Abstract
The present study aimed to investigate the variation in the metal(loid)s composition, phytochemicals, enzymatic and non-enzymatic antioxidants activity of pot marigold grown in different treatments of soil and fly ash (FA), i.e., control (soil 100% + 0% FA), 20% FA (soil 80% + 20% FA), 40% FA (soil 60% + 40% FA), 60% FA (soil 40% + 60% FA), 80% FA (soil 20% + 80% FA), and 100% FA (soil 0% + 100% FA). The plant leaves from each treatment were harvested and screened for various metal(loid)s concentrations (Fe, Cu, Mn, Ni, Co, Zn, Cd, Mg, Ca, and As). Further, the harvested leaf samples were extracted using three solvents (ethanol, methanol, and water), and their extract yield percentage, total flavonoid, and phenolic profile, total antioxidants (1,1-Diphenyl-2-picrylhydrazyl (DPPH) assay, ABTS (2,2’-Azinobis-(3-Ethylbenzthiazolin-6-Sulfonic Acid), Ferric reducing power assay (FRAP)), and antioxidant enzymatic activity (Superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), and catalase (CAT)) were determined. The results revealed that a high accumulation of Fe, Cu, Ni, Co, Zn, and Cr with bioaccumulation factor (BAF > 1) was detected at 40% FA treatment. Furthermore, the phenolic and flavonoid content were increased by 35.44 and 160.13% respectively whereas, DPPH and ABTS activity (with low IC50) was decreased by 82.67 and 31.87% in ethanolic leaf extract at 60% FA application compared to the control. Additionally, at high FA application (100% FA), the parameters such as FRAP value, SOD, CAT, APX, and POD activity were increased by 42.42%, 313.98%, 388.76%, 415.11%, and 177.54% with respect to control soil. The findings suggested that FA application (up to 60%) in soil induced increased production of secondary metabolites, and total antioxidant activity compared to control soil.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
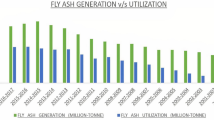
In today’s scenario, with an increasing population all around the globe, there is an increasing demand for electricity which requires coal as a chief source. Combustion of coal produces massive amounts of fly ash (FA), which, if not properly managed, poses an environmental hazard all over the world. In India, the generation of FA is about 106.36 million tons, of which 84.22 million tons are utilized in the year 2020–2021 (CEA, 2020–21). For proper management of FA, its bulk utilization in various sectors including cement, sanitary, bricks, roads and embankments, mine filling, concrete industry, etc. has been described (Varshney et al. 2020; Ahmad et al. 2021). Though numerous FA has already been utilized in different sectors, the remaining percent has been left out as toxic waste in the environment resulting in air, water, and soil pollution. Therefore, there is a necessity for long-term approaches to increase its potential application in many industries. Rather than dumping it as waste, methods for its eco-friendly use in agriculture are presently being explored (Jambhulkar et al. 2018).
FA has been effectively evaluated for the increase in growth and antioxidant status of a variety of agricultural crops such as Oryza nivara, Oryza rufipogon, Vigna mungo, Ricinus communis, and Beta vulgaris, (Bisoi et al. 2017; Panda and Bhattacharya 2019; Panda et al. 2020b; Shakeel et al. 2020). In recent studies, FA has also been shown to be effective as a soil additive for cultivating high-value medicinal plant species (Ahmad et al. 2021; Panda et al. 2021). Low doses of FA as soil amendment increases the nutrient availability and plant biomass but high doses of FA in the soil cause increased production of antioxidant enzymes present in the plants (Pandey et al. 2016; Gajic et al. 2018). The optimum dose of FA-amended soil tends to increase the physical, biological, and chemical characteristics of the soil. A low concentration of FA increases the availability of various micro and macronutrients such as K, Na, Fe, Mg, and Ca and causes increased biomass and plant yield (Shakeel et al. 2021).
FA also contains heavy metals such as Ni, Zn, Cd, Mo, Pb, and Co other than essential nutrients. Heavy metals in contaminated soil cause a variety of physiological and metabolic alterations in plants (Varshney et al. 2020). The most common symptoms of heavy metal toxicity include reduction in plant growth, decreased seed germination and photosynthesis, suppression or activation of the antioxidative defense system with advancing senescence processes, and plant death. Heavy metal stress also causes the overproduction of reactive oxygen species (ROS) such as H2O2, O2*, 1O2, and OH* which leads to cellular damage in plants including membrane damage by lipid peroxidation, cell disintegration, and cell death (Upadhyay et al. 2021). When plants are exposed to heavy metals, the equilibrium between ROS generation and antioxidative system detoxification is disturbed, resulting in oxidative stress conditions (Kaya et al. 2019a, 2019b). The tolerance mechanisms of plants to mitigate the enhanced production of ROS are a measure of various plant species. Generally, plants adopted two main strategies to combat heavy metals stress using antioxidative machinery-i) including enzymatic and ii) non-enzymatic antioxidants (Upadhyay et al. 2021). The enzymatic antioxidant machinery includes superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), peroxidase (POD), guaiacol peroxidase (GPX), glutathione-S-transferase (GST) whereas non-enzymatic antioxidants include carotenoids, proline, phenolics, flavonoids, DPPH, FRAP, ABTS, etc. (Varshney et al. 2020). Plant resistance to metal stress is frequently linked to enhanced antioxidative defense, or the ability to scavenge ROS (Varshney et al. 2020). Therefore, a plant's antioxidant defense's ability to elucidate heavy metal tolerance mechanisms is critical for understanding the plant response to heavy metals in the soil environment (Ahmad et al. 2017). However, few studies have been reported on the oxidative metabolism in medicinal important plants under FA stress but no studies have been conducted on the impact of FA on the phytochemical and antioxidant capacity of Calendula officinalis in metal stress conditions till now.
Pot marigold (Calendula officinalis L.) is a promising species used in remediating heavy metals present in contaminated sites (Saffari and Saffari 2020). It is an annual, herbaceous, and ornamental plant of the Asteraceae family and Genus Calendula. It is an edible and aromatic herb used for a variety of industrial applications such as dyes, fabrics, foods, and cosmetics. The richness in flavonoids, terpenoids, saponins, carotenoids, and minerals makes calendula an efficient source of herbal medicine. This plant possesses a high concentration of polyphenols that have the potential to act as an antioxidant, anticancer, antiviral, antimicrobial, anti-inflammatory activity, and free radical scavenger (Bedoya et al. 2020). The most important polyphenols (flavonoids and phenolics) have various biological properties. These bioactive compounds act against free radicals and reduce the oxidative stress caused by heavy metals in plants thereby protecting plant growth (Dominic et al. 2022). Therefore, the present study was designed to explore the variation in metal(loid)s composition, phenolic, and flavonoid profile, and antioxidant potentials of leaf extracts of Calendula officinalis grown in different treatments of soil and FA.
Materials and Methods
Sample Collection and Experimental Design
FA used in this study was collected from National Thermal Power Corporation (NTPC), Dadri, Uttar Pradesh, India, while soil samples were attained from Organic Farm, Amity University, Noida, India. Earthen pots having 25 cm diameter containing a total of 4 kg of air-dried soil-FA mixtures were used for the experiment. The pot experimental study was conducted at an Organic Farm, Amity Institute of Organic Agriculture, Amity University Uttar Pradesh, Noida, India. For the study, FA and soil were blended in different ratios (w/w) and a pre-requisite amount was employed in each pot. Different combinations of soil and FA were prepared with six different treatments. A total of 20 pot replicates were maintained for each treatment. The standardized treatments include-
-
1.
Treatment 1: 100% soil (control)
-
2.
Treatment 2: 20% FA + 80% soil
-
3.
Treatment 3: 40% FA + 60% soil
-
4.
Treatment 4: 60% FA + 40% soil
-
5.
Treatment 5: 80% FA + 20% soil
-
6.
Treatment 6: 100% FA
Plant Material
The Calendula officinalis seeds were procured and certified from CSIR- NISCAIR Herbarium and Museum (RHMD), Pusa Campus, IARI, New Delhi, India. Five seeds of Calendula officinalis were propagated in each pot at a depth of 2 cm at the required distances to avoid germination failure. Before sowing, a light application of tap water was done to each pot to maintain the required moisture content. The plants in each treatment were allowed to grow and were irrigated with the same volume of water at regular intervals of 2 days. No additional doses of FA or any other co-application were done at any developmental stage of the plant. After germination, plants were thinned into a single plant for maintaining one plant in each pot for each treatment. The plant leaves from all treatments were harvested after the flowering in the Calendula plants. The leaves were taken to the lab and cleaned with running water and then with double distilled water. After that, the leaves were air-dried at ambient temperature for 7 days before being pulverized with an electric grinder. The powdered leaf material was packed in an airtight container for further extraction process.
