Abstract
Salt toxicity in agricultural soils is a principal abiotic constraint that limits crop growth, development, and yield. The employment of potential selection markers for screening salt-tolerant wheat cultivars is crucial for conventional breeding programs and molecular biology approaches that may ensure sustainable wheat production under saline soils. The current experiment explored the tolerance potential of ten wheat cultivars to salt stress (150 mM) by utilizing various growth, biomass, physiological, and biochemical traits. Salt stress significantly abated growth-related parameters, leaf relative water content (LRWC), SPAD, gas exchange attributes, total soluble proteins (TSP), and anthocyanins in all wheat cultivars. The drop in these attributes was more visible alongside higher oxidative stress mirrored as excessive accumulation of oxidative stress markers such as superoxide radicals (O2⋅‒), methylglyoxal (MG), hydrogen peroxide (H2O2), malondialdehyde (MDA), and higher lipoxygenase (LOX) activity in salt-sensitive cultivars than salt-tolerant cultivars. Salinity stress caused disequilibrium in ionic uptake with an apparent decline in K, P, and Ca content with a concomitant increase in the accumulation of Na in both leaves and roots of all wheat cultivars, with a more visible effect in salt-sensitive cultivars. Further, salt-tolerant cultivars displayed greater root Na content. Salt-sensitive cultivars failed to maintain the K/Na ratio under salt toxicity. In contrast, salt-tolerant cultivars displayed better growth, gas exchange attributes, and strengthened antioxidant systems alongside lower oxidative stress. Moreover, salt-tolerant cultivars exhibited a higher accumulation of osmolytes, hydrogen sulfide, and nitric oxide. Therefore, these physiological and biochemical markers could be promising for screening tolerant wheat cultivars under salinity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity in agricultural soils, either caused by natural or anthropic factors, adversely affects plant growth, development, and yield by interfering with essential physiological and biochemical processes (Evelin et al. 2019; Akbar et al. 2021). The detrimental effects of salt stress occur in two successive phases: (i) osmotic stress and (ii) ionic toxicity (Na and Cl) and followed by the subsequent impact of secondary stresses such as oxidative stress and nutritional imbalances (Arzani 2008; Munns and Tester 2008). Excessive toxic ions (Na and Cl) cause metabolic dysfunction via impaired enzyme function, the integrity of the cell and organelle membranes, and photosynthetic apparatus (Arzani and Ashraf 2016).
Osmotic adjustment is one trait that can alleviate some of the deleterious effects of stress by maintaining cell turgor and water content as leaf water potential declines (Flower and Ludlow 1987). In other words, osmotic and ion toxicity effects were thought to be spatially and temporally separated. This spatial and temporal separation suggested that early salinity stress responses are due to general osmotic or water deficit stress and that sodium-specific responses (i.e., ion sequestration or exclusion) are induced later (Van Zelm et al. 2020). Excluding Na from the cells and maintaining the K/Na ratio under saline conditions are pivotal physiological mechanisms for plant salinity tolerance (Ebrahim et al. 2020; Rahimi et al. 2021).
Under a saline environment, plants experience oxidative stress through the immoderate accumulation of reactive oxygen species (ROS), which damages various vital components of plant cells, such as membranes, proteins, lipids, pigments, and nucleic acids (Chawla et al. 2013). Plants restrain excessive ROS production and oxidative damage through convoluted enzymatic [superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX)] and non-enzymatic (ascorbic acid (AsA), glutathione (GSH), phenolics, flavonoids) antioxidant systems (Desoky et al. 2020). However, antioxidant potential varies among different cultivars/varieties of the same plant species (Rasheed et al. 2014). Therefore, a robust antioxidant system defines plant tolerance to salinity stress.
Salinity also induces dicarbonyl stress in plants through excessive methylglyoxal (MG) production (Mahmud et al. 2020). Methylglyoxal is a cytotoxic compound synthesized endogenously through glycolysis and several other biochemical processes that can disrupt cellular structures such as proteins, carbohydrates, lipids, and nucleic acids (Hasanuzzaman et al. 2017). The glyoxalase system comprised of two enzymes, glyoxalase I (Gly I) and glyoxalase II (Gly II), is involved in the detoxification of overly produced MG by converting it into D-lactate in association with reduced glutathione (GSH) (Parvin et al. 2020). Glutathione is a significant component of the plant defense system primarily involved in ROS and MG detoxification and supports growth, development, protein activation, and gene expression under normal and abiotic stress conditions (Yousuf et al. 2012; Nahar et al. 2015a, b). Therefore, synchronization between the antioxidant and glyoxalase systems is essential for plant salinity tolerance. A substantial amount of literature has been published on the changes in the glyoxalase system of plants under salinity stress (Upadhyaya et al. 2011; Sankaranarayanan et al. 2017; Parvin et al. 2021).
Considering the climate change scenario and the increasing global population, it is urgent to unravel the underlying mechanisms of salinity tolerance in plants for developing crops that can sustain in salt-polluted soils and ensure sufficient yield production. To date, scarce data have examined salinity-mediated modulation in glutathione metabolism, detoxification of cytotoxic compounds, signaling molecules, and photosynthesis for the characterization of wheat cultivars. Hence, we are reporting the use of critical physiological and biochemical selection criteria for establishing the salinity tolerance potential of wheat cultivars. This information can be incorporated into different breeding programs to produce salt-tolerant wheat cultivars.
Materials and Methods
Plant Material and Growth Conditions
Ten wheat cultivars, namely LU-26, Punjab-85, Akbar-2019, Inqlab-91, WL-711, Anaj-17, Chakwal-86, SA-42, Parvaaz-94, and Barani-83, were used in the present screening experiment. Seeds were procured from Ayub Agricultural Research Institute, Faisalabad, Pakistan. Seeds of all cultivars were soaked in deionized water for 12 h to obtain uniform germination. Later, seeds were sown in 25 × 15 cm plastic pots filled with 5 kg of thoroughly washed sterilized sand. The experiment was conducted in a completely randomized design (CRD), having four replicates with two pots per replication. Three plants were kept in each pot after seed germination. Full-strength Hoagland’s solution was provided to the plants on the 12th day of the experiment. On the twenty-fifth day, salt toxicity of NaCl (Merck KGaA, Darmstadt, Germany) was introduced by dissolving salt in the nutrient solution in increments of 50 mM to attain the required level of 150 mM. Control plants were only supplied with nutrient solution. Plants were harvested after forty-five days of germination and excised into shoots and roots to measure fresh weights using an electronic weighing scale (AND, Model: DH-V 300A). Leaf samples were quickly kept in liquid nitrogen and stored at − 20 °C for further biochemical analysis. For dry weights measurement, plants were oven-dried at 70 °C for seven days. Shoot and root length were measured using a measuring tape. Leaf area was measured using Gardner et al. (1985) formula:
The experimental site was the botanical garden, Botany Department, Government College University Faisalabad, situated at coordinates 31°38′97.1"N 73°02′35.9"E.
Gas Exchange Parameters and Pigments
A portable infrared gas analyzer (LI-COR Lincoln, NE, USA) was used to measure the stomatal conductance (gs), photosynthesis (A), water use efficiency (A/E), and transpiration rate (E). All measurements were recorded between 10:00 and 12:00 PM. The specifications of the instrument were as follows; photosynthetically active radiation (PAR) was 1084 μmol m−2 s−1, leaf temperature 29 to 33 °C and leaf chamber volume gas flow rate 295 mL min−1, leaf chamber molar gas flow rate (U) 402 μmol s−1 and ambient pressure 97.95 kPa.
Chlorophyll a, b, total chlorophyll (a + b), and carotenoid content were estimated by Lichtenthaler (1987) protocol. The leaf sample (50 mg) was chopped and immersed in 80% aqueous acetone for a day in an airtight container. The OD of the solution was recorded at 470, 646.8, and 663.2 nm spectrophotometrically (Hitachi U-2900).
A chlorophyll content meter (CL-01, Hansatech Instruments, UK) was used to measure leaf chlorophyll content. For this purpose, three fully expanded young leaves from the top were selected, and SPAD values were taken.
Nitric Oxide (NO), Hydrogen Sulfide (H2S), and Glutathione Content
Leaf NO content was measured by employing the protocol of Zhou et al. (2005). Leaf material (0.5 g) was pulverized in 3 mL 50 mM acetic acid buffer (pH 3.6). Centrifugation of the homogenate was done at 10,000×g for 20 min to collect the supernatant. The collected supernatant was mixed with 100 mg of charcoal and filtered. Afterward, 1 mL of Griess reagent was mixed with 1 mL of supernatant and kept at room temperature for half-hour. A spectrophotometer was used to measure the absorbance of the reaction mixture at 540 nm.
The H2S content was measured following the protocol of Nashef et al. (1977). Fresh leaf material (0.5 g) was ground in 50 mM potassium phosphate buffer (pH 7.5). The mixture contained 0.1 mL supernatant, 20 µL of 20-mM 5,5′-dithiobis (2-nitrobenzoic acid), and 0.19 mL extraction buffer. The OD of the sample mixture was taken at 412 nm.
Leaf material (0.5 g) was ground in a mixture containing 3 mL of 5% metaphosphoric acid and 1 mM of EDTA. The supernatant was taken after centrifugation of the homogenate. Afterward, the reaction solution for reduced glutathione (GSH) determination was prepared by adding 5,5-dithiobis (2-nitrobenzoic acid, DTNB) and 50 mM potassium phosphate buffer (pH 7.5). To determine oxidized glutathione (GSSG), 2-vinylpyridine was added to remove GSH. The absorbance of the mixture was recorded at 412 nm (Hasanuzzaman and Fujita 2011).
Methylglyoxal (MG) and Cytosolutes
The leaf sample (0.5 g) was ground in 10 ml of 5% perchloric acid and centrifuged for MG determination. The supernatant was taken and mixed with charcoal and sodium bicarbonate and used for N-acetyl-l-cysteine-based assay. The absorbance was recorded at 288 nm (Nahar et al. 2016).
