Abstract
Regulated deficit irrigation (RDI) is a technique used to save water, increase water use efficiency (WUE) and nutrients in plants. This practice can be enhanced when combined with soil management. In addition, enhancing root architecture and improving the absorption of water and nutrients in deeper zones can be achieved through the application of phosphorus at depth. There is a scarcity of studies that evaluate the integrated effect of different management practices of fertilization and water on the fruit quality of tomato plants intended for the industry. Thus, the objective was to investigate the postharvest responses of fruit quality and cell wall metabolism of the industry tomato ‘Heinz 9553’. Two irrigation frequencies (IF) (one and seven days) were implemented in the plots and three soil management models (conventional fertilization + limestone [FL]; conventional fertilization + limestone + gypsum [FLG]; conventional fertilization + limestone + gypsum + phosphorus applied in depth [FLGP]) were implemented in the subplots. Variables that affects the quality parameters (reducing sugars, phenolic compounds, carotenoids, pectinolytic enzymes) were evaluated. The results showed that the combined management FLG and IF of seven days provided increase in soluble solids, reducing sugars, soluble solids/titratable acidity ratio, dry matter, phenolic compounds and total carotenoids in the fruits. It was also found that the firmness of the pulp was higher in the seven-day IF treatment and was inversely related to the activity of the enzymes pectin methylesterase and polygalacturonases. The combined fertilization and water management strategies promoted improvements in quality and postharvest cell wall firmness of the industrial tomato ‘Heinz 9553’. Therefore, the combined soil and water management strategies FLG and FLGP with IF of seven days are recommended for field cultivation of tomato for industrial processing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Low water and nutrients availability are the most limiting factors for the crop plants production (Ali et al. 2019). Studies investigating the increase in crop yields and the maintenance of fruit quality worldwide have focused using genetic engineering; however, the optimization and development of management strategies mainly for water and nutrients has been used with great success (Shao et al. 2008; Medyouni et al. 2021), thus requires further investigation to optimize yield of crops such as tomato and promote non-GMO crops for consumption.
In Brazil, about 36% of the total tomato production is destined to the industry (Almeida Neta et al. 2019). However, to obtain high yields and ensure the maintenance of fruit quality, tomato cultivation requires a high availability of water and nutrients during its entire cycle (Chapagain and Orr 2009; Yang et al. 2020). Abiotic factors associated with water availability and nutrition have affected the yield and quality of industrial tomatoes (Villas Boas et al. 2017). Therefore, it is essential to develop strategies and management that aim to optimize the efficiency of water and nutrient use by the plant, without compromising the production and final quality of the fruit.
The application of regulated deficit irrigation (RDI) is a technique, applied in the growth phase, in which water is made available throughout the root zone in an amount lower than potential evapotranspiration, causing moderate stress with minimal or no effects on production (Dodd 2009). In RDI, the adoption of specific irrigation frequencies (IF) is implemented to optimize water utilization by the plant, thereby enhancing its overall water use efficiency (Khapte et al. 2019; Fara et al. 2019).
However, the variation in soil water availability generates a complex network of morphological and physiological changes in the plant and fruit (Morales et al. 2015). Fruit ripening, for example, involves a cascade of biochemical and physiological events in the cell wall metabolism (Quinet et al. 2019) that determine their firmness. The pectins play a significant role in forming the complex and heterogeneous set of polysaccharide compounds that constitute the primary cell wall of tomato (Xie et al. 2017). The depolymerization of these structural domains and the solubilization of pectic polysaccharides causes the modification of the cell wall polysaccharides and finally the disassembly of its architecture, as a result of the combined action of the enzymes pectin methylesterases (PME, E.C. 3.1.1.11) and polygalacturonase (PG, EC 3.2.1.15) (Xie et al. 2017). Like many other fleshy fruits, tomatoes go through different development processes, ending with ripening and softening (firmness loss), which ultimately determine the quality of the fruit and the shelf life of the product (Liu et al. 2021a, b).
Furthermore, several studies have shown that the adoption of this practice coupled with correct IF, promotes improvement in water use efficiency (WUE), photosynthetic rates, higher stomatal conductance, increases yield and improves desirable traits of tomato fruit, such as total soluble solids, soluble sugars, soluble solids/titratable acidity ratio and bioactive compounds (Kirda et al. 2004; Fara et al. 2019; Liu et al. 2021a, b; Medyouni et al. 2021). The increments in these characteristics are achieved by maintaining the stomatic opening for more time throughout the day, providing a greater and better distribution and allocation of photoassimilates (Chai et al. 2016). The effects of RDI and IF are optimized in soils with properly corrected acidity because they improve the nutrient supply of the crop, facilitate greater root development and consequently increase the active zone of root uptake (Fara et al. 2019).
The combination of gypsum and agricultural limestone, for correction of soil acidity, are fundamental for the construction of the soil chemical profile and improvement of root growth. This occurs because the application of limestone allows the correction of soil acidity in the superficial layer, besides promoting the supply of sulfur (S), calcium (Ca) and magnesium (Mg) to the plant. While gypsum, due to its high mobility, is transported through the soil profile to the subsurface layers, promoting the correction of acidity and root development in this zone (Saeed and Ahmad 2009; Fara et al. 2019).
Plants typically obtain S from the soil solution as sulfate ions (SO42−). These ions might be transported or stored in different parts of the plant. Sulfate can transfer from roots to shoots and other plant tissues through sulfate transporters. The xylem transports sulfate and water to the aerial regions of the plant. Sulfur is an essential component of metabolic activities like the production of proteins and amino acids. S is found in proteins, the amino acids cysteine (Cys) and methionine (Met), vitamins (biotin and thiamin), cofactors (Co-A and S-adenosyl methionine, SAM), and a variety of secondary metabolites (Mazid et al. 2011). In order to support plant growth and vital processes, sulfur compounds are distributed in different tissues according to parameters including organ demand and environmental conditions (Capaldi et al. 2015).
Calcium (Ca) is acquired by plants from the soil through the roots. It is present in the soil solution as calcium ions (Ca2+). Ca localization primarily happens in mature root tissues and mid-cortical cells (Pesacreta et al. 2021; Acharya and Pesacreta 2022). Both passive and active transport systems are used to transfer it across the plant. Through transpiration or other transporters, calcium is transferred through the xylem with water. It is essential for signal transmission, the activation of enzymes, and the formation of cell walls. Based on demand, developmental phases, and physiological needs, calcium distribution is controlled. When it is required for processes like fruit growth or seed generation, it is carried to certain tissues or organs (White and Broadley 2003).
Plants primarily obtain magnesium (Mg) from the soil through their roots. It is found as magnesium ions (Mg2+) in the soil solution. The root cell membranes have specific transporters that aid in magnesium absorption. Mg2+ can be transported throughout the plant or briefly retained in vacuoles once they have entered the roots. Mg is transported by both passive and active methods, using either plasmodesmata to move from cell to cell. From the roots to the shoots, magnesium is carried by the xylem together with water. Mg is an essential element for the stability of cell membranes, enzyme activity, and the production of chlorophyll in plants (Guo et al. 2016). Its distribution is controlled based on physiological needs, developmental phases, and demand to ensure it reaches the precise tissues or organs where it is required (Xie et al. 2021).
Phosphorus (P) is an essential macronutrient that plays an important role mainly in the initial growth and metabolism of plants (Higo et al. 2020). Additionally, P is a fundamental constituent of nucleotides such as ATP (adenosine triphosphate), an important molecule whose main function is to act as an energy source at the cellular level to carry out metabolic processes (De Col et al. 2017).
Phosphorus (P) is predominantly taken up by plants from the soil as inorganic phosphate (Pi) by means of certain transporters in their root cell membranes. Pi can be temporarily stored or moved around the plant once it has entered the root cells (Acharya and Pesacreta 2023). Pi may move more easily from roots to shoots and other plant components thanks to phosphate transporters. Pi is carried from plant roots to aerial structures via the xylem along with water. Pi is an essential component of protein, DNA, and energy transport inside plants. Pi undergoes enzymatic processes as it reaches the aerial sections before being transformed into organic molecules. Pi is allocated effectively for growth, development, and vital physiological processes by being regulated according to organ demand, developmental phases, and environmental factors (Raghothama 2005).