Metal(loid)s Concentration Analysis
After harvesting, the soil and FA samples from each treatment were air-dried. Air-dried soil samples were grounded into thin powder and passed through a 2 mm sieve. Plants were harvested, washed, separated, and oven-dried at 70 °C. Dried plant leaves from each treatment were ground into fine particles. For the estimation of metal(loid)s concentration, the wet digestion method was performed following the standard protocol (Kotelnikova et al. 2022). 1 g of each soil and leaf sample was mixed in 10 ml aquaregia solution (HNO3/HCl 1:3 ratio) (Pandey and Bhattacharya 2019). Concentrated H2O2 (35%) was further added to this solution for the breakdown of organic matter and then again digested in a 10 ml aquaregia solution. The solution was kept overnight and then heated at 120 °C for two hours and 80 °C for 4 h till the solution became colorless (Yu et al. 2019). The digested material was cooled, and filtered, and then distilled water was added to make a final volume of 50 ml. After wet digestion, the concentration of various metal(loid)s (Fe, Cu, Mn, Ni, Co, Zn, Cd, Mg, Ca, and As) in different treatments of soil and FA and the plant leaves grown in the same treatment were determined using Inductively coupled plasma mass spectrophotometer (ICP-MS, Agilent 7900, Santa Clara, USA). For ensuring quality control, CL51-014CR was used as NISSRM-certified reference material. The multielement standard 2A calibration standards were used for checking the accuracy of results. The analytical variations were observed within ± 10% of the repeated standard samples at a 95% confidence interval with a 2-coverage factor. Blank and internal standards were maintained for quality assurance of the elemental analysis. All the samples were calculated as mean ± SD with replicates (n = 3).
The bioaccumulation factor (BAF) was evaluated for the leaf sample of Calendula officinalis according to the equation of Pandey et al. (2016).
Extract Preparation and Extract Yield
The powdered samples were extracted with different solvents (60–80 °C) by hot percolation technique in soxhlet apparatus (Tanco SEE-4 Soxhlet Extraction Unit PLT-175 Series, Ghitorni, Delhi, India) until the solvent became colorless. The sequential extraction of plant leaves was carried out in various batches using three different solvents with increasing polarity in the soxhlet apparatus. The solvents taken for the extraction of leaves were ethanol, methanol, and distilled water. The extraction was performed in order of polarization of solvents used in the order: ethanol (E) < methanol (M) < distilled water. 20 g powdered leaf and flower material were packed, kept in a soxhlet extractor, and extracted with 500 ml of different solvents for 8 h by providing constant heating. The temperature of soxhlet extraction was maintained on a heating mantle with thermostat control. The extracts in the round bottom flask were then transferred to another flask and were concentrated by distilling the solvent in the soxhlet apparatus. All the solvents were separated from the extract in 4 to 5 h. The concentrated extracts were transferred to a glass petri plate, oven-dried until the removal of the remaining solvent, and then stored at 4 °C for further use (Akhtar et al. 2022). The concentrated extracts were evaluated for the percentage extractive yield using the below formula:
Phytochemical Analysis
Qualitative Analysis
The ethanol, methanol, and aqueous leaf extracts of Calendula officinalis obtained were subjected to phytochemical investigation using the qualitative test to unveil the existence of alkaloids, saponins, steroids, flavonoids, tannins, carbohydrates, coumarins, anthraquinones, triterpenoids, phylobatannins, glycosides, and phenols using the method defined by Roghini and Vijayalakshmi (2018). Wagner's test was used to confirm the presence of alkaloids in the crude plant extracts. The presence of carbohydrates and flavonoids was revealed by Molish’s test and the alkaline reagent test. A ferric chloride test was used to evaluate the presence of phenolic compounds and tannins. The existence of glycosides, steroids, and triterpenoids was assessed by Keller- Killiani test, Salkowski test, and Horizon test. The froth formation test was used to detect saponins. The occurrence of anthraquinones, phylobatannins, and coumarins in the crude plant extracts was detected by Borntrager’s test, HCl test, and NaOH test, respectively.
Quantitative Analysis
Total Phenolic (TPC) and Flavonoid Content (TFC)
The total phenolic content was determined spectrophotometrically using the method described by Singleton and Rossi (1965). 0.1 ml extract (1 mg/ml) was dissolved in 0.75 ml Folin and Ciocalteu reagent (1:10) and 1 ml sodium carbonate (8% w/v) was added after 5 min. The solution was mixed and agitated for homogenization and the final volume was made up to 3 ml. The solution was kept for 30 min in dark at room temperature. The optical density was taken at 765 nm. The total phenolic content was expressed in mg/g gallic acid equivalent (GAE) of dry extract. The aluminum chloride colorimetric method was performed for the determination of total flavonoid content (Jiao and Wang 2000). In this method, 1 ml (1 mg/ml stock) leaf extract was dissolved in 3 ml methanol and 0.2 ml of 10% (w/v) aluminum chloride solution with 0.2 ml of 1 M potassium acetate was further added to it in a test tube. The final volume was made up to 5.6 ml. The solution was kept for 30 min at room temperature. The optical density was taken at 415 nm against solvent as a blank. The values were expressed in mg quercetin equivalent (QE)/g of dry extract. The experiments were performed in triplicates with 3 replicates for each treatment.
Determination of Free Radical Scavenging Activity
DPPH free Radical Scavenging Activity
1, 1-Diphenyl-2-picrylhydrazyl (DPPH) assay was determined to assess the free radical scavenging activity of the plant extract following the method described by Chhabra et al. (2021). For this method, 0.15 ml of different concentrations of test extract (200, 150, 100, 80, 60, 40, 20, 10 µg/ml) was added to each test tube. To this, 2.85 ml of 0.1 mM DPPH in methanol was added to each test tube. All the test tubes containing the mixture were allowed to shake for a few seconds for mixing and kept in dark for 30 min at room temperature. The optical density of all the reaction mixtures was measured at 517 nm using UV–Visible Spectrophotometer. A control containing 1 ml methanol and 3 ml of 0.1 mM DPPH was also prepared. The IC50 value was calculated from the graph plotting concentration versus inhibition percentage. DPPH scavenging activity was calculated using the following formula.
Ferric Reducing Antioxidant Power (FRAP) Assay
The total antioxidant capacity of different plant extracts was evaluated using the FRAP assay by Benzie and Strain (1999) method. The antioxidant capacity of the extracts converts ferric (Fe3+) ions to ferrous (Fe2+) ions, forming the blue color complex TPTZ (tripyridyl triazine) with an increase in OD at 593 nm. Before use, FRAP reagent was prepared by mixing 10:1:1 (v/v/v) ratios of 300 mM sodium acetate buffer (pH 3.6), 10 mM TPTZ in 40 mM HCl, and 20 mM FeCl3.6H2O solution. In a test tube, 100 µl of plant extract was mixed with 3400 ul of FRAP reagent and mixed thoroughly, and incubated at 370C for half an hour. The absorbance was measured at 593 nm by using a spectrophotometer. A standard curve was prepared by using different concentrations (100–1000 µM) of FeSO4 solution. The FRAP value was calculated by using the linear calibration curve and expressed in mM Fe (II)/g of extract. The experiment for each extract was performed in triplicates.
ABTS (2,2′-Azinobis-(3-Ethylbenzthiazolin-6-Sulfonic Acid)) Assay
ABTS radical scavenging activity procedure was followed according to the method of Re et al. (1999). The ABTS stock solution was prepared by the reaction of a 7 mm ABTS reagent and 2.4 mm potassium persulphate solution. The working solution was prepared by using the same stock mixtures in equal volumes and allowing them to react in dark at room temperature for 14–16 h. The fresh solution of ABTS was prepared by further diluting with methanol in the ratio (1:60) to get an absorbance of 0.705 ± 0.01 units at 734 nm. For the experiment, plant extracts at different concentrations were added with 1 ml of the freshly prepared ABTS solution and allowed to react for 7 min in dark. The absorbance was taken at 734 nm using a spectrophotometer. ABTS radical scavenging activity was calculated as percentage inhibition and the IC50 value was calculated using the following formula:
Antioxidant Enzyme Activity
Superoxide Dismutase (SOD, EC 1.1.5.1.1)
SOD activity was evaluated by the method of Giannopolitis and Ries (1977). The activity was measured by its ability to inhibit the photochemical reduction of nitro blue tetrazolium (NBT). The reaction mixture (3 ml) contained 100 mM methionine, 50 mM phosphate buffer, 1 mM NBT, 1 mM Ethylenediamine tetra-acetic acid (EDTA), 1 mM riboflavin, and 0.1 ml of enzyme extract. The blue formazone produced by NBT photoreduction was measured with an increase in absorbance at 560 nm. The SOD activity was expressed as µmoles/min/mg of protein.
