Abstract
Sophora davidii is a multipurpose, nitrogen-fixing shrub species. Phosphorus deficiency in the acidic soil of Southwest China has seriously affected its survival and growth, especially in the seedling stage. Evidence suggests that arbuscular mycorrhizal fungi (AMF) may improve the stress tolerance of plants. However, there is limited information on the systematic effects of AMF on phosphorus deficiency in S. davidii seedlings. We investigated the effects of three phosphorus levels (0.5, 0.25, 0 mmol/L) and two mycorrhizal inoculation (with Funneliformis mosseae and without Funneliformis mosseae) treatments on the growth and physiological performance of S. davidii using factorial design. The results showed that low-phosphorus stress significantly limited the growth of S. davidii seedlings and negatively affected their physiological properties. However, inoculation with F. mosseae significantly improved the plant height and shoot dry weight, promoted root growth, increased chlorophyll contents and osmoregulation substance contents, increased protective enzyme activity, and significantly reducing the accumulation of malondialdehyde, alleviated oxidative stress induced by low-phosphorus stress, improved the IAA and GA3 contents, and alleviated the negative effects of low-phosphorus stress. AMF-induced enhancement of aboveground growth and plant physiological characteristics is dependent on the P level and its impact on roots is regardless of phosphorus status. AMF inoculation significantly promoted the absorption of nitrogen and phosphorus in the roots (0.25- and 0-mmol/L treatments), thereby maintaining a higher biomass and relieving stress in S. davidii seedlings under low-phosphorus conditions. Our results demonstrated that AMF inoculation is useful for the promotion and cultivation of S. davidii in the karst area of Southwest China under low-phosphorus stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus is a macroelement that is necessary for plant growth and development. It is a component of important compounds such as phospholipids, nucleic acids, and nucleoproteins and participates in physiological and biochemical processes, such as plant metabolism, photosynthesis, and respiration (Blank 2012; Kumar et al. 2015). According to statistics, more than 5.7 billion hectares of arable soil worldwide are deficient in available phosphorus (Yamaji et al. 2017). For example, phosphorus is present in soil solutions at concentrations of 0.1–10 µM/L in tropical soils, which is much lower than the adequate concentration needed for the optimal growth of many crops (Liu et al. 2016). Plants absorb phosphorus in the form of acid phosphates (H2PO4−, HPO42−) (Kazadi et al. 2022). However, these two forms are easily immobilized with soil particles or metal ions (Al3+, Ca2+, Fe2+, etc.), which reduces the absorption and utilization of soil phosphorus by plants (Baghbani-Arani et al. 2021; Li et al. 2011). The application of phosphorus fertilizer can alleviate the shortage of soil available phosphorus to a certain extent. However, phosphorus has low mobility in the soil and is easily fixed. Studies have reported that a long-term low-phosphorus environment reduces plant nodule development and nitrogen fixation ability and increases flower shedding and plant dwarfism. Under low-phosphorus stress, the plant seedling emergence rate is low, and seed size and yield are rapidly reduced (Zhang et al. 2010). The decrease in plant growth and productivity caused by low-phosphorus stress is the main challenge facing current production practices. Therefore, how to increase the available phosphorus content in the soil and improve phosphorus use efficiency through biological measures is a key problem to be solved in current crop production.
Rhizosphere microorganisms dissolve and mineralize poorly soluble organic and inorganic phosphates in soil (Sharma et al. 2013; Oliveira et al. 2009); thus, they can improve the absorption and utilization of phosphorus by crops. In particular, arbuscular mycorrhizal fungi (AMF) can form a mutualistic relationship with 80% of terrestrial plants (Bahadur et al. 2019). Plants deliver carbohydrates to mycorrhizal fungi, which deliver nutrients and water to plants to improve host plant resistance to biotic and abiotic stresses (Abdel Hamed Abdel Latef et al. 2016). Various plant studies have shown that arbuscular mycorrhiza (AM) formation increases antioxidant enzyme activity, reduces the accumulation of malondialdehyde and hydrogen peroxide (Benhiba et al. 2015), and enhances the accumulation of soluble sugars in host plants (Jia et al. 2019). Increases in host plant biomass and leaf photosynthesis promote phosphorus absorption and utilization (Diao et al. 2021). It has also been reported that AMF hyphae can produce gibberellins (GA) and cytokinins (CTKs) and can also synthesize auxin (indole-3-acetic acid, IAA) and ethylene, which play an important role in the symbiosis between AMF and plants and are beneficial for plant adaptation to low-phosphorus environments (Feng et al. 2020; Campo and Segundo 2020). Therefore, exploring the effects and mechanisms of mycorrhizae on plant growth and physiology is of great significance for improving phosphorus utilization efficiency, coping with adversity, and promoting agricultural development.
Sophora davidii is a multipurpose, nitrogen-fixing shrub species belonging to the family Leguminosae (Zhang et al. 2021; Ying et al. 2021). Owing to its high feed, medicinal, and ecological protection value, it has been extensively planted as a feed and ecological protection shrub to provide forage and reduce water and soil loss in the Loess Plateau and southwest karst areas of China (Ying et al. 2021). However, its physical dormancy (hard seed), low seedling emergence rate, and low seedling growth rate make seedling propagation difficult (Zhao et al. 2022). The acidic soil in the south exhibits strong phosphorus fixation and low soil nutrient availability (N, P) (Liang et al. 2022). The shortage of phosphorus, especially in the acidic soil of southwestern China, is a key factor affecting the survival and rapid growth of S. davidii seedlings. Therefore, new approaches to improve the survival and growth performance of S. davidii seedlings and enhance their adaptability to low-phosphorus stress are necessary for animal husbandry and ecological environment protection in southwestern China. Previous studies indicated that low-phosphorus stress (Zhao et al. 2021) and drought stress (Zhao et al. 2022) can affect the growth of S. davidii seedlings. AMF have been shown to improve phosphorus absorption and utilization in many shrubs (Liu et al. 2021). However, there are few reports on the use of AMF to explore the phenotypic adaptations and physiological responses of S. davidii. To further improve the stress resistance of S. davidii seedlings, a pot experiment was carried out to study the effects of AMF on the growth response, root morphology, and physiological responses of S. davidii seedlings under low phosphorus. These findings provide a theoretical basis for using AMF to fully promote and utilize S. davidii resources.