However, the unavailability and/or immobility of P in the soil limits plant growth in the early stages (Higo et al. 2020), by reducing maximum root development and hence final yield of tomato (Zhu et al. 2017, 2018). In order to optimize the availability of P, especially in the early stages of growth, the application of P can be made locally, close to the roots (Ma et al. 2020) or in the subsurface to stimulate root growth. In turn, plant roots adapt to specific nutrient patches in the soil by either growing in proportion to them, a process known as morphological plasticity, or by increasing nutrient uptake rates, a process known as physiological plasticity (Zhou et al. 2017). Better initial root development, and consequently better establishment of the root system, play important roles in the acquisition of resources such as water and nutrients (Hodge 2004; Ma et al. 2020), thus, strongly influencing plant growth, and improving the commercial characteristics of the fruit (Coutinho Edson et al. 2014).
Therefore, the integrated use of soil and water management strategies can increase the productivity and quality of tomato fruit. The individual effect of soil and water management have been widely studied, whereas few literatures refer to the combined effect of these factors, especially on the quality of tomato fruit destined for processing. Thus, the objective was to investigate the responses in quality and postharvest cell wall metabolism of industrial tomato ‘Heinz 9553’ to combined management strategies aiming to optimize the use of water and nutrients by the plant.
Material and Methods
Experimental Design and Description of Plant Material
The commercial tomato (Solanum lycopersicum L.) hybrid Heinz 9553 (H9553) (Heinz Seed©) was used. H9553 displays concentrated ripeness (Luz et al. 2016), exhibits determinate growth, and reaches full maturity within a range of 110–120 days. Sowing was done in polypropylene trays of 128 cells arranged in an agricultural nursery of galvanized steel and dimensions of 6.4 m wide by 18.0 m long with 3.5 m high, closed in 45º, with monofilament screen, mesh for 50% shade.
The experiment was in randomized blocks design, with 4 repetitions in a split-plot scheme. Two irrigation frequencies (IF—one and seven days) were implemented in the plots and three soil management models (conventional fertilization + limestone [FL]; conventional fertilization + limestone + gypsum [FLG]; conventional fertilization + limestone + gypsum + phosphorus applied in depth [FLGP]) were implemented in the subplots. The experimental area was composed of 24 experimental units of 13.5 m2 (4.5 m × 3 m). The experimental units were arranged in 4 single rows of 4.5 m long and 3.0 m wide, with 1.0 m between rows, 0.3 m between plants and 1.1 m wide between experimental units. Each experimental unit consisted of 64 plants, with the central 24 plants used for evaluations. The single rows on the extremities and two plants on the edges of the central rows were considered as borders.
The experiment was performed from March to August 2021, in the experimental area of the Teaching, Research and Extension Technical-operational Unit (UEPE) belonging to the Department of Plant Science, Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brazil (20º 45’ S and 42° 51’ W, altitude 693 m).
Soil Preparation and Planting
The initial physicochemical characteristics of the soil were determined (Table 1). The physical and chemical characteristics of the soil were evaluated according to the methodology proposed by Claessen et al. (1997). P, K, Fe, Zn, Mn and Cu were extracted with Mehlich; Ca, Mg and Al were extracted with KCl−1 mol L−1; Potential acidity was measured at pH 7.0 extracted with calcium acetate obtained from 0.5 mol L−1; B was extracted in hot water.
The analysis of SB, SCC, t, V, m, OM, thick sand, thin sand, clay, silt was performed according to Teixeira et al. (2017). Dolomitic limestone was applied to the soil surface 130 days before transplanting and incorporated to a depth of 0.25 m with a mouldboard plow followed by light harrowing. Limestone and gypsum were applied manually. After these operations, on 74 days before transplanting gypsum was added in a single dose to the soil surface in the experimental units that involved soil management with limestone and gypsum. The complementation of P in depth applied in the planting furrow at 0.30 m depth, was done the day after transplanting the seedlings, in the form of triple superphosphate, in the amounts of 4.35 (kg plot−1), 1.35 (kg plot−1) and 163.5 (g m−1 linear), respectively.
After soil preparation and twenty-five days after emergence, seedlings 12–15 cm tall, with 4–6 true leaves, were transplanted to the experimental growing area. Seedling production, transplanting, weed, disease and pest control were performed according to Alvarenga et al. (2004).
Irrigation Management
Irrigation management was determined by crop evapotranspiration (ETc). The crop water demand and irrigation frequencies (IF) adopted were calculated following the estimated ETc, based on the adjustment coefficients in relation to the reference evapotranspiration (ET0) (Table 2). The water balance was used to calculate the net irrigation blade. Equations 1 and 2 were used to estimate crop evapotranspiration (Allen and Pereira 2009; Delazari et al. 2016).
where: ETc is crop evapotranspiration, in mm d−1; ET0, reference evapotranspiration, in mm d−1; Kc, crop coefficient (dimensionless); Kcb, basal crop coefficient (dimensionless); Ke, soil evaporation coefficient (dimensionless). Ks, stress coefficient (dimensionless).
The tomato cycle was divided into phenological phases and the Kcb values were adjusted according to the developmental stage of the crop. The initial, intermediate and final Kcb were 0.15, 1.15 and 0.70, respectively (Allen et al. 1998).
The irrigation method used was localized drip irrigation. The Naan Dan Jain Irrigation equipment, AmnonDrip model, self-compensating and antidrain operating at a real flow rate of 1.60 L h−1 was used. The irrigation system operated with a uniformity of 99.4%, as determined by the Christiansen Uniformity Coefficient (CUC) (Mantovani et al. 2013).
Weather Conditions
The meteorological data were obtained through an automatic agrometereological station E 4000 (IRRIPLUS) equipped with sensors that measure temperature (°C), relative humidity (%), wind speed (m s−1), solar radiation (W m−2 dia−1) and precipitation (mm). The data were collected and used for irrigation management calculations. The experiment was conducted during the dry season, with only one instance of rainfall occurring throughout the experimental period. In this particular scenario, the following procedure was implemented: The amount of rainfall was subtracted, and a reduced water depth was applied based on calculations provided by the local meteorological station.
Fruit Quality Analysis
Harvesting occurred at 128 days after transplanting. The fruit were harvested manually at the “red ripe” maturity stage was visually determined, which corresponds to fruit with more than 90% of the red surface (Gupta et al. 2019). Ten plants from each experimental unit were sampled. Then, another sampling and previous selection of the fruit was made adopting criteria of size uniformity, ripeness degrees and sanity (fruit without signs of diseases and pests) of the plant material.
Pulp Firmness
Pulp firmness was determined with a digital penetrometer (Fruit hardness tester® mod. FR-5120) using a stainless steel cylinder probe with a diameter of 8 mm. For this analysis, five tomatoes were used, taking two readings in opposite positions at the equatorial region of the fruit (totaling 10 measurements per repetition, Al-Dairi et al. 2021). The results are expressed in Newtons (N).
Soluble Solids Content and Titratable Acidity
Tomato fruit were processed in an analytical mill (IKA® A11 basic), then filtered and homogeneous juice from the pulp was obtained. The determination of soluble solids was performed using a portable digital refractometer (Atago® mod PR-201α) and the results expressed as percentage (Sinha et al. 2019). The acidity was determined by titrating 10 mL of the tomato filtrate with 0.05 N NaOH to pH 8.2, according to Tigist et al. (2013). Titratable acidity was expressed as a percentage (%), assuming citric acid as the predominant acid in the tomato juice.
Dry Matter
Fresh tomato fruit were weighed on a semi-analytical balance (precision of 0.0001 g) in duplicates for each repetition within the respective treatments. Then, the material was placed in a forced air oven at 65 °C for 72 h until reaching a constant weight. After this time, it was weighed again (Ronga et al. 2017). The result was expressed as a percentage of dry matter (% DM), and calculated from the following formula:
where: %DM is percentage of tomato fruit dry matter (%); DM is the weight of tomato fruit after drying (g); FM is the weight of fresh tomato fruit (g).
Extraction Preparation
The preparation of the extract followed the methodology described by Araújo et al. (2020) with few modifications. Approximately 2 g of fresh pulp from the previously cut tomato fruit were weighed. The samples were ground and homogenized in boiling 80% ethanol. The supernatant was recovered, filtered, and centrifuged at 13.000 rpm for 10 min. Then extract was pooled and equilibrated to known volume with 80% ethanol and subsequently used for quantification of reducing sugars (RS) and total phenolic compounds (PC).