Catalase (CAT, EC 1.11.1.6)
CAT activity was estimated by Cakmak and Marschner (1992). The reaction mixture contained enzyme extract, 20 mM H2O2, 0.1 M phosphate buffer, pH 7.0, and 0.1 mM EDTA. The decrease in absorbance was measured for 2 min at 240 nm. The reaction mixture without plant extract was taken as blank. The CAT activity was designated as µmoles of H2O2 oxidized /min/mg of protein.
Ascorbate Peroxidase (APX, EC 1.11.1.1)
APX activity was calculated by the method of Nakano and Asada method (1981). The reaction mixture contained 50 mM phosphate buffer (pH 7.8), 1 mM EDTA, 100 Mm ascorbic acid, 30% hydrogen peroxide (H2O2), and enzyme extract. The decrease in absorbance was recorded at 290 nm at a 30-s interval for 2 min. The APX activity was expressed as µmol ascorbate oxidized/min/mg of protein.
Peroxidase (POD, EC 1.11.1.7)
POD activity was evaluated following the procedure of Kar and Mishra (1976) by observing an increase in absorbance at 420 nm for 5 min. The reaction mixture contained 50 mM phosphate buffer (pH 7.8), 30% H2O2, 5% pyrogallol, and enzyme extract. Enzyme activity was expressed as µmoles/ min/mg of protein. All enzyme assays were performed in triplicates with 3 replicates for each treatment.
Statistical Analysis
Initially, the Shapiro–Wilk test was used to ensure that all the experimental data possess normal distribution. All phytochemical parameters, antioxidant, and antimicrobial activity results were calculated as mean ± S.D., and all experiments were carried out in triplicates. Using SPSS Version 22 software, one-way ANOVA was applied, followed by Duncan’s Multiple Range Test (DMRT), to determine the statistical difference between treatment means. Using Origin Pro 2022 software, a Principal Component Analysis (PCA) was used to assess the relationship between total phenolic and flavonoid content, DPPH, FRAP, ABTS, and antioxidant enzyme activity in the leaves of Calendula officinalis cultivated in various soil and FA treatments. In addition to PCA analysis, Pearson’s correlation analysis plot was determined using Origin Pro 2022 software between these parameters to standardize the variables, which are not measured on the same scale.
Results
Metal(loid)s Concentration in Soil and FA Treatments and Plant Leaves Grown in the Same Treatments
The concentration of various elements (Fe, Cu, Mn, Ni, Co, Zn, Cd, Mg, Ca, and As) was determined in all soil and FA treatments after harvesting (Table S1). According to one-way ANOVA at P < 0.05, the metal(loid)s concentration showed significant variation with the treatments of FA in soil. In the current study, the concentration of all metal(loid)s in different treatments of soil and FA increased significantly (P < 0.05) with increasing FA concentration (Table S1). The heavy metals Cu and Zn were detected within the permissible range in all soil and FA combinations while Mn and As (range 270–525, 6.83–20) (Kabata-Pendias and Pendias 2011) was found lower than the allowable limit with concentrations of 210.65 mg/kg, and 4.96 mg/kg respectively at 100% FA treatment. However, the concentration of Ni and Co were found toxic at 120.16 mg/kg and 51.08 mg/kg respectively whereas Mn concentration was found deficient at 210.65 mg/kg at high (100% FA) application (Range of Ni 1–110 mg/kg, Mn 270–525 mg/kg and Co 11.3–50 mg/kg) (Kabata-Pendias and Pendias 2011). Hence, FA incorporation in the soil increases nutrient availability (Ca, Mg, Zn, Cu, Zn, and Fe) in agricultural soil making it available for plant growth and development (Panda et al. 2018).
Furthermore, a gradual increase in all metal(loid)s concentrations (Fe, Cu, Mn, Ni, Co, Zn, Cd, Mg, Ca, and As) was observed in plant leaves grown at high FA (100% FA) application except Mg which was found lower at 100% FA application compared to garden soil (Table S2). The results showed that the concentration of Cu (70.01 mg/kg), Ni (105.63 mg/kg), Zn (128.69 mg/kg), and Cd (5.23 mg/kg) in the plant leaves grown in 100% FA doses was found in the phytotoxic range 20–100, 40–246, 100–500, and 5–30 respectively as compared with the reference standard (Kabata-Pendias, 2011) (Table S2). A comparative study of each metal(loid)s concentration of all treatments of soil after harvesting and the plant leaves grown in the same treatments indicated a gradual increase in the metal(loid)s concentration with increasing FA application in soil.
Bioaccumulation Factor (BAF) Analysis
In the current study, BAF values of Fe, Cu, Mn, Ni, Co, Zn, Cr, Cd, Mg, Ca, and As in the plant leaves grown under different FA treatments were calculated after harvesting (Table 1). Based on the results obtained, the BAF value of Cu, Fe, Ni, Co, Zn, Cd, and Cr showed greater than one (> 1), and Mn, Cd, Mg, Ca, and As were smaller than one (< 1). In Calendula officinalis, a high accumulation of Fe, Cu, Ni, Co, Zn, and Cr with BAF (> 1) was detected at 40% FA treatment. This research indicates that this plant can be utilized to remediate metals such as Fe, Cu, Ni, Co, Zn, and Cr from FA, however, the remedial potential was higher in 40% FA treatment. Thus, the increase in growth and development of Calendula officinalis was observed at a low dose of FA application in soil (40%) without showing any toxicity symptoms.
The Percentage Yield of Leaf Extracts of Calendula officinalis Grown in FA-Amended Soil
The percentage yield of leaf extracts of Calendula officinalis cultivated in different soil and FA treatments using 3 different solvents is displayed in Fig. 1. Different solvents displayed significant variations in the total dry weight of plant leaves and the extract yields. In the extraction of Calendula officinalis powdered leaves, the aqueous extract had the maximum percentage of extractive potential followed by methanol and ethanol extracts, respectively. The minimum extractive potential was observed with ethanol in all soil and FA treatments.
Phytochemical Analysis
For this study, three different extracts (ethanol, methanol, and aqueous) from leaves of Calendula officinalis were evaluated for phytochemical constituents. The results revealed that the leaf extract of Calendula officinalis is a rich source of alkaloids, flavonoids, coumarins, flavonoids, phenols, tannins, glycosides, steroids, and triterpenoids (Table 2). Saponins and phylobatannins are only present in methanol and aqueous extract while anthraquinones are present only in aqueous extract. In this study, methanol extract showed to be a good solvent system for extracting a wide variety of phytoconstituents from Calendula officinalis leaves.
Estimation of Total Phenolic Content (TPC) and Flavonoid Content (TFC)
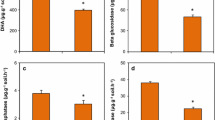
Results displayed that TPC in extracts, expressed in gallic acid equivalents (GAE)/g dry weight of the plant, varied significantly at P < 0.05 and ranged from 8.48 to 13.02 mg/g GAE in aqueous extracts, 10.76 to 16.25 mg/g GAE in methanol extracts, and 12.95 to 17.54 mg/g GAE in ethanol extracts (Fig. 2a). Among the three extracts, the phenolic content was increased by 35.44% in the ethanolic extract of Calendula officinalis followed by 51.02% in the methanolic extract and 53.53% in the aqueous extract at 60% FA compared to control soil. TFC was calculated as mg Quercetin equivalent/g as displayed in Fig. 2b significant increase (P < 0.05) in TFC among different leaf extracts of Calendula officinalis was observed, ranging from, and 9.68 to 20.62 mg QE/g for aqueous extracts, 10.51 to 23.75 mg QE/g for methanol extracts, 11.84 to 30.8 mg QE/g for ethanol extracts. Among three extracts, the flavonoid content was found to be maximum by 1.6-fold in ethanolic extract followed by methanol (1.25-fold) and aqueous extract (1.13-fold) at 60% FA treatment compared to control. However, there was a decrease in phenolic and flavonoid content at high applications (80% and 100% FA), which could be attributed to the increasing concentration of heavy metals (Ni, Cd, Co, Cu, and Zn). In the current study, ethanol extract was a good solvent for the phenolics and flavonoid components from plant leaves.