Materials and Methods
Experimental Materials
The S. davidii seeds used in this study were preserved in the laboratory of Guizhou University, China. The arbuscular mycorrhizal inoculum (AMF, Funneliformis mosseae) was obtained from the College of Horticulture, Yangtze University, China.
Experimental Design
The pot experiment was conducted from April to July 2021 in the greenhouse of Guizhou University using a factorial design, including phosphorus and inoculation treatments. Plump and uniform seeds of S. davidii were soaked and germinated. At 14 days after germination, seedlings with uniform growth were transplanted into 4.5-L plastic pots with quartz sand and vermiculite (1:1), with 10 plants in each pot. The roots were inoculated with 10 g of AMF-containing culture soil (AM, available phosphorus was 50.2 mg/kg) or high-temperature sterilized (121 °C, 2 h) AMF culture soil (NAM), and plants were allowed to grow for 60 days. During the experimental growth period, the temperature in the greenhouse was controlled at 20–25 °C, and the humidity was 60%. During this period, Hoagland nutrient solution containing 0.5-mmol/L KH2PO4 (Zhao et al. 2021) was added every two days to ensure that the plant nutrient and water requirements were met. Root samples of three seedlings were randomly selected for the detection of AMF colonization. After successful colonization, three phosphorus treatments were set for the AM and NAM treatment groups: P0.5 (0.5-mmol/L KH2PO4 Hoagland nutrient solution), P0.25 (0.25-mmol/L KH2PO4 Hoagland nutrient solution), and P0 (0-mmol/L KH2PO4 Hoagland nutrient solution). Each treatment was replicated 7 times. HCl and NaOH were used to adjust the pH value of the nutrient solution to 6.0 ± 0.1, and KH2PO4 was used as a phosphorus source (Zhao et al. 2021). Samples were taken after 21 days of low-phosphorus stress treatment and stored at − 80 °C.
Index Determination Methods
Measurement of AMF Colonization
Seedling roots were rinsed with running tap water and then with distilled water several times. Samples were randomly cut into 1-cm-long root fragments. The small root segments were immersed in 10% (w/v) KOH solution and placed in a water bath at 90 °C for 30 min. After washing the residual KOH with distilled water, the root segments were stained in trypan blue at 90 °C for 30 min. Samples were then decolorized with a mixture of lactic acid and glycerin (v/v = 1:1) three times (Chen et al. 2020). The AMF colonization rate was calculated according to the magnifying cross method. The colonization rate of AMF is determined as follows: AMF colonization rate = (hyphae intersection + vesicle intersection + arbuscular intersection)/total intersection × 100%.
Measurement of Growth Indexes
The plant height of five plants per pot was measured with a ruler. Root and leaf samples were scanned with an Epson Perfection V800 photo scanner. The root parameters (total root length, root surface area, root volume, average root diameter, and root tip number) were analyzed using Win-RHIZO software (Regent Instructions Inc., Quebec, Canada). The fresh seedlings were divided into shoots and roots, placed in an oven at 105 °C for 20 min and dried at 65 °C to a constant weight followed by weighing. The root-shoot ratio was calculated using the formula: root-shoot ratio = dry weight of the roots (mg)/dry weight of the shoot part (mg).
Measurement of Physiological Indexes
The contents of proline (Pro), soluble sugar (SS), soluble protein (SP), and malondialdehyde (MDA) in the roots were determined by ninhydrin colorimetry, anthrone colorimetry, Coomassie brilliant blue G-250 staining, and thiobarbituric acid assay, respectively. The activity levels of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and acid phosphatase (ACP) in the roots were determined by nitroblue tetrazolium assay, guaiacol assay, UV–Visible spectrophotometry, and p-nitrophenyl phosphate (PNPP), respectively. These methods were performed in accordance with the experimental methods of Zhao et al. (2021) and Li (2000).
Chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoid contents in the leaves were determined according to the method of Li (2000).
Measurement of Endogenous Hormones
Auxin (indole-3-acetic acid, IAA), gibberellin (GA3), and brassinolide (BR) levels in the leaves and roots were determined using high-performance liquid chromatography (Cao et al. 2014; Tarkowski et al. 2009).
Measurement of Mineral Elements
Powdered root, stem, and leaf samples were sieved through a 0.45-mm sieve, and the N and P concentrations were determined using the Kjeldahl method and molybdenum-stibium colorimetry (Bao 2000), respectively.
Data Processing
Microsoft Excel 2010 was used for data sorting, and Tukey’s HSD method was used to perform variance analysis on the various S. davidii parameters (SPSS Inc., Chicago, version 20.0). All data are reported as the mean ± standard error (SE). SigmaPlot 14.0 was used to draw the graphs.