Reducing Sugars
The content of reducing sugars was quantified following adaptations of the 3,5-dinitrosalicylic acid (DNS) method proposed by Gonçalves et al. (2010). For quantification, a 0.25 mL aliquot was taken from the ethanolic extract, added 0.25 mL of deionized water and 0.5 mL of DNS reagent. Tubes containing the solution were heated in a boiling water bath for 5 min. After cooling, 4 mL of deionized water was added and the absorbance was read in a spectrophotometer at 540 nm (GENESYS TM UV–VIS Thermo Scientific). The results were expressed as %RS on fresh mass (FM) basis using the standard fructose curve (0–1.0 mg).
Phenolic Compounds
Total phenolic compound content was quantified following the methodology proposed by Fu et al. (2011) with some modifications. For quantification, a 0.2 mL sample was taken from the ethanolic extract, added 1 mL of Folin-Ciocalteu reagent (a mixture of phosphomolybdate and phosphotungstate) and kept at room temperature. Subsequently, 0.8 mL of calcium carbonate (7.5%) was added, stirred, and were incubated in a dark environment for 30 min. After this procedure the absorbance was read at 760 nm in a spectrophotometer (GENESYSTM UV–VIS Thermo Scientific). The results were expressed as mg gallic acid 100 mg−1 MF using standard gallic acid curve.
Total Carotenoids
The content of total carotenoids in fruit was obtained according to the methodology performed by Araújo et al. (2020) with some modifications. Weighed 0.75 g of the fruit in 10 mL of cooled 80% acetone. After that, the preparation was incubated in the dark for 24 h at 4 °C. The extract was obtained by filtering the material and the absorbance read at 470 in a spectrophotometer (GENESYSTM UV–VIS Thermo Scientific). The absorbance coefficients were determined from calibration curves prepared with the lycopene standard (Strati; Oreopoulou, 2011). The content of total carotenoids was expressed as mg 100 g−1 FM, following the formula proposed by Rodriguez-Amaya (2001).
Determination of Calcium (Ca2+) and Magnesium (Mg2+) in Fruit
The preparation of tomato fruit samples consisted of drying in an oven with forced air circulation for 72 h at a temperature of 65 ºC. Then, the samples were weighed (approximately 0.5 g) on precision scales and ground in stainless steel knife mills. The quantification of Ca and Mg was done by 4:1 nitroperchloric digestion. The reading was done in an optical emission spectrometer with inductively coupled plasma (ICP- OES; Perkin Elmer Model Optima 8300 DV®) following the methodology proposed by Sarruge and Haag (1974). The results were expressed as dag kg −1 de DM.
Extraction and Analysis of Pectinolytic Enzymes
Extraction and pectin methylesterase (PME, EC3.1.1.11) activity was performed as described by Bu et al. (2013) with modifications. Frozen fruit tissues (0.5 g) were ground and homogenized in mortar with a pestle, containing solution composed of 2 mL of 8.8% NaCl and 10 g L−1 PVPP. The extract solution was centrifuged for 15 min at 14,000 rpm. The supernatant was then collected and adjusted to pH 7.5 with 1 M NaOH and then used for the enzyme activity assay.
The solution for the reaction was composed of 0.6 mL of pectin solution (Sigma®, from citrus peel; 0.5% aqueous solution adjusted to pH 7.5 using 1 M NaOH), 0.2 mL of 0.01% (m/v) bromothymol blue solution, 0.75 mL of 3 mM phosphate buffer (pH 7.5), 0.1 mL of enzyme extract. After that, the absorbance was read immediately at 620 nm in a spectrophotometer for 2 min (GENESYSTM UV–VIS Thermo Scientific). The rate of decrease from 0 to 2 min was used to determine the PME activity. Enzyme activity was expressed as µmol min−1 mg protein−1. The activity calculation was performed against a standard curve of polygalacturonic acid described by Hagerman and Austin (1986). Total proteins in the enzymatic preparation were determined by the Bradford method (Bradford 1976).
Extraction and polygalacturonase (PG, EC 3.2.1.15) activity was performed as described by Bu et al. (2013) with modifications. Tissues of the frozen fruit (0.5 g) were ground and homogenized with a mortar and pestle and then extracted by adding 2 mL of 37.5 mM sodium acetate buffer (pH 5.0). The extract solution was centrifuged for 15 min at 14,000 rpm. The supernatant was collected and used to determine the enzyme activity.
The solution for the PG reaction was made from the mixture containing 0.2 mL 37.5 mM sodium acetate buffer (pH 5.0), 0.225 mL of 0.25% (w/v) polygalacturonic acid previously diluted in 100 mM sodium acetate buffer (pH 5.0), 0.1 mL of the enzyme extract. The mixture was incubated at 37 °C for 15 min and the reaction was stopped by adding 0.5 mL of 3,5-dinitrosalicylic acid (DNS) and immersion in a boiling water bath for 5 min. After cooling, 1 mL of distilled water was added and the absorbance was read at 540 nm in a spectrophotometer (GENESYSTM UV–VIS Thermo Scientific). The enzymatic activity was expressed as µmol min−1 mg protein−1. Total proteins of the enzyme preparation were determined by the Bradford method (Bradford 1976).
Statistical Analysis
Data were subjected to the test for normality (Shapiro–Wilk) and homogeneity of variances (Bartlett). The data were subjected to a mean test by the ExpDes.pt package (Ferreira et al. 2021). A canonical variable analysis and confidence ellipses (p ≤ 0.01) were performed to study the interrelationship between variables and the factors using the candisc package (Friendly et al. 2013). Statistical analyses was performed by the R software (R Core Team 2021).
Results
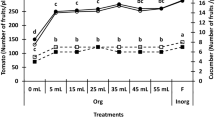
The soluble solids content was higher in fruit under soil management with conventional fertilization + limestone + gypsum (FLG) and irrigation frequency (IF) of seven days (Fig. 1A). The titratable acidity did not differ (Fig. B). The content of reducing sugars was higher in the combined treatment of FLG and IF for seven days (Fig. 1C). The soluble solids/titratable acidity ratio was higher in fruit submitted to FI for seven days, with no difference between soil managements (Fig. 1D). The control treatment was conventional fertilization + limestone [FL] with daily irrigation. This treatment was chosen because it practically represents the management adopted by industrial tomato producers in the country’s major production regions.
Soluble solids (A), Titratable acidity (B) Reducing sugars (C) and soluble solids/titratable acidity ratio (D) of tomato fruit subjected to different soil managements (conventional fertilization + limestone [FL]; conventional fertilization + limestone + gypsum [FLG]; conventional fertilization + limestone + gypsum + phosphorus applied at depth [FLGP]) and irrigation frequencies (one and seven days).Values are mean ± standard deviation (n = 4). Equal capital letters do not differ among irrigation frequencies and lower case letters among soil managements, according to Tukey’s test (p ≤ 0.05)
A The amount of calcium (Ca2+) in tomato fruit was lower only in the combined treatment by FL and the IF of seven days (Fig. 2A). Magnesium (Mg2+) did not differ in any of the treatments. (Fig. 2B).
Calcium (A) and magnesium (B) content of tomato fruit subjected to different soil managements and irrigation frequencies. Values are mean ± standard deviation (n = 4). Equal capital letters do not differ among irrigation frequencies and lower case letters among soil managements, according to Tukey’s test (p ≤ 0.05)
Pulp firmness was higher in tomato fruit under combined management with FLG and IF for seven days (Fig. 3A). The percentage of dry matter was higher in fruit managed combined with FL and FLG and IF of seven days (Fig. 3B). PG activity was lower in fruit under combined management with FLG, FLGP and IF of seven days (Fig. 3C). PME activity was lower in all treatments with seven days IF managements (Fig. 3D).
Pulp firmness (A), dry matter (B), and enzymatic activity of pectin methylesterase (C) and polygalacturonase (D) of tomato fruit subjected to different soil managements and irrigation frequencies. Values are mean ± standard deviation (n = 4). Equal capital letters do not differ among irrigation frequencies and lower case letters among soil managements, according to Tukey’s test (p ≤ 0.05)
The content of total carotenoids and phenolic compounds was higher in the combined treatment of by FLG and in all managements with IF of seven days (Fig. 4A and 4B).