Cytosolutes such as free proline (Bates et al. 1973), glycine betaine (Grieve and Grattan 1983), phenolics (Wolfe et al. 2003), flavonoids (Zhishen et al. 1999), total soluble sugars (Dubois et al. 1956), total free amino acids (Hamilton and Van Slyke 1943), and anthocyanins (Mita et al. 1997) were measured from fresh leaf material.
Superoxide Radicals (O2 ⋅‒), Malondialdehyde (MDA), and Hydrogen Peroxide (H2O2)
The production rate of O2⋅‑ was determined using the protocol of Yang et al. (2011). The leaf sample (0.5 g) was finely ground and mixed with hydroxylamine hydrochloride and incubated at 25 °C for 20 min. After incubation, the reaction solution was mixed with 17 mM sulfanilamide and 7 mM naphthylamine, and absorbance was monitored at 530 nm.
The methodology of Velikova et al. (2000) was employed for the determination of H2O2. Leaf material (0.5 g) was ground in 10 mL of 5% TCA. The homogenate was centrifuged at 11,000×g. Half-milliliter supernatant was taken and reacted with 50 mM potassium phosphate buffer (pH 7.5) and potassium iodide (KI). The reaction mixture was allowed to incubate at room temperature (25 °C) for half-hour. The absorbance was recorded at 390 nm. For evaluation of MDA content, the leaf sample (0.5 g) was ground in 5% TCA (10 mL) and centrifuged. The supernatant (0.5 mL) taken was mixed with 2 mL 0.5% thiobarbituric acid substance (TBA) and incubated at 95 °C for half-hour. The OD of the mixture was monitored at 532 and 600 nm (Heath and Packer 1968).
DPPH Radical Scavenging Activity
2,2 Diphenyl-picrylhydrazyl (DPPH) radical scavenging activity was assessed by employing the protocol of Sharma and Bhat (2009). The reduction of DPPH absorption was monitored spectrophotometrically at 515 nm. Ascorbic acid was used as a positive control. The calculation was carried out by the following equation:
Estimation of Total Soluble Proteins (TSP) and Enzymes Activities
Fresh leaf material was finely crushed in liquid nitrogen and 10 mL of 0.5 M potassium phosphate buffer (pH 7.5) was added. The homogenized was centrifuged at 10,000×g for 20 min at 4 °C. Supernatant collected was used for the measurement of TSP (Bradford 1976) and enzyme activities. Superoxide dismutase (SOD) activity was monitored using the protocol of van Rossum et al. (1997). Peroxidase (POD) and catalase (CAT) activities were determined by the method of Chance and Maehly (1955). For APX activity, Nakano and Asada (1981) protocol was employed, and a reduction in absorption was taken at 290 nm for 120 s. The activity of lipoxygenase (LOX) was determined at 234 nm on a spectrophotometer in the presence of linoleic acid as a substrate as defined by Doderer et al. (1992).
Glutathione-S-Transferase (GST) Activity, Ascorbic Acid (AsA), and Ions Determination
Hasanuzzaman and Fujita (2011) protocol was followed for measuring the GST activity. Constituents of the reaction mixture were enzyme extract, 1.5 mM GSH, and 1 mM 1-chloro-2,4-dinitrobenzene. The OD was recorded at 340 nm for 60 s on a spectrophotometer.
Leaf material (0.5 g) was homogenized in 10 mL of 6% TCA and filtered for the determination of AsA. Filtrate (2 mL) taken was mixed with 1 mL of 2% 2,4-dinitrophenylhydrazine, followed by the addition of 1 drop of 10% thiourea (prepared in 70% ethanol). The samples were placed in a water bath for 30 min at 95 °C. The samples were then allowed to cool down in an ice bath, followed by adding 2.5 mL of 80% H2SO4. The absorbance of the samples was recorded at 530 nm (Mukherjee and Choudhuri 1983).
Plant dry samples (0.1 g) were acid-digested following the protocol of Allen et al. (1986). A flame photometer (Sherwood, model 360) was used to measure the potassium (K), calcium (Ca), and sodium (Na) content. The methodology of Jackson (1962) was employed to estimate phosphorus (P) content.
Statistical Analysis
Data obtained from a completely randomized experiment with three replicates were statistically interpreted using Minitab software version 20.3. Graphs of the data were generated in Origin-Pro 2021 software. Significance among means was calculated using the least significance test (LSD) at P ≤ 0.05.
Results
Growth Attributes
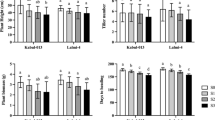
Salinity stress imposition manifested apparent aberration in growth-related traits of all wheat cultivars. Exposure to 150 mM salinity caused a remarkable (P ≤ 0.001) reduction in shoot and root length, fresh and dry biomass, leaf area, and the number of tillers. Data revealed that cultivars also varied significantly (P ≤ 0.001) under salinity stress for these attributes (Table 1, S1).
Statistical data for shoot length divulged that cultivars WL-711 (60.73%), Anaaj-17 (55.42%), Barani-83 (49.02%), and Chakwal-86 (45.68%) exhibited maximum reduction, while the minimum reduction was observed in cultivars Punjab-85 (9.69%), LU-26 (10.51%), Inqlab-91 (16.02%), and Akbar-2019 (18.37%). A moderate reduction was noted in cultivars SA-42 (25.28%) and Parvaaz-94 (33.27%) (Table S1).
For root length, the maximum reduction was evident in cultivars Anaaj-17 (61.99%), WL-711 (54.41%), Chakwal-86 (53.89%), and Barani-83 (48.28%). On the other hand, Akbar-2019, Inqlab-91, Punjab-85, and LU-26 exhibited lower reduction by 8.67%, 10.96%, 15.25%, and 16.48%, respectively, for this parameter. A mild reduction was evident in cultivars SA-42 (37.06%) and Parvaaz-94 (37.53%) under salt toxicity (Table S1).
Salt toxicity conspicuously (P ≤ 0.001) decreased the number of tillers in all wheat cultivars. Wheat cultivars responded widely to salinity stress. Maximum reduction in the number of tillers was observed in cultivars Chakwal-86 (57.14%), WL-711 (54.55%), Anaaj-17 (50.00%), and Barani-83 (50.00%). A slight reduction was evident in Akbar-2019 (6.00%), Punjab-85 (9.52%), LU-26 (10.53%), and Inqlab-91 (11.76%) under salt stress. Cultivars SA-42 (28.57%) and Parvaaz-94 (29.41%) showed moderate reduction (Table S1).
The leaf area of cultivars WL-711 (52.08%), Anaaj-17 (49.91%), Barani-83 (46.06%), and Chakwal-86 (45.35%) was distinctly reduced under salt toxicity. However, the leaf area of cultivars LU-26 (11.83%), Punjab-85 (14.41%), Inqlab-91 (14.81%), and Akbar-2019 (15.51%) was least affected by salt toxicity. A modest reduction was evident in leaf area of cultivars SA-42 (29.87%) and Parvaaz-94 (31.52%) under stress (Table S1).
Shoot and Roots Weights
Shoot fresh weight (SFW) of stressed plants was clearly (P ≤ 0.001) reduced in Barani-83 (74.95%), WL-711 (74.10%), Anaaj-17 (67.76%), and Chakwal-86 (65.26%), whereas, LU-26 (11.11%), Punjab-85 (11.23%), Inqlab-91 (11.34%), and Akbar-2019 (14.58%) showed lower reduction for this attribute. Salt toxicity moderately reduced the SFW of SA-42 (44.84%) and Parvaaz-94 (46.56%) (Table 1).
Cultivars Barani-83 (64.38%), Chakwal-86 (63.85%), WL-711 (48.94%), and Anaaj-17 (45.13%) showed considerably (P ≤ 0.001) higher reduction percentages for shoot dry weight (SDW) under salt stress. In contrast, Inqlab-91 (10.98%), LU-26 (18.08%), Punjab-85 (20.86%), and Akbar-2019 (21.31%) showed lower reduction values for this variable. In contrast, SA-42 (33.76%) and Parvaaz-94 (34.19%) displayed a moderate reduction in SDW under salt stress (Table 1).
Salinity stress significantly (P ≤ 0.001) attenuated root fresh weight of WL-711 (63.24%), Barani-83 (60.93%), Anaaj-17 (58.98%), and Chakwal-86 (58.52%) as compared with cultivars LU-26 (8.82%), Akbar-2019 (12.72%), Inqlab-91 (20.99%), and Punjab-85 (23.27%) exhibiting least reduction under salt stress as compared to their respective control plants. Cultivars SA-42 (42.80%) and Parvaaz-94 (44.08%) showed a moderate reduction in this parameter (Table S1).
Salt toxicity considerably (P < 0.001) reduced the root dry weight of all wheat cultivars. Plants of Akbar-2019 (7.94%), Punjab-85 (8.18%), Inqlab-91 (12.99%), and LU-26 (19.70%) were least affected by salt toxicity and displayed maximum root dry weight, whereas WL-711 (67.00%), Barani-83 (55.87%), Anaaj-17 (53.94%), and Chakwal-86 (53.80%) were failed to produce better root dry weight under salt stress. Cultivars Parvaaz-94 and SA- 42 showed moderate reductions of 28.19% and 32.82%, respectively (Table S1).
Leaf Relative Water Content (LRWC), Photosynthetic Pigments, and SPAD Values
Salt toxicity substantially (P ≤ 0.001) reduced the LRWC of cultivars Anaaj-17 (37.58%), Barani-83 (36.23%), WL-711 (33.17%), and Chakwal-86 (28.63%) in contrast to cultivars Punjab-85 (5.98%), LU-26 (8.59%), Akbar-2019 (9.85%), and Inqlab-91 (10.25%) that showed lower reduction percentages. In cultivars Parvaaz-94 (18.06%) and SA-42 (19.67%), salt toxicity caused a moderate reduction in LRWC (Table 1).