Results and Analysis
Root AMF Colonization Rate
Significant mycorrhizal colonization was observed in mycorrhizae-inoculated seedlings. Under the P0.5 (Fig. 1B), P0.25 (Fig. 1C), and P0 (Fig. 1D) conditions, mycorrhizal hyphae formed different morphological structures, indicating that the AMF could form a symbiotic relationship with the seedlings. Inoculation with AMF significantly increased the AMF colonization rate in the roots of S. davidii seedlings (P < 0.05) (Fig. 1E). The lower the phosphorus concentration was, the higher the AMF infection rate (P < 0.05) of S. davidii seedlings, and the AMF infection rates showed the following trend: P0 > P0.25 > P0.5.
Development of arbuscular mycorrhizal fungus (AMF) in S. davidii seedling roots visualized by Trypan blue staining. A The root of a non-inoculated plant. B Inoculated roots under the P0.5 treatment, C P0.25 treatment, and D P0 treatment, and E colonization rate of mycorrhizal S. davidii seedlings. Ih, intraradical hyphae; Ar, arbuscule; Vs, vesicles; A, B, C and D, G: × 400). NAM, non-AMF-inoculated; AM, AMF inoculated. Colonization rate** means P < 0.01. Values are means ± SE (n = 3). Bar: 200 μm
Effects of AMF on the Growth Indexes of S. davidii Under Low-Phosphorus Stress
Plant Height and Biomass
With the intensification of low-phosphorus stress (inoculated or not inoculated with AMF), plant height and shoot dry weight showed a downward trend, while root-shoot ratio showed an upward trend, and the root dry weight showed a trend of first increasing and then decreasing (Table 1). The root dry weight significantly decreased in P0 treatments. Low phosphorus stress significantly inhibited the increase of S. davidii seedlings. Compared with NAM, inoculation with AMF significantly increased the plant height and shoot dry weight under the P0.25 and P0 treatments and significantly increased the root dry weight under the P0.5 and P0 treatments (P < 0.05).
Root System Architecture
Low phosphorus stress significantly affected root growth, and AMF inoculation significantly improved root development (Fig. 2). Without AMF inoculation, the total root length, total root surface area, root volume, root tip number, and root hair number of S. davidii first increased and then decreased with increasing low-phosphorus stress intensity, and all were the largest under the P0.25 treatment, with values significantly larger than those under the other treatments (P < 0.05) (Table 2). Compared with NAM, inoculation with AMF significantly increased the total root length, total root surface area, root volume, root tip number (except the P0.25 treatment), and root hair number of S. davidii in the P0, P0.25, and P0.5 treatments (P < 0.05). Inoculation with AMF, phosphorus stress, and the interaction of AMF × P resulted show no significant difference in root diameter (P > 0.05). After inoculation with AMF, the total root length, total root surface area, root volume, root tip number, and root hair number were the largest under the P0.25 treatment (Table 2).
Effects of AMF on the Physiological Indexes of S. davidii Under Low-Phosphorus Stress
Chlorophyll Content
Without AMF inoculation, the chlorophyll a, chlorophyll b, and carotenoid contents of S. davidii significantly decreased with increasing low-phosphorus stress intensity (P < 0.05) (Table 3). Compared with NAM, inoculation with AMF significantly increased the chlorophyll a, chlorophyll b, and carotenoid contents of S. davidii in the P0.25 and P0 treatments (P < 0.05).
Osmotic Regulatory Substance Content
Without AMF inoculation, the contents of proline, soluble sugar, and soluble protein in S. davidii increased with the intensification of low-phosphorus stress, all reaching a maximum in the P0 treatment, with levels significantly higher than those in the P0.5 and P0.25 treatments (P < 0.05). Compared with NAM, inoculation with AMF significantly increased the proline, soluble sugar, and soluble protein contents under the P0.25 and P0 treatments (Table 4). After inoculation with AMF, the maximum contents of proline, soluble sugar, and soluble protein were 127.62 ug/g, 63.94 mg/g, and 30.72 mg/g, respectively, in the P0 treatment (Table 4).
Protective Enzyme Activity and Malondialdehyde Content
Without AMF inoculation, as the low-phosphorus stress intensified, the activities of acid phosphatase, superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) of S. davidii significantly increased (Table 5). The activities of acid phosphatase, SOD, POD, and CAT in the P0 treatment were the highest and were significantly higher than those in the P0 and P0.5 treatments (P < 0.05). The malondialdehyde (MDA) content significantly increased in the P0.25 treatment and was the highest in the P0 treatment, at 43.13 nmol/g (Table 5). Inoculation with AMF, phosphorus stress, and the AMF × P interaction had significant effects on acid phosphatase, SOD, POD, CAT, and MDA (P < 0.05). Compared with NAM, inoculation with AMF significantly increased the activity of acid phosphatase, SOD, POD, and CAT under the P0.25 and P0 treatments and significantly decreased the MDA content under the P0.25 and P0 treatments (P < 0.05) (Table 5).
Effects of AMF on Endogenous Hormones in S. davidii Under Low Phosphorus Stress
Without AMF inoculation, the contents of IAA, GA3, and BR in S. davidii roots first increased and then decreased with the intensification of low-phosphorus stress, and the contents of IAA, GA3, and BR in leaves first decreased and then increased (Fig. 3). The contents of IAA, GA3, and BR in roots were the largest under the P0.25 treatment and were significantly higher than those under the other treatments (P < 0.05) (Fig. 3A, B, and C). The contents of GA3 and BR in leaves in the P0.5 treatment were the highest, at 402.33 pg/mL and 128.46 ng/L, respectively, and were significantly higher than those in the P0 treatment (P < 0.05) (Fig. 3E and F). Compared with NAM, inoculation with AMF significantly increased the content of BR in the roots, the content of IAA in the roots and leaves under the P0 treatment, the content of GA3 in the roots under the P0 and P0.25 treatments, and the content of GA3 in the leaves under the P0.25 treatment (P < 0.05).