Data denotes mean ± SD (n = 3). Significant differences were observed at P < 0.05 as compared to the control (One way ANOVA followed by Tukey test using SPSS Version 22). Letters marked by different letters above the bar are significantly different.
DPPH Free Radical Scavenging Activity
The DPPH free radical scavenging activity of three different extracts of Calendula officinalis leaves grown in various soil and FA treatments was determined in Fig. 3a. In this study, the DPPH activity was found to be decreased with increased FA application up to 60% FA treatments but increased at high FA application (80% and 100% FA). The IC50 value (the amount of antioxidants required to reduce the concentration of DPPH by 50%) is inversely associated with the increased antioxidant compound. The extract possesses a low IC50 value indicating more antioxidant power. The aqueous extract demonstrated elevated DPPH inhibition concentration (IC50) of plant extract followed by methanol and ethanol extract. For DPPH inhibition, the IC50 values of ethanol extract ranged from 10 to 100 µg/ml, 50 to 150 µg/ml for methanol extract, and 70 to 380 µg/ml for aqueous extract (Fig. 3a). The lowest IC50 was found in the ethanolic extract (82.67%), followed by methanol extract (60.75%), and aqueous extract (60.75%) of Calendula officinalis at 60% FA treatment compared to their control plants. The ethanol extract possesses the lowest IC50 value in all soil and FA treatments.
a DPPH (IC50 µg/ml), b FRAP (mm Fe (II)/g of extract), and c) ABTS (IC50 µg/ml) assay of Calendula officinalis leaf (ethanol, methanol, and aqueous) extracts grown in different treatments of soil and FA. Data represent mean ± SD (n = 3). Significant differences were observed at P < 0.05 as compared to the control (One way ANOVA followed by Tukey test using SPSS version 22). Letters marked by different letters above the bar are significantly different
Ferric Reducing Antioxidant Power (FRAP) Assay
Three leaf extracts (ethanol, methanol, and aqueous) of Calendula officinalis cultivated in all FA treatments were subjected to FRAP assay to evaluate the reducing power which causes reduction of Fe3+ to Fe2+ form (Fig. 3b). A significant difference (P < 0.05) in FRAP values was observed in all leaf extracts of Calendula officinalis cultivated in various treatments of soil and FA. From the results obtained, the ethanol leaf extract of Calendula officinalis grown in 100% FA treatment produced a maximum FRAP value (0.52-fold) followed by methanol (0.41-fold) and aqueous extract (0.58-fold) with respect to their control plants (Fig. 3b).
ABTS or Trolox Equivalent Antioxidant Capacity (TEAC) Assay
All three-leaf extracts of Calendula officinalis grown in varied soil and FA treatments were tested for ABTS radical scavenging activities. (Fig. 3c). In the present study, the aqueous extract possesses IC50 value ranging between 45 and 90 µg/ml, methanol extract ranged between 25 and 50 µg/ml and ethanol extract ranges between 25 and 50 µg/ml (Fig. 3c). The ethanol extract exhibited the maximum ABTS scavenging activity compared to the other two extracts. The lowest IC50 was observed in the ethanolic extract (31.87%), followed by methanol extract (43.74%), and aqueous extract (44.70%) of Calendula officinalis at 60% FA treatment compared to their control plants.
Antioxidant Enzyme Activity
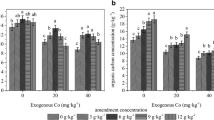
In the current study, the antioxidant enzyme activities of leaf (ethanol, methanol, and aqueous) extracts of Calendula officinalis exhibited significant variations under different FA treatments compared with control soil. The inflection of antioxidant enzymes originates from their enhanced activity to resist oxidative stress produced in plant tissues. For instance, the ethanol extract of Calendula officinalis obtained from 100% FA application showed increased SOD activity by 3.13-fold compared to plants grown in control soil to detoxify superoxide radicals that were probably induced by FA exposure. The aqueous extract of Calendula officinalis obtained from 100% FA treatment showed relatively modest levels of SOD activity compared to ethanol and methanol extract in all soil and FA treatments (Fig. 4). APX and POD activity also displayed a similar pattern, the plants grown in 100% FA showed relatively increased enzyme activity when compared to plants grown in control soil, suggesting an increase of 4.14-fold, and 1.77-fold in APX and POD activity, respectively (Fig. 4). The activity of enzymes like CAT was significantly increased (P < 0.05) in plants exposed to a high level of FA (100% FA). The activity of CAT in the ethanolic extract of Calendula officinalis at 100% FA was increased by 3.88-fold compared to control plants. In contrast, a significant increase in protein content by 0.93-fold, 0.82-fold, and 2.32-fold respectively in ethanolic, methanolic and aqueous extract was observed at 60% FA application in comparison to control plants (Fig. 4). However, a significant drop of 10.77%, 23.24%, and 6.52% in ethanol, methanol and aqueous extract of Calendula officinalis was seen in the protein content at high FA (100% FA) application.
Spider-shaped visual plot of a ethanol, b methanol, and c aqueous extract showing changes in antioxidant enzyme activities in Calendula officinalis leaf grown in different treatments of soil and FA. The black circle with a radius of 1 cm represents APX, CAT, POD, protein, and SOD concentration of leaf extracts of Calendula officinalis. Data are mean ± S.D (n = 3). APX Ascorbate peroxidase; CAT Catalase; SOD Superoxide Dismutase; POD- Peroxidase
Multivariate Statistical Analysis (PCA and Correlation Analysis)
Pearson's correlation plot was used to evaluate the correlation of phytochemical contents (TPC and TFC), enzymatic antioxidants (CAT, SOD, APX, POD, Protein content), and non-enzymatic antioxidants (DPPH, FRAP, and ABTS) with the concentration of metal(loid) in the leaves of Calendula officinalis (Fig. 5). For this study, correlation analysis of all the studied parameters was performed on the ethanol extract (best solvent). The correlation plot is represented in Fig. 5 and the analysis was executed using the mean value of each variable. The IC50 values of DPPH and ABTS in different extracts show a negative correlation with all phytochemicals (TPC and TFC) present in ethanol extract, indicating a positive relationship between the non-enzymatic antioxidants and phytochemicals (as IC50 values are inversely correlated to antioxidant activity). Total phenolic and flavonoids are positively correlated with enzymatic antioxidants (SOD, CAT, POD, APX, and protein content). Additionally, all the studied parameters (TPC, TFC, DPPH, FRAP, ABTS, APX, SOD, CAT, POD, and protein) are positively associated with the majority of metal(loid) concentration in the plant leaves. This suggests that Calendula officinalis can able to survive at high FA application and mitigate the oxidative stress caused by high concentrations of heavy metals in FA.
Pearson correlation coefficient plot to determine the relationship of total phenolic and flavonoids, protein, enzymatic antioxidants (SOD, CAT, POD, and APX), and non-enzymatic antioxidants (ABTS, DPPH, and FRAP) with metal(loid)s concentration in leaves of Calendula officinalis grown under different treatments of soil and FA. Note: blue color indicates a negative correlation, orange color indicates a positive correlation. The number represents the Pearson correlation. The darker color represents the strong correlation between variables. Correlation is significant at *P < = 0.05; **P < = 0.01; ***P < = 0.001. TPC Total phenolic content; TFC Total flavonoid content; DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical scavenging activity; ABTS (2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) free radical scavenging activity; FRAP Ferric reducing antioxidant power; CAT catalase; APX Ascorbate peroxidase; SOD Superoxide dismutase; POD Peroxidase
Further, principal component analysis was executed in determining the relationship between the above-mentioned phytochemicals and enzymatic and non-enzymatic antioxidant activity, and loading plots and scores are depicted in Fig. 6. In the factor plane, the biplot created between PC1 and PC2 signified a clear summary of assembling treatments. PC1 displays 63.16% of the variation in the original data, while PC2 reveals a 26.19% variance. A strong positive correlation was observed between FRAP activity and enzymatic antioxidants (SOD, CAT, POD, and APX). The combination of 80% FA and 100% FA treatments and 40% FA and 60% FA treatments are detached from other treatments and are existed in the identical quarter (Fig. 6). These FA treatments are effectively isolated from the other treatments, according to the biplot, due to the creation of a group of positively correlated variables, which include factors such as SOD, CAT, DPPH, FRAP, APX, Mn, As, Fe, and Co in the same quarter containing 80% and 100% FA treatment whereas, TPC, TFC, POD, FRAP, protein, Ni, Al, Zn, Ca, and Cd present in another quarter containing 40% and 60% FA treatments. This study discovered that the phytoconstituent and antioxidant activity of leaves of Calendula officinalis were increased with increased heavy metal concentration at high FA application to cope with the metal stress in the soil environment. The connection between phenolics and antioxidant activities is crucial for grouping factors/ variables based on correlation and understanding how these variables contribute to the antioxidant activity of plant extracts grown under various soil and FA treatments.