Salinity toxicity resulted in the substantial (P ≤ 0.001) diminution of chlorophyll pigments in wheat cultivars. A significant (P ≤ 0.001) variation among cultivars was also evident. Toxicity of NaCl caused eminent (P ≤ 0.001) reduction of Chl a content in cultivars, Chakwal-86, Barani-83, Anaaj-17, and WL-711 by 82.98%, 74.88%, 71.01%, and 70.12%, whereas cultivars LU-26, Inqlab-91, Punjab-85, and Akbar-2019 were least affected with reduction percentages of 17.23%, 19.49%, 21.27%, and 25.51%, respectively. Cultivars SA-42 (36.59%) and Parvaaz-94 (37.26%) displayed a moderate reduction in Chl a content under salt toxicity.
Chlorophyll b content also showed a substantial (P ≤ 0.001) decline under salt toxicity with a significant (P ≤ 0.001) variable response among cultivars. Cultivars LU-26 (4.69%), Inqlab-91 (5.77%), Punjab-85 (9.69%), and Akbar-2019 (13.49%) exhibited less reduction in chl b content as compared with cultivars Barani-83 (73.32%), WL-711 (70.94%), Chakwal-86 (70.61%), Anaaj-17 (64.17%) under salt stress. Salt toxicity caused a moderate reduction in chl b content in cultivars Parvaaz-94 (38.71%) and SA-42 (41.88%) under salt stress.
Total chlorophyll contents was significantly reduced in cultivars Chakwal-86 (78.98%), Barani-83 (74.31%), WL-711 (70.43%), and Anaaj-17 (68.79%). Cultivars LU-26 (11.98%), Inqlab-91 (13.73%), Punjab-85 (16.64%), and Akbar-2019 (20.80%) manifested lower reduction in total chlorophyll contents. However, a moderate reduction in total chlorophyll contents was observed in Parvaaz-94 (37.81%) and SA-42 (38.83%) under salt stress (Table S1).
Salt stress distinguishably (P < 0.001) reduced the carotenoid contents of all wheat cultivars. The responses of wheat cultivars varied remarkably for this variable. Cultivars Anaaj-17, Barani-83, WL-711, and Chakwal-86 showed higher reduction percentages of 38.34%, 36.85%, 34.78% and 31.98%, respectively. However, cultivars LU-26 (7.42%), Inqlab-91 (8.32%), Akbar-2019 (10.08%), and Punjab-85 (10.45%) manifested the lowest abridge in carotenoid contents. A moderate reduction was evident in SA-42 (28.61%) and Parvaaz-94 (27.40%) under salt stress (Table S1).
Salt toxicity resulted in a significantly higher reduction in SPAD values of cultivars WL-711 (79.60%), Chakwal-86 (72.24%), Barani-83 (71.31%), and Anaaj-17 (69.58%). Cultivars Inqlab-91, Punjab-85, Akbar-2019, and LU-26 exhibited 7.72%, 11.79%, 14.40%, and 14.65% reduction in SPAD values under salt stress, respectively. A moderate reduction was evident in cultivars Parvaaz-94 (50.12%) and SA-42 (55.28%) under salt stress (Table 1).
Cyto-Solutes
Proline levels significantly (P ≤ 0.001) augmented in cultivars Punjab-85 (240.61%), Inqlab-91 (238.44%), Akbar-2019 (235.55%), and LU-26 (232.85%) than cultivars Barani-83 (30.99%), Anaaj-17 (47.02%), WL-711 (47.20%), and Chakwal-86 (54.35%) under salt toxicity. SA- 42 (171.18%) and Parvaaz-94 (181.00%) experienced a moderate increase in proline levels under salinity (Table S1).
Likewise, cultivars LU-26 (139.09%), Punjab-85 (107.42%), Inqlab-91 (107.31%), and Akbar-2019 (87.90%) accumulated more (P ≤ 0.001) glycine betaine (GB) content than cultivars WL-711 (17.54%), Anaaj-17 (18.72%), Chakwal-86 (21.16%), and Barani-83 (22.04%). A moderate rise was observed in SA-42 (64.23%) and Parvaaz-94 (69.02%) under salt stress (Table S1).
Total free amino acids (TFAA) considerably (P < 0.001) increased in cultivars Punjab-85, Akbar-2019, LU-26, and Inqlab-91 showing percentages of 93.19%, 82.46%, 66.94%, and 56.23%, respectively, under salt stress. A moderate increase in this attribute was evident in cultivars Parvaaz-94 (25.79%) and SA-42 (24.52%). In contrast, cultivars Barani-83 (50.29%), Chakwal-86 (55.21%), WL-711 (56.90%), and Anaaj-17 (58.57%) showed considerable reduction in TFAA content under salt stress (Table S1).
Salt toxicity prominently (P ≤ 0.001) increased total soluble sugars (TSS) to 105.99%, 102.92%, 96.03%, and 67.99% in LU-26, Akbar-2019, Inqlab-91, and Punjab-85, respectively. A minimum increase in TSS was noted in Anaaj-17 (12.09%), Barani-83 (16.38%), Chakwal-86 (19.38%), and WL-711 (24.32%). This attribute manifested a moderate increase in cultivars SA-42 (35.84%) and Parvaaz-94 (43.14%) (Table S1).
Nitric Oxide (NO) and Hydrogen Sulfide (H2S)
Under salt toxicity, nitric NO content increased appreciably (P ≤ 0.001) to 141.23%, 118.70%, 104.63%, and 100.74% in cultivars LU-26, Punjab-85, Inqlab-91, WL-711, and Akbar-2019, respectively, whereas cultivars Anaaj-17, Chakwal-86, WL-711, and Barani-83 manifested inconspicuous increase of 5.13%, 6.19%, 6.90%, and 9.60%, respectively. Plants of SA-42 (55.45%) and Parvaaz-94 (61.29%) experienced a modest increase for this attribute (Fig. 1D).
Effect of salt stress on lipoxygenase (LOX) activity, malondialdehyde (MDA), hydrogen peroxide (H2O2), nitric oxide (NO), hydrogen sulfide (H2S), methylglyoxal (MG), superoxide radical (O2⋅‑), total soluble proteins (TSP), 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) activity in ten wheat cultivars
A considerable (P ≤ 0.001) increase in the H2S content of cultivars Punjab-85 (250.11%), Akbar-2019 (237.64%), LU-26 (172.82%), and Inqlab-91 (159.13%) was evident under salt toxicity as compared to cultivars WL-711 (19.85%), Chakwal-86 (39.93%), Anaaj-17 (44.89%), and Barani-83 (51.88%) exhibiting the lowest increase in H2S content under salt stress. A mediocre upsurge in H2S content was observed in Parvaaz-94 (80.31%) and SA-42 (98.56%) under salt toxicity (Fig. 1E).
Gas Exchange Characteristics
Photosynthesis (A) was least affected in cultivars LU-26 (4.37%), Punjab-85 (4.81%), Inqlab-91 (8.09%), and Akbar-2019 (8.56%), whereas it was markedly (P ≤ 0.001) reduced in cultivars Chakwal-86 (55.64%), WL-711 (44.14%), Barani-83 (36.45%), and Anaaj-17 (33.58%) under salt toxicity. Data revealed a moderate reduction in photosynthesis of Parvaaz-94 (17.34%) and SA-42 (18.06%) under salt toxicity (Table 1).
Transpiration rate (E) was slightly decreased in cultivars Akbar-2019, Punjab-85, Inqlab-91, and LU-26 to 30.64%, 35.27%, 36.51%, and 40.30%, respectively, whereas a considerable (P ≤ 0.001) decline was noted in cultivars WL-711, Chakwal-86, Anaaj-17, and Barani-83 to 79.88%, 77.96%, 77.85%, and 77.78%, respectively, under salt toxicity. A moderate reduction was evident in SA-42 (47.66%) and Parvaaz-94 (48.04%) under salt toxicity (Table 1).
Stomatal conductance (gs) was severely (P ≤ 0.001) hampered in Barani-83 (65.12%), WL-711 (64.37%), Anaaj-17 (63.49%), and Chakwal-86 (60.24%) under salt toxicity. The minimum reduction was evident in cultivars LU-26 (45.27%), Akbar-2019 (47.65%), Punjab-85 (50.30%), and Inqlab-91 (53.98%), while the temperate decline was recorded in Parvaaz-94 (56.50%) and SA-42 (58.94%) plants experienced salt toxicity (Table 1).
Water use efficiency (A/E) also declined to a significant (P ≤ 0.001) extent, with maximum reduction evident in cultivars WL-711 (86.86%), Anaaj-17 (84.33%), Barani-83 (84.32%), and Chakwal-86 (84.31%) than cultivars Akbar-2019 (39.71%), Punjab-85 (40.74%), LU-26 (43.48%), and Inqlab-91 (46.43%). A mild reduction was observed in the A/E of Parvaaz-94 (61.74%) and SA-42 (61.02%) under salinity (Table 1). Salt toxicity caused a considerable (P ≤ 0.001) decline in intrinsic water use efficiency (A gs−1) of cultivars WL-711 (54.01%), Barani-83 (53.41%), Chakwal-86 (52.36%), and Anaaj-17 (50.26%) than cultivars Punjab-85 (38.89%), Akbar-2019 (39.41%), LU-26 (39.68%), and Inqlab-91 (40.60%). A modest decrease was noticed in SA-42 (43.54%) and Parvaaz-94 (43.84%) plants under salt toxicity (Table S1).
