:19. https://doi.org/10.1155/2019/8060316
Debnath P, Mondal A, Sen K, Mishra D, Mondal NK (2020) Genotoxicity study of nano Al2O3, TiO2 and ZnO along with UV-B exposure: An Allium cepa root tip assay. Sci Total Environ 713:136592. https://doi.org/10.1016/j.scitotenv.2020.136592
Dobias J, Bernier-Latmani R (2013) Silver release from silver nanoparticles in natural waters. Environ Sci Technol 47(9):4140–4146. https://doi.org/10.1021/es304023p
Feizi H, Kamali M, Jafari L, Moghaddam PR (2013) Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill). Chemosphere 91(4):506–511. https://doi.org/10.1016/j.chemosphere.2012.12.012
Guha T, Das H, Mukherjee A, Kundu R (2021) Elucidating ROS signaling networks and physiological changes involved in nanoscale zero valent iron primed rice seed germination sensu stricto. Free Radic Biol Med 171:11–25. https://doi.org/10.1016/j.freeradbiomed.2021.05.005
Honchar L, Mazurenko B, Shutyi O, Pylypenko V, Rakhmetov D (2021) Effect of pre-seed and foliar treatment with nano-particle solutions on seedling development of tiger nut (Cyperus Esculentus L.) plants. Agron Res 19(S1):767–776. https://doi.org/10.15159/ar.21.021
Janmohammadi M, Sabaghnia N (2015) Effect of pre-sowing seed treatments with silicon nanoparticles on germinability of sunflower (Helianthus annuus). Bot Lith 21(1):13–21. https://doi.org/10.1515/botlit-2015-0002
Khan I, Awan SA, Rizwan M, Akram MA, Zia-ur-Rehman M, Wang X, Zhang X, Huang L (2023) Physiological and transcriptome analyses demonstrate the silver nanoparticles mediated alleviation of salt stress in pearl millet (Pennisetum glaucum L). Environ Pollut 318:120863. https://doi.org/10.1016/j.envpol.2022.120863
Kittler S, Greulich C, Diendorf J, Koller M, Epple M (2010) Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater 22(16):4548–4554. https://doi.org/10.1021/cm100023p
Kumar D, Dhankher OP, Tripathi RD, Seth CS (2023) Titanium dioxide nanoparticles potentially regulate the mechanism(s) for photosynthetic attributes, genotoxicity, antioxidants defense machinery, and phytochelatins synthesis in relation to hexavalent chromium toxicity in Helianthus annuus L. J Hazard Mater 454:131418. https://doi.org/10.1016/j.jhazmat.2023.131418/cm100023p
Laware SL, Raskar S (2014) Effect of titanium dioxide nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion. Int J Curr Microbiol App Sci 3(7):749–760
Lakshmi SJ, Bai RR, Sharanagouda H, Ramachandra CT, Nadagouda S, Doddagoudar SR (2017) Biosynthesis and characterization of ZnO nanoparticles from spinach (Spinacia oleracea) leaves and its effect on seed quality parameters of greengram (Vigna radiata). Int J Curr Microbiol Appl Sci 6(9):3376–3384. https://doi.org/10.20546/ijcmas.2017.609.416
Lowry O, Rosebrough N, Farr AL, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Ma C, White JC, Dhankher OP, Xing B (2015) Metal-based nanotoxicity and detoxification pathways in higher plants. Environ Sci Technol 49(12):7109–7122. https://doi.org/10.1021/acs.est.5b00685
Maclachlan S, Zalik S (1963) Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can J Bot 41(7):1053–1062. https://doi.org/10.1139/b63-088
Mahakham W, Theerakulpisut P, Maensiri S, Phumying S, Sarmah AK (2016) Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci Total Environ 573:1089–1102. https://doi.org/10.1016/j.scitotenv.2016.08.120
Mahakham W, Sarmah AK, Maensiri S, Theerakulpisut P (2017) Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci Rep 7(1):1–21. https://doi.org/10.1038/s41598-017-08669-5
McCready RM, Guggolz J, Silviera V, Owens HS (1950) Determination of starch and amylose in vegetables. Anal Chem 22(9):1156–1158. https://doi.org/10.1021/ac60045a016
Mondal NK, Chowdhury A, Dey U, Mukhopadhya P, Chatterjee S, Das K, Datta JK (2014) Green synthesis of silver nanoparticles and its application for mosquito control. Asian Pac J Trop Dis 4(1):S204–S210. https://doi.org/10.1016/S2222-1808(14)60440-0
Mondal A, Sen K, Mondal A, Mishra D, Debnath P, Mondal NK (2022) Bio-fabricated silver nanoparticles for controlling dengue and filaria vectors and their characterization, as well as toxicological risk assessment in aquatic mesocosms. Environ Res 212:113309. https://doi.org/10.1016/j.envres.2022.113309
Nair PMG, Chung IM (2014) Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ Sci Pollut Res 21:8858–8869. https://doi.org/10.1007/s11356-014-2822-y
Nair PMG, Chung IM (2015) Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna radiata L.). Acta Physiol Plant 37(1):1–11. https://doi.org/10.1007/s11738-014-1719-1