Principal Component Analysis (PCA) showing loading plots demonstrating the relationship between phytochemicals, antioxidant activity, and metal(loid)s concentration of leaves of Calendula officinalis grown under different treatments of soil and FA. TPC Total phenolic content; TFC Total flavonoid content; DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical scavenging activity; ABTS (2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) free radical scavenging activity; FRAP Ferric reducing antioxidant power; APX Ascorbate peroxidase; CAT Catalase; SOD Superoxide dismutase; POD peroxidase
Discussion
Fly ash (FA) as a soil ameliorant is a rapidly growing and promising area used in agricultural soil research since it is utilized as a fertilizer to increase the growth and productivity of several important crop species (Ahmad et al. 2017). FA is a source of essential micronutrients for plants including K, Mg, Ca, Fe, S, etc. FA produced from the coal combustion process also releases a huge amount of toxic elements, such as lead (Pb), nickel (Ni), zinc (Zn), and manganese (Mn). The degree of toxic elements in FA is dependent upon the mineralogy, combustion process, particle size distribution, combustion temperature, and extent of weathering of FA (Gajić et al. 2018). Generally, plants absorb heavy metals through their roots and concentrate these metal(loid)s at different plant parts including stems, leaves, and flowers (Panda et al. 2018). After the roots, the highest accumulation of metal(loid)s occurs in the leaves of the plant. In the present study, the metal(loid)s concentration in soil and FA treatments and Calendula leaves grown in different treatments of soil and FA was determined. The results of the present study showed that FA incorporation in the soil increases the nutrient availability (Ca, Mg, Zn, Cu, Zn, and Fe) in agricultural soil making it available for plant growth and development (Table S1). However, at high (80% and 100%) FA application, Calendula leaves showed chlorosis and necrosis which might be due to the accumulation of metal(loid)s (Ni, Co, Cd, Cr, Cu, As, and Zn) from FA (Table S2). In this study, Calendula officinalis survived in FA containing a high concentration of Mg, Ni, Co, Mn, Zn, Ca, Cu, Cd, As, and Fe (Table S2). Previously, it was observed that the Calendula officinalis can phytoremediate toxic concentrations of heavy metals (Ni, Co, Cd, and Zn) in FA and survive in various environmental conditions (Afrousheh et al. 2015). Similar findings of increased heavy metal concentration in plant parts due to various FA amendments in soil have been reported (Alvarenga et al. 2017). Similarly, the increased concentration of FA leads to impaired growth and development of Sida acuta Burm. f. and Cassia tora (L.) Roxb has also been reported (Panda et al. 2020a).
The bioaccumulation factor (BAF) was observed mainly to understand the accumulation behavior of heavy metals in the above-ground plant tissues. The BAF value (> 1) reflects how effectively plant leaves uptake and absorbs metals from the soil. In Calendula officinalis, a high accumulation of Fe, Cu, Ni, Co, Zn, and Cr with BAF (> 1) was detected at 40% FA treatment (Table 1). This research indicates that this plant can be utilized to remediate metals such as Fe, Cu, Ni, Co, Zn, and Cr from FA, however, the remedial potential was higher in 40% FA treatment. Thus, it was demonstrated that plants can serve as a good tool for phytoremediation in the vicinity of FA. Similar observations were made by Kumari et al. (2011) that reported increased bioaccumulation of Fe, Zn, Al, Si, Cu, Pb, Cr, As, and Ni in the Pteris Vittata L leaves grown in the vicinity of FA. Upadhyay et al. (2021) observed that 40% FA supplemented with manure was found ideal for chickpea growth due to the increased value of BAF and TF (Zn, Fe, and Mn).
Numerous methods were employed to extract and isolate various phytochemicals from plant parts. Phytochemical components typically occurred in a very low concentration in plants. The extraction yield and quality of herbal extracts were primarily influenced by extraction techniques, phytochemical nature, particle size, composition, solvent nature, and the presence of interfering substances (Nile et al. 2017). In this study, soxhlet extraction was used to extract biologically important components from the leaves of Calendula officinalis by using three different solvent systems such as ethanol, methanol, and water in the order of their increased polarity. The results in the present study revealed that Calendula officinalis leaves had the maximum percentage of extractive potential in the aqueous extract followed by methanol and ethanol extracts, respectively (Fig. 1). Similar results were achieved in accordance with previous studies in which aqueous solvent was shown to be the most effective solvent for extracting polar chemical components from a variety of medicinal plants (Adnan et al. 2020; Bohara et al. 2020). However, the aqueous extract was commonly used by various researchers for the phytochemical studies of Calendula officinalis (Kuntal et al. 2019). The polarity of the solvents employed in the experiment causes considerable differences in the dry weight of the extracts and yield between different organic solvents (Truong et al. 2019).
Medicinal plants belonging to the Asteraceae family are a source of phytochemical components mainly phenols and flavonoids which are responsible for therapeutic and medicinal properties. The results revealed that methanolic leaf extract of Calendula officinalis obtained from all soil and FA treatments confirmed the presence of alkaloids, flavonoids, coumarins, flavonoids, phenols, tannin, glycosides, steroids, and triterpenoids (Table 2). Prior studies related to the phytoconstituent analysis of Calendula officinalis reported similar findings in methanolic extracts (Sagar et al. 2014). Individual phytochemical components present in this species perform a chief role in biological processes; for instance, flavonoids are known for antioxidant activity, steroids are involved in anti-inflammatory reactions, alkaloids are responsible for antimicrobial, antispasmodic and analgesic properties, saponins possess antifungal, antioxidant, antipyretic, and antimicrobial activity (Chatoui et al. 2016). The presence of a significant number of phytochemical components, therefore, confers the medicinal potential of plant species including antioxidant and antimicrobial activities.
Antioxidant compounds such as phenolic and flavonoid compounds are abundant in plants. The natural antioxidants contain hydroxyl groups and are accountable for scavenging ROS generation. The total phenolic and flavonoid content is one of the phytoconstituent assessments to understand the antioxidative potential of the plants. In the present study, the quantitative analysis of total phenolics and flavonoids showed that among the three extracts, the phenolic and flavonoid content was increased in the ethanolic extract of Calendula officinalis followed by methanolic extract and aqueous extract at 60% FA compared to control soil (Fig. 2a, b). However, there was a decrease in phenolic and flavonoid content at high FA applications (80% and 100% FA), which could be attributed to the increasing concentration of heavy metals (Ni, Cd, Co, Cu, and Zn). At a high FA application rate, this could have eventually resulted in the disruption of plant metabolism. The ability of a plant to resist and control oxidative stress induced by free radicals is determined by its overall phenolic and flavonoid content (Saeed et al. 2020). Phenolic compounds containing hydroxyl groups give off electrons or hydrogen atoms to combat free radicals, whereas the hydroxyl atom in flavonoids is critical for scavenging these free radicals (Kerdsomboon et al. 2020). At 100% FA treatment, heavy metals such as Ni, Cd, Co, Cu, and Zn were present in larger amounts with concentrations of 105.63, 47.91, 128.69, 5.23, and 70.01 mg/kg respectively, which causes enhanced ROS production and induced cellular damage, resulting in morphological alterations in the plant (Shakeel et al. 2020). This leads to the stimulation of the plant antioxidant systems to neutralize these ROS species triggered by heavy metals (Varshney et al. 2021). Kisa et al. (2016) observed an increase in phenolic compounds with increased heavy metals (Cd, Cu, and Pb) concentration in the Zea mays leaves. Cu stress increased polyphenol and flavonoid content in leaves and roots of Lycopersicon esculentum Mill. (Badiaa et al. 2020). According to Chen et al. 2019, increased Cd and Zn concentration resulted in increased generation of TPC and TFC in the leaves and roots of Kandelia obovate. In relevance to our findings, antioxidant production, such as phenolic and flavonoid compounds decreased when the toxic concentration of Cd and Zn in the leaves increased. These occurrences could be stress-related or the manifestation of a stress-response mechanism adaptation to Cd and Zn accumulation (Sakurai et al. 2019).