Effects of AMF on endogenous hormones of S. davidii seedlings under low-phosphorus stress. Values are means ± SE (n = 3). Different lowercase letters indicate significant differences at P < 0.05. NAM, non-AMF inoculated; AM, AMF inoculated. AMF × P, interaction between AMF inoculation and phosphorus stress. ns, P > 0.05; * P < 0.05; ** P < 0.01. A, B, and C represent the contents of IAA, GA3, and BR in the root, respectively. D, E, and F represent the contents of IAA, GA3, and BR in the leaf, respectively
Effects of AMF on the Nitrogen and Phosphorus Contents in S. davidii Under Low-Phosphorus Stress
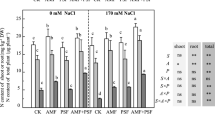
Low-phosphorus stress had a significant effect on the nitrogen content of leaves, stems, and roots of S. davidii seedlings (P < 0.05), and there was an interaction between AMF and phosphorus stress (Fig. 4 A, B, and C). Without AMF inoculation, as low-phosphorus stress intensified, the nitrogen content of roots first decreased and then increased, the nitrogen content of stems increased, and the nitrogen content of leaves first increased and then decreased. The root nitrogen content was the largest in the P0.5 treatment, which was significantly higher than those of the other treatments (P < 0.05). Both stem and leaf nitrogen contents were the smallest in the P0.5 treatment. AMF inoculation significantly increased root nitrogen contents (P < 0.05) (P0.25 and P0 treatment) and significantly decreased stem nitrogen content in the P0 treatment and leaf nitrogen content in all treatments (P < 0.05).
Effects of AMF on nitrogen and phosphorus contents in leaves, stems, and roots of S. davidii seedlings under low-phosphorus stress. Values are means ± SE (n = 3). Different lowercase letters indicate significant differences at P < 0.05. NAM, non-AMF-inoculated; AM, AMF-inoculated. AMF × P, interaction between AMF inoculation and phosphorus stress. ns, P > 0.05; * P < 0.05; ** P < 0.01
AMF inoculation, low-phosphorus stress, and the AMF × P interaction had a significant effect on the phosphorus content of the roots, stems, and leaves of S. davidii seedlings (P < 0.05) (Fig. 4 D, E, and F). The phosphorus content in roots did not change significantly under low-phosphorus stress, while the phosphorus content in stems and leaves decreased significantly under P0 treatment. Compared with NAM, inoculation with AMF significantly increased the phosphorus content of roots and stems in the P0.25 and P0 treatments and significantly increased the phosphorus content of leaves in the P0 treatment (P < 0.05).
Discussion
The AMF colonization rate is an important indicator of whether AMF have established a symbiotic relationship with host plants. It can measure the ecological adaptability of AMF and, to a certain extent, also determine plant growth and stress resistance. The results of this study showed that low-phosphorus stress (inoculation with AMF) increased the AMF colonization rate of S. davidii, which is similar to the findings for Faidherbia albida by Hailemariam et al. (2018) and for Oryza sativa by Wissuwa et al. (2020). Mycorrhizal plants grown under low-phosphorus stress are more responsive and dependent on AMF (Wissuwa et al. 2020). Therefore, in a low-phosphorus environment, inoculation of AMF can facilitate a good symbiotic relationship with plant roots, thereby enhancing the survival of plants under stress.
Effects of AMF on the Growth Mechanism of S. davidii Seedlings Under Low-Phosphorus Stress
Phosphorus is an essential mineral element for plants, accounting for 0.2% of the dry weight of plant cells, and plant cell growth requires a large amount of phosphorus (Schachtman et al. 1998). Phosphorus deficiency in the soil is the main limiting factor for plant growth. From this experiment, the S. davidii seedlings under P0.5, P0.25, and P0 conditions were significantly different in growth performance, and the plant height and shoot dry weight decreased with the intensification of low-phosphorus stress, indicating that high P is required to accelerate the plant growth process. Plant roots are the link between the soil and the plant itself and are the most important organ for the absorption of water and nutrients from the soil environment. A good root system is a prerequisite for plants to adapt to low-phosphorus stress. During the process of sensing the changes in nutrients in the environment, roots can produce morphological and physiological changes to cope with environmental stress (Mei et al. 2010). The root morphology of S. davidii, including the root dry weight, total root length, root surface area, root tip number, root volume, root tip number, and root hair number, showed a trend of first increasing and then decreasing with the aggravation of low-phosphorus stress. These variables all reached maximum values under the P0.25 treatment, with levels significantly higher than those under the P0.5 and P0 treatments. Yang et al. (2018) found that the phosphorus-efficient Fagopyrum tataricum variety had more root vigor, a higher root biomass, and more developed root systems, which was consistent with the results of this study. This is because under low-phosphorus stress conditions (P0.25), to obtain the phosphorus nutrient elements needed for growth, S. davidii transports more carbohydrates to the roots, increases the biomass of underground roots, increases the root-shoot ratio, promotes root growth, and forms a well-developed root system by increasing the root length, total root surface area, and number of root hairs, guaranteeing effective phosphorus absorption (Zhang et al. 2013). However, the P0 treatment inhibited the root morphological characteristics of S. davidii. The main function of AMF is to provide mineral elements, especially phosphorus. Colonization by AMF had positive effects on the growth parameters (plant height and shoot dry weight) of S. davidii seedlings under low-phosphorus stress, especially under the P0.25 treatment and P0 treatment. The plant height and shoot dry weight significantly increased, suggesting that AMF-induced enhancement in plant growth is dependent on the substrate P level; the lower the P level is, the more obvious the enhancement effect. Similar effects were previously reported in other species, Poncirus trifoliata (L.) Raf. (Wu et al. 2015) and Medicago sativa L. (Liu et al. 2020). This may be attributed to the more developed root system increasing nutrient uptake to maintain the high biomass of S. davidii under low-phosphorus stress (Wu et al. 2010).