Oxidative Stress Markers
Salt toxicity resulted in significantly (P ≤ 0.001) excessive generation of superoxide radicals (O2⋅‾) in Chakwal-86 (542.56%), Barani-83 (395.78%), WL-711 (336.01%), and Anaaj-17 (310.93%) than in Inqlab-91 (14.18%), Akbar-2019 (34.45%), LU-26 (41.55%), Punjab-85 (96.62%). An average accumulation was noted in SA-42 (231.39%) and Parvaaz-94 (236.73%) (Fig. 1G).
Likewise, hydrogen peroxide (H2O2) was also considerably (P ≤ 0.001) increased by salt toxicity in Barani-83 (168.58%), Chakwal-86 (158.96%), Anaaj-17 (143.70%), and WL-711 (142.77%) than in LU-26 (61.61%), Akbar-2019 (77.18%), Inqlab-91 (87.30%), and Punjab-85 (90.78%). An average increase in H2O2 levels was shown by Parvaaz-94 (114.34%) and SA-42 (127.54%) plants under salt toxicity (Fig. 1C).
Malondialdehyde (MDA) content showed a substantial (P ≤ 0.001) increase under salt toxicity in Chakwal-86 (258.20%), Anaaj-17 (231.10%), WL-711 (193.73%), and Barani-83 (173.88%) than Akbar-2019 (16.20%), Punjab-85 (52.68%), LU-26 (62.66%), and Inqlab-91 (97.37%). On the other hand, SA-42 (152.66%) and Parvaaz-94 (156.54%) moderately accumulated MDA content under salt toxicity (Fig. 1B).
Salt toxicity caused a considerable (P ≤ 0.001) rise in lipoxygenase (LOX) activity of cultivars Anaaj-17 (147.22%), Chakwal-86 (118.79%), Barani-83 (116.49%), and WL-711 (103.98%), compared to Inqlab-91 (26.97%), Punjab-85 (35.67%), Akbar-2019 (26.88%), and LU-26 (40.39%) that showed minimal percentages. A moderate increase was recorded in SA-42 (67.97%) and Parvaaz-94 (76.30%) (Fig. 1A).
Methylglyoxal (MG) and Total Soluble Proteins (TSP)
For MG, WL-711 (402.54%), Anaaj-17 (381.26%), Chakwal-86 (357.31%), and Barani-83 (340.25%) exhibited greater (P ≤ 0.001) values than Inqlab-91 (110.60%), Punjab-85 (115.88%), LU-26 (118.97%), and Akbar-2019 (123.56%) under salt toxicity. SA-42 (271.13%) showed a moderate increase in MG content, followed by Parvaaz-94 (272.98%) under salt toxicity (Fig. 1F).
Salt toxicity remarkably (P ≤ 0.001) curtailed the TSP content of cultivars WL-711 (64.36%), Chakwal-86 (61.48%), Barani-83 (56.94%), and Anaaj-17 (48.62%), whereas cultivars Punjab-85 (20.94%), Inqlab-91 (23.95%), Akbar-2019 (25.36%), and LU-26 (29.49%) showed a minimal reduction for this attribute. Results showed that SA-42 (41.37%) and Parvaaz-94 (41.71%) were moderately affected by salt toxicity for this variable (Fig. 1H).
Antioxidant Compounds
Ascorbic acid content increased significantly (P ≤ 0.001) in Akbar-2019 (202.79%), LU-26 (197.11%), Punjab-85 (175.61%), and Inqlab-91 (159.44%) than in Barani-83 (67.35%), Anaaj-17 (73.67%), WL-711 (82.48%), and Chakwal-86 (90.25%) cultivars under salt toxicity. Ascorbic acid content was moderately increased in Parvaaz-94 (119.00%) and SA-42 (134.18%) under salt-polluted environment (Table S1).
Anthocyanins decreased considerably (P ≤ 0.001) in wheat plants under salt toxicity. The maximum drop in anthocyanins was noted in cultivars Chakwal-86 (64.45%), Barani-83 (52.69%), WL-711 (49.74%), and Anaaj-17 (42.54%), whereas the minimum fall in anthocyanins was depicted by cultivars LU-26 (9.31%), Punjab-85 (10.53%), Inqlab-91 (11.84%), and Akbar-2019 (17.88%) compared to control plants under salt toxicity. Cultivars SA-42 (26.47%) and Parvaaz-94 (35.17%) performed moderately for this parameter under salt toxicity (Table S1).
Cultivars Chakwal-86 (9.84%), Anaaj-17 (10.03%), Barani-83 (11.19%), and WL-711 (12.96%) plants showed lower accumulation of flavonoids content under salt stress, whereas cultivars Punjab-85 (97.63%), Akbar-2019 (78.15%), LU-26 (65.52%), and Inqlab-91 (65.52%) manifested noticeably (P ≤ 0.001) higher flavonoids content under salinity. SA-42 (36.42%) and Parvaaz-94 (38.04%) manifested moderate accumulation of flavonoids under salinity (Table S1).
Phenolics were discernibly (P ≤ 0.001) increased in cultivars LU-26 (176.31%), Punjab-85 (146.16%), Akbar-2019 (131.51%), and Inqlab-91 (117.75%) than in cultivars WL-711 (18.81%), Barani-83 (27.02%), Anaaj-17 (29.02%), and Chakwal-86 (33.68%) under salt stress as compared to their respective controls. A moderate increase in phenolics was found in SA-42 (79.84%) and Parvaaz-94 (80.11%) under salt toxicity (Table S1).
Enzyme Assays
Cultivars Punjab-85 (68.80%), Akbar-2019 (56.17%), LU-26 (40.22%), and Inqlab-91 (36.75%) displayed significantly (P ≤ 0.001) higher superoxide dismutase (SOD) activity relative to cultivars WL-711 (4.25%), Chakwal-86 (5.79%), Barani-83 (6.63%), and Anaaj-17 (7.46%) as compared to their respective controls under salt toxicity. Parvaaz-94 (15.35%) and SA-42 (17.27%) performed moderately for SOD under stress (Fig. 2A).
When experiencing salt toxicity, cultivars Inqlab-91 (149.37%), LU-26 (133.48%), Punjab-85 (123.34%), and Akbar-2019 (122.28%) showed a more significant increase in peroxidase (POD) activity, and cultivars Barani-83 (32.21%), Anaaj-17 (36.81%), WL-711 (47.49%), and Chakwal-86 (60.25%) showed the least increment for this variable. Imposition of salt toxicity caused a moderate increase in Parvaaz-94 (98.20%) and SA-42 (110.95%) (Fig. 2B).
A similar trend was observed in catalase (CAT) and ascorbate peroxidase (APX) activities under salt toxicity. For CAT activity, cultivars Inqlab-91, Punjab-85, Akbar-2019, and LU-26 exhibited 99.08%, 98.90%, 79.93%, and 66.48% increase relative to cultivars Anaaj-17, WL-711, Chakwal-86, and Barani-83 showing 15.66%, 20.25%, 24.85%, and 29.31% increase, respectively, compared to control plants. A moderate increase was noted in SA-42 (47.65%) and Parvaaz-94 (48.12%) (Fig. 2C).
For APX activity, cultivars Inqlab-91, Akbar-2019, Punjab-85, and LU-26 displayed 150.46%, 138.54%, 136.51%, and 135.04% increase and cultivars Chakwal-86, WL-711, Barani-83, and Anaaj-17 showed 33.32%, 36.85%, 39.71%, and 40.46% increment, respectively. The APX activities of SA-42 (86.09%) and Parvaaz-94 (90.67%) showed a modest increase under salt stress (Fig. 2E).
A sharp increase in glutathione-S-transferase (GST) activity was observed in cultivars Akbar-2019 (261.55%), Punjab-85 (205.29%), Inqlab-91 (184.00%), and LU-26 (179.75%) than cultivars WL-711 (37.38%), Anaaj-17 (39.16%), Barani-83 (49.59%), and Chakwal-86 (60.59%) under salt toxicity in reference to their untreated control plants. The activity of GST under salt toxic conditions was moderately elevated in SA-42 (128.53%) and Parvaaz-94 (144.88%) as compared to other cultivars and control plants (Fig. 2D).
Salt toxicity impressively (P ≤ 0.001) increased the DPPH activity of cultivars Akbar-2019, Inqlab-91, Punjab-85, and LU-26 to 91.95%, 90.38%, 80.12%, and 77.74%, whereas cultivars Barani-83, Anaaj-17, WL-711, and Chakwal-86 showed minor increase of 3.49%, 14.80%, 16.31%, 20.22%, respectively, for this attribute. Cultivars Parvaaz-94 (35.67%) and SA-42 (42.23%) showed an average increase in this regard when compared to other cultivars under salt stress (Fig. 1I).
Glutathione Pool
Reduced glutathione (GSH) activity of cultivars Akbar-2019, Inqlab-91, Punjab-85, and LU-26 significantly (P ≤ 0.001) increased to 199.63%, 196.82%, 191.49%, 1and 90.79%, respectively, under salt toxicity, whereas Anaaj-17, Barani-83, WL-711, and Chakwal-86 exhibited 64.36%, 84.20%, 95.04%%, and 99.19% increase in this attribute. GSH activity increased modestly in Parvaaz-94 (112.79%) and SA-42 (119.51%) under salt toxicity (Fig. 2F).
Oxidized glutathione (GSSG) values showed a marked (P ≤ 0.001) increase in cultivars Akbar-2019 (91.35%), Punjab-85 (73.20%), Inqlab-91 (68.66%), and LU-26 (68.02%) compared to Anaaj-17 (19.26%), Chakwal-86 (32.30%), WL-711 (38.09%), and Barani-83 (38.54%) under salt toxicity. GSSG values of Parvaaz-94 and SA-42 increased by 46.80% and 49.23% (moderate increase as compared to other cultivars), respectively (Fig. 2G).