In the present study, the ethanol extract possesses the lowest IC50 value of DPPH and ABTS in all soil and FA treatments, indicating that the test extract has a great ability to serve as a free radical scavenger and detoxify ROS generated by heavy metals in FA (Sonter et al. 2021). Ethanol extract of leaves grown in 60% FA treatment was found efficient for successfully scavenging the ROS species and preventing the plant from oxidative damage caused by heavy metals from FA (Fig. 3a, c). This increased antioxidant potential (DPPH and ABTS) from control to 60% FA treatments in the plant leaves could be due to the increased concentration of phytoconstituents, such as total phenolics, and total flavonoids (Benslama and Harrar 2016). Our findings are in accordance with previous studies that suggest that the amount of secondary metabolites (phenolics, flavonoids, and saponins) corresponds to the medicinal plants’ ability to detoxify free radicals (Turumtay et al. 2014). Our findings are also consistent with the results of Ibrahim et al. 2017 indicating the enhanced production of TPC, TFC, total saponins, DPPH, and FRAP activity under Cd and Cu treatment. Excessive amounts of Cu and Zn can be harmful owing to the generation of ROS and disproportion in the cellular redox state, creating deficiency symptoms. Similarly, Haddadi et al. (2019), observed that the increased levels of Ni, Cu, Mn, Zn, Cr, and Fe in maize leaves indicated that the heavy (toxic) metals in cattle manure amended soil are more readily available to plants causing an increased level of antioxidant activity (DPPH, FRAP, and ABTS).
In the current study, it was noticed that reducing power (FRAP value) increases with increasing FA application in soil (Fig. 3b). The reducing power of a compound/extract reveals its ability to donate electrons (Kumar et al. 2013). The increase in antioxidant activity at 100% FA application might be due to the plant’s tolerance to high heavy metal content in FA (Varshney et al. 2021). Because the FRAP assay only detects nonenzymatic (reductants) antioxidants in a sample, there is an intriguing link between metal concentration and the FRAP value obtained, which is true for all redox metals studied. Antioxidant systems and their importance in plant adaptation to heavy metal pollution and climatic challenges have been extensively studied, with a focus on leaf responses (Gjorgieva et al. 2013; Ibrahim et al. 2017).
Heavy metals in contaminated soil cause the overproduction of ROS which causes oxidative stress in plants (Bisoi et al. 2017). Plants have antioxidant defense systems that use both enzymatic and non-enzymatic antioxidants to control and scavenge ROS (Qadir et al. 2020). The mitigation of oxidative damage and enhanced resistance to heavy (toxic) metal stress due to FA is directly linked to an efficient antioxidant system, and plants with high antioxidant capacity show less sensitivity to metal toxicity (Randelovic et al. 2016). Plant tolerance potential to FA is directly associated with an increase in antioxidants to eliminate toxic ROS (Bisoi et al. 2017). The antioxidant enzyme activity of the plant Calendula officinalis was assessed to check whether the leaf part of this plant possesses free radical scavenging capability generated under oxidative stress conditions triggered by FA containing heavy metals. The present study showed that all the enzymatic antioxidants (SOD, CAT, APX, and POD) in leaves of Calendula officinalis increased significantly with increased FA application in the soil in all the test extracts (Fig. 4). This increase in antioxidant enzyme activity in Calendula officinalis could be a reason for plant response to toxic ROS caused by increased heavy metals concentration such as Ni, Cd, Co, Cu, and Zn at high FA application. Zn is a redox-inactive metal that eases the stress of ROS while also activating the antioxidant pool and disrupting metabolic equilibrium (Pramanick et al. 2017). Whereas Cu is needed for various antioxidant enzymatic activities in preventing oxidative damage to plants but chronic Cu toxicity can lead to severe intoxication (Padhy et al. 2016). Heavy metals in FA can produce oxidative stress in paddy crops, which causes antioxidant enzyme activity to increase to offset the detrimental effects of oxidative stress and prevent phytotoxic damage (Singh and Pandey 2012). The earlier study of Georgiadou et al. (2018) revealed that the reduction in antioxidant enzyme activity in plant tissues exposed to excessive Ni might cause the deficiency of essential metals for the formation of these enzyme molecules. Several authors have explored innumerable views on various facets of antioxidant activities from biosynthesis to stress defense. The results of this study were in agreement with the earlier findings which indicated an increased antioxidant enzyme activity of Oryza nivara and Ricinus communis L. at high FA application (Bisoi et al. 2017; Panda et al. 2020b). They noted enhanced levels of SOD, GR, and MDA activity in the plants with an increase in FA application in soil. SOD is one of the essential enzymes required by plants as an antioxidant defense system which provides the first level of defense against oxidative stress. SOD causes the dismutation of O2− into H2O2 with the release of molecular oxygen as a reaction product, which is thereafter eliminated by APX and CAT activities (Rajput et al. 2021). In addition to CAT, APX sequest H2O2 and this reaction is a vital process for the ability of plants to withstand combined stress (Zandalinas et al. 2017). The considerable increase in APX activity induced by FA stress shows that H2O2 can be effectively scavenged under these circumstances. In addition, ascorbate (AsA) serves as the electron donor for APX to dismutase H2O2 to H2O2 with the consequent generation of monodehydroascorbate (MDHA), a univalent oxidant of ascorbate (AA) (Bisoi et al. 2017).
The present study showed that low application of FA in soil did not trigger oxidative stress in plants whereas high FA application resulted in a disturbance in plant metabolic processes, distortion in the cell membrane, and inhibiting plant growth. In the current study, the same effect was also detected under high FA application in the form of decreased protein content (Fig. 4). The decrease in protein content was detected at high FA application which might be due to increased protease activity affecting the plant growth and increased generation of toxic ROS (Panda et al. 2018). It was previously reported that treatment with increasing doses of Zn and Cu resulted in a substantial decrease in total protein content, presumably owing to the breakdown of several proteins (Duman and Ozturk 2010). Similar findings were reported in various crop species such as Triticum aestivum, Oryza nivara, and Cymbopogon citratus (Wang et al. 2014; Bisoi et al. 2017; Panda et al. 2018). Moreover, a positive correlation was observed between all the studied parameters (TPC, TFC, DPPH, FRAP, ABTS, APX, SOD, CAT, POD, and protein) and the majority of metal(loid) concentration in the plant leaves (Fig. 5). This suggests that Calendula officinalis can able to survive at high FA application and mitigate the oxidative stress caused by high concentrations of heavy metals in FA.
Conclusions
The use of FA in agriculture may provide a viable alternative for its proper disposal without causing deleterious effects on the environment. The current study observed that Calendula officinalis grown in different treatments of soil and FA showed a promising result and can be tried further under different field trials up to 40% FA application. In the current study, it is reported that the high application of FA causes increased production of TPC and TFC (up to 60% FA), enzymatic antioxidants (SOD, CAT, APX, POD), and non-enzymatic antioxidants (DPPH, FRAP, and ABTS) due to high metal content (Fe, Cu, Ni, Co, Zn, and Cr) in FA. The activation of various antioxidative enzymes in Calendula officinalis under high FA application suggests that this plant can withstand oxidative stress. Based on the higher BAF value (> 1), this research indicates that Calendula officinalis can be utilized to remediate metals such as Fe, Cu, Ni, Co, Zn, and Cr from FA, however, the remedial potential was higher in a low dose of FA (40% FA) amended soil. Calendula officinalis possess a wide variety of medicinal properties, therefore a future investigation is required to evaluate the bioactive components under FA treatments. It may be concluded that there is sufficient scope for the safe utilization of FA in the long-term growth of medicinal plants, as well as its prospective application in the phytoremediation of heavy metal-polluted soil. However, optimization of FA doses will need extensive field or pot trials and may vary depending on soil type, source of FA, and plant species. Furthermore, the long-term influence of FA treatment on soil quality and the environment should be carefully studied.