Effects of AMF on the Physiological Mechanisms of S. davidii Seedlings Under Low Phosphorus Stress
Chlorophyll is the most important pigment involved in photosynthesis and has the functions of absorbing, transmitting, and transforming light energy. Within a certain range, the chlorophyll content is proportional to the photosynthetic rate, which directly reflects the level of plant photosynthetic capacity. In this experiment, low-phosphorus stress reduced the chlorophyll a, chlorophyll b, and carotenoid contents in the leaves of S. davidii without AMF inoculation. Inoculation with AMF significantly increased the chlorophyll contents in the P0.25 and P0 treatments, similar to findings in Chili by Elahi et al. (2013). Studies have shown that the increase in chlorophyll content may be related to the absorption of phosphorus and magnesium by AMF (Doubková et al. 2013). In our study, inoculation with AMF significantly increased the leaf phosphorus content in the P0 treatment compared with that in NAM. More phosphorus and chlorophyll contents in leaves provide the basis for maintaining higher photosynthetic capacity.
Plants show a series of physiological adaptation mechanisms under low phosphorus to adapt to these adverse environmental conditions (Soumya et al. 2021). In this study, the contents of proline, soluble sugar, and soluble protein in the roots of S. davidii without AMF inoculation increased with increasing phosphorus stress intensity, and inoculation with AMF further increased the content of the above osmotic regulators. These results show that under low-phosphorus stress, AMF inoculation is conducive to the reduction in the osmotic potential of S. davidii by these substances; thus, the osmotic potential of the cell is maintained, and the cell is protected so that the plant can adapt to the adversity (Zhao et al. 2021). Acid phosphatase is an enzyme induced by plant roots according to the amount of external phosphorus. When external phosphorus is low, the activity of acid phosphatase in plants increases, thereby increasing the effective phosphorus concentration in the rhizosphere (Gaume et al. 2001). In this study, the acid phosphatase in the roots of S. davidii without AMF inoculation was significantly increased under the low phosphorus environment and reached the maximum value under the P0 treatment, which is similar to findings in soybean by Nadira et al. (2014). AMF inoculation further significantly increased the acid phosphatase activity in roots, possibly because of the symbiotic colonization between AMF and plant roots in root cortex cells to obtain the required carbohydrates; at the same time, mineral nutrients such as N, P, and K can also be transferred from the soil to the root cortex and secrete phosphatases from organophosphorus compounds to hydrolyze phosphate (Smith et al. 2011).
Superoxide dismutase, peroxidase, and catalase are key enzymes involved in plant stress resistance in the protective enzyme system. They can scavenge the oxygen free radicals generated by the disturbance in plant tissues through oxidation, thereby reducing damage to plants and protecting plants (Gao et al. 2020). In this study, the activities of superoxide dismutase, peroxidase, and catalase in roots of S. davidii without AMF inoculation increased to a certain extent under low-phosphorus stress, which is similar to findings in wheat by Wang et al. (2019). AMF inoculation further significantly increased superoxide dismutase, oxidase, and catalase activities under low-phosphorus stress, which shows that the symbiosis between AMF and S. davidii can improve the activity of protective enzymes under low-phosphorus stress, enhance adaptation to a low-phosphorus environment, and maintain a stable biomass (Antunes et al. 2012).
Malondialdehyde is the product of membrane lipid peroxidation. When plants are under stress, the accumulation of malondialdehyde increases, which can aggravate cell membrane damage and damage membrane lipids (Lian et al. 2018). In this experiment, AMF inoculation significantly inhibited the malondialdehyde contents in roots, which is closely related to the increase of osmotic regulators, such as proline, soluble sugar, and soluble protein, as well as the increase in the activities of key protective enzymes, such as POD, SOD, and CAT. These substances eliminate oxygen free radicals in plant tissues and reduce plant damage under stress.
Effects of AMF on the Endogenous Hormones of S. davidii Seedlings Under Low-Phosphorus Stress
Endogenous hormones, as important regulators of plant metabolism, are involved in a series of physiological and biochemical processes (Rubio et al. 2008). In this experiment, low-phosphorus stress significantly decreased the contents of BR, GA3, and IAA (except for the P0 treatment) in leaves and significantly increased the contents of BR (except for the P0 treatment), GA3, and IAA in roots of S. davidii without AMF inoculation. This is because under a low-phosphorus environment, plants can induce root structure changes and improve phosphorus utilization efficiency by transporting the accumulated GA3, BR, and IAA in the leaves to the roots (Jiang et al. 2007). AMF inoculation significantly increased leaf and root IAA contents under the P0 treatment, significantly increased root GA3 and BR contents under the P0.25 and P0 treatments and significantly decreased leaf BR contents under the P0.25 and P0 treatments. It has been reported that AMF inoculation significantly increased the content of IAA in the roots and leaves of Catalpa bungei C.A.Mey., significantly increased the contents of GA3 and BR in roots, and significantly decreased the contents of GA3 and BR in leaves to regulate plant growth and improve stress resistance (Chen et al. 2020).