Total Carotenoids (A), and Phenolic compounds (B) of tomato fruit subjected to different soil managements and irrigation frequencies. Values are mean ± standard deviation (n = 4). Equal capital letters do not differ among irrigation frequencies and lower case letters among soil managements, according to Tukey’s test (p ≤ 0.05)
The variance accumulated by the two canonical variables (Can1 and Can 2) was 90.6%, so there is a distinction between the types of management and the IF (Fig. 5). Can1 is positively correlated with the activity of the enzymes PG, PME and the Ca and Mg present in tomato fruit; and negatively with titratable acidity (TA), soluble solids/titratable acidity ratio (RATIO), total carotenoids (TC), reducing sugars (RS), phenolic compounds (PC), firmness (FIRM) and dry matter (DM). On the other hand, Can2 showed a strong negative correlation, mainly with the Ca content.
Canonical variable analysis and confidence ellipses (p ≤ 0.01) for the treatments consisting of different soil managements (conventional fertilization + limestone [FL]; conventional fertilization + limestone + gypsum [FLG]; conventional fertilization + limestone + gypsum + phosphorus applied at depth [FLGP]) and irrigation frequencies (one and seven days) Ca Calcium, Mg Magnesium, PME Pectin methylesterase, PG Polygalacturonase, TA Titratable acidity, Ratio, SS Soluble solids, FIRM Firmness, DM Dry matter, TC Total carotenoids, RS Reducing sugars, FEN Phenolic compounds
Discussion
The increase in the soluble solids content of tomato fruit subjected to water management, adopting the IF of seven days and soil management with FLG is due to the low dilution of soluble solids caused by the possible reduced transport of water (Nangare et al. 2016). Increasing the soluble solids content in fruits destined for industry is one of the main objectives to be achieved by the sector, as it increases industrial yield, and identity and quality standards (IQS) are more easily achieved (Wei et al. 2018a, b). The higher the SS content, the lower the energy required to evaporate the water from the fruits in the preparation of extracts, sauces and concentrated juices (Bennett 2012; Dariva et al. 2021).
Titratable acidity did not differ, most likely because treatments with soil management and a seven-day IF had no effect on the osmotic adjustment process, which involves the active production of organic acids under controlled water stress (Hou et al. 2020). Compared to sugar metabolism, malic and citric acid metabolism (major acids found in tomatoes) involves enzyme-catalyzed biochemical pathways including carboxylation of phosphoenolpyruvate (PEP), decarboxylation of oxaloacetate, the tricarboxylic acid cycle (TCA) and the glyoxylate cycle (Etienne et al., 2013; Hou et al. 2020) which still remains poorly understood in the literature on the physiological mechanisms involved behind acid accumulation responses to RDI strategies. The non-influence of management on titratable acidity, in contrast to the increase in SS under IF of seven days, promoted an increase in the ratio, indicating an increase in the perception of sweetness of the fruit from the greater allocation of carbohydrates (Wang and Frei 2011; Hou et al. 2017). According to Mian et al. (2021) tomatoes that have soluble solids/titratable acidity ratio greater than 10 are considered to have good flavor. In our study, the values ranged from 11.92 to 13.01.
The increase in reducing sugars in the combined treatments with FLG and IF of seven days can be explained by the increase in the activity of carbohydrate metabolism enzymes that increase the degradation of disaccharides during the period of fruit maturation, generating greater mobilization and accumulation of glucose and fructose in the fruits (Ruan et al. 2010; Ripoll et al. 2014; Hou et al. 2020).
Improvements in tomato fruit quality in relation to increases in SS, sugars, and consequently better soluble solids/titratable acidity ratio, can be explained by the reduction of lateral shoots and reproductive growth in plants submitted to RDI. These reductions possibly improve the draining activity in tomato fruit, so the carbohydrate that would be directed to the sprouts is redirected to the fruit, culminating in the increase of assimilates in them. (Patanè and Cosentino 2010; Wei et al. 2018a, b; Liu et al. 2021a, b). Additionally, moderate regulated deficit irrigation (RDI) can induce a greater accumulation of starch during early fruit development, with greater conversion of it into sugars from the increase in the activity of carbohydrate catabolizing enzymes that modulate the sugar concentration in tomatoes during ripening (Zegbe-Domınguez et al. 2003; Sun et al. 2014; Liu et al. 2021a, b). The balance between these two hormones stimulates the activity of invertase enzymes, which catabolize carbohydrates and trigger the increase in sugars in fruits (Ruan et al. 2010; Wei et al. 2018a, b).
The lower Ca2+ content in tomato fruit in the combined management with FL and FI of seven days can be explained by the lower mass flow rate of water in the xylem, which is mainly influenced by transpiration and plant growth rates. (Hocking et al. 2016; Reitz et al. 2021), in addition to the availability of the nutrient in the soil. This indicates that soil managements FLG and FLGP increased the levels and availability of Ca2+ in the soil, regardless of the IF adopted. Previous studies have shown that the exclusive application of limestone provided improvements in soil chemical attributes, including reductions in pH, exchangeable Al3+, H + Al and cationic micronutrients (Fe, Mn, Cu and Zn), reducing possible toxic effects on plant development and microbial growth (Carmeis Filho et al. 2017). However, in a recent study Bossolani et al. (2020) reported that the application of lime and gypsum together provided greater availability of N, P, Ca2+ and S-SO42− when compared to the application of lime alone.
Although gypsum does not directly affect soil pH, these changes in the availability of the aforementioned nutrients increase the pH due to the exchange reactions of S-SO42− ligands with terminal hydroxides associated with Al and Fe oxides, which displace OH− and promote partial neutralization of soil acidity. Improvements in crop yields due to gypsum application are mainly due to increased Ca2+ and S solubility in the soil and, consequently, plant availability and/or reduced availability of Al3+ in the soil, especially in deep layers (Caires et al. 2011). Due to the thermodynamics of ion exchange and the properties of Ca2+, gypsum can also potentially increase the leaching of Mg2+ and K+ to deep layers (Zoca and Penn 2017). Therefore, it is reasonable to suggest that with decrease of IF (seven days) and combined soil management practices (FLG and FLGP), it possibly maintains the nutritional status and supply of calcium and magnesium in tomato fruit based on maintenance and better moisture distribution along the soil profile (Liu et al. 2011; Chai et al. 2016), favoring a greater zone of root growth and, consequently, absorption of water and nutrients (Cui et al. 2009; Fara et al. 2019).
The higher pulp firmness in tomato fruit of the combined treatments with FLG and IF of seven days is possibly related to the lower activity of the pectinolytic enzymes PG and PME, which are the main enzymes that affect tomato firmness during ripening (Wei et al. 2018b). The lower activity of PG and PME resulting in greater firmness of tomato fruit is that PME catalyzes the demethylation of pectin and generates pectic acid, a substrate for PG. PG, in turn, depolymerizes the polygalacturonic acid chain (Xie et al. 2017). The depolymerization of these structural domains and the solubilization of pectic polysaccharides causes the modification of the cell wall polysaccharides and, finally, the disassembly of their architecture, as a result of the combined action of these enzymes (Tieman et al. 1992; Brummell and Harpster 2001; Bu et al. 2013; Kumar et al. 2021). However, as calcium is one of the main constituents of pectin in the cell wall and helps to stabilize the plasma membrane (Hocking et al. 2016), adequate amounts of this nutrient in tomato fruit contribute to stabilization, causing a persistent inhibition of pectic polysaccharide hydrolysis and strongly suppressing PG-mediated pectin release from cell walls (Rushing and Huber 1987; Huber et al. 2001).
Pectin methylesterase enzymes belong to large multigene families in all plant species examined so far. For example, in Arabidopsis thaliana, 66 ORFs (Open Reading Frames) have been annotated as putative full-length pectin methylesterases, representing 6.81% of all active carbohydrate-active enzymes (CAZymes) and expansins in the species. In Populus trichocarpa, there are 89 ORFs and 5.46%, respectively, while these numbers appear to be substantially smaller (35 ORFs and 3.14%) in Oryza sativa (Pelloux et al. 2007). In Fragaria vesca, Malus domestica, Pyrus bretschneideri, Prunus mume, Prunus persica, and Rosa chinensis, a total of 54, 78, 79, 57, 66, and 53 pectin methylesterase genes were individually identified. These genes were named as FvPME, MdPME, PbPME, PmPME, PpPME, and RcPME, respectively (Xue et al. 2020). The firmness of the fruit decreases as the pectin content increases, and this was observed in tomato fruits in this study. Similar results were reported in strawberries after the downregulation of the FaPG1, PL, and FaβGal4 genes (Paniagua et al. 2016), as well as in peaches (Liu et al. 2018).