References
Adnan M, Oh KK, Azad MOK, Shin MH, Wang MH, Cho DH (2020) Kenaf (Hibiscus cannabinus L.) leaves and seed as a potential source of the bioactive compounds: effects of various extraction solvents on biological properties. Life 10:223. https://doi.org/10.3390/life10100223
Afrousheh M, Shoor M, Tehranifar A, Safari VR (2015) Phytoremediation potential of copper contaminated soils in Calendula officinalis and effect of salicylic acid on the growth and copper toxicity. Int Lett Chem Phys Astron 50:159–68. https://doi.org/10.18052/www.scipress.com/ILCPA.50.159
Ahmad P, Ahanger MA, Alyemeni MN et al (2017) Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 255:79–93. https://doi.org/10.1007/s00709-017-1132-x
Ahmad G, Khan AA, Mohamed HI (2021) Impact of the low and high concentrations of fly ash amended soil on growth, physiological response, and yield of pumpkin (Cucurbita moschata Duch. Ex Poiret L.). Environ Sci Pollut Res 28:17068–17083. https://doi.org/10.1007/s11356-020-12029-8
Akhtar MS, Rafiullah M, Shehata WA, Hossain A, Ali M (2022) Comparative phytochemical, thin layer chromatographic profiling and antioxidant activity of extracts from some Indian herbal drugs. J Bioresour Bioprod 7(02):128–134. https://doi.org/10.1016/j.jobab.2022.01.001
Alvarenga P, Palma P, Mourinha C, Farto M, Dôres J, Patanita M et al (2017) Recycling organic wastes to agricultural land as a way to improve its quality: a field study to evaluate benefits and risks. Waste Manag 61:582–592. https://doi.org/10.1016/j.wasman.2017.01.004
Badiaa O, Yssaad HAR, Topcuoglu B (2020) Effect of heavy metals (copper and zinc) on proline, polyphenols, and flavonoids content of tomato (Lycopersicon esculentum Mill.). Plant Archives 20(1):2125–2137
Bedoya R, Cristina L, LÃpez G, MarÃn K, Nathalia P (2020) Extraction of metabolites from Calendula officinalis and evaluation of their colorant and antibacterial capacity. Revista Colombiana de BiotecnologÃa 22(1):60–69. https://doi.org/10.15446/rev.colomb.biote.v22n1.79999
Benslama A, Harrar A (2016) Free radicals scavenging activity and reducing the power of two Algerian Sahara medicinal plants extracts. Int J Herbal Medicine 4(6):158–161
Benzie IFF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–23
Bisoi SS, Mishra SS, Barik J, Panda D (2017) Effects of different treatments of fly ash and mining soil on growth and antioxidant protection of Indian wild rice. Int J Phytorem 19(5):446–452
Bohara P, Dulta K, Chauhan P, Thakur K, Seth A, Chauhan PK (2020) Impact of different solvents on phytoconstituents, antioxidants, and FTIR analysis of Diplazium esculentum leaf extract from the Himalayan region. Plant Archives 20(2):6057–6063
Cakmak I, Marschner H (1992) Magnesium deficiency and highlight intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227. https://doi.org/10.1104/pp.98.4.1222
CEA (2020–2021) Report on fly ash generation at coal/lignite-based thermal power stations and its utilization in the country for the year 2020–2021. Central Electricity Authority, New Delhi, India
Chatoui K, Talbaoui A, Aneb M, Bakri Y, Harhar H, Tabyaoui M (2016) Phytochemical screening, antioxidant and antibacterial activity of Lepidium sativum seeds from Morocco. J Mater Environ Sci 7(8):2938–2946
Chen S, Wang Q, Lu H, Li L, Yang D, Liu J, Yan C (2019) Phenolic metabolism and related heavy metal tolerance mechanism in Kandelia obovata under Cd and Zn stress. Ecotoxicol Environ Saf 169:134–143. https://doi.org/10.1016/j.ecoenv.2018.11.004
Chhabra A, Velvijayan VB, Mohan S, Dahiya P (2021) Alteration in chemical composition and antioxidant defense potential of essential oil of Jatropha curcas L. grown in fly ash amended soil. Energy, Ecology, and Environment 6:566–575. https://doi.org/10.1007/s40974-021-00210-9
Dominic S, Hussain AJ, Saleem MH, Alshaya H, Jan BL, Ali S, Wang X (2022) Variation in the primary and secondary metabolites, antioxidant and antibacterial potential of tomatoes, grown in soil blended with different concentration of fly ash. Plants 11:551. https://doi.org/10.3390/plants11040551
Duman F, Ozturk F (2010) Nickel accumulation and its effect on biomass, protein content and antioxidative enzymes in roots and leaves of watercress (Nasturtium officinale R. Br.). J Environ Sci 22:526–532. https://doi.org/10.1016/S1001-0742(09)60137-6
Gajić G, Djurdjević L, Kostić O, Jarić S, Mitrović M, Pavlović P (2018) Ecological potential of plants for phytoremediation and eco restoration of fly ash deposits and mine wastes. Front Environ Sci 6:1–24. https://doi.org/10.3389/fenvs.2018.00124
Georgiadou EC, Kowalska E, Patla K, Kulbat K, Smolińska B, Leszczyńska J, Fotopoulos V (2018) Influence of heavy metals (Ni, Cu, and Zn) on nitro-oxidative stress responses, proteome regulation and allergen production in basil (Ocimum basilicum L.) plants. Front Plant Sci 9:862–876. https://doi.org/10.3389/fpls.2018.00862
Giannopolitis CN, Ries SK (1977) Superoxide dismutase: occurrence in higher plants. Plant Physiol 115:159–169. https://doi.org/10.1104/pp.59.2.309
Gjorgieva D, Kadifkova Panovska T, Ruskovska T, Bačeva K, Stafilov T (2013) Influence of heavy metal stress on antioxidant status and DNA damage in Urtica dioica. BioMed Res Int. https://doi.org/10.1155/2013/276417
Haddadi MA, Al-Dalain SY, Al-Tabbal JA, Bani-Hani NB, Jaradat DMM, Obeidat M, Al-Ramamneh EADA (2019) In vitro antioxidant activity, macronutrients and heavy metals in leaves of maize (Zea mays L.) plants grown at different levels of cattle manure amended soil in Jordan valley. Pak J Bot 51(3):933–940
Ibrahim MH, Kong YC, Mohd Zain NA (2017) Effect of cadmium and copper exposure on growth, secondary metabolites and antioxidant activity in the medicinal plant Sambung Nyawa (Gynura procumbens (Lour.) Merr). Molecules 22:1623. https://doi.org/10.3390/molecules22101623
Jambhulkar HP, Shaikh SMS, Kumar MS (2018) Fly ash toxicity, emerging issues and possible implications for its exploitation in agriculture; Indian scenario: a review. Chemosphere 213:333–344
Jiao H, Wang SY (2000) Correlation of antioxidant capacities to oxygen radical scavenging enzyme activities in blackberry. J Agric Food Chem 48(11):5672–5676
Kabata-Pendias A, Pendias H (2011) Trace elements in soils and plants, 4th edn. CRC Press, Boca Raton
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenol oxidase activities during rice leaf senescence. Plant Phys 57(2):315–319
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2019a) Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol Plant 168(2):345–360. https://doi.org/10.1111/ppl.13012
Kaya C, Okant M, Ugurlar F, Alyemeni MN, Ashraf M, Ahmad P (2019b) Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 225:627–638. https://doi.org/10.1016/j.chemosphere.2019.03.026
Kerdsomboon K, Chumsawat W, Auesukaree C (2020) Effects of Moringa oleifera leaf extracts and its bioactive compound gallic acid on reducing toxicities of heavy metals and metalloid in Saccharomyces cerevisiae. Chemosphere 270:1–10. https://doi.org/10.1016/j.chemosphere.2020.128659
Kisa D, Elmastaş M, Öztürk L et al (2016) Responses of the phenolic compounds of Zea mays under heavy metal stress. Appl Biol Chem 59:813–820. https://doi.org/10.1007/s13765-016-0229-9
Kotelnikova AD, Rogova OB, Karpukhina EA, Solopov AB, Levin IS, Levkina VV, Proskurnin MA, Volkov DS (2022) Assessment of the structure, composition, and agrochemical properties of fly ash and ash-and slug waste from coal-fired thermal power plants for their possible use as soil ameliorants. J Clean Prod 333:1–15. https://doi.org/10.1016/j.jclepro.2021.130088
Kumar A, Gul MZ, Zeeshan A, Bimolata W, Qureshi IA, Ghazi IA (2013) Differential antioxidative responses of three different rice genotypes during bacterial blight infection. Aust J Crop Sci 7:1893–1900
Kumari A, Lal B, Pakade YB, Chand P (2011) Assessment of bioaccumulation of heavy metal by Pteris vittata L. growing in the vicinity of fly ash. Int J Phytoremed 13(8):779–787. https://doi.org/10.1080/15226514.2010.525561
Kuntal DAS, Someswar DEB, Karanth T (2019) Phytochemical screening and metallic ion content and its impact on the antipsoriasis activity of aqueous leaf extracts of Calendula officinalis and Phlebodium decumanum in an animal experiment model. Turk J Pharm Sci 16(3):292–302. https://doi.org/10.4274/tjps.galenos.2018.44265