Oracz K, Karpinski S (2016) Phytohormones signaling pathways and ROS involvement in seed germination. Front Plant Sci 7:864. https://doi.org/10.3389/fpls.2016.00864
Pereira ADES, Oliveira HC, Fraceto LF (2019) Polymeric nanoparticles as an alternative for application of gibberellic acid in sustainable agriculture: a field study. Sci Rep 9(1):1–10. https://doi.org/10.1016/j.fbio.2021.100978
Pérez-de-Luque A (2017) Interaction of nanomaterials with plants: what do we need for real applications in agriculture? Front Environ Sci. https://doi.org/10.3389/fenvs.2017.00012
Prażak R, Święciło A, Krzepiłko A, Michałek S, Arczewska M (2020) Impact of Ag nanoparticles on seed germination and seedling growth of green beans in normal and chill temperatures. Agriculture 10(8):312. https://doi.org/10.3390/agriculture10080312
Rai-Kalal P, Jajoo A (2021) Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol Biochem 160:341–351. https://doi.org/10.1016/j.plaphy.2021.01.032
Rashid H, Khan MA, Amin A, Nawab K, Hussain N, Bhowmik PK (2008) Effect of Parthenium hysterophorus L., root extracts on seed germination and growth of maize and barley. Am J Plant Sci Biotech 2(2):51–55
Rauf A, Khan IA, Alnasser SM, Shah SUA, Rahman MM (2022) Phytochemical analysis and in vitro and in vivo pharmacological evaluation of Parthenium hysterophorus Linn. Evid Based Complem Altern Med 2022:6088585. https://doi.org/10.1155/2022/6088585
Song K, He X (2021) How to improve seed germination with green nanopriming. Seed Sci Technol 49(2):81–92. https://doi.org/10.15258/sst.2021.49.2.01
Stefano DE, Tsopmo A, Oliviero T, Fogliano V, Udenigwe CC (2019) Bioprocessing of common pulses changed seed microstructures, and improved dipeptidyl peptidase-IV and α-glucosidase inhibitory activities. Sci Rep 9(1):1–13. https://doi.org/10.1038/s41598-019-51547-5
Tefera T (2002) Allelopathic effects of Parthenium hysterophorus extracts on seed germination and seedling growth of Eragrostis tef. J Agron Crop Sci 188(5):306–310. https://doi.org/10.1046/j.1439-037X.2002.00564.x
Tomacheski D, Pittol M, Simões DN, Ribeiro VF, Santana RMC (2017) Impact of silver ions and silver nanoparticles on the plant growth and soil microorganisms. Glob J Environ Sci Manag 3(4):341–350. https://doi.org/10.22034/gjesm.2017.03.04.001
Tymoszuk A (2021) Silver nanoparticles effects on in vitro germination, growth, and biochemical activity of tomato, radish, and kale seedlings. Materials 14(18):5340. https://doi.org/10.3390/ma14185340
Vishwakarma K, Shweta UN, Singh J, Liu S, Singh VP, Prasad SM, Chauhan DK, Tripathi DK, Sharma S (2017) Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front Plant Sci 8:1501. https://doi.org/10.3389/fpls.2017.01501
Wu YX, von Tiedemann A (2002) Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ Pollut 116(1):37–47. https://doi.org/10.1016/S0269-7491(01)00174-9
Xu L, Zhu Z, Sun DW (2021) Bioinspired nanomodification strategies: moving from hemical-based agrosystems to sustainable agriculture. ACS Nano 15(8):12655–12686. https://doi.org/10.1021/acsnano.1c03948
Yuen CWM, Ku SKA, Choi PSR, Kan CW, Tsang SY (2005) Determining functional groups of commercially available ink-jet printing reactive dyes using infrared pectroscopy. Res J Text Appar 9(2):26–38. https://doi.org/10.1108/RJTA-09-02-2005-B004
Acknowledgements
The authors express their sincere thanks to all the faculty members including technical staff of the Department of Environmental Science and University Science Instrumentation Centre (USIC), The University of Burdwan, for their moral and technical support.
Funding
This work was supported by Swami Vivekananda Merit cum Means Fellowship (WBP211642404696), Govt. of West Bengal, West Bengal, India.
Author information
Authors and Affiliations
Contributions
RK contributed to conceptualization, methodology, investigation, formal analysis, writing—original draft. AM contributed to methodology, investigation, formal analysis, writing—review and editing. NKM contributed to conceptualization, formal analysis, resources, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koley, R., Mondal, A. & Mondal, N.K. Green synthesized silver nanoparticles mediated regulation on hydrolytic enzymes and ROS homeostasis promote growth of three pulses (Cicer arietinum L., Pisum sativum L., and Vigna radiata L.). Energ. Ecol. Environ. 8, 537–555 (2023). https://doi.org/10.1007/s40974-023-00293-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40974-023-00293-6