Effects of AMF on the Mineral Elements of S. davidii Seedlings Under Low-Phosphorus Stress
Phosphorus and nitrogen are essential nutrients for plant growth and are generally limited in karst shrubland ecosystems (Zhang et al. 2015). Nitrogen is an important part of plant proteins and related enzymes and plays a major role in plant growth. In this study, low-phosphorus stress significantly decreased the nitrogen content in roots and increased the nitrogen content in the stems and leaves of S. davidii without AMF inoculation, while the changes in nitrogen content in the leaves and stems were smaller than those in the roots. This is similar to findings in Zea mays L. by Rafique et al. (2020). This may have been caused by the slow growth of plant cells due to the lack of phosphorus and the increase in nitrogen content in leaves and stems to maintain growth. Under external stress, to maintain normal growth, plants may choose to transport more nitrogen to the aboveground parts to meet the needs of photosynthesis for nitrogen (Kunio et al. 1997). AMF inoculation significantly increased the nitrogen content in roots (P0.25 and P0 treatments) and decreased the nitrogen content in leaves and stems. This is because AMF can help plant roots obtain nitrogen from the soil, so plants will allocate more carbohydrates to AMF and then obtain more N through AMF, resulting in an increase in the nitrogen content of plant roots, which is similar to findings in Lolium multiflorum by Liu et al. (2019).
Phosphorus is the second most important nutrient after nitrogen that limits crop growth. This nutrient is involved in a range of plant processes, such as photosynthesis, respiration, energy production, and nucleic acid biosynthesis, and is a component of some plant structures, such as phospholipids (Elgharably and Nafady 2021). In this study, low-phosphorus stress significantly decreased the phosphorus content of the leaves and stems of S. davidii without AMF inoculation (P0 treatment). This is similar to findings in Zea mays L. by Rafique et al. (2020). Plants obtain phosphorus from the external environment through the root system. Under phosphorus deficiency, plant growth is inhibited, which reduces the acquisition of phosphorus in the soil by the plant; therefore, the phosphorus content in plant leaves and stems decreases. When phosphorus is deficient, plant photosynthetic products are preferentially distributed to the underground parts, especially the root tips, to obtain phosphorus, so the phosphorus content of the roots remains unchanged. AMF inoculation significantly increased the phosphorus content of roots and stems (P0.25 and P0 treatments) and significantly increased the phosphorus content of leaves (P0 treatment). Relevant studies have shown that AMF can absorb phosphorus elements within 10 cm of the soil surface through extra root hyphae and then transport the absorbed phosphorus elements to root epidermal cells, where these elements are eventually absorbed by plant cells (Nottingham et al. 2013). AMF can significantly improve the uptake of phosphorus by plants, especially in phosphorus-deficient environments (Mathur et al. 2018). Under a low-phosphorus stress environment, the reduction in phosphorus contents in plant leaves and stems will have a significant impact on plant growth, thus affecting photosynthesis and other physiological processes. Inoculation with AMF can alleviate this adverse effect of phosphorus stress and enhance the adaptation of S. davidii seedlings to phosphorus stress.
Conclusion
This study demonstrated that low-phosphorus stress inhibited the material accumulation of S. davidii, affected the root morphology, increased the contents of osmotic regulatory substances and the activity of protective enzymes, and altered the contents of hormones to adapt to the stressful environment. However, when the low-phosphorus stress intensity was further increased under the P0 treatment, the regulatory effect was severely weakened. Under low-phosphorus stress, F. mosseae could form a good symbiotic relationship with S. davidii seedlings, and AMF inoculation significantly increased the chlorophyll content, improved the growth of S. davidii seedlings, and further significantly increased osmotic regulatory substance contents and protective enzyme activities, alleviating low phosphorus-induced oxidative stress. Inoculation with AMF under low-phosphorus stress significantly increased IAA, GA3, and BR levels in the roots, promoted root growth, and improved the absorption of N and P in seedlings, which was conducive to plant adaptation to a low-phosphorus environment. Therefore, for ecological restoration and forage improvement using S. davidii plantings in acidic soils in the karst region of southwest China, inoculation with AMF may be a good strategy to stabilize the yields of S. davidii. However, further research on the molecular mechanism by which AMF improves the low-phosphorus resistance of S. davidii will be needed.