Polygalacturonases are encoded by large multigene families (Yang et al. 2018). This gene family has been identified in various plants, including Arabidopsis, Oryza sativa, Brassica rapa, Populus, cucumber, watermelon, tomato, mango, apple, and peach (Zhang et al. 2019). In strawberry fruits, two different genes have been described: FaPG1 and FaPG2 (AY280662) (Quesada et al. 2009). Among the 54 SlPGs identified in tomato fruits, members in clades A and B are involved in fruit and abscission zone development, while members from clades C, D, and F are involved in flowering development (Dautt-Castro et al. 2019).
Thus, the results of this study provide convincing evidence that the synergistic effect of soil and water management strategies contribute to lower PG and PME activity, generating less pectin solubilization by hydrolytic enzymes, as a result of adequate Ca2+ supply in the fruits and the increased allocation of water to these bodies. This indicates that the fruits managed with FLG and FLGP have superior physical qualities, that is, they tend to be firmer and less predisposed to suffer mechanical damage during transport. Firm tomatoes are considered an essential attribute for the industry as they tolerate long-distance transport to processing sites without pericarp rupture (Dariva et al. 2021).
The increase in the percentage of dry matter indicates that the IF of seven days combined with soil management techniques was probably due to the optimization and improvement in water use efficiency (WUE) by the plants submitted to the adopted strategies. Concomitantly, the application of RDI is also accompanied by a reduction in the osmotic potential of the fruits, suggesting that active metabolism of solutes may have resulted in an increase in the accumulation of water and dry matter in the fruit (Ripoll et al. 2014). The reflection of this is the increase in the quality of tomatoes, that is, as dry matter is one of the key variables to describe the amount of fixed carbon (soluble and insoluble solids, mainly sugars, acids, pectic substances and other polysaccharides, in addition to nutrients inorganic substances) to the detriment of the amount of water applied by the plant (Foolad 2007; Chai et al. 2016; Villas Boas et al., 2017); we can infer that the higher amount of carbon fixed in our study reflected in a higher yield of tomatoes produced, per unit of water supplied. Previous studies have shown that the adoption of management strategies that aim to increase the WUE of the plants maintains or improves the dry matter of tomato fruit (Li et al. 2019; Liu et al. 2021a, b), as they increase productivity (Fara et al. 2019).
The increase in total carotenoids and phenolic compounds when submitted to FLG soil treatment and in all managements with IF of seven days can be explained by the regulated oxidative stress induced in plants by RDI, which led to an increase in the production of reactive oxygen species (ROS), which possibly stimulates the synthesis and accumulation of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX) and guaiacol peroxidase (GPOX), or non-enzymatic substances such as lycopene, β-carotene and vitamins in tomato fruit (Ripoll et al. 2014; Hou et al. 2020). RDI strategies are known to trigger increased synthesis of carotenoids (Fanciullino et al. 2014; Dariva et al. 2021) and phenolic compounds (Fumar et al., 2015). In our study, we observed that the adoption of RDI, given the increase in IF (seven days) influenced the increase in the content of phenolic compounds and total carotenoids, in all treatments, especially when combined with FLG. Higher levels of carotenoids were also reported by Coyago-Cruz et al. (2022) in tomato fruit when subjected to RDI strategies.
Our results showed high positive scores obtained in Can1, indicating that treatments with daily IF, regardless of the type of management adopted, resulted in high activities of PME and PG, lower pulp firmness, as well as a low content of phenolic and carotenoids. Here we present that shows that the increase in firmness of tomato fruits is related to the decrease in the activity of pectinolytic enzymes when subjected to RDI management. In contrast, the negative scores in Can1 presented in the canonical variable analysis, were obtained by the managements with FLG and FLGP and FI of seven days, and indicate a production of firmer fruits, with higher content of carotenoids, phenolic compounds, reducing sugars, and dry matter, as well as higher ratio. The high positive score on Can2 indicates a strong negative relationship of Ca, with FL managements with IF of seven days. A schematic diagram with the main biochemical changes in tomatoes for industrial processing influenced by combined soil and water management was created (Fig. 6).
Illustration of the main biochemical changes in tomato for industrial processing influenced by combined soil and water management. On the right the treatment composed conventional fertilization + limestone + irrigation frequencies of one day (FL + IF1), and the left conventional fertilization + limestone + gypsum + irrigation frequencies of seven days (FLG + IF7)
Transpiration is crucial for proper tomato plant growth, enabling the transfer of vital nutrients like S, Ca, Mg, and P from roots to various plant parts, especially fruits. Sulfur supports amino acids for protein synthesis, calcium aids in cell wall growth and fruit quality, magnesium is essential for chlorophyll and photosynthesis, and phosphorus is involved in metabolic processes and energy transport. The source-sink connection refers to the flow of sugars, phenolics, carotenoids, and other organic chemicals within the tomato plant. Sugars are primarily produced through photosynthesis in the leaves and serve as a source for developing fruits, where they are utilized for growth and ripening processes. The presence of soluble sugars like glucose and fructose enhances the flavor and sweetness of tomatoes, impacting their marketability. Phenolics and carotenoids, secondary metabolites responsible for color, flavor, and antioxidant properties, are crucial for attracting customers and determining the overall market value of tomatoes.
Transpiration facilitates the movement of organic substances like soluble sugars, phenolics, and carotenoids within the plant, from source leaves to growing fruits. Adequate water supply and optimal transpiration rates are essential for proper distribution and accumulation of these chemicals in the fruits, ultimately affecting their taste, color, flavor, and market value. Watering tomatoes every seven days promotes the growth of deeper roots, leading to more efficient nutrient absorption and reduced water usage. Deeper roots access a larger moisture reserve in the soil, allowing plants to develop healthier and more robustly. This deeper root system also enhances drought resistance, making the plants adaptable to water shortages and dry conditions. Moreover, less frequent irrigation results in significant water savings due to reduced evaporation and overall water usage. Additionally, tomatoes harvested from plants with deeper root systems have improved nutrient density and longer shelf life, enhancing the fruits’ quality.
It is possible to enhance post-harvest features of industrial tomatoes without genetic modification by comparing Flavr Savr tomatoes with a non-GMO approach of intensive fertilization and minimal irrigation. Flavr Savr tomatoes were genetically modified to delay ripening and extend shelf life, while the non-GMO approach focuses on nutrient availability and water management. Both methods aim to improve post-harvest qualities, but GMO crops raise concerns about environmental impacts and consumer acceptance, while the non-GMO strategy aligns with sustainable practices, reducing resource usage. GMO crops require approval and labeling, adding regulatory considerations, whereas the non-GMO approach may encounter fewer obstacles. In conclusion, Flavr Savr tomatoes rely on genetic modification for specific benefits, while the non-GMO strategy optimizes cultural practices, enhances fruit quality, extends shelf life, and promotes sustainable agriculture.
Conclusion
The combined soil and water management strategies promoted an increase in post-harvest quality of industrial tomato variety Heinz 9553. The lower activity of pectin methylesterase and polygalacturonase and resulted in higher firmness in fruits with FLG and IF management of seven days. The combination of soil and water management with FLG and IF of seven positively influenced soluble solids, reducing sugars, soluble solids/titratable acidity ratio, firmness, dry matter, phenolic compounds and total carotenoids of tomato. Therefore, the combined soil and water managements FLG and FLGP with seven days IF are recommended for field cultivation of tomato for industrial processing.
Data Availability
All data generated or analyzed during this study will be provided upon request to the corresponding author.
Code Availability
Not applicable.