Salt stress caused a visible increase in the GSH: GSSG ratio of wheat cultivars. Plants of cultivars Punjab-85 (72.15%), LU-26 (71.69%), Akbar-2019 (67.73%), and Inqlab-91 (66.70%) showed higher increase percentages than cultivars WL-711 (35.28%), Parvaaz-94 (37.62%), Barani-83 (40.02%), Chakwal-86 (46.17%), and SA-42 (46.71%) under salt stress (Fig. 2H).
Ions Uptake
The spike of salt concentration in the growth medium caused a remarkable (P ≤ 0.001) increase in root Na of cultivars LU-26 (1000%), Punjab-85 (868.42%), Inqlab-91 (831.58%), and Akbar-2019 (828.57%) under salt stress as compared to controls. Cultivars Barani-83 (268.75%), Chakwal-86 (306.67%), Anaaj-17 (472.73%), and WL-711 (530.00%) showed less accumulation of Na in their roots. Salt-treated plants of Parvaaz-94 and SA-42 displayed an average uptake of Na in roots with percentages of 716.67% and 743.75%, respectively, evaluated against their respective controls (Fig. 3B).
Leaf Na content spiked significantly (P ≤ 0.001) in cultivars Anaaj-17 (3075.00%), WL-711 (3062.50%), Barani-83 (2030.00%), and Chakwal-86 (2000%) than cultivars Punjab-85 (290.00%), Akbar-2019 (388.89%), LU-26 (507.14%), and Inqlab-91 (525.00%) compared to their counterparts when grown under salt toxicity. SA-42 (1076.92%) and Parvaaz-94 (1221.10%) salt-stressed plants showed an average increase for this parameter as compared to other cultivars (Fig. 3A).
In contrast, imposition of salt toxicity prominently (P ≤ 0.001) trivialized leaf K content in cultivars Barani-83 (49.04%), Chakwal-86 (48.91%), Anaaj-17 (46.68%), and WL-711 (44.36%). However, cultivars Inqlab-91 (21.12%), Akbar-2019 (23.00%), Punjab-85 (25.78%), and LU-26 (27.01%) maintained their leaf K content with a nominal decrease under salt toxicity, whereas leaf K contents of SA-42 (33.50%) and Parvaaz-94 (35.31%) were decreased modestly (Fig. 3C). Similarly, salt toxicity caused visible (P ≤ 0.001) attenuation of root K content in cultivars Chakwal-86 (60.69%), Barani-83 (58.77%), WL-711 (53.95%), and Anaaj-17 (51.87%). However, cultivars Punjab-85 (7.26%), Inqlab-91 (13.80%), Akbar-2019 (16.95%), and LU-26 (19.04%) were least affected by salt toxicity for this variable in reference to the control plants, whereas an average reduction was visible in SA-42 (43.14%) and Parvaaz-94 (44.03%) under salt stress (Fig. 3D).
Salt toxicity caused a considerable (P ≤ 0.001) drop in the K/Na ratio in both leaves and roots of wheat plants. In leaves, the decrease was conspicuous in cultivars Anaaj-17 (98.59%), WL-711 (98.48%), Chakwal-86 (98.19%), and Barani-83 (98.15%) than cultivars Punjab-85 (81.35%), Akbar-2019 (85.07%), Inqlab-91 (87.75%), and LU-26 (89.41%). Data examined showed that the K/Na ratio of salt-stressed SA-42 (94.69%) and Parvaaz-94 (95.58%) was also notably disturbed (Fig. 3E). In roots, cultivars SA-42, Parvaaz-94, WL-711, Anaaj-17, Chakwal-86, LU-26, Akbar-2019, Inqlab-91, and Punjab-85 exhibited 96.00%, 95.34%, 94.28%, 94.08%, 93.99%, 93.01%, 91.93%, 91.01%, and 90.66% decline in K/Na ratio, respectively, as compared to control plants under salt stress (Fig. 3F).
Root P content of cultivars Anaaj-17 (89.10%), WL-711 (87.16%), Chakwal-86 (82.15%), and Barani-83 (79.68%) showed a significant (P ≤ 0.001) decline under salt toxicity. Cultivars Punjab-85 (45.28%), LU-26 (47.77%), Akbar-2019 (55.78%), and Inqlab-91 (56.70%) showed minimal reduction percentages for this variable compared to control plants. SA-42 (76.30%) and Parvaaz-94 (76.31%) were moderately affected by salt toxicity (Fig. 4B). Similarly, cultivars WL-711 (94.41%), Barani-83 (89.39%), Anaaj-17 (88.36%), and Chakwal-86 (82.89%) exhibited considerable (P ≤ 0.001) decline in leaf P content under salt toxicity compared to their respective controls. Cultivars Punjab-85 (29.41%), Akbar-2019 (35.77%), LU-26 (36.66%), and Inqlab-91 (43.02%) were least affected by salt toxicity showing a minimal reduction in leaf P content. However, there was a slight decrease in leaf P content of SA-42 (65.55%) and Parvaaz-94 (69.24%) under salt stress (Fig. 4A).
A noteworthy (P ≤ 0.001) decline was evident in the root Ca content of cultivars WL-711 (76.71%), Barani-83 (76.51%), Chakwal-86 (74.52%), and Anaaj-17 (70.65%) than cultivars Akbar-2019 (42.92%), Inqlab-91 (49.47%), Punjab-85 (50.66%), and LU-26 (51.10%) exhibiting minimum decline percentages under salt toxicity. A nominal reduction of 58.38% and 60.54% was recorded in cultivars Parvaaz-94 and SA-42 under stress, respectively (Fig. 3H). Leaf Ca content was also eminently (P ≤ 0.001) reduced under salt toxicity in cultivars Barani-83 (63.43%), Chakwal-86 (62.70%), Anaaj-17 (60.75%), and WL-711 (59.24%). Cultivars Punjab-85 (14.94%), Akbar-2019 (15.79%), Inqlab-91 (24.40%), and LU-26 (26.28%) manifested the least reduction values for this variable under salt stress. Cultivars SA-42 (34.97%) and Parvaaz-94 (39.52%) performed moderately (slight reduction) under stress as compared to other cultivars (Fig. 3G).
Based on the above findings, cultivars Akbar-2019, Punjab-85, and LU-26 were grouped as salt-tolerant, and cultivars WL-711, Barani-83, Chakwal-86, and Anaaj-17 were placed in the salt-sensitive group. Cultivars SA-42 and Parvaaz-94 were categorized as moderately salt-sensitive cultivars, and Inqlab-91 was observed as a moderately salt-tolerant cultivar. Our findings were further assisted by hierarchical cluster analysis illustrating salt-tolerant cultivars in cluster A, salt-sensitive cultivars in cluster B, moderately salt-sensitive cultivars in cluster C, and moderately salt-tolerant cultivars in cluster D (Fig. 5).
Discussion
Salinity inhibits plant growth due to osmotic stress and excess ion toxicity. Salt-induced osmotic stress causes physiological drought, which results in restricted plant growth (Gupta and Huang 2014; Liu et al. 2015). Our results manifested reduced plant growth and biomass of wheat cultivars administered with salt toxicity (Table 1, S1). However, the reduction was more prominent in salt-sensitive cultivars than in salt-tolerant cultivars. This reduction in growth parameters could be ascribed to perturbed nutrient uptake and excessive accumulation of Na ions, causing ion-specific toxicity and higher osmotic stress (Hussain et al. 2015; Nadeem et al. 2022). Moreover, salinity stress hampers normal cell division, elongation, and enlargement in plants, reducing leaf area (Farooq et al. 2015; Oney-Birol 2019; Tanveer et al. 2020), which could also be a reason for reduced photosynthesis due to the lesser leaf area available to capture light. Consistent with the variable response of different wheat cultivars in the current study, several scientists also reported genotypic variations for salinity tolerance in different crops (Moustafa et al. 2021; Omrani et al. 2022; Quamruzzaman et al. 2022).
Photosynthetic pigments mainly determine plant photosynthetic performance under normal and stressed conditions (Sherin et al. 2022). Both stomatal and non-stomatal reasons can modulate the photosynthetic performance of plants (Akbar et al. 2021). In the current study, salt toxicity markedly reduced chlorophyll a, b, and total chlorophyll content of wheat cultivars, with a more visible reduction evident in salt-sensitive cultivars. This reduction in chlorophyll pigments might be due to impaired biosynthesis or increased degradation via stress-induced activation of chlorophyllase activity and/or due oxidative stress (Muhammad et al. 2021). Increased oxidative stress due to the excessive generation of reactive oxygen species (ROS) in plants under environmental constraint can also deteriorate chlorophyll pigments (Ahmad et al. 2022). Our results are in consonance with previous literature (Taïbi et al. 2016; Luis Castañares and Alberto Bouzo 2019).
In the present study, gas exchange attributes such as photosynthetic rate (A), stomatal conductance (gs), transpiration rate (E), and water use efficiency (WUE) of wheat plants showed remarkable reduction under salt toxicity. Our results agree with the findings of Shaheen et al. (2013) and Hatam et al. (2020). This reduction in photosynthetic rate could be due to the declined gs and E, hindering transpiration pull and nutrient uptake. Surfeit accumulation of Na and deficit of K in plants under salinity stress results in chlorophyll degradation and disturbs thylakoid membranes. The photosynthetic rate also correlated positively with the leaf and root K content of wheat plants. Moreover, increased accumulation of hydrogen peroxide (H2O2) and malondialdehyde (MDA) exerted conspicuously detrimental effects on A, gs, E, WUE, and SPAD (Fig. 7).