References
Abdel Hamed Abdel Latef A, Hashem A, Rasool S, Fathi Abd E, Egamberdieva D et al (2016) Arbuscular mycorrhizal symbiosis and abiotic stress in plants: a review. J Plant Biol 59:407–426
Antunes PM, Franken P, Schwarz D, Matthias CR, Miranda H (2012) Linking soil biodiversity and human health: do arbuscular mycorrhizal fungi contribute to food nutrition? Oxford University Press
Baghbani-Arani A, Modarres-Sanavy S, Poureisa M (2021) Improvement the soil physicochemical properties and fenugreek growth using zeolite and vermicompost under water deficit conditions. J Soil Sci Plant Nut 21:1213–1228
Bahadur A, Batool A, Nasir F, Jiang SJ, Qin MS et al (2019) Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int J Mol Sci 20(17):4199. https://doi.org/10.3390/ijms20174199
Bao SD (2000) Soil agrochemical analysis, 3rd edn. China Agriculture Press, Beijing
Benhiba L, Fouad MO, Essahibi A, Ghoulam C, Qaddoury A (2015) Arbuscular mycorrhizal symbiosis enhanced growth and antioxidant metabolism in date palm subjected to long-term drought. Trees 29(6):1725–1733
Blank LM (2012) The cell and P: from cellular function to biotechnological application. Curr Opin Biotech 23(6):846–851. https://doi.org/10.1016/j.copbio.2012.08.002
Campo S, Segundo BS (2020) Systemic induction of phosphatidylinositol-based signaling in leaves of arbuscular mycorrhizal rice plants. SCI Rep-UK 10(1):15896
Cao X, Jia J, Zhang C, Li H, Liu T, Jiang X, Polle A, Peng C, Luo ZB (2014) Anatomical, physiological and transcriptional responses of two contrasting poplar genotypes to drought and re-watering. Physiol Plant 151:480–494
Chen W, Meng P, Feng H, Wang C (2020) Effects of arbuscular mycorrhizal fungi on growth and physiological performance of Catalpa bungei CA Mey. under drought stress. Forests 11(10):1117
Diao F, Dang Z, Cui X, Xu J, Guo W (2021) Transcriptomic analysis revealed distinctive modulations of arbuscular mycorrhizal fungi inoculation in halophyte Suaeda salsa under moderate salt conditions[J]. Environ Exp Bot 183:104337
Doubková P, Vlasáková E, Sudová R (2013) Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant Soil 370:149–161
Elahi F, Mridha M, Aminuzzaman F (2013) Role of AMF on plant growth, nutrient uptake, arsenic toxicity and chlorophyll content of chili grown in arsenic amended soil. Bangladesh J Agric Res 37(4):635–644
Elgharably A, Nafady NA (2021) Inoculation with Arbuscular mycorrhizae, Penicillium funiculosum and Fusarium oxysporum enhanced wheat growth and nutrient uptake in the saline soil. Rhizosphere. https://doi.org/10.1016/j.rhisph.2021.100345
Feng Z, Liu X, Zhu H, Yao Q (2020) Responses of arbuscular mycorrhizal symbiosis to abiotic stress: a lipid-centric perspective. Front Plant Sci 11:578919
Gao WQ, Lü LH, Srivastava AK, Wu QS, Kuca K (2020) Effects of mycorrhizae on physiological responses and relevant gene expression of peach affected by replant disease. Agronomy 10(2):186
Gaume A, Machler F, León CD, Narro L, Frossard E (2001) Low-P tolerance by maize (Zea mays L.) genotypes: Significanceof root growth, and organic acids and acidphosphatase root exudation. Plant Soil 228:253–264
Hailemariam M, Birhane E, Gebresamuel G, Gebrekiros A, Desta Y, Alemayehu A et al (2018) Arbuscular mycorrhiza effects on Faidherbia albida (Del.) A. Chev. growth under varying soil water and phosphorus levels in Northern Ethiopia. Agroforest Syst 92:485–498
Jia TT, Wang J, Chang W, Fan XX, Sui X, Song FQ (2019) Proteomics analysis of E. angustifolia seedlings inoculated with arbuscular mycorrhizal fungi under salt stress. Int J Mol 20(3):788. https://doi.org/10.3390/ijms20030788
Jiang C, Gao X, Liao L, Harberd NP, Fu X (2007) Phosphate starva-tion root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol 145(4):1460–1470
Kazadi AT, Lwalaba JLW, Ansey BK, Muzulukwau JM, Katabe GM, Karul MI, Baert G, Haesaert G, Mundende RPM (2022) Effect of phosphorus and arbuscular mycorrhizal fungi (AMF) inoculation on growth and productivity of maize (Zea mays L.) in a tropical ferralsol. Gesunde Pflanz 74:159–165. https://doi.org/10.1007/s10343-021-00598-8
Kumar V, Singh D, Sangwan P, Gill PK (2015) Management of environmental phosphorus pollution using phytases: current challenges and future prospects. Springer, India
Kunio O, Koji C, Kiyoshi M (1997) Suitable level of nitrogen fertilizer for tea (Camellia sinensis L.) plants in relation to growth, photosynthesis, nitrogen uptake and accumulation of free amino acids. Jpn J Crop Sci 66(2):279–287
Li HS (2000) Principles and techniques of plant physiological biochemical experiment. Beijing
Li H, Huang G, Meng Q, Ma L, Yuan L, Wang F et al (2011) Integrated soil and plant phosphorus management for crop and environment in China. A Rev Plant Soil 349:157–167
Lian H, Cheng Q, Li Z, Cong Z, Li H, Zhang S (2018) Lanthanum nitrate improves phosphorus-use efficiency and tolerance to phosphorus-deficiency stress in Vigna angularis seedlings. Protoplasma 256(2):383–392
Liang Y, Pan F, Jiang Z, Li Q, Pu J, Liu K (2022) Accumulation in nutrient acquisition strategies of arbuscular mycorrhizal fungi and plant roots in poor and heterogeneous soils of karst shrub ecosystems. BMC Plant Biol 22(1):1–12
Liu WL, Zhang YL, Jiang SS, Deng Y, Christie P, Murray PJ, Li XL, Zhang JL (2016) Arbuscular mycorhizal fungi in soil and roots respond differently to phosphorus inputs in an intensively managed calcareous agricultural soil. Sci Rep 6:24902. https://doi.org/10.1038/srep24902
Liu M, Che Y, Wang L, Zhao Z, Zhang Y et al (2019) Rice straw biochar and phosphorus inputs have more positive effects on the yield and nutrient uptake of Lolium multiflorum than arbuscular mycorrhizal fungi in acidic Cd-contaminated soils. Chemosphere 235:32–39