References
Acharya A, Pesacreta TC (2022) Localization of seed-derived and externally supplied nutrients in peanut seedling root. Theor Exp Plant Physiol. https://doi.org/10.1007/s40626-021-00227-9
Acharya A, Pesacreta TC (2023) P-ring: The conserved nature of phosphorus enriched cells in seedling roots of distantly related species. Plant Signal Behav. https://doi.org/10.1080/15592324.2023.2217389
Al-Dari M, Pathare PB, Al-Yahyai R (2021) Effect of postharvest transport and storage on color and firmness quality of tomato. Horticulturae 7:163. https://doi.org/10.3390/horticulturae7070163
Ali A, Lin SL, He JK, Kong FM, Yu JH, Jiang HS (2019) Climatic water availability is the main limiting factor of biotic attributes across large-scale elevational gradients in tropical forests. Sci Total Environ 647:1211–1221. https://doi.org/10.1016/j.scitotenv.2018.08.072
Allen RG, Pereira LS (2009) Estimating crop coefficients from fraction of ground cover and height. Irrig Sci 28:17–34. https://doi.org/10.1007/s00271-009-0182-z
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. Fao, Rome 300:D05109
Almeida Neta MN, Mota WFD, Pegoraro RF, Pacheco MC, Batista CM, Soares MDC (2019) Agronomic yield and quality of industrial tomatoes under NPK doses. Revista Brasileira De Engenharia Agrícola e Ambiental 24:59–64. https://doi.org/10.1590/1807-1929/agriambi.v24n1p59-64
Alvarenga, M. A. R., Alvarenga, M., Carvalho, J. G., Bastos, A. R. R., Vale, F. X. R., Zambolim, L., Zambolim, E. M., Lima, L. A., Faquin, V., 2004. Tomate: Produção em campo, em casa-de-vegetação e em hidroponia. Lavras: UFLA. 400p.
Araújo NO, Véras MLM, Santos MNS, Araújo FF, Tello JPJ, Finger FL (2020) Sucrose degradation pathways in cold-induced sweetening and its impact on the non-enzymatic darkening in sweet potato root. Food Chem. https://doi.org/10.1016/j.foodchem.2019.125904
Bennett AB (2012) Taste: Unraveling tomato flavor. Curr Biol 22:R443–R444. https://doi.org/10.1016/j.cub.2012.04.017
Bogale A, Nagle M, Latif S, Aguila M, Müller J (2016) Regulated deficit irrigation and partial root-zone drying irrigation impact bioactive compounds and antioxidant activity in two select tomato cultivars. Sci Hortic 213:115–124. https://doi.org/10.1016/j.scienta.2016.10.029
Bossolani JW, Crusciol CAC, Merloti LF, Moretti LG, Costa NR, Tsai SM, Kuramae EE (2020) Long-termlimestoneand gypsum amendment increase nitrogen fixation and decrease nitrification and denitrification gene abundances in the rhizosphere and soil in a tropical no-till intercropping system. Geoderma. https://doi.org/10.1016/j.geoderma.2020.114476
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brummell DA, Harpster MH (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47:311–339. https://doi.org/10.1023/A:1010656104304
Bu J, Yu Y, Aisikaer G, Ying T (2013) Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruit. Postharvest Biol Technol 86:337–345. https://doi.org/10.1016/j.postharvbio.2013.07.026
Caires EF, Joris HAW, Churka S (2011) Long-term effects of limestone and gypsum additions on no-till corn and soybean yield and soil chemical properties in southern Brazil. Soil Use Manag 27:45–53. https://doi.org/10.1111/j.1475-2743.2010.00310.x
Capaldi FR, Gratão PL, Reis AR, Lima LW, Azevedo RA (2015) Sulfur metabolism and stress defense responses in plants. Tropical Plant Biol 8:60–73. https://doi.org/10.1007/s12042-015-9152-1
Carmeis Filho AC, Penn CJ, Crusciol CA, Calonego JC (2017) Limestone and phosphogypsum impacts on soil organic matter pools in a tropical Oxisol under long-term no-till conditions. Agric Ecosys Environ 241:11–23
Chai Q, Gan Y, Zhao C, Xu HL, Waskom RM, Niu Y, Siddique KH (2016) Regulated deficit irrigation for crop production under drought stress. A review. Agron Sustain Develop 36:1–21. https://doi.org/10.1007/s13593-015-0338-6
Chapagain AK, Orr S (2009) An improved water footprint methodology linking global consumption to local water resources: A case of Spanish tomatoes. J Environ Manage 90:1219–1228. https://doi.org/10.1016/j.jenvman.2008.06.006
Claessen, M. E. C., Barreto, W. O., Paula, J. L., Duarte, M. N. (1997). Manual de métodos de análise de solo. 2.ed. EMBRAPA. Centro Nacional de Pesquisa de Solos. 212p.
Coutinho Edson, L. M., Orioli Júnior, V., da Silva, E. J., Coutinho Neto, A. M., Cardoso, S. S., 2014. Nutrición, producción y calidad de frutos de tomate para procesamiento en función de la fertilización con fósforo y potasio. Agrociencia, 18, 40–46 http://www.scielo.edu.uy/scielo.php?script=sci_arttext&pid=S230115482014000200005&lng=es&tlng=es.
Coyago-Cruz E, Corell M, Moriana A, Hernanz D, Stinco CM, Mapelli-Brahm P, Meléndez-Martínez AJ (2022) Effect of regulated deficit irrigation on commercial quality parameters, carotenoids, phenolics and sugars of the black cherry tomato (Solanum lycopersicum L.) ʽSunchocolaʼ. J Food Compos Anal. https://doi.org/10.1016/j.jfca.2021.104220
Cui N, Du T, Kang S, Li F, Hu X, Wang M, Li Z (2009) Relationship between stable carbon isotope discrimination and water use efficiency under regulated deficit irrigation of pear-jujube tree. Agric Water Manag 96:1615–1622. https://doi.org/10.1016/j.agwat.2009.06.009
Dariva FD, Pessoa HP, Copati MGF, Almeida GQ, Castro Filho MN, Picoli EAT, Nick C (2021) Yield and fruit quality attributes of selected tomato introgression lines subjected to long-term deficit irrigation. Sci Hortic. https://doi.org/10.1016/j.scienta.2021.110426
Dautt-Castro M, López-Virgen AG, Ochoa-Leyva A, Contreras-Vergara CA, Sortillón-Sortillón AP, Martínez-Téllez MA, González-Aguilar GA, Casas-Flores JS, Sañudo-Barajas A, Kuhn DN, Islas-Osuna MA (2019) Genome-wide identification of mango (Mangifera indica L.) polygalacturonases: Expression analysis of family members and total enzyme activity during fruit ripening. Front Plant Sci 10:969. https://doi.org/10.3389/fpls.2019.00969
de Castro A, Vilas Boas A, Page D, Giovinazzo R, Bertin N, Fanciullino AL (2017) Combined effects of irrigation regime, genotype, and harvest stage determine tomato fruit quality and aptitude for processing into puree. Front Plant Sci 8:1725. https://doi.org/10.3389/fpls.2017.01725
De Col V, Fuchs P, Nietzel T, Elsässer M, Voon CP, Candeo A, Schwarzländer M (2017) ATP sensing in living plant cells reveals tissue gradients and stress dynamics of energy physiology. Elife. https://doi.org/10.7554/eLife.26770.001
Delazari FT, Giovanelli LB, Gomes RS, Junior RM, Lima JDO, Freitas EM, Silva DJH (2016) Irrigation water management during the ripening of tomato aiming fruit quality. Afr J Agric Res 11:4525–4531. https://doi.org/10.5897/AJAR2016.11673
Dodd IC (2009) Rhizosphere manipulations to maximize ‘crop per drop’during deficit irrigation. J Exp Bot 60:2454–2459. https://doi.org/10.1093/jxb/erp192
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Fanciullino AL, Bidel LPR, Urban L (2014) Carotenoid responses to environmental stimuli: integrating redox and carbon controls into a fruit model. Plant Cell Environ 37:273–289. https://doi.org/10.1111/pce.12153
FAOSTAT, 2020. Food and Agriculture Organization of the United Nations. Production and Trade Statistics.
Fara SJ, Delazari FT, Gomes RS, Araújo WL, Silva DJH (2019) Stomata opening and productiveness response of fresh market tomato under different irrigation intervals. Sci Hortic 255:86–95. https://doi.org/10.1016/j.scienta.2019.05.025
EB Ferreira PP Cavalcanti DA Nogueira 2021 ExpDes: Experimental Designs Package. R package version 1.2.1
Foolad MR (2007) Genome mapping and molecular breeding of tomato. Int J Plant Genom. https://doi.org/10.1155/2007/64358
Friendly, M., Fox, J., Friendly, M. M., 2013. Visualizing generalized canonical discriminant and canonical correlation analysis. R Package ‘‘candisc’’, version: 0.6–5.