The present investigation showed significantly elevated Na accumulation and reduced K, P, and Ca uptake in the leaves and roots of all wheat cultivars under salt stress. However, salt-sensitive cultivars were most affected in this regard. Various reports align with our findings (Zhu et al. 2020; Kafi et al. 2021). Potassium (K) is a requisite for normal plant metabolic functions. However, excessive Na levels in the growth medium compete with K for its uptake and decrease K/Na ratio resulting in reduced plant growth (Hussain et al. 2015). Several studies suggested that the accumulation of Na and K both influence light-driven reactions by modulating the stacking of grana in the chloroplast (Sudhir and Murthy 2004; Tränkner et al. 2018). Calcium (Ca) is majorly involved in plant signaling as a secondary messenger and protects plasma membrane integrity. In this study, the decrease in Ca content could be ascribed to the displacement of Ca by Na on the cellular binding sites (Shahid et al. 2020). Principal component analysis depicted that Na accumulation in leaves or roots is strongly negatively correlated with nutrient (K, Ca, and P) acquisition in salt-stressed plants (Fig. 6).
Principal component analysis among studied growth and biomass-related attributes, nutrient ions, and Na ions in both leaves and roots of ten wheat cultivars under salt stress. Abbreviations: SL (shoot length), RL (root length), SFW (shoot fresh weight), SDW (shoot dry weight), RFW (root fresh weight), RDW (root dry weight), K-L (leaf potassium content), K-R (root potassium content), Ca-L (leaf calcium content), Ca-R (root calcium content), P-L (leaf phosphorus content), P-R (root phosphorus content), Na-L (leaf sodium content), and Na-R (root sodium content)
Salt toxicity altered leaf relative water content (LRWC) in all wheat cultivars. Salt-sensitive cultivars exhibited a prominent reduction in LRWC under salt toxic conditions. However, salt-tolerant wheat cultivars showed minimum reduction for this parameter. Salt stress imposes osmotic stress that reduces water uptake through roots resulting in decreased LRWC of leaves (Roychoudhury et al. 2021). Relative water content is a significant determinant of metabolic activities and survival of leaves that could be employed for the discrimination of salt-sensitive and tolerant plants under salt toxicity (Sarker and Oba 2020; Haddoudi et al. 2021).
To maintain their osmotic potential, plants regulate ionic homeostasis and accumulate various osmolytes such as sugars, proline, glycine betaine, and polyols (Nahar et al. 2016). Our results displayed a marked accumulation of proline, glycine betaine (GB), and total soluble sugars (TSS) in salt-tolerant wheat cultivars than in salt-sensitive cultivars under salt toxicity. Proline plays a considerable role in the osmotic adjustment of plants under salt toxicity. Moreover, proline also acts as a non-enzymatic antioxidant that attenuates the negative impact of salt-induced ROS in plants (El Moukhtari et al. 2020). Various reports have shown increased proline accumulation under stress conditions (Wang et al. 2015; Zhang et al. 2015; Zegaoui et al. 2017). Sugars are also reported to play a role as an osmoprotectant and scavenger of ROS under stress conditions (Sami et al. 2016). Many reports have indicated that GB acts as an osmoprotectant and could be involved in the changing lipid and fatty acid composition, thus improving membrane fluidity of thylakoid membrane under stress conditions (Chen and Murata 2011; Mäkelä et al. 2019; Zulfiqar et al. 2019).
Salt toxicity significantly decreased total soluble protein (TSP) content in salt-sensitive wheat cultivars. However, salt-tolerant cultivars showed a slight decrease in their TSP content. This reduction in TSP might be due to the denaturation of enzymes involved in protein synthesis or the decreased availability of amino acids under salt toxicity (Hassanpour et al. 2013; Shaki et al. 2018). The higher levels of TSP and TSS content in salt-tolerant cultivars than in salt-sensitive cultivars indicates their adaptation to salt toxicity. It is reported that soluble proteins are involved in cellular osmoregulation under salt stress and provide a storage form of nitrogen (Ahmad et al. 2016). Total free amino acids (TFAA) can neutralize the ROS and maintain the osmotic status of the plant under stress. In the present experiment, TFAA increased significantly in salt-tolerant cultivars under salt stress. In contrast, we found a marked decline in TFAA content in salt-sensitive cultivars, suggesting that salt-sensitive wheat cultivars were compromised to elicit an efficient adaptive osmoprotectant mechanism under salt toxicity.
Salt toxicity causes the excessive generation of ROS such as singlet oxygen, hydrogen peroxide (H2O2), superoxide (O2⋅‒), and hydroxyl radicals that deteriorate major cellular components such as nucleic acids, proteins, and membranes (Ashraf et al. 2019). Our results demonstrated that salt-sensitive wheat cultivars displayed significantly higher accumulation of O2⋅‒ and H2O2 under salt toxic conditions. ROS-induced damage to membranes is measured in the form of malondialdehyde (MDA), a by-product of lipid peroxidation of membranes (Rasheed et al. 2014). In our study, MDA levels increased significantly under salt toxicity in salt-sensitive cultivars. However, the salt-tolerant cultivars showed a non-significant increase in MDA level under salt stress manifesting minimal oxidative damage to cellular membranes and other components. Salt-sensitive cultivars also showed a substantial increase in lipoxygenase (LOX) activity under salt toxicity. It is reported that higher LOX activity generates oxidative stress and damage to membranes (Nahar et al. 2015a, b). Pearson’s correlation analysis depicted a positive correlation between LOX activity and MDA under salt toxicity (Fig. 7).
Pearson’s correlation analysis performed among different studied attributes of wheat cultivars under salt stress. Abbreviations: RWC (relative water content), NT (number of tillers), H2S (hydrogen sulfide), NO (nitric oxide), TSP (total soluble proteins), MG (methylglyoxal), GB (glycine betaine), O2•‑ (superoxide radical), ASA (ascorbic acid), Chl a (chlorophyll a), Chl b (chlorophyll b), H2O2 (hydrogen peroxide), MDA (malondialdehyde), TSS (total soluble sugars), TFAA (total free amino acids), DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate), SOD (superoxide dismutase), POD (peroxidase), CAT (catalase), APX (ascorbate peroxidase), GST (glutathione-S-transferase), LOX (lipoxygenase), GSH (reduced glutathione), GSSH (oxidized glutathione), A (photosynthetic rate), E (transpiration rate), gs (stomatal conductance), WUE (water use efficiency), iWUE (intrinsic water use efficiency), SPAD (soil plant analysis development chlorophyll meter)
In the present investigation, salt toxicity caused an eminent increase in the DPPH radical scavenging activity of salt-tolerant wheat cultivars. Our results are in parallel with the previous study of Golkar et al. (2019). It has been reported that increasing stress intensity resulted in increased DPPH radical scavenging percentage in cabbage plants (Hagen et al. 2009). Moreover, DPPH activity in wheat plants has been reported to correspond with the level of stress tolerance (Kiani et al. 2021).
Plants possess robust antioxidant systems to preclude the overproduction of ROS caused by salt toxicity (Masood et al. 2006; Acosta-Motos et al. 2017). The antioxidant system is based on enzymatic [superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), glutathione-S-transferase (GST)] and non-enzymatic antioxidants [ascorbic acid (AsA), glutathione (GSH), phenolics, flavonoids] (Garcia-Caparros et al. 2021). Increased activities of these antioxidants under stress conditions are known to play a substantial role in plant stress tolerance (Hasanuzzaman et al. 2020). In the present study, salt toxicity caused a considerable rise in SOD, POD, CAT, and APX activities alongside increased accumulation of AsA, GSH, phenolics, and flavonoids. The SOD catalyzes the conversion of O2 to H2O2, serving as the first line of defense in plants under stress (Alscher et al. 2002). This converted H2O2 is still toxic to the cellular environment and is further dismutated to H2O and O2 by CAT (Sofo et al. 2015). Likewise, APX utilizes ascorbate as an electron donor and converts H2O2 into H2O (Pandey et al. 2017).
Methylglyoxal (MG) is a cytotoxic compound generated via different plant metabolic reactions. Methylglyoxal being oxidative in nature, can cause damage to nucleic acid and other ultrastructural components of the cell (Kaur et al. 2016). Yadav et al. (2005) reported that stress conditions could cause a two to six fold increase in plant MG production. Similarly, our results exhibited a considerable increase in MG levels of salt-sensitive plants under salt toxicity. Plants detoxify excess MG through the glyoxalase system (Gly I and Gly II enzymes) that works co-ordinately with GSH under stress conditions (Nahar et al. 2015a, b). We observed a minimum increase in GSH content of salt-sensitive wheat cultivars exposed to salt toxic conditions. However, salt-tolerant cultivars notably enhanced GSH level that is accountable for detoxifying cellular MG. We also found increased GSSG content in salt-tolerant plants, which could be due to the GSH reaction with ROS or elevated GPX and GST activity, as GST is involved in catalyzing the tripeptide glutathione (GSH) conjugation to a range of endo- and xenobiotics and reduces ROS-induced stress (Kumar and Trivedi 2018). Consistently, we also recorded higher GST activity in salt-tolerant wheat cultivars than in salt-sensitive cultivars under salt toxicity.
Nitric oxide (NO) is a gaseous molecule that acts as a signaling molecule in plants and mediates different defense responses under adverse environmental conditions (Sharma et al. 2020). Besides, hydrogen sulphide (H2S) is another gaseous transmitter involved in many physiological processes in plants (Zhang et al. 2021). Hydrogen sulphide is also reported to impart stress tolerance in many crops (Xiao et al. 2020; Zhou et al. 2020; Srivastava et al. 2022). It has been reported that H2S enhanced salt stress tolerance in alfalfa plants by regulating ion channels on the plasma membrane, inhibiting K efflux, and maintaining the K/Na ratio (Lai et al. 2014). Our results showed a significant increase in NO and H2S content of salt-stressed wheat plants (Fig. 2B, G). However, a more prominent increase was evident in salt-sensitive wheat cultivars. Various reports showed that H2S strengthened the antioxidant defense system, as evident in our study (Luo et al. 2015; Kaya et al. 2019; Li et al. 2020). Correlation analysis showed a positive correlation of H2S and NO with the antioxidant system of wheat plants under salt toxicity (Fig. 7).