Liu JY, Liu XS, Zhang QB, Li SY, Aun YL, Lu WH, Ma CH (2020) Response of alfalfa growth to arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria under different phosphorus application levels. AMB Expr. https://doi.org/10.1186/s13568-020-01137-w
Liu TY, Hao L F, Bai S L, Wang Y L (2021) Ecoenzymatic stoichiometry and microbial nutrient limitation of shrub rhizosphere soils in response to arbuscular mycorrhizal fungi inoculation. J Soil Sediment: 1–13
Mathur S, Sharma MP, Jajoo A (2018) Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J Photochem Photobiol B Biol 180:149–154
Mei Y, Ding G, Shi L, Feng J, Xu F, Meng J (2010) Quantitative trait loci for root morphology in response to low phosphorus stress in Brassica napus. Theor Appl Genet 121(1):181–193
Nadira UA, Ahmed IM, Zeng JB, Bibi N, Cai SG et al (2014) The changes in physiological and biochemical traits of Tibetan wild and cultivated barley in response to low phosphorus stress(Plant nutrition)[J]. Soil Sci Plant Nutr 60(6):832–842
Nottingham AT, Turner BL, Winter K, Chamberlain PM, Stott A, Tanner EVJ (2013) Root and arbuscular mycorrhizal mycelial interactions with soil microorganisms in lowland tropical forest. Fems Microbiol Ecol 85(1):37–50
Oliveira CA, Alves VMC, Marriel IE, Scotti MR, Carneiro NP et al (2009) Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol Biochem 41:1782–1787
Rafique M, Orta B, Rizwan M, Chaudhary H J, Munis M (2020) Residual effects of biochar and phosphorus on growth and nutrient accumulation by maize (Zea mays L.) amended with microbes in texturally different soils. Chemosphere, 238
Rubio V, Bustos R, Trigoyen ML, Cardona-López X, Rojas-Triana M, Paz-Ares J (2008) Plant hormones and nutrient signaling. Plant Mol Biol 69(4):361–373
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116(2):447–453
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2(1):587
Smith SE, Jakobsen I, Gronlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156(3):1050–1105
Soumya PR, Singh D, Sharma S, Singh AM, Pandey R (2021) Evaluation of diverse wheat (Triticum aestivum) and triticale (× Triticosecale) genotypes for low phosphorus stress tolerance in soil and hydroponic conditions. J Soil Sci Plant Nut 21(2):1236–1251
Tarkowski P, Ge L, Yong JWH, Tan SN (2009) Analytical methods for cytokinins. Trends Anal Chem 28:323–335
Wang J, Qin Q, Pan J, Sun L, Song K (2019) Transcriptome analysis in roots and leaves of wheat seedlings in response to low-phosphorus stress. Sci Rep-UK 9(1):19802
Wissuwa M, Gonzalez D, Watts-Williams SJ (2020) The contribution of plant traits and soil microbes to phosphorus uptake from low-phosphorus soil in upland rice varieties. Plant Soil 448(1):523–537
Wu QS, Zou YN, He XH (2010) Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol Plant 32(2):297–304
Wu QS, Srivastava AK, Li Y (2015) Effects of mycorrhizal symbiosis on growth behavior and carbohydrate metabolism of trifoliate orange under different substrate P levels. J Plant Growth Regul 34(3):499–508
Yamaji N, Takemoto Y, Miyaji T, Mitani-Ueno N, Oshida KTY, Ma JF (2017) Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature 541:92–95
Yang TC, Zhang YQ, Ma XX, Chen W, Dong L et al (2018) Screening genotypes and identifying indicators of different Fagopyrum tataricum varieties with low phosphorus toleranc. J Appl Ecol 29(9):2997–3007
Ying C, Dan T, Jia L, Xu B, Jjya B et al (2021) Diversity and distribution of Sophora davidii rhizobia in habitats with different irradiances and soil traits in Loess Plateau area of China. Syst Appl Microbiol 44(4):126224
Zhang D, Liu C, Cheng H, Kan G, Cui S et al (2010) Quantitative trait loci associated with soybean tolerance to low phosphorus stress based on flower and pod abscission. Plant Breeding 129:243–324
Zhang YK, Chen FJ, Chen XC, Long LZ, Gao K, Yuan LX, Zhang FS, Mi GH (2013) Genetic improvement of root growth contributes to efficient phosphorus acquisition in maize (Zea mays L.). J Integr Agr 12(6):1098–1111
Zhang W, Zhao J, Pan F, Li D, Chen H, Wang K (2015) Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 391:77–91
Zhang ZJ, Dong SW, Gao DD, Du XY, Xie YQ, Xia XS, Li RT (2021) Unusual matrine-adenine hybrids isolated from Sophora davidii and their inhibitory effects on human cytomegalovirus. Phytochemistry 190:112842–112842
Zhao X, Huang L J, Zhao L L, Wang P C, Sun X F (2021) Transcriptome analysis of Sophora davidii leaves in response to low-phosphorus stress. J Plant Growth Regul 1–13
Zhao X, Huang LJ, Sun XF, Zhao LL, Wang PC (2022) Transcriptomic and metabolomic analyses reveal key metabolites, pathways and candidate genes in Sophora davidii (Franch.) skeels seedlings under drought stress. Front Plant Sci 13:1–19. https://doi.org/10.3389/fpls.2022.785702
Funding
This work was funded through projects of Science and Technology Project of Guizhou Province (QKHZC[2021]YB155, QKHPTRC[2021]5636, QKHJC[2020]1Z026) and the National Natural Science Foundation of China (32260340, 32060391).
Author information
Authors and Affiliations
Contributions
LLZ and PCW conceived and designed research. LTW, KKC, and HS conducted experiments. KKC, LTW, and PCW analyzed the data. LLZ, KKC, and LTW wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Handling Editor: Nudrat Aisha Akram.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, LL., Wang, Lt., Chen, K. et al. Effects of Arbuscular Mycorrhizal Fungi on the Growth and Physiological Performance of Sophora davidii Seedling Under Low-Phosphorus Stress. J Plant Growth Regul 43, 2383–2395 (2024). https://doi.org/10.1007/s00344-024-11273-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-024-11273-3