Gonçalves C, Rodriguez-Jasso RM, Gomes N, Teixeira JA, Belo I (2010) Adaptation of dinitrosalicylic acid method to microtiter plates. Anal Methods 2:2046–2048. https://doi.org/10.1039/C0AY00525H
Guo W, Nazim H, Liang Z, Yang D (2016) Magnesium deficiency in plants: An urgent problem. Crop J 42:83–91. https://doi.org/10.1016/j.cj.2015.11.003
Gupta A, Pandey R, Sinha R, Chowdhary A, Pal RK, Rajam MV (2019) Improvement of post-harvest fruit characteristics in tomato by fruit-specific over-expression of oat arginine decarboxylase gene. Plant Growth Regul 88:61–71. https://doi.org/10.1007/s10725-019-00488-0
Helyes L, Lugasi A, Pek Z (2012) Effect of irrigation on processing tomato yield and antioxidant components. Turk J Agric for 36:702–709
Higo M, Azuma M, Kamiyoshihara Y, Kanda A, Tatewaki Y, Isobe K (2020) Impact of phosphorus fertilization on tomato growth and arbuscular mycorrhizal fungal communities. Microorganisms 8:178. https://doi.org/10.3390/microorganisms8020178
Hocking B, Tyerman SD, Burton RA, Gilliham M (2016) Fruit calcium: transport and physiology. Front Plant Sci 7:569. https://doi.org/10.3389/fpls.2016.00569
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24. https://doi.org/10.1111/j.1469-8137.2004.01015.x
Hou M, Jin Q, Lu X, Li J, Zhong H, Gao Y (2017) Growth, water use, and nitrate-15N uptake of greenhouse tomato as influenced by different irrigation patterns, 15N labeled depths, and transplant times. Front Plant Sci 8:666. https://doi.org/10.3389/fpls.2017.00666
Hou X, Zhang W, Du T, Kang S, Davies WJ (2020) Responses of water accumulation and solute metabolism in tomato fruit to water scarcity and implications for main fruit quality variables. J Exp Bot 71:1249–1264. https://doi.org/10.1093/jxb/erz526
Huber DJ, Karakurt Y, Jeong J (2001) Pectin degradation in ripening and wounded fruits. Rev Bras Fisiol Veg 13:224–241. https://doi.org/10.1590/S0103-31312001000200009
Khapte PS, Kumar P, Burman U, Kumar P (2019) Deficit irrigation in tomato: Agronomical and physio-biochemical implications. Sci Hortic 248:256–264. https://doi.org/10.1016/j.scienta.2019.01.006
Kirda C, Çetin M, Dasgan Y, Topçu S, Kaman H, Ekici B, Ozguven AI (2004) Yield response of greenhouse grown tomato to partial root drying and conventional deficit irrigation. Agric Water Manag 69:191–201. https://doi.org/10.1016/j.agwat.2004.04.008
Kumar N, Tokas J, Raghavendra M, Singal HR (2021) Impact of exogenous salicylic acid treatment on the cell wall metabolism and ripening process in postharvest tomato fruit stored at ambient temperature. Int J Food Sci Technol 56:2961–2972. https://doi.org/10.1111/ijfs.14936
Li Q, Wei M, Li Y, Feng G, Wang Y, Li S, Zhang D (2019) Effects of soil moisture on water transport, photosynthetic carbon gain and water use efficiency in tomato are influenced by evaporative demand. Agric Water Manag. https://doi.org/10.1016/j.agwat.2019.105818
Liu L, Gan Y, Bueckert R, Van Rees K (2011) Rooting systems of oilseed and pulse crops I: Temporal growth patterns across the plant developmental periods. Field Crop Res 122:256–263. https://doi.org/10.1016/j.fcr.2011.04.002
Liu H, Qian M, Song C, Li J, Zhao C, Li G, Wang A, Han M (2018) Down-regulation of PpBGAL10 and PpBGAL16 delays fruit softening in peach by reducing polygalacturonase and pectin methylesterase activity. Front Plant Sci 9:1015. https://doi.org/10.3389/fpls.2018.01015
Liu J, Hu T, Feng P, Yao D, Gao F, Hong X (2021a) Effect of potassium fertilization during fruit development on tomato quality, potassium uptake, water and potassium use efficiency under deficit irrigation regime. Agric Water Manag. https://doi.org/10.1016/j.agwat.2021.106831
Liu J, Hu T, Feng P, Yao D, Gao F, Hong X (2021b) Effect of potassium fertilization during fruit development on tomato quality, potassium uptake, water and potassium use efficiency under deficit irrigation regime. Agric Water Manag. https://doi.org/10.1016/j.agwat.2021.106831
Luz JM, Bittar CA, Oliveira RC, Nascimento AR, Nogueira AP (2016) Desempenho e divergência genética de genótipos de tomate para processamento industrial. Hortic Bras 34:483–490. https://doi.org/10.1590/S0102-053620160406
Ma Q, Chen L, Du M, Zhang Y, Zhang Y (2020) Localized and moderate phosphorus application improves plant growth and phosphorus accumulation in Rosa multiflora thumb. ex murr. via efficient root system development. Forests 11:570
Mantovani EC, Delazari FT, Dias LE, Assis IR, Vieira GH, Landim FM (2013) Yield and water use efficiency for two sweet potato cultivars depending on irrigation depths. Hortic Bras 31:602–606. https://doi.org/10.1590/S0102-05362013000400015
Mazid M, Khan TA, Mohammad F (2011) Response of crop plants under sulphur stress tolerance: A holistic approach. J Stress Physiol Biochem 7:23–57
Medyouni I, Zouaoui R, Rubio E, Serino S, Ahmed HB, Bertin N (2021) Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food Sci Nutr 9:1949–1960. https://doi.org/10.1002/fsn3.2160
Mian S, Constantino LV, Nunes MP, Ventura MU, Spinosa WA, Hata NN, Gonçalves LS (2021) Post-harvest quality and sensory acceptance of Italian tomatoes grown under organic, integrated and conventional management. Hortic Bras 39:417–424. https://doi.org/10.1590/s0102-0536-20210411
Morales RGF, Resende LV, Bordini IC, Galvão AG, Rezende FC (2015) Caracterização do tomateiro submetido ao déficit hídrico. Scientia Agraria 16:9–17
Nangare DD, Singh Y, Kumar PS, Minhas PS (2016) Growth, fruit yield and quality of tomato (Lycopersicon esculentum Mill.) as affected by deficit irrigation regulated on phenological basis. Agric Water Manag 171:73–79. https://doi.org/10.1016/j.agwat.2016.03.016
Paniagua C, Blanco-Portales R, Barceló-Muñoz M, García-Gago JA, Waldron KW, Quesada MA, Muñoz-Blanco J, Mercado JA (2016) Antisense down-regulation of the strawberry β-galactosidase gene FaβGal4 increases cell wall galactose levels and reduces fruit softening. J Exp Bot 67:619–631. https://doi.org/10.1093/jxb/erv462
Patanè C, Cosentino SL (2010) Effects of soil water deficit on yield and quality of processing tomato under a Mediterranean climate. Agric Water Manag 97:131–138. https://doi.org/10.1016/j.agwat.2009.08.021
Pelloux J, Rusterucci C, Mellerowicz EJ (2007) New insights into pectin methylesterase structure and function. Trends Plant Sci 12:267–277. https://doi.org/10.1016/j.tplants.2007.04.001
Pesacreta TC, Acharya A, Hasenstein KH (2021) Endogenous nutrients are concentrated in specific tissues in the Zea mays seedling. Protoplasma 258:863–878. https://doi.org/10.1007/s00709-021-01606-4
Quesada MA, Blanco-Portales R, Posé S, García-Gago JA, Jiménez-Bermúdez S, Muñoz-Serrano A, Caballero JL, Pliego-Alfaro F, Mercado JA, Muñoz-Blanco J (2009) Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol 150:1022–1032. https://doi.org/10.1104/pp.109.138297
Quinet M, Angosto T, Yuste-Lisbona FJ, Blanchard-Gros R, Bigot S, Martinez JP, Lutts S (2019) Tomato fruit development and metabolism. Front Plant Sci 10:1554. https://doi.org/10.3389/fpls.2019.01554
R Core Team. (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raghothama KG (2005) Phosphorus and plant nutrition: an overview. Phosphorus 46:353–378. https://doi.org/10.2134/agronmonogr46.c11
Reitz NF, Shackel KA, Mitcham EJ (2021) Differential effects of excess calcium applied to whole plants vs. excised fruit tissue on blossom-end rot in tomato. Sci Hortic. https://doi.org/10.1016/j.scienta.2021.110514