Conclusion
In conclusion, salt stress negatively affected wheat plant growth, biomass, LRWC, chlorophyll pigments, ion uptake, and gas exchange attributes. The salinity also increased oxidative stress by excessively generating ROS and MG content in stressed plants. It also modulated the antioxidant and MG detoxification systems of wheat plants. However, the negative impact of salt toxicity in salt-sensitive cultivars was more considerable than salt-tolerant cultivars. Salt-tolerant cultivars showed tolerance to salinity due to their better antioxidant and MG detoxification system, restricted excessive Na uptake and accumulation in aerial parts accompanied by maintained K/Na ratio in both root and shoot, better photosynthetic performance, and higher accumulation of cyto-solutes in plant tissues. Therefore, the aforementioned growth, physiological and biochemical parameters can be employed to screen salt-tolerant wheat cultivars under salt toxic conditions.
References
Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7(1):18
Ahmad P, Abdel Latef AA, Hashem A, Abd-Allah EF, Gucel S, Tran LSP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347
Ahmad S, Mfarrej MFB, El-Esawi MA, Waseem M, Alatawi A, Nafees M, Saleem MH, Rizwan M, Yasmeen T, Anayat A (2022) Chromium-resistant Staphylococcus aureus alleviates chromium toxicity by developing synergistic relationships with zinc oxide nanoparticles in wheat. Ecotoxicol Environ Saf 230:113142
Akbar A, Ashraf MA, Rasheed R, Ali S, Rizwan M (2021) Menadione sodium bisulphite regulates physiological and biochemical responses to lessen salinity effects on wheat (Triticum aestivum L.). Physiol Mol Biol Plants 27(5):1135–1152
Allen S, Grimshaw H, Rowland A (1986) Chemical analysis. In: Moore PD, Chapman SB (eds) Methods in plant ecology. Blackwell, Oxford, pp 285–344
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53(372):1331–1341
Arzani A (2008) Improving salinity tolerance in crop plants: a biotechnological view. In Vitro Cell Dev Biol-Plant 44(5):373–383
Arzani A, Ashraf M (2016) Smart engineering of genetic resources for enhanced salinity tolerance in crop plants. Crit Rev Plant Sci 35(3):146–189
Ashraf MA, Asma HF, Iqbal M (2019) Exogenous menadione sodium bisulfite mitigates specific ion toxicity and oxidative damage in salinity-stressed okra (Abelmoschus esculentus Moench). Acta Physiol Plant 41(12):1–12
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Castañares JL, Bouzo CA (2019) Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic Plant J 5(2):79–87
Chance B, Maehly A (1955) Assay of catalases and peroxidases. Elsevier, Amsterdam
Chawla S, Jain S, Jain V (2013) Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J Plant Biochem Biotechnol 22(1):27–34. https://doi.org/10.1007/s13562-012-0107-4
Chen TH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34(1):1–20
Desoky E-SM, El-maghraby LM, Awad AE, Abdo AI, Rady MM, Semida WM (2020) Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata). Sci Hortic 272:109576
Doderer A, Kokkelink I, van der Veen S, Valk BE, Schram A, Douma AC (1992) Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta (BBA)- Protein Struct Mol Enzymol 1120(1):97–104
Dubois M, Gilles KA, Hamilton JK, Pt R, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Ebrahim F, Arzani A, Rahimmalek M, Sun D, Peng J (2020) Salinity tolerance of wild barley Hordeum vulgare ssp. spontaneum. Plant Breed 139(2):304–316
El Moukhtari A, Cabassa-Hourton C, Farissi M, Savouré A (2020) How does proline treatment promote salt stress tolerance during crop plant development? Front Plant Sci 11:1127
Evelin H, Devi TS, Gupta S, Kapoor R (2019) Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges. Front Plant Sci 10:470
Farooq M, Hussain M, Wakeel A, Siddique KH (2015) Salt stress in maize: effects, resistance mechanisms, and management. Rev Agron Sustain Dev 35(2):461–481
Flower D, Ludlow M (1987) Variation among accessions of pigeonpea (Cajanus cajan) in osmotic adjustment and dehydration tolerance of leaves. Field Crops Res 17(3–4):229–243
Garcia-Caparros P, De Filippis L, Gul A, Hasanuzzaman M, Ozturk M, Altay V, Lao MT (2021) Oxidative stress and antioxidant metabolism under adverse environmental conditions: a review. Bot Rev 87(4):421–466
Gardner FP, Pearce RB, Mitchell RL (2017) Physiology of crop plants. Scientific publishers
Golkar P, Taghizadeh M, Yousefian Z (2019) The effects of chitosan and salicylic acid on elicitation of secondary metabolites and antioxidant activity of safflower under in vitro salinity stress. Plant Cell Tissue Organ Cult (PCTOC) 137(3):575–585
Grieve C, Grattan S (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70(2):303–307
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics. https://doi.org/10.1155/2014/701596
Haddoudi L, Hdira S, Hanana M, Romero I, Haddoudi I, Mahjoub A, Ben Jouira H, Djébali N, Ludidi N, Sanchez-Ballesta MT (2021) Evaluation of the morpho-physiological, biochemical and molecular responses of contrasting Medicago truncatula lines under water deficit stress. Plants 10(10):2114
Hagen SF, Borge GIA, Solhaug KA, Bengtsson GB (2009) Effect of cold storage and harvest date on bioactive compounds in curly kale (Brassica oleracea L. var. acephala). Postharvest Biol Technol 51(1):36–42
Hamilton PB, Van Slyke DD, Lemish S (1943) The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. Biol Chem 150:231–250
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143(3):1758–1776
Hasanuzzaman M, Nahar K, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H, Fujita M (2017) Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int J Mol Sci 18(1):200
Hasanuzzaman M, Bhuyan MB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9(8):681
Hassanpour H, Khavari-Nejad RA, Niknam V, Najafi F, Razavi K (2013) Penconazole induced changes in photosynthesis, ion acquisition and protein profile of Mentha pulegium L. under drought stress. Physiol Mol Biol Plants 19(4):489–498
Hatam Z, Sabet MS, Malakouti MJ, Mokhtassi-Bidgoli A, Homaee M (2020) Zinc and potassium fertilizer recommendation for cotton seedlings under salinity stress based on gas exchange and chlorophyll fluorescence responses. S Afr J Bot 130:155–164
Hussain RA, Ahmad R, Waraich EA, Nawaz F (2015) Nutrient uptake, water relations, and yield performance lf different wheat cultivars (Triticum aestivum L.) under salinity stress. J Plant Nutr 38(13):2139–2149
Jackson ML (1969) Soil chemical analysis-advanced course. M.L. Jackson, Madison
Kafi M, Nabati J, Ahmadi-Lahijani MJ, Oskoueian A (2021) Silicon compounds and potassium sulfate improve salinity tolerance of potato plants through instigating the defense mechanisms, cell membrane stability, and accumulation of osmolytes. Commun Soil Sci Plant Anal 52(8):843–858
Kaur C, Sharma S, Singla-Pareek SL, Sopory SK (2016) Methylglyoxal detoxification in plants: role of glyoxalase pathway. Indian J Plant Physiol 21(4):377–390
Kaya C, Akram NA, Sürücü A, Ashraf M (2019) Alleviating effect of nitric oxide on oxidative stress and antioxidant defence system in pepper (Capsicum annuum L.) plants exposed to cadmium and lead toxicity applied separately or in combination. Sci Hortic 255:52–60
Kiani R, Arzani A, Mirmohammady Maibody S (2021) Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front Plant Sci 12:646221
Kumar S, Trivedi PK (2018) Glutathione S-transferases: role in combating abiotic stresses including arsenic detoxification in plants. Front Plant Sci 9:751
Lai D, Mao Y, Zhou H, Li F, Wu M, Zhang J, He Z, Cui W, Xie Y (2014) Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Sci 225:117–129
Li J, Shi C, Wang X, Liu C, Ding X, Ma P, Wang X, Jia H (2020) Hydrogen sulfide regulates the activity of antioxidant enzymes through persulfidation and improves the resistance of tomato seedling to copper oxide nanoparticles (CuO NPs)-induced oxidative stress. Plant Physiol Biochem 156:257–266
Lightenthaler H (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu P, Yin L, Wang S, Zhang M, Deng X, Zhang S, Tanaka K (2015) Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ Exp Bot 111:42–51
Luo Z, Li D, Du R, Mou W (2015) Hydrogen sulfide alleviates chilling injury of banana fruit by enhanced antioxidant system and proline content. Sci Hortic 183:144–151
Mahmud JA, Hasanuzzaman M, Khan MIR, Nahar K, Fujita M (2020) Aminobutyric acid pretreatment confers salt stress tolerance in Brassica napus L. by modulating reactive oxygen species metabolism and methylglyoxal detoxification. Plants 9(2):241
Mäkelä PS, Jokinen K, Himanen K (2019) Roles of endogenous glycinebetaine in plant abiotic stress responses. In: Hossain MA, Kumar V, Burritt DJ, Fujita M, Mäkelä PSA (eds) Osmoprotectant-mediated abiotic stress tolerance in plants. Springer, Berlin
Masood A, Shah NA, Zeeshan M, Abraham G (2006) Differential response of antioxidant enzymes to salinity stress in two varieties of Azolla (Azolla pinnata and Azolla filiculoides). Environ Exp Bot 58(1–3):216–222
Mita S, Murano N, Akaike M, Nakamura K (1997) Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for β-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J 11(4):841–851
Moustafa ES, Ali MM, Kamara MM, Awad MF, Hassanin AA, Mansour E (2021) Field screening of wheat advanced lines for salinity tolerance. Agronomy 11(2):281
Muhammad I, Shalmani A, Ali M, Yang Q-H, Ahmad H, Li FB (2021) Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front Plant Sci 11:615942