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nile SH, Nile AS, Keum YS (2017) Total phenolics, antioxidant, antitumor, and enzyme inhibitory activity of Indian medicinal and aromatic plants extracted with different extraction methods. 3 Biotech 7(1):76. https://doi.org/10.1007/s13205-017-0706-9
Padhy RN, Nayak N, Dash-mohini RR, Rath S, Sahu RK (2016) Growth, metabolism, and yield of rice cultivated in soils amended with fly ash and cyanobacteria and metal loads in plant parts. Rice Sci 23(1):22–32
Panda D, Panda D, Padhan B, Biswas M (2018) Growth and physiological response of lemongrass (Cymbopogon citratus (D.C.) Stapf.) under different levels of fly ash-amended soil. Int J Phytoremediat 20(6):538–544. https://doi.org/10.1080/15226514.2017.1393394
Panda D, Mandal L, Barik J (2020a) Phytoremediation potential of naturally growing weed plants grown on fly ash amended soil for restoration of fly ash deposits. Int J Phytorem 22(1):1–9. https://doi.org/10.1080/15226514.2020.1754757
Panda D, Mandal L, Barik J, Padhan B, Bisoi SS (2020) Physiological response of metal tolerance and detoxification in castor (Ricinus communis L.) under fly ash-amended soil. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e04567
Panda D, Barik JR, Barik J, Behera PK, Dash D (2021) Suitability of Brahmi (Bacopa monnieri L.) cultivation on fly ash-amended soil for better growth and oil content. Int J Phytoremediat 23(1):72–79. https://doi.org/10.1080/15226514.2020.1791052
Pandey SK, Bhattacharya T (2019) Mobility, ecological risk and change in surface morphology during sequential chemical extraction of heavy metals in fly ash: a case study. Environ Technol Innov 13:373–382
Pandey SK, Bhattacharya T, Chakraborty S (2016) Metal phytoremediation potential of naturally growing plants on fly ash dumpsite of Patratu thermal power station, Jharkhand. India Int J Phytoremed 18(1):87–93
Pramanick P, Chakraborty A, Raychaudhuri SS (2017) Phenotypic and biochemical alterations in relation to MT2 gene expression in Plantago ovata Forsk under zinc stress. Bio Metals 30(2):171–184. https://doi.org/10.1007/s10534-017-9990-4
Qadir SU, Raja V, Siddiqui WA, Alyemeni MN, Ahmad P (2020) Foliar concentrations of selected elements, assessment of oxidative stress markers and role of antioxidant defense system is associated with fly ash stress tolerance in Withania somnifera. J Plant Growth Regul 40(4):1450–1465. https://doi.org/10.1007/s00344-020-10200-6
Rajput VD, Harish SRK, Verma KK, Sharma L, Quiroz-Figueroa FR, Meena M, Gour VS, Minkina T, Sushkova S, Mandzhieva S (2021) Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology (basel) 10(4):267. https://doi.org/10.3390/biology10040267
Randelovic D, Gajic G, Mutic J et al (2016) Ecological potential of Epilobium dodonaei Vill. for restoration of metalliferous mine wastes. Ecol Eng 95:800–810. https://doi.org/10.1016/j.ecoleng.2016.07.015
Re R, Pellegrini N, Proteggente A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Roghini R, Vijayalakshmi K (2018) Phytochemical screening, quantitative analysis of flavonoids and minerals in ethanolic extract of Citrus paradise. Int J Pharm Sci Res 9:4859–4864. https://doi.org/10.19080/JDVS.2019.09.555768
Saeed Z, Iqbal S, Younas U, Pervaiz M, Bukhari SM, Zaidi A (2020) Variation in the antioxidant potential of Vigna unguiculata grown in pure and amended soil. Kuwait J Sci 47:2–14
Saffari VR, Saffari M (2020) Effects of EDTA, citric acid, and tartaric acid application on growth, phytoremediation potential, and antioxidant response of Calendula officinalis L. in a cadmium-spiked calcareous soil. Int J Phytoremed. https://doi.org/10.1080/15226514.2020.1754758
Sagar R, Sahoo HB, Kar B, Mishra NK, Mohapatra R, Sarangi SP (2014) Pharmacological evaluation of Calendula officinalis L. on bronchial asthma in various experimental animals. Int J Nutr Pharmacol Neurol Dis 4:95–103. https://doi.org/10.4103/2231-0738.129595
Sakurai M, Tomioka R, Hokura A, Terada Y, Takenaka C (2019) Distributions of cadmium, zinc, and polyphenols in Gamblea innovans. Int J Phytorem 21(3):217–223. https://doi.org/10.1080/15226514.2018.1524840
Shakeel A, Khan AA, Hakeem KR (2020) Growth, biochemical, and antioxidant response of beetroot (Beta vulgaris L.) grown in fly ash amended soil. SN Appl Sci 2:1378. https://doi.org/10.1007/s42452-020-3191-4
Shakeel A, Khan AA, Alharby HF, Bamagoos AA, Tombuloglu H, Hakeem KR (2021) Evaluation of coal fly ash for modulating the plant growth, yield, and antioxidant properties of Daucus carota (L.): a sustainable approach to coal waste recycling. Sustainability 13:5116. https://doi.org/10.3390/su13095116
Singh JS, Pandey VC (2012) Fly ash application in nutrient poor agriculture soils: Impact on methanotrophs population dynamics and paddy yields. Ecotoxicol Environ Saf 89:43–51
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Sonter S, Mishra S, Dwivedi MK, Singh PK (2021) Chemical profiling, in vitro antioxidant, membrane stabilizing and antimicrobial properties of wild-growing Murraya paniculata from Amarkantak (M.P.). Scientific Reports. https://doi.org/10.1038/s41598-021-87404-7
Truong DH, Nguyen DH, Ta NTA, Bui AV, Do TH, Nguyen HC (2019) Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J Food Qual. https://doi.org/10.1155/2019/8178294
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines 5(3):93. https://doi.org/10.3390/medicines5030093
Turumtay EA, İslamoğlu F, Çavuş D, Şahin H, Turumtay H, Vanholme B (2014) Correlation between phenolic compounds and antioxidant activity of Anzer tea (Thymus praecox Opiz subsp. caucasicus var. caucasicus). Ind Crop Prod 52:687–694
Upadhyay SK, Ahmed M, Srivastava AK, Abhilash PC, Sharma B (2021) Optimization of eco-friendly novel amendments for sustainable utilization of Fly ash based on growth performance, hormones, antioxidant, and heavy metal translocation in Chickpea (Cicer arietinum L.) plant. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.129216
Varshney A, Mohan S, Dahiya P (2020) Growth and antioxidant responses in plants induced by heavy metals present in fly ash. Energy Ecol Environ 6:92–110. https://doi.org/10.1007/s40974-020-00191-1
Varshney A, Mohan S, Dahiya P (2021) Assessment of leaf morphological characteristics, phenolic content, and metal(loid)s concentrations in Calendula officinalis L. grown on fly ash amended soil. Ind Crops Prod 174:1–9. https://doi.org/10.1016/j.indcrop.2021.114233
Wang S, Shi X, Sun H, Chen Y, Pan H, Yang X, Rafiq T (2014) Variations in metal tolerance and accumulation in three hydroponically cultivated varieties of Salix integra treated with lead. PLoS ONE 9:108–568
Yu CL, Deng Q, Jian S, Li J, Dzantor EK, Hui D (2019) Effects of fly ash application on plant biomass and element accumulations: a meta-analysis. Environ Pollut 250:137–142. https://doi.org/10.1016/j.envpol.2019.04.013
Zandalinas SI, Balfagón D, Arbona V, Gómez-cadenas A (2017) Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Front Plant Sci Sci 8:1–10. https://doi.org/10.3389/fpls.2017.00953
Acknowledgements
The authors would like to thank Amity University Uttar Pradesh, Noida, for providing the facilities to carry out this work.
Funding
Financial support from the Council of Science and Technology (CST), Department of Science and Technology, UP, Government of India through the research grant (CST/AAS/D-1549) to Dr. Sumedha Mohan and Dr. Praveen Dahiya is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
AV: Experimental design, Writing- original draft and software, Data curation/PD: Conceptualization, Methodology, Project administration, Funding acquisition, Review, and Editing/SM: Conceptualization, Methodology, Project administration, Funding acquisition, Validation, Review and Editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest in this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Handling Editor: Sudhir K. Sopory.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Varshney, A., Dahiya, P. & Mohan, S. Antioxidant Activity of Pot Marigold (Calendula officinalis L.) in Response to Metal(loid) Induced Oxidative Stress from Fly Ash Amended Soil. J Plant Growth Regul 42, 5928–5944 (2023). https://doi.org/10.1007/s00344-023-10977-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-10977-2