Ripoll J, Urban L, Staudt M, Lopez-Lauri F, Bidel LP, Bertin N (2014) Water shortage and quality of fleshy fruits—making the most of the unavoidable. J Exp Bot 65:4097–4117. https://doi.org/10.1093/jxb/eru197
Rodriguez-Amaya DB (2001) A guide to carotenoid analysis in foods, vol 71. ILSI press, Washington
Ronga D, Zaccardelli M, Lovelli S, Perrone D, Francia E, Milc J, Pecchioni N (2017) Biomass production and dry matter partitioning of processing tomato under organic vs conventional cropping systems in a Mediterranean environment. Sci Hortic 224:163–170. https://doi.org/10.1016/j.scienta.2017.05.037
Ruan YL, Jin Y, Yang YJ, Li GJ, Boyer JS (2010) Sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Mol Plant 3:942–955. https://doi.org/10.1093/mp/ssq044
Rushing JW, Huber DJ (1987) Effects of NaCl, pH and Ca2+ on autolysis of isolated tomato fruit cell walls. Physiol Plant 70:78–84. https://doi.org/10.1111/j.1399-3054.1987.tb08700.x
Saeed R, Ahmad R (2009) Vegetative growth and yield of tomato as affected by the application of organic mulch and gypsum under saline rhizosphere. Pak J Bot 41:3093–3105
Sarruge JR, Haag HP (1974) Análises químicas em plantas. Esalq, Piracicaba, p 56
Shao HB, Chu LY, Jaleel CA, Zhao CX (2008) Water-deficit stress-induced anatomical changes in higher plants. CR Biol 331:215–225. https://doi.org/10.1016/j.crvi.2008.01.002
Sinha SR, Singha A, Faruquee M, Jiku M, Sayem A, Rahaman M, Kader MA (2019) Post-harvest assessment of fruit quality and shelf life of two elite tomato varieties cultivated in Bangladesh. Bullet Natl Res Cent 43:1–12. https://doi.org/10.1186/s42269-019-0232-5
Strati IF, Oreopoulou V (2011) Process optimisation for recovery of carotenoids from tomato waste. Food Chem 129:747–752. https://doi.org/10.1016/j.foodchem.2011.05.015
Sun Y, Holm PE, Liu F (2014) Alternate partial root-zone drying irrigation improves fruit quality in tomatoes. Hortic Sci 41:185–191
Teixeira, P. C., Donagemma, G. K., Fontana, A., Teixeira, W. G., 2017. Manual de métodos de análise de solo. 3. ed. Brasília, DF: Embrapa, 2017.
Tieman DM, Harriman RW, Ramamohan G, Handa AK (1992) An antisense pectin methylesterase gene alters pectin chemistry and soluble solids in tomato fruit. Plant Cell 4:667–679. https://doi.org/10.1105/tpc.4.6.667
Tigist M, Workneh TS, Woldetsadik K (2013) Effects of variety on the quality of tomato stored under ambient conditions. J Food Sci Technol 50:477–486. https://doi.org/10.1007/s13197-011-0378-0
Tonetto de Freitas S, McElrone AJ, Shackel KA, Mitcham EJ (2014) Calcium partitioning and allocation and blossom-end rot development in tomato plants in response to whole-plant and fruit-specific abscisic acid treatments. J Exp Bot 65:235–247. https://doi.org/10.1093/jxb/ert364
Wang Y, Frei M (2011) Stressed food–The impact of abiotic environmental stresses on crop quality. Agr Ecosyst Environ 141:271–286. https://doi.org/10.1016/j.agee.2011.03.017
Wang Y, Liu F, Andersen MN, Jensen CR (2010) Improved plant nitrogen nutrition contributes to higher water use efficiency in tomatoes under alternate partial root-zone irrigation. Funct Plant Biol 37:175–182. https://doi.org/10.1071/FP09181
Wei Y, Zhou D, Wang Z, Tu S, Shao X, Peng J, Tu K (2018a) Hot air treatment reduces postharvest decay and delays softening of cherry tomato by regulating gene expression and activities of cell wall-degrading enzymes. J Sci Food Agric 98:2105–2112. https://doi.org/10.1002/jsfa.8692
Wei Z, Du T, Li X, Fang L, Liu F (2018b) Interactive effects of elevated CO2 and N fertilization on yield and quality of tomato grown under reduced irrigation regimes. Front Plant Sci 9:328. https://doi.org/10.3389/fpls.2018.00328
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 924:487–511. https://doi.org/10.1093/aob/mcg164
Xie F, Yuan S, Pan H, Wang R, Cao J, Jiang W (2017) Effect of yeast mannan treatments on ripening progress and modification of cell wall polysaccharides in tomato fruit. Food Chem 218:509–517. https://doi.org/10.1016/j.foodchem.2016.09.086
Xie K, Cakmak I, Wang S, Zhang F, Guo S (2021) Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J 92:249–256. https://doi.org/10.1016/j.cj.2020.10.005
Xue C, Guan SC, Chen JQ, Wen CJ, Cai JF, Chen X (2020) Genome wide identification and functional characterization of strawberry pectin methylesterases related to fruit softening. BMC Plant Biol 20:1–17. https://doi.org/10.1186/s12870-019-2225-9
Yang Y, Yu Y, Liang Y, Anderson CT, Cao J (2018) A profusion of molecular scissors for pectins: classification, expression, and functions of plant polygalacturonases. Front Plant Sci 9:1208. https://doi.org/10.3389/fpls.2018.01208
Yang X, Zhang P, Wei Z, Liu J, Hu X, Liu F (2020) Effects of CO2 fertilization on tomato fruit quality under reduced irrigation. Agric Water Manag. https://doi.org/10.1016/j.agwat.2019.105985
Yemm EW, Cocking EC, Ricketts RE (1955) The determination of amino-acids with ninhydrin. Analyst 80:209–214. https://doi.org/10.1039/AN9558000209
Zegbe-Domınguez JA, Behboudian MH, Lang A, Clothier BE (2003) Deficit irrigation and partial rootzone drying maintain fruit dry mass and enhance fruit quality in ‘Petopride’ processing tomato (Lycopersicon esculentum, Mill.). Sci Hortic 98:505–510. https://doi.org/10.1016/S0304-4238(03)00036-0
Zhang S, Ma M, Zhang H, Zhang S, Qian M, Zhang Z, Luo W, Fan J, Liu Z, Wang L (2019) Genome-wide analysis of polygalacturonase gene family from pear genome and identification of the member involved in pear softening. BMC Plant Biol 19:1–12. https://doi.org/10.1186/s12870-019-2168-1
Zhou C, Jiang W, Li Y, Hou X, Liu A, Cai L (2017) Morphological plasticity and phosphorus uptake mechanisms of hybrid Eucalyptus roots under spatially heterogeneous phosphorus stress. J for Res 28:713–724. https://doi.org/10.1007/s11676-016-0335-x
Zhu Q, Ozores-Hampton M, Li Y, Morgan K, Liu G, Mylavarapu RS (2017) Effect of phosphorus rates on growth, yield, and postharvest quality of tomato in a calcareous soil. HortScience 52:1406–1412
Zhu Q, Ozores-Hampton M, Li YC, Morgan KT (2018) Phosphorus application rates affected phosphorus partitioning and use efficiency in tomato production. Agron J 110:2050–2058. https://doi.org/10.2134/agronj2018.03.0152
Zoca SM, Penn C (2017) An important tool with no instruction manual: a review of gypsum use in agriculture. Adv Agron 144:1–44. https://doi.org/10.1016/bs.agron.2017.03.001
Acknowledgements
We are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES—financial code 001), to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), to the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), to the Universidade Federal da Paraíba and the Universidade Federal de Viçosa for the financing and technical support for this research.
Funding
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial assistance to this study.
Author information
Authors and Affiliations
Contributions
J.V.S.M. and E.D.P. designed the experiments; N.O.A., F.F.A. and T.I.S., performed experiments and wrote the paper; D.J.H.S., S.M.S. W.S.R. and T.J.D. supervised the work. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Handling Editor: Anket Sharma.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva Martins, J.V., Pereira, E.D., de Araújo, N.O. et al. A Combined Soil and Water Management Strategy to Improve the Nutrition and Marketability of Tomato Variety ‘Heinz 9553’. J Plant Growth Regul 43, 500–515 (2024). https://doi.org/10.1007/s00344-023-11105-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11105-w