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651
Nadeem M, Anwar-ul-Haq M, Saqib M, Maqsood M, He Z (2022) Ameliorative effect of silicic acid and silicates on oxidative, osmotic stress, and specific ion toxicity in spring wheat (Triticum aestivum L.) genotypes. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-022-00812-0
Nahar K, Hasanuzzaman M, Alam M, Fujita M (2015a) Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plant. https://doi.org/10.1093/aobpla/plv069
Nahar K, Hasanuzzaman M, Alam M, Fujita M (2015b) Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol Plant 59(4):745–756
Nahar K, Hasanuzzaman M, Fujita M (2016) Roles of osmolytes in plant adaptation to drought and salinity. In: Iqbal N, Nazar R, Khan NA (eds) Osmolytes and plants acclimation to changing environment: Emerging omics technologies. Springer, New Delhi
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Nashef AS, Osuga DT, Feeney RE (1977) Determination of hydrogen sulfide with 5,5′-dithiobis-(2-nitrobenzoic acid), N-ethylmaleimide, and parachloromercuribenzoate. Anal Biochem 79(1):394–405
Omrani S, Arzani A, Esmaeilzadeh Moghaddam M, Mahlooji M (2022) Genetic analysis of salinity tolerance in wheat (Triticum aestivum L.). PLoS ONE 17(3):e0265520
Oney-Birol S (2019) Exogenous L-carnitine promotes plant growth and cell division by mitigating genotoxic damage of salt stress. Sci Rep 9(1):1–12
Pandey S, Fartyal D, Agarwal A, Shukla T, James D, Kaul T, Negi YK, Arora S, Reddy MK (2017) Abiotic stress tolerance in plants: myriad roles of ascorbate peroxidase. Front Plant Sci 8:581. https://doi.org/10.3389/fpls.2017.00581
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22(6):4056–4075. https://doi.org/10.1007/s11356-014-3739-1
Parvin K, Nahar K, Hasanuzzaman M, Bhuyan MB, Mohsin SM, Fujita M (2020) Exogenous vanillic acid enhances salt tolerance of tomato: insight into plant antioxidant defense and glyoxalase systems. Plant Physiol Biochem 150:109–120
Parvin K, Hasanuzzaman M, Mohsin SM, Nahar K, Fujita M (2021) Coumarin improves tomato plant tolerance to salinity by enhancing antioxidant defence, glyoxalase system and ion homeostasis. Plant Biol 23(S1):181–192. https://doi.org/10.1111/plb.13208
Quamruzzaman M, Manik SN, Livermore M, Johnson P, Zhou M, Shabala S (2022) Multidimensional screening and evaluation of morpho-physiological indices for salinity stress tolerance in wheat. J Agron Crop Sci. https://doi.org/10.1111/jac.12587
Rahimi E, Nazari F, Javadi T, Samadi S, da Silva JAT (2021) Potassium-enriched clinoptilolite zeolite mitigates the adverse impacts of salinity stress in perennial ryegrass (Lolium perenne L.) by increasing silicon absorption and improving the K/Na ratio. J Environ Manage 285:112142
Rasheed R, Ashraf MA, Parveen S, Iqbal M, Hussain I (2014) Effect of salt stress on different growth and biochemical attributes in two canola (Brassica napus L.) cultivars. Commun Soil Sci Plant Anal 45(5):669–679
Roychoudhury A, Singh A, Aftab T, Ghosal P, Banik N (2021) Seedling priming with sodium nitroprusside rescues Vigna radiata from salinity stress-induced oxidative damages. J Plant Growth Regul 40(6):2454–2464
Sami F, Yusuf M, Faizan M, Faraz A, Hayat S (2016) Role of sugars under abiotic stress. Plant Physiol Biochem 109:54–61
Sankaranarayanan S, Jamshed M, Kumar A, Skori L, Scandola S, Wang T, Spiegel D, Samuel MA (2017) Glyoxalase goes green: the expanding roles of glyoxalase in plants. Int J Mol Sci 18(4):898
Sarker U, Oba S (2020) The response of salinity stress-induced A tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front Plant Sci 11:559876
Shaheen S, Naseer S, Ashraf M, Akram NA (2013) Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. J Plant Interact 8(1):85–96
Shahid MA, Sarkhosh A, Khan N, Balal RM, Ali S, Rossi L, Gómez C, Mattson N, Nasim W, Garcia-Sanchez F (2020) Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 10(7):938
Shaki F, Maboud HE, Niknam V (2018) Growth enhancement and salt tolerance of Safflower (Carthamus tinctorius L.), by salicylic acid. Curr Plant Biol 13:16–22
Sharma OP, Bhat TK (2009) DPPH antioxidant assay revisited. Food Chem 113(4):1202–1205
Sharma A, Soares C, Sousa B, Martins M, Kumar V, Shahzad B, Sidhu GP, Bali AS, Asgher M, Bhardwaj R (2020) Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: a review on molecular and biochemical aspects. Physiol Plant 168(2):318–344
Sherin G, Aswathi KR, Puthur JT (2022) Photosynthetic functions in plants subjected to stresses are positively influenced by priming. Plant Stress. https://doi.org/10.1016/j.stress.2022.100079
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16(6):13561–13578
Srivastava V, Chowdhary AA, Verma PK, Mehrotra S, Mishra S (2022) Hydrogen sulfide-mediated mitigation and its integrated signaling crosstalk during salinity stress. Physiol Plant 174(1):e13633
Sudhir P, Murthy S (2004) Effects of salt stress on basic processes of photosynthesis. Photosynthetica 42(4):481–486
Taïbi K, Taïbi F, Abderrahim LA, Ennajah A, Belkhodja M, Mulet JM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot 105:306–312
Tanveer K, Gilani S, Hussain Z, Ishaq R, Adeel M, Ilyas N (2020) Effect of salt stress on tomato plant and the role of calcium. J Plant Nutr 43(1):28–35
Tränkner M, Tavakol E, Jákli B (2018) Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol Plant 163(3):414–431
Upadhyaya CP, Venkatesh J, Gururani MA, Asnin L, Sharma K, Ajappala H, Park SW (2011) Transgenic potato overproducing L-ascorbic acid resisted an increase in methylglyoxal under salinity stress via maintaining higher reduced glutathione level and glyoxalase enzyme activity. Biotechnol Lett 33(11):2297–2307
van Rossum MW, Alberda M, van der Plas LH (1997) Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci 130(2):207–216
Van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Annu Rev Plant Biol 71:403–433
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(1):59–66
Wang H, Tang X, Wang H, Shao H-B (2015) Proline accumulation and metabolism-related genes expression profiles in Kosteletzkya virginica seedlings under salt stress. Front Plant Sci 6:792
Wolfe K, Wu X, Liu RH (2003) Antioxidant activity of apple peels. J Agric Food Chem 51(3):609–614
Xiao Y, Wu X, Sun M, Peng F (2020) Hydrogen sulfide alleviates waterlogging-induced damage in peach seedlings via enhancing antioxidative system and inhibiting ethylene synthesis. Front Plant Sci 11:696
Yadav SK, Singla-Pareek SL, Reddy M, Sopory S (2005) Methylglyoxal detoxification by glyoxalase system: a survival strategy during environmental stresses. Physiol Mol Biol Plants 11(1):1
Yang HY, Shi GX, Qiao XQ, Tian XL (2011) Exogenous spermidine and spermine enhance cadmium tolerance of Potamogeton malaianus. Russ J Plant Physiol 58(4):622–628
Yousuf PY, Hakeem KUR, Chandna R, Ahmad P (2012) Role of glutathione reductase in plant abiotic stress. In: Parvaiz Ahmad MNV, Prasad, (eds) Abiotic stress responses in plants. Springer, New York
Zegaoui Z, Planchais S, Cabassa C, Djebbar R, Belbachir OA, Carol P (2017) Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J Plant Physiol 218:26–34
Zhang Z-P, Miao M-M, Wang C-L (2015) Effects of ALA on photosynthesis, antioxidant enzyme activity, and gene expression, and regulation of proline accumulation in tomato seedlings under NaCl stress. J Plant Growth Regul 34(3):637–650
Zhang J, Zhou M, Zhou H, Zhao D, Gotor C, Romero LC, Shen J, Ge Z, Zhang Z, Shen W (2021) Hydrogen sulfide, a signaling molecule in plant stress responses. J Integr Plant Biol 63(1):146–160
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4):555–559
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56(422):3223–3228
Zhou H, Chen Y, Zhai F, Zhang J, Zhang F, Yuan X, Xie Y (2020) Hydrogen sulfide promotes rice drought tolerance via reestablishing redox homeostasis and activation of ABA biosynthesis and signaling. Plant Physiol Biochem 155:213–220
Zhu J, Fan Y, Shabala S, Li C, Lv C, Guo B, Xu R, Zhou M (2020) Understanding mechanisms of salinity tolerance in barley by proteomic and biochemical analysis of near-isogenic lines. Int J Mol Sci 21(4):1516
Zulfiqar F, Akram NA, Ashraf M (2020) Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 251(1):1–17
Acknowledgements
This research work has been financially supported by the Higher Education Commission of Pakistan.
Author information
Authors and Affiliations
Contributions
MAA: contributed to conceptualization, project administration, supervision, and writing—original draft. AH: contributed to formal analysis, software, validation, and writing—review & editing. RR: contributed to data curation, formal analysis, methodology, and writing—original draft. IH: contributed to conceptualization and writing—review & editing. UF: contributed to supervision, methodology, and writing—review & editing. MR: contributed to formal analysis, supervision, software, validation, and writing—review & editing. SA: contributed to project administration and writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest regarding this paper.
Additional information
Handling Editor: Francesca Cardinale.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashraf, M.A., Hafeez, A., Rasheed, R. et al. Evaluation of Physio-Morphological and Biochemical Responses for Salt Tolerance in Wheat (Triticum aestivum L.) Cultivars. J Plant Growth Regul 42, 4402–4422 (2023). https://doi.org/10.1007/s00344-023-10905-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-